MoV2O8 nanostructures: controlled synthesis and lithium storage mechanism†
Zhigang
Yin
,
Ying
Xiao
,
Xia
Wang
,
Wei
Wang
,
Di
Zhao
and
Minhua
Cao
*
Key Laboratory of Cluster Science, Ministry of Education of China, Beijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials Department of Chemistry, Beijing Institute of Technology, Beijing 100081, P. R. China. E-mail: caomh@bit.edu.cn; Tel: +86-10-68918648
First published on 23rd November 2015
Abstract
A facile two-step strategy involving a solvothermal method and a subsequent calcining treatment was successfully developed for the preparation of MoV2O8 nanorods in the absence of any surfactants. Acetic acid was chosen as the solvent to provide an acidic environment. The as-synthesized MoV2O8 nanorods were evaluated as an anode material in lithium ion batteries, which showed excellent lithium storage performance in terms of its specific capacity, rate performance, and cycling stability. It could deliver a specific capacity of over 1325 mA h g−1 after 50 cycles at 0.2 A g−1, which is much higher than that of bulk MoV2O8 (617 mA h g−1). When the cell was cycled at a current density as high as 10.0 A g−1, it still maintained a high specific capacity of around 570 mA h g−1. The phase transformation, intercalation–deintercalation and partial redox processes are responsible for the lithium storage mechanism of MoV2O8 based on ex situ X-ray diffraction, X-ray photo electron spectroscopy and transmission electron microscopy studies, highlighting a new lithium storage mechanism for ternary metal oxides.
1. Introduction
Recently, tremendous research effort has been triggered in investigating power sources with high specific energy density and long cycle life to meet the current demand in the expanding market of portable electronic products.1–4 Lithium ion batteries (LIBs) are the most rapidly growing high-energy storage device due to their high volumetric and gravimetric capacity, enabling smaller and lighter battery packs.5–7 Commercially available LIBs generally use layered transition metal oxides such as LiCoO2 or Li2Mn2O4 as cathode materials and graphitized carbon as anode materials.8,9 As is well known, a graphite-based anode possesses a favorably low potential for lithium ion insertion/extraction, but its low theoretical capacity (372 mA h g−1) and rate capacity make it difficult to meet the requirements for the large-scale applications of LIBs in the near future.10 Hence, developing high-performance anode materials for LIBs is highly desirable.Although a lot of progress has been made in developing new anode materials for LIBs, these anode materials still suffer from severe capacity fading, low capacity and high cost, which have seriously hindered the development of LIBs. Among all of the reported materials, transition metal oxides have attracted much attention due to their low cost, environmental benignity, and higher theoretical capacity, resulting in them having been widely exploited as high-capacity anode materials for LIBs.11,12 In particular, mixed metal oxides can synergistically improve the electrochemical properties including the reversible capacity, and mechanical stability.13–15 Recently, several research groups have reported the synthesis of double metal oxide nano-/micro-meter materials and their lithium storage properties. It should be noted that their results were encouraging. For example, Christie et al. reported the fabrication of interconnected CoMoO4 submicrometer particles, which could deliver a reversible capacity of 990 mA h g−1 at a current density of 100 mA g−1, with 100% capacity retention between the 5th and 50th cycles.16 AB2O4 (A = Fe, Co, Ni, Mn, B = Fe, Co, Ni, Zn, Mn, Fe, etc. A ≠ B) spinel compounds have emerged as promising anode materials and have been investigated extensively for use in next-generation LIBs.17–25
Furthermore, a considerable number of vanadates, such as Co3V2O8, Zn3V2O8 and RVO4 (R = In, Fe, Cu), have been investigated for lithium insertion materials for many years. For instance, Yang et al. have prepared multilayered Co3V2O8 nanosheets, which showed high reversible capacity (1114 mA h g−1 retained after 100 cycles) and excellent rate performance (361 mA h g−1 at a high current density of 10 A g−1) for lithium storage.26 Gan et al. synthesized hexagonal Zn3V2O8 nanosheets, which displayed an excellent lithium storage performance for LIBs with a reversible capacity as high as 1103 mA h g−1 at a current density of 200 mA g−1.27 InVO4 was tested by Reddy et al.28 with negligible capacity fading between the 2nd and 50th cycles and a high capacity of 1241 mA h g−1 at the end of the 20th cycle, close to its theoretical capacity. Yang et al. investigated Fe2VO4 as an anode material, and the reversible specific capacity with 1 C was about 225 mA h g−1, which was close to its theoretical specific capacity.29 Zhang et al. synthesized hollow Cu3V2O7(OH)2·2H2O nanostructures, which were used as a cathode material in primary LIBs. Electrochemical measurements showed that the sponge-like hollow structure exhibited a higher discharge capacity of 513 mA h g−1 at the current density of 100 mA g−1.30
