Tuning the surface oxygen concentration of {111} surrounded ceria nanocrystals for enhanced photocatalytic activities†
Adnan
Younis
*,
Dewei
Chu
*,
Yusuf Valentino
Kaneti
and
Sean
Li
School of Materials Science and Engineering, University of New South Wales, Sydney, 2052, NSW, Australia. E-mail: a.younis@unsw.edu.au; d.chu@unsw.edu.au; Fax: +61 (0)2 9385 6565; Tel: +61 (0)2 9385 0763
First published on 16th November 2015
Abstract
For oxide semiconductors, the morphology, particle size and oxygen vacancies are usually considered as key influential parameters for photocatalytic degradation of organic pollutants/dyes. It is widely accepted that cation doping not only modifies their phase and microstructures but also introduces variations in oxygen vacancy concentration. Herein, we report the fabrication of sub-10 nm sized pure and indium doped CeO2 nanocrystals (NCs) via a facile, green hydrothermal method for the investigation of photocatalytic activities. X-ray diffraction and transmission electron microscopy were employed to examine the crystal phase and morphology of the as-prepared nanocrystals. Raman and X-ray photoelectron spectroscopy techniques were implemented to investigate the presence and variations in oxygen vacancy concentration in un-doped and indium doped CeO2 nanocrystals. The photocatalytic activity results revealed that 10 at% doping is the optimal indium doping level to demonstrate superior dye removal efficiency (∼40%) over un-doped and doped CeO2 NCs. Moreover, the 10% In-doped CeO2 nanocrystals expressed excellent cycling stability and superior photocatalytic performance toward other dye pollutants. Finally, on the basis of our findings, a possible photocatalytic mechanism in which indium doping can generate more surface oxygen vacancies in the ceria lattice which delay the electron–hole recombination rates, thus increasing the lifetime of electron–hole separation for enhanced photocatalytic performances was proposed.
Introduction
Many organic pollutants that originate from the textile industry are toxic and non-biodegradable in nature, hence causing serious threats of water contamination and environmental pollution. Semiconductor-based photocatalysts have shown great potential for effective degradation of compounds causing air and water pollution. The process of photocatalysis mainly relies on (1) the generation of charge carriers photonically, (2) efficient transfer of electrons or holes; and (3) their recombination rates to determine the efficiency of photocatalysis. The photocatalytic activity of oxide semiconductors can be tuned by simply regulating the recombination of charge carriers. It is generally believed that the presence of oxygen vacancies on/near the surface of the photocatalyst can inhibit the recombination of electron–hole pairs thus facilitating the photocatalysis process.1,2 Thus, controlling the size, shape, dimensionality, and surface states of oxide semiconductors is crucial for optimizing the catalytic reactivity, selectivity and stability.Until now, the majority of photocatalytic studies have been mainly focused on titanium dioxide (TiO2) with comparatively few reports on other oxide semiconductors. To this end, the quest of a diverse set of high-performance photocatalysts is beneficial for both fundamental and applied photocatalyses. In particular, CeO2 (ceria) is a unique catalyst which can take and release oxygen through reversible shifts between Ce3+ and Ce4+. In fact, the actual importance of oxygen uptake/release is determined not only by the OSC, but also by the rates of the redox cycles. Usually, the oxidation rate of cerium dominates over its reduction rate; therefore various efforts have been made to promote its reducibility by adjusting the nature and density of oxygen vacancies.3
The performance of CeO2 nanoparticles/nanocrystals strongly depends on the morphology and the particle size distribution. Usually, the CeO2 (100) crystal face has the highest surface energy and reactivity when compared to its low-index crystal planes (111) and (110). This difference in surface energies originates from the instability of the top-layer oxygen and is located at the bridging positions between two cerium ions. Therefore, CeO2 morphologies exposed by the (100) face usually demonstrate superior catalytic and oxygen storage capabilities.4,5 Although, the (100) face has superior photocatalytic properties by having higher surface energy, the long term stability of the (100) surface remains a challenge. Therefore, it would be of great interest if the photocatalytic performance of the most stable (111) plane of CeO2 nanocrystals can be improved. On the other hand, the exact surface structures of nano-scaled CeO2 still remain less explored and there are only a few reports on the well-defined (111) CeO2 surface for excellent photocatalytic applications. Doping transitional metal ions into CeO2 has been considered as an effective approach to modify its physical and chemical properties. In particular, indium (In) is considered as an efficient metal to produce a large number of electrons due to vacant d-orbitals and has the ability to hinder photo-generated charges. In3+ also has a comparable ionic radius with Ce3+/4+, so their doping levels in ceria can be easily controlled. Furthermore, the doping of indium can introduce more oxygen vacancies into CeO2, which can act as reaction sites to improve photocatalytic properties. The doping of indium can also narrow the bandgap of CeO2, which is beneficial for visible light photocatalysis. Owing to the aforementioned advantages, it would be of great interest to explore the role of In incorporation into the CeO2 matrix to study the variations in oxygen vacancy concentration and eventually their photocatalytic activities.
Herein, we have been able to prepare high-surface-area, thermally stable and monodisperse nanostructured CeO2 nanocrystals with varying In doping concentrations (0 at%, 5 at%, 10 at% and 15 at%) by self-assembly of individual nanoparticles in a liquid crystal phase. The effect of In doping on the crystal structure, morphologies and oxygen vacancy concentration was investigated. Moreover, we researched the photocatalytic activities of all the products under the irradiation of ultraviolet light. To the best of our knowledge, the present report is a first attempt towards the study of indium (In) doping on the catalytic activities of high surface area occupied CeO2 nanocrystals.
Materials and methods
Synthesis of CeO2 nanocrystals
All chemicals were purchased from Sigma and used without further purification. CeO2 nanocrystals were prepared by using a solvothermal process whose details are reported elsewhere.6–8 In a typical synthesis, 15 ml of 16.7 mmol l−1 cerium(III) nitrate and indium nitrate hydrate aqueous solutions were added into a 50 ml autoclave, and then a 15 ml mixed solution of toluene, oleic acid (OLA, 0.6 ml, OLA/Ce 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added. Subsequently, tert-butylamine (0.15 ml) was added into the autoclave under an ambient atmosphere. The sealed autoclave was heated at 200 °C for 36 h and then cooled to room temperature. The resulting product was isolated by centrifugation and washed three times with ethanol and deionized water and dried at 80 °C for 24 h. The product was calcined in air at 180 °C for 1 h. The preparations of various In-doped CeO2 nanocrystals were carried out using the same procedure with different weight percentage ratios. The solid solutions are denoted as pure CeO2 (CS-0), 5 at% In-doped CeO2 (CS-05), 10 at% In-doped CeO2 (CS-10) and 15 at% In-doped CeO2 (CS-15) respectively.
1) was added. Subsequently, tert-butylamine (0.15 ml) was added into the autoclave under an ambient atmosphere. The sealed autoclave was heated at 200 °C for 36 h and then cooled to room temperature. The resulting product was isolated by centrifugation and washed three times with ethanol and deionized water and dried at 80 °C for 24 h. The product was calcined in air at 180 °C for 1 h. The preparations of various In-doped CeO2 nanocrystals were carried out using the same procedure with different weight percentage ratios. The solid solutions are denoted as pure CeO2 (CS-0), 5 at% In-doped CeO2 (CS-05), 10 at% In-doped CeO2 (CS-10) and 15 at% In-doped CeO2 (CS-15) respectively.
Characterization
The phase composition of the samples was characterized by X-ray powder diffraction (Philips X'pert Multipurpose X-ray Diffraction System (MRD) with Cu Kα). The transmission electron microscopy (TEM) images were obtained using a Philips CM 200 transmission electron microscope operating at 200 kV. The Raman spectra of all the samples in the region of 200–800 cm−1 were recorded using a Renishaw inVia Raman microscope system equipped with a Spectra-Physics model 127 helium–neon laser operating at 35 mW for the 633 nm output as the excitation source. The photoluminescence (PL) spectrum was measured using an RF-5301PC spectrofluorophotometer (Shimadzu, Kyoto, Japan). The X-ray photoelectron spectroscopy (XPS) was performed on an ESCALAB 250Xi spectrometer using a monochromatized Al K alpha X-ray source (hv = 1486.6 eV) with 20 eV pass energy.Photocatalytic degradation of dyes
Two organic dyes Methyl Orange (MO) and Methyl Blue (MB) were used to record the photocatalytic activities of the prepared materials. A 200 ml quartz round bottom flask was placed 15 cm away from the light source containing a mixture of 15 mg of the catalyst and 100 mL of a 15.5 mg l−1 dye solution. First, the reaction mixture was stirred in the dark for 30 min to ensure complete equilibration of the adsorption/desorption of the organic dye by the catalyst. The mixture was then irradiated with 110 W UV light source for 20 min, and 5 mL aliquots were collected, followed by centrifugation to remove the catalyst particles. The supernatant was used to determine the concentration of the residual dye by UV-vis spectroscopy based on the absorbance at 464 nm for MO and 610 nm for MB. This procedure was performed in triplicate for each sample. To study the photodecomposition of the dye at higher temperature, 20 mg of the catalyst was stirred in a 200 mL quartz round-bottom flask containing 100 mL of a 30 mg l−1 MO solution.Results and discussion