MoV2O8, a promising host for metal ion intercalation, has outstanding advantages due to its layered structure (the inset in Fig. 1b), which can improve its ability to intercalate ions in a wide range of sites. However, the complex nature of molybdates makes them difficult to synthesize, especially their nanostructures. Until now, there have been few reports on MoV2O8 nanostructures and their application as anode materials for LIBs. Therefore, it was of great significance to develop a simple yet effective method to prepare nanoscale MoV2O8. Herein we demonstrated a mild, facile route towards the synthesis of MoV2O8 nanorods through a solvothermal method followed by a subsequent calcining treatment in air. A Mo–V-based precursor was first prepared using the solvothermal process, and then the precursor was subjected to a calcining treatment to form the final product. The resultant MoV2O8 nanorods exhibited high capacity, excellent cycling stability and perfect rate capability as an anode material for LIBs. The lithium storage mechanism of the MoV2O8 nanorods was also investigated in detail, which was different from those of other vanadates. To the best of our knowledge, this is the first report on the lithium storage of MoV2O8 nanorods. It is believed that the MoV2O8 nanorods could be a promising candidate as an anode material because of their high capacity and rate performance.
2. Experimental
Synthesis of MoV2O8 nanorods
All chemical regents used in this work were analytical grade and were used without further purification. Typically, 0.3 mmol of molybdenyl acetylacetonate [MoO2(acac)2] was added to 30 mL of acetic acid at room temperature to form a transparent solution. Meanwhile, 0.6 mmol of vanadyl acetylacetonate [VO(acac)2] was added to another 30 mL of acetic acid. After being ultrasonically treated for several minutes, the resultant two solutions were mixed in one beaker under magnetic stirring followed by a heating process. Finally the formed clear solution was transferred into an 80 mL Teflon-lined stainless steel autoclave. The autoclave was sealed tightly and maintained at 200 °C for 36 h. Afterwards, the autoclave was cooled to room temperature naturally. The resultant precipitate was collected through centrifugation, washed with water and ethanol several times, and dried using the freeze drying method. Finally, the product was selectively calcined at different temperatures for 3 h under atmospheric air to obtain the final products.For comparison, bulk MoV2O8 was also prepared (Fig. S1†). In a typical process, 0.143 mmol of (NH4)6Mo7O24·4H2O, 2.0 mmol of NH4VO3 and 6.0 mmol of urea were added to 10 mL of deionized water at room temperature with magnetic stirring to form a transparent solution, which then was transferred to a 50 mL centrifuge tube and was rapidly frozen. The freeze drying was carried out for 24 h. Finally the powder was calcined at 600 °C in air for 12 h to yield the final product.
Characterizations
Powder X-ray diffraction (XRD) measurements were collected from 5° to 80° (2θ) with a scanning step of 5° min−1 using Cu-Kα (λ = 1.54178 Å) incident radiation (40 kV, 50 mA) on a Shimadzu XRD-6000 instrument. The general size and morphology of the products were characterized using field-emission scanning electron microscopy (FE-SEM, Hitachi S-4800). Transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED) were carried out on a H-8100 transmission electron microscope operating at a 200 kV accelerating voltage. X-ray photo electron spectra (XPS) were recorded on an ESCALAB 250 spectrometer (Perkin-Elmer) to characterize the surface composition. The Brunauer–Emmett–Teller (BET) surface area was estimated using a Belsorp-max surface area detecting instrument through N2 physisorption at 77 K. The thermogravimetry (TG) analysis was performed from 25 to 700 °C in air with a heating rate of 10 °C min−1 using a DTG-60AH instrument.Electrochemical measurements
The electrochemical measurements of the as-prepared MoV2O8 sample were recorded by using standard CR2025 coin cells at room temperature. To prepare the working electrode, the as-prepared active material (80%), carbon black (10%) and carboxymethylcellulose sodium (10%) were mixed and ground in a mortar. Ultrapure water was used as the solvent and the resultant slurry was then uniformly pasted on a Cu foil current collector. The coated electrode was dried at 120 °C for 24 h under vacuum before being assembled into a coin cell in an argon-filled glove box. The mass loading of the pure active materials is about 0.8–1.04 mg. A 1 M solution of LiPF6 in ethylene carbonate (EC)/dimethyl carbonate (DMC)/diethyl carbonate (DEC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, vol%) was used as the non-aqueous electrolyte. A pure lithium metal slice was used as both the counter and the reference electrodes. Galvanostatic charge–discharge tests of the assembled coin cell were performed on a LAND CT2001A with a cutoff voltage of 0.005–3.00 V versus Li+/Li. Cyclic voltammetry (CV) was performed using an electrochemical workstation (CHI660D) with a scanning rate of 0.5 mV s−1 in the frequency range of 100 kHz–0.005 Hz at room temperature.