Structural analysis
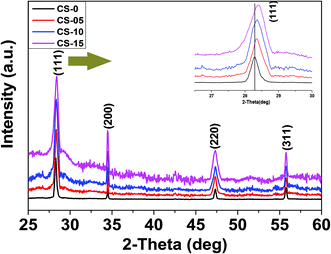
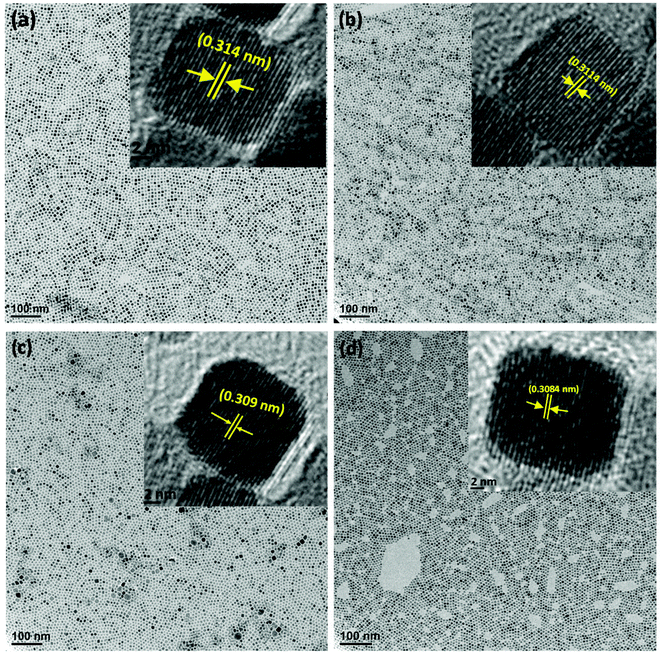
The X-ray diffraction (XRD) patterns of all the synthesized samples (CS-0, CS-05, CS-10 and CS-15) are depicted in Fig. 1. Each of the XRD patterns is indexed as CeO2 with a face-centered cubic (FCC) fluorite structure (JCPDS 34-0394), and no diffraction peaks corresponding to In-related impurity phases were detected. Samples with a higher In doping concentration revealed a shift in the (111) peak towards higher 2θ values. Also, by increasing the In content, the calculated crystallite size and lattice parameter decreased slightly (ESI, Table 1†) which may be attributed to the difference in the ionic radius of Ce3+ (0.115 nm) and In (0.094 nm).Transmission electron microscopy (TEM) was utilized to determine the morphologies of the as-prepared samples. From Fig. 2a–d, it can be seen that the CeO2 nanocrystals are well separated by forming a monodisperse array. All the samples preserved the nano-cubic type morphology with a slight variation in d-spacing with increasing In doping. The lattice fringes in the insets of Fig. 2a–d are clearly visible with a d-spacing of 0.314, 0.3114, 0.309 and 0.3084 nm for CS-0, CS-05, CS-10 and CS-15, respectively. These d-spacings are attributed to the (111) planes of ceria (JCPDS 34-0394) which match well with the XRD results.
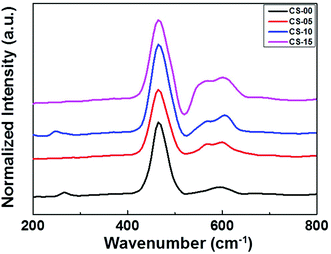
It is generally believed that the interface and the grain boundaries are the active regions in CeO2 based materials for the formation of Ce3+ and oxygen vacancies.9 So, the local atomic structure and grain orientation near the grain boundaries determine the possibilities of defect formation near the grain boundary region. Careful examination of the HRTEM images revealed that the shape of CeO2 nanocrystals transformed from nearly perfect cubic to irregular/de-shaped cubic by increasing the In content which may generate line defects, containing oxygen vacancies as one of the point defects. This is an assumption that defects may be located in these regions. To understand the defect induced changes in the local structure of CeO2 nanocrystals and to characterize oxygen vacancies, Raman spectroscopy was carried out and the corresponding spectra are shown in Fig. 3. Usually bulk ceria has an intense Raman band at 464 cm−1, which is attributed to the Raman active vibrational mode (F2g) of the fluorite-type structure.10 All the samples show a strong band around this region, confirming the fluorite structure in all the un-doped and doped CeO2 samples. Another band at ∼595 cm−1 was found which can be related to the oxygen vacancies and/or point defects due to the presence of Ce3+ in the CeO2 lattice and defects caused by small size effects/point defects.11 By increasing the In concentration, the intensity of this band increases which may be due to the formation of Ce3+ ions in order to accommodate In3+ ions in the Ce host.12 Another band around ∼565 cm−1 was also found whose intensity increases with increasing In doping (CS-05, CS-10 and CS-15) which can be attributed to the extrinsic oxygen vacancies generated by the substitution of tetravalent Ce4+ with trivalent In3+.13 A number of previous studies14,15 have attributed the band gap narrowing of CeO2 as a result of additional states introduced within the band gap of CeO2. These additional states may have originated from the increased concentration of Ce3+ induced by the defect levels generated in the CeO2 lattice and oxygen vacancy defect states. On the basis of Raman spectroscopy, it can be concluded that indium doping can introduce more defects/oxygen vacancies into the CeO2 lattice. Therefore, it is suspected that the introduction of oxygen vacancies or their increased density at the surface of individual CeO2 nanocrystals can inhibit the recombination of electron–hole pairs to improve their performance as efficient photocatalysts.
The oxidation/valence state of the elements and the surface composition of CS-00 and CS-10 samples have been analysed by the XPS technique. The Ce 3d core-level X-ray photoelectron spectra consisting of peaks corresponding to the Ce3+ and Ce4+ states have been Gaussian fitted as shown in Fig. 4a and b. The percentage composition of Ce3+ and Ce4+ has been calculated using the following equations:
| Ce3+ = v0 + v′ + u0 + u′ | (1) |
| Ce4+ = v + v′′ + v′′′ + u + u′′ + u′′′ | (2) |
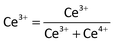 | (3) |
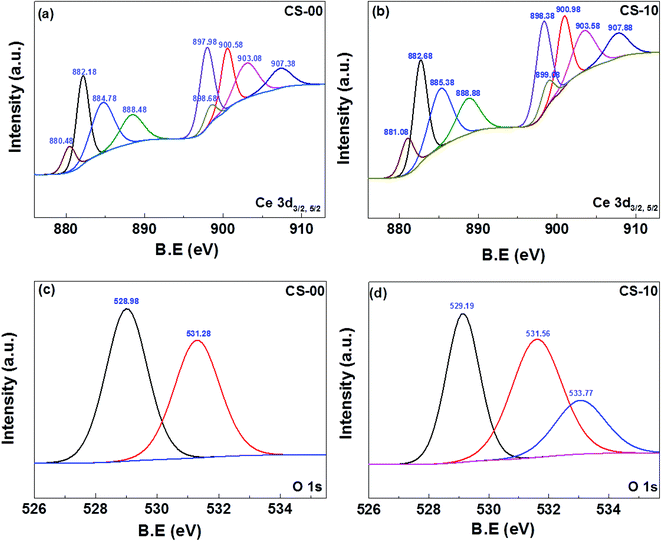 | ||
| Fig. 4 XPS core-level spectrum of (a, b) Ce 3d of CeO2 and In-doped CeO2. (c) O 1s of CeO2. (d) O 1s of In-doped CeO2 nanocrystals. | ||
To further strengthen the aforementioned arguments about oxygen vacancies, a series of surface depth profiling XPS experiments were conducted and the concentration of Ce3+ on the surface and at various depths below the surface was recorded. For these measurements, all the samples (CS-0, CS-05, CS-10 and CS-15) were dispersed in ethanol followed by drop-coating on silicon wafers to form their subsequent thin films. To conduct the surface profiling experiments, the thin film samples were etched by using an argon (Ar) ion beam with an accelerating voltage of 3 keV. The etching duration was varied from 0 seconds to 90 seconds, 180 seconds and finally to 270 seconds. The variations in the Ce3+/Ce4+ ratio at various sample depths extracted from XPS data were calculated and are plotted in Fig. S1.†
From Fig. S1,† it is clear that the concentration of Ce3+/Ce4+ (oxygen vacancies) on the surface of all the samples was much higher as compared to their bulk counterpart. A significant decrease in the concentration of oxygen vacancies was observed by increasing the etching time. This trend provides a strong evidence to support the presence of a higher concentration of oxygen vacancies on the surface of CeO2 samples. Furthermore, their concentration is relatively much lower in the bulk part of the sample. The Ce3+/Ce4+ ratio on the surface of all the samples (with no etching) is plotted separately in the inset of Fig. S1† for better understanding. The sample CS-15 shows the highest concentration of oxygen vacancies as compared to other samples and its Ce3+/Ce4+ ratio is almost double that of the CS-0 sample. Therefore on the basis of these results, it can be concluded that the incorporation of indium into the CeO2 lattice can promote the formation of more oxygen vacancies on the surface of CeO2.
Photocatalytic performance
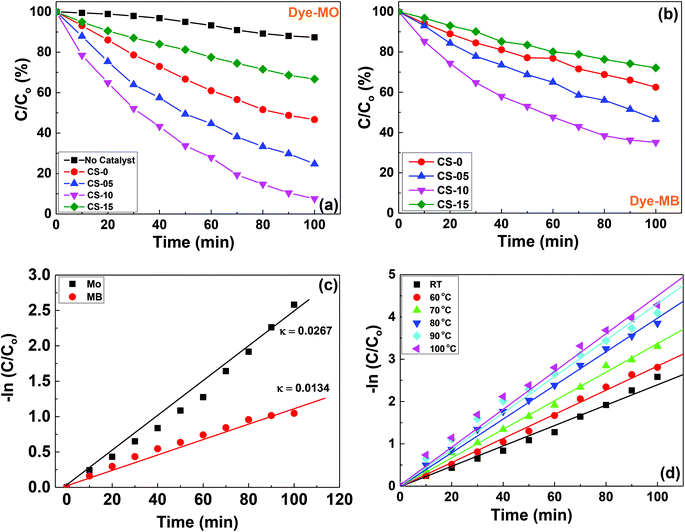
The photocatalytic properties of the as-prepared CeO2 nanocrystals (CS-0, CS-05, CS-10 and CS-15) were evaluated in terms of photodegradation of anionic (Methyl Orange, MO) and cationic (Methyl Blue, MB) dyes. 2.5 mg of each sample was used as the photocatalyst. In this study, the pH of the dye solution was maintained at 6, which is closer to the pH of the dye wastewater. The photocatalytic degradation efficiencies of the anionic dye (MO) in a quartz reactor over 100 min having samples (CS-0, CS-05, CS-10 and CS-15) are shown in Fig. 5a. It can be seen that the samples with different In doping concentrations exhibit higher photocatalytic activities compared to pure ceria. The CS-10 sample demonstrated the highest photocatalytic activity by expressing ∼50% higher degradation compared to pure CS-0. However, a declining trend in the photocatalytic efficiency was also observed with higher amounts of oxygen vacancies at room temperature. In other words, when the doping concentration in CeO2 nanocrystals increases up to 10% (CS-05 and CS-10), a pronounced dye degradation efficiency was observed as compared to un-doped CeO2 nanocrystals (CS-0). By further increasing the doping level to 15% (CS-15), a decrease in activity was observed.It is a known fact that the electron–hole pair separation is closely related to the availability of oxygen vacancies in the catalyst. The In doping in CeO2 nanocrystals can induce/generate more oxygen vacancies (as confirmed from Raman spectroscopy), so the probability of the cations sitting in close proximity to each other will increase. Therefore, all the existing mobile oxygen vacancies come together to form deep traps and as a consequence, small micro-domains may be generated due to the ordering/re-arrangement of the isolated cations and oxygen vacancies within the lattice.18,19 Although the increased concentration of deep traps favours a fast electron–hole recombination rate, however, beyond a certain level, their presence can suppress the photocatalytic efficiency13 (as shown in Fig. 5a for the case of CS-15). The poor photocatalytic performance of sample CS-15 may also be due to the presence of high In doping which may act as charge recombination centres resulting in the decrease of the photocatalytic activity. Therefore, the achievement of an optimum level of oxygen vacancies by incorporating In into the Ce lattice is mandatory to achieve an enhanced photocatalytic performance. Therefore, an optimum level of oxygen vacancies by incorporating In into the Ce lattice is mandatory to achieve better photocatalytic performance. On the basis of the present findings, 10% indium doping in the ceria lattice is the optimum level, which provides the optimum number of oxygen vacancies for achieving the highest photocatalytic activity.