1, vol%) was used as the non-aqueous electrolyte. A pure lithium metal slice was used as both the counter and the reference electrodes. Galvanostatic charge–discharge tests of the assembled coin cell were performed on a LAND CT2001A with a cutoff voltage of 0.005–3.00 V versus Li+/Li. Cyclic voltammetry (CV) was performed using an electrochemical workstation (CHI660D) with a scanning rate of 0.5 mV s−1 in the frequency range of 100 kHz–0.005 Hz at room temperature.
3. Results and discussion
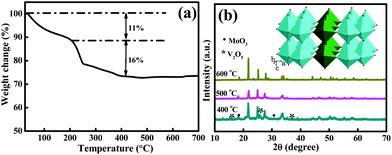
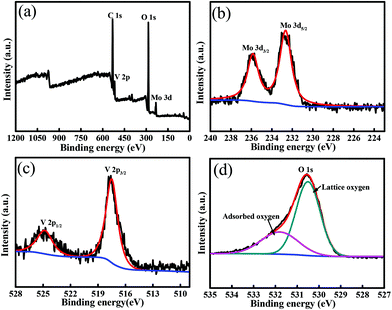
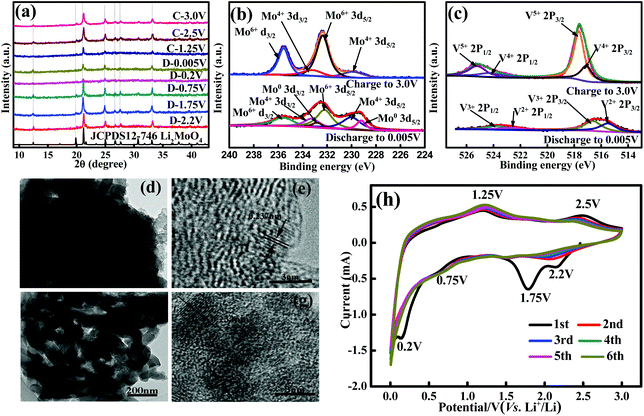
As described in the Experimental section, the synthesis of the MoV2O8 nanorods involved two steps, a solvothermal process followed by a calcining treatment. The first step resulted in the formation of a Mo–V-based precursor, which was then calcined to form the target product via the second step. The calcination temperature was determined through TG analysis of the Mo–V-based precursor, which was performed from room temperature to 700 °C at a ramp rate of 10 °C min−1 in air. As shown in Fig. 1a, the TG curve exhibits two main regions over the temperature ranges 25–200 °C and 200–450 °C, which involve a total weight loss of 27%. The weight loss of about 11% between 25 °C and 200 °C is ascribed to the release of adsorbed water and coordinated water,27 while between 200 and 450 °C, there is a quick weight loss of 16%, which usually is regarded as the decomposition process of the precursor. Over this temperature range, the precursor is decomposed into a MoV2O8 phase, which will be proven through XRD measurements below. From 450 °C, no weight loss is observed, indicating that 450 °C is the lowest temperature for the formation of a pure MoV2O8 phase. Following the TG results, we tried different calcining temperatures. Fig. S2a† shows an XRD pattern of the precursor, which clearly indicates its amorphous structure. When the precursor is calcined at 400 °C, the resultant sample is a mixture of MoV2O8, MoO3 and V2O5 (Fig. 2b). Interestingly, if the calcining temperature is increased to 500 °C or 600 °C, a pure MoV2O8 phase is obtained (JCPDS Card no. 74-0050). This result is consistent with the above TG analysis.The XPS analysis was further used to elucidate the chemical composition and oxidation state of the resultant sample and here we use the sample obtained at 500 °C as an example. As shown in Fig. 2a, the survey spectrum further confirms that this sample consists of the elements Mo, V, and O. The high-resolution Mo 3d spectrum (Fig. 2b) shows two obvious signals at ca. 232.6 and 235.8 eV, which can be attributed to the Mo 3d5/2 and Mo 3d3/2 of Mo6+, in good agreement with previous reports.31,32Fig. 2c displays the high-resolution V 2p spectrum, which