The effectiveness of CeO2 nanocrystals (CS-0, CS-05, CS-10 and CS-15) as a test model substrate in determining the photocatalytic degradation efficiencies of the cationic dye (MB) was also tested and the results are shown in Fig. 5b. Interestingly, the dye degradation pattern of MB remains the same as for MO and the catalyst CS-10 showed the highest photocatalytic activity among all the catalysts. However, the overall catalytic efficiency for all the catalysts was lower compared to the degradation of anionic dyes. The surface state of the CeO2 could depend on the pH of the medium. If the pH of the solution is greater than the isoelectric point of ceria (6.4–6.5),20 the surface becomes negatively charged and when it is lower than the pH of the solution, the surface becomes positively charged. Also, if the doped material has a higher isoelectric point (indium oxide isoelectric point is (8.5–8.7)21) than the pH value of the dye, the surface is assumed to be positively charged. Therefore, the anionic dyes have a greater affinity toward the catalyst, which may be a potential reason for the higher photodegradation efficiency of the CeO2 based nanocrystals toward anionic dyes.
Based on these results, it can be concluded that CeO2 nanocrystals are a good catalyst towards the photodecomposition of both anionic and cationic dyes, but anionic dyes have a greater affinity toward the catalyst, resulting in a much higher photodegradation.
The kinetics of MO and MB decolourisation at room temperature are presented in Fig. 5c, and are found to follow pseudo-first order reaction. The decomposition rates of MO and MB over CS-10 samples can be calculated as 0.00267 min−1 and 0.0134 min−1, respectively, signifying that the photodegradation process of MO is much faster than that of MB. It is commonly believed that at high temperatures, the oxygen vacancies may become mobile and the probability to form deep trap sites is very low. Therefore, the degradation of MO with CS-10 was carried out at elevated temperatures as shown in Fig. 5d. It can be clearly observed that at high temperatures, the catalyst, CS-10 degraded the dye faster than at room temperature. The enhanced photocatalytic activity at high temperatures can also be attributed to the enhanced oxygen ion mobility and possible lattice (phonon) scattering effects. At high temperatures, the ions/atoms start to vibrate strongly thereby increasing the scattering probability of electrons/holes within the lattice, hence leading to the recombination of photo-generated carriers.
By exposing ceria nanocrystals to UV radiation, electrons are excited from the O2p valence band to an empty or partially empty conduction band (Ce4f), forming the electron–hole pair (Ce4+(e−) and O2−(h+) pair).14 The photo-generated holes get trapped by oxygen ions, whereas electrons were locally surrounded by Ce ions. The photo-generated electrons reduce adsorbed electron acceptors (e.g., O2) by forming superoxide radicals, and the holes oxidize organic substances adsorbed on the catalyst or react with water forming hydroxyl radicals, which can also be involved in the decomposition of the organic substrates.22 For elevated temperatures, the effect of increased lattice oxygen ion mobility surpasses the lattice vibration effect which is beneficial for the photo-generated electron and hole pairs, and as a consequence, superior photocatalytic activity was observed.
To gain more insights into the thermo-photocatalytic performance of CS-10, the catalytic activities of all the samples in the absence of light (but at high temperature 100 °C) were investigated. The thermocatalytic performances of all the samples were very low as compared to the results obtained under UV irradiation as shown in Fig. S2.†
The enhanced photocatalytic activity at high temperatures (under UV light) can be attributed to the enhanced oxygen ion mobility and possible lattice (phonon) scattering effects. At high temperatures, the ions/atoms start to vibrate strongly thereby increasing the scattering probability of electrons/holes within the lattice. As a result, the scattering of electrons/holes increases within the lattice leading to the recombination of photogenerated carriers.13,23 This is further supported by a previous report on Y-doped CeO2 where the increase in the photocatalytic activity at elevated temperatures was also attributed to the presence of higher amounts of oxygen vacancies and the enhanced oxygen ion mobility.13 Another study conducted by Li et al.23 had also indicated an increase in the separation of charge carriers at higher temperatures. At elevated temperatures, ions/atoms tend to vibrate strongly, leading to more phonons which subsequently increase the scattering probabilities of ions/electrons with phonons. As, In-doped CeO2 samples exhibit a higher amount of oxygen vacancies (based on the Raman and XPS data), the oxygen ion mobility in these samples was also improved by increasing temperature.24 The improved mobility of the lattice oxygen ions rather than lattice vibrations leads to the improved separation of photogenerated electrons and holes, which ultimately enhances the photocatalytic activity of the In-doped samples. Furthermore, the rate constants for the photocatalytic activity of CS-10 at room temperature and 100 °C for all three different conditions (in the absence of light, with visible light radiation and UV light radiation) were calculated and are plotted in Fig. S3.†
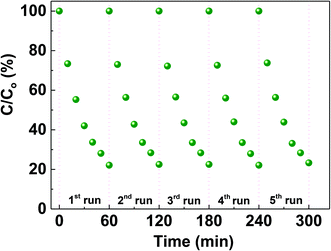
To assess the efficiency of the CS-10 samples for repetitive use, the degradation of MO was carried out repeatedly for five cycles as shown in Fig. 6. It is clearly observed that the nature of degradation remains unaltered and the inherent efficiency of CS-10 persisted (less than 5% decrease from its initial activity during the photodegradation process) without self-degradation.
Mechanism
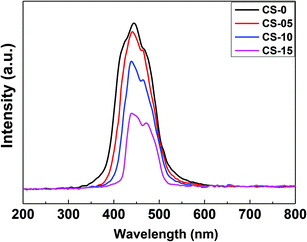
To observe the probability of electron–hole pair recombination in the catalyst, the photoluminescence (PL) spectra of CeO2 nanocrystals were recorded as shown in Fig. 7. The PL spectrum of pure CeO2 nanocrystals shows a strong and sharp blue emission centred at 420–435 nm, and a rather weak shoulder at ca. 465–470 nm. The appearance of the blue emission is considered to be associated with the defect levels localized between the O 2p and the Ce 4f bands. Since the PL emission spectra originated from the recombination of excited electrons and holes, a higher PL intensity would imply a higher recombination rate of electron–hole pairs under light irradiation.25 The CS-0 sample exhibited the highest PL intensity, indicating that it could provide the fastest recombination rate of electrons and holes. However, the intensity of the PL emission of the CeO2 nanocrystals gradually decreased with increasing In content, suggesting that the In doping could delay the recombination rate of the photo-generated charge carriers.In order to calculate the band-gap energies of the as-prepared samples, the Schuster–Kubelka–Munk absorption function was plotted against the photon energy (hv), according to the equation:25,26
| ahv1/2 = A(hv − Eg) | (4) |
| Ecb(CeO2) = χ(CeO2) − EC − 0.5Eg(CeO2) | (5) |
| Evb(CeO2) = Eg(CeO2) − Ecb(CeO2) | (6) |
In this study, the amount of In dopant greatly influences the photocatalytic activity of the produced CeO2 nanocrystals. The photocatalytic activity of the sample increases with increasing In content before it reaches a maximum value of 10 at% In doping. Then, the activity obviously decreases with increasing In content above 10 at%. This is due to a small amount of In3+, which can act as an electron acceptor (from In3+ to In2+) and/or a hole donor (from In3+ to In4+) to facilitate charge carrier localization and thus, prolonged separation by trapping at energy levels close to the conduction or valence bands, respectively,25,28 as schematically illustrated in Fig. 8.
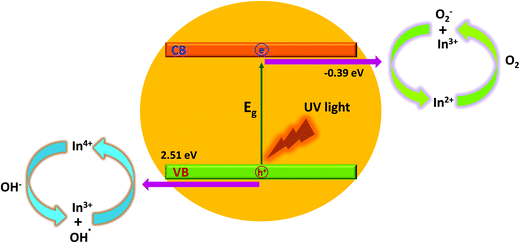 | ||
| Fig. 8 Schematic diagram of the degradation mechanisms for the MO dye with indium doped CeO2 nanocrystals under ultraviolet-visible light irradiation. | ||
Based on crystal field theory, In2+ and In4+ are relatively unstable compared to In3+, and this likely leads to the transfer of the trapped charge carriers from In2+ or In4+ to the adsorbed O2 and surface hydroxyl (–OH) to regenerate In3+.28,29 The newly produced active species (such as ˙OH and O2−) will initiate the subsequent photocatalytic reactions.
It is important to note that the photocatalytic activity of In-doped CeO2 nanocrystals is strongly dependent on the concentration of the In dopant since the In3+ ions can serve not only as a mediator of interfacial charge transfer but also as a recombination centre.26 In this study, the optimal concentration of the In doping is 10 at%. Above this concentration, In3+ ions steadily become recombination centres and the photocatalytic activity steadily decreases through quantum tunnelling.28–30 Another possible reason for the enhancement in the photocatalytic activity of CS-10 is that In doping also induces oxygen vacancies (as confirmed by the XPS and Raman spectroscopy results), which are highly advantageous for the separation of photo-induced charges. The increase in the oxygen vacancy concentration can also generate an increment in the surface hydroxyl amount, which is beneficial for photocatalytic reactions31 to improve waste water treatment efficiencies.