shows two peaks with binding energies at 524.8, and 517.5 eV, which are associated with V 2p1/2 and V 2p3/2, respectively.26,33 The high-resolution O 1s spectrum (Fig. 2d) can be resolved into two peaks at 529.5 and 531.6 eV. The former is ascribed to the lattice-oxygen and the latter is related to the adsorbed-oxygen.34,35 Combining these results with that from XRD, it can be deduced that a pure MoV2O8 phase can be synthesized using the current approach.
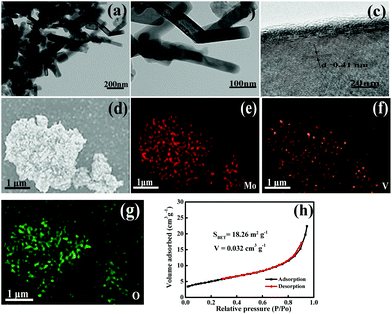
Fig. 3 presents representative FE-SEM images of the resultant products. It can be clearly seen from Fig. 3a and b that the sample obtained at 500 °C is composed of relatively uniform nanorods and that some nanorods have twinned together to form nanorod bundles. The nanorods have lengths ranging from 150 to 300 nm, and diameters in the range of 20–30 nm. Although the MoV2O8 nanorods are obtained via the calcination treatment of the precursor at 500 °C, they almost maintains the morphology of the Mo–V-based precursor (Fig. S2a and b†). However, when the calcination temperature was increased to 600 °C, MoV2O8 nanobelts were formed instead of nanorods, as is clearly disclosed by Fig. 3c and d. The nanobelts typically have an average length of ca. 10 μm and thickness of ca. 100 nm. The morphology evolvement may be due to further growth of the nanorods at higher calcination temperature. From the above results, we can see that the calcined samples retain the one-dimensional structure of the precursor. In our experiments, acetic acid was used as the solvent to prepare the precursor. Patzke et al. reported previously that weak acetic acid can induce the formation of small-size MoO3-based nanorods.36,37 In fact, we also tried other solvents, such as ethylene glycol and N,N-dimethylformamide. As shown in Fig. S4,† the former results in the formation of larger irregular particles and the latter, similar to acetic acid, also leads to the formation of nanorods but with a much larger average diameter.
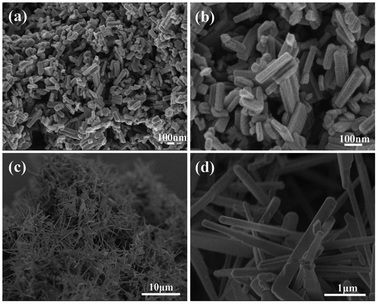 | ||
| Fig. 3 (a and b) SEM images of MoV2O8 nanorods obtained at 500 °C. (c and d) SEM images of MoV2O8 nanobelts obtained at 600 °C. | ||
The microstructure of the as-prepared MoV2O8 nanorods was further investigated through TEM measurements. The low-magnification TEM images show the nanorod morphology (Fig. 4a and b), which is consistent with the above SEM observations. A high-resolution TEM (HRTEM) image of an individual nanorod is shown in Fig. 4c, in which the lattice fringes are clearly visible. The distance between neighboring fringes is found to be 0.41 nm, which corresponds to the (201) planes of orthorhombic MoV2O8.38 Surface-scanning element mapping clearly reveals that these elements are homogeneously distributed (Fig. 4