Summary
In summary, CeO2 nanocrystals with different indium doping concentrations have been successfully fabricated for waste water treatment (photodegradation of organic dyes) by a facile and green hydrothermal approach. Under UV light irradiation, In doping can significantly increase the photocatalytic performance of CeO2 nanocrystals with the best efficiency obtained for 10 at% indium doping. The doped ceria nanocrystals showed higher photocatalytic activities compared to un-doped ceria because of lower band gap energies and higher concentrations of oxygen vacancies on the surface (as confirmed by depth profiling XPS measurements). The PL results confirmed that indium doping could be effective in delaying electron–hole recombination, thereby increasing the lifetime of the electron–hole separation. The presence of In3+ may also contribute to the In3+/In2+ and In4+/In3+ additional levels in CeO2, which leads to a decrease in band gap energies. Moreover, the improved photocatalytic efficiency of an organic dye at high temperatures was also realized by significant enhancement in oxygen ion conductivity. The present work is expected to provide a thrust on the utilization of un-tested and unconventional dopants such as indium for tailoring multifunctional properties of rare earth oxide materials.Acknowledgements
The authors would like to acknowledge the financial support from the Australian Research Council Projects of DP140104373, DP150103006.References
- J. Wang, P. Liu, X. Fu, Z. Li, W. Han and X. Wang, Relationship Between Oxygen Defects And The Photocatalytic Property Of Zno Nanocrystals In Nafion Membranes, Langmuir, 2009, 25, 1218–1223 CrossRef CAS PubMed.
- X. Q. Gong, A. Selloni, M. Batzill and U. Diebold, Steps On Anatase TiO2 (101), Nat. Mater., 2006, 5, 665–670 CrossRef CAS PubMed.
- X. Liu, K. Zhou, L. Wang, B. Wang and Y. Li, Oxygen Vacancy Clusters Promoting Reducibility and Activity of Ceria Nanorods, J. Am. Chem. Soc., 2009, 31, 3140–3141 CrossRef PubMed.
- H. X. Mai, L. D. Sun, Y. W. Zhang, R. Si, W. Feng, H. P. Zhang, H. C. Liu and C. H. Yan, Shape-Selective Synthesis and Oxygen Storage Behavior of Ceria Nanopolyhedra, Nanorods, and Nanocubes, J. Phys. Chem. B, 2005, 109, 24380–24385 CrossRef CAS PubMed.
- J. Zhang, H. Kumagai, K. Yamamura, S. Ohara, S. Takami, A. Morikawa, H. Shinjoh, K. Kaneko, T. Adschiri and A. Suda, Extra-Low-Temperature Oxygen Storage Capacity of CeO2 Nanocrystals with Cubic Facets, Nano Lett., 2011, 11, 361–364 CrossRef CAS PubMed.
- F. Dang, K. Kato, H. Imai, S. Wada, H. Haneda and M. Kuwabara, Characteristics of Multilayered Nanostructures of CeO2 Nanocrystals Self-Assembled on an Enlarged Liquid–Gas Interface, Cryst. Growth Des., 2011, 11, 4129–4134 CAS.
- A. Younis, D. Chu, I. Mihail and S. Li, Interface-Engineered Resistive Switching: CeO2 Nanocubes as High-Performance Memory Cells, ACS Appl. Mater. Interfaces, 2013, 5, 9429–9434 CAS.
- A. Younis, D. Chu, C. M. Li, T. Das, S. Sehar, M. Manefield and S. Li, Interface Thermodynamic State-Induced High-Performance Memristors, Langmuir, 2014, 30, 1183–1189 CrossRef CAS PubMed.
- B. Choudhury, P. Chetri and A. Choudhury, Oxygen defects and formation of Ce3+ affecting the photocatalytic performance of CeO2 nanoparticles, RSC Adv., 2014, 4, 4663–4671 RSC.
- Z. Wu, M. Li, J. Howe, H. M. Meyer and S. H. Overbury, Probing Defect Sites on CeO2 Nanocrystals with Well-Defined Surface Planes by Raman Spectroscopy and O2 Adsorption, Langmuir, 2010, 26, 16595–16606 CrossRef CAS PubMed.
- M. Sarkar, M. R. Rajkumar, S. Tripathy and S. Balakumar, Effect Of Gd Dopant Concentration On The Defect Engineering In Ceria Nanostructures, Mater. Res. Bull., 2012, 47, 4340–4346 CrossRef.
- G. Yuji, T. Kazuyuki, O. Takahisa and O. Y. M. Shinya, Synthesis Of Y2O3 -Doped CeO2 Nanocrystals And Their Surface Modification, J. Phys.: Conf. Ser., 2009, 165, 012041 CrossRef.
- A. D. Liyanage, S. D. Perera, K. Tan, Y. Chabal and K. J. Balkus, Synthesis, Characterization, and Photocatalytic Activity of Y-Doped CeO2 Nanorods, ACS Catal., 2014, 4, 577–584 CrossRef CAS.
- P. Patsalas, S. Logothetidis, L. Sygellou and S. Kennou, Structure-Dependent Electronic Properties Of Nanocrystalline Cerium Oxide Films, Phys. Rev. B: Condens. Matter, 2003, 68, 035104 CrossRef.
- S. A. Ansari, M. M. Khan, M. O. Ansari, S. Kalathil, J. Lee and M. H. Cho, Band gap engineering of CeO2 nanostructure using an electrochemically active biofilm for visible light applications, RSC Adv., 2014, 4, 16782 RSC.
- C. Santra, S. Rahman, S. Bojja, O. O. James, D. Sen, S. Maity, A. K. Mohanty, S. Mazumder and B. Chowdhury, Barium, Calcium And Magnesium Doped Mesoporous Ceria Supported Gold Nanoparticle For Benzyl Alcohol Oxidation Using Molecular O2, Catal. Sci. Technol., 2013, 3, 360–370 CAS.
- J. C. Dupin, D. Gonbeau, P. Vinatier and A. Levasseur, Systematic XPS Studies Of Metal Oxides, Hydroxides And Peroxides, Phys. Chem. Chem. Phys., 2000, 2, 1319–1324 RSC.
- T. Mori, J. Drennan, J. H. Lee, J. G. Li and T. Ikegami, Oxide Ionic Conductivity And Microstructures Of Sm- Or La-Doped Ceo2-Based Systems, Solid State Ionics, 2002, 154–155, 461–466 CrossRef CAS.
- T. Mori, Y. Wang, J. Drennan, G. Auchterlonie, J. G. Li and T. Ikegami, Influence Of Particle Morphology On Nanostructural Feature And Conducting Property In Sm-Doped Ceo2 Sintered Body, Solid State Ionics, 2004, 175, 641–649 CrossRef CAS.
- K. S. Lin and S. Chowdhury, Synthesis, Characterization, and Application of 1-D Cerium Oxide Nanomaterials: A Review, Int. J. Mol. Sci., 2010, 11, 3226 CrossRef CAS PubMed.
- M. Kosmulski and J. Coll, Pristine Points of Zero Charge of Gallium and Indium Oxides, J. Colloid Interface Sci., 2001, 238, 225–227 CrossRef CAS PubMed.
- S. Malato, J. Blanco, A. Vidal and C. Richter, Photocatalysis With Solar Energy At A Pilot-Plant Scale: An Overview, Appl. Catal., B, 2002, 37, 1–15 CrossRef CAS.
- Y. Li, Q. Sun, M. Kong, W. Shi, J. Huang, J. Tang and X. Zhao, Coupling Oxygen Ion Conduction to Photocatalysis in Mesoporous Nanorod-like Ceria Significantly Improves Photocatalytic Efficiency, J. Phys. Chem. C, 2011, 115, 14050–14057 CAS.
- S. Kim, J. S. Lee, C. Mitterbauer, Q. M. Ramasse, M. C. Sarahan, N. D. Browning and H. J. Park, Anomalous Electrical Conductivity of Nanosheaves of CeO2, Chem. Mater., 2009, 21, 1182–1186 CrossRef CAS.
- D. Channei, B. Inceesungvorn, N. Wetchakun, S. Ukritnukun, A. Nattestad, J. Cen and S. Phanichphant, Photocatalytic Degradation Of Methyl Orange By CeO2 And Fe-Doped CeO2 Films Under Visible Light Irradiation, Sci. Rep., 2014, 4, 5757, DOI:10.1038/srep05757.
- N. Boonprakob, N. Wetchakun, S. Phanichphant, D. Waxler, P. Sherrell, A. Nattestad, J. Chen and B. Inceesungvorn, Enhanced Visible-Light Photocatalytic Activity Of G-C3N4/Tio2 Films, J. Colloid Interface Sci., 2014, 417, 402–409 CrossRef CAS PubMed.
- Y. Xu and M. A. A. Schoonen, The Absolute Energy Positions Of Conduction And Valence Bands Of Selected Semiconducting Minerals, Am. Mineral., 2000, 85, 543–556 CrossRef CAS.
- J. Yu, Q. Xiang and M. Zhou, Preparation, Characterization And Visible-Light-Driven Photocatalytic Activity Of Fe-Doped Titania Nanorods And First-Principles Study For Electronic Structures, Appl. Catal., B, 2009, 90, 595–602 CrossRef CAS.
- W. Choi, A. Termin and M. R. Hoffmann, The Role Of Metal Ion Dopants In Quantum-Sized Tio2: Correlation Between Photoreactivity And Charge Carrier Recombination Dynamics, J. Phys. Chem., 1994, 98, 13669–13679 CrossRef.
- M. Zhou, J. Yu and B. Cheng, Effects Of Fe-Doping On The Photocatalytic Activity Of Mesoporous Tio2 Powders Prepared By An Ultrasonic Method, J. Hazard. Mater., 2006, 137, 1838–1847 CrossRef CAS PubMed.
- L. Jing, B. Xin, F. Yuan, L. Xue, B. Wang and H. Fu, Effects of Surface Oxygen Vacancies on Photophysical and Photochemical Processes of Zn-Doped TiO2 Nanoparticles and Their Relationships, J. Phys. Chem. B, 2006, 110, 17860–17865 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nr06588g |
| This journal is © The Royal Society of Chemistry 2016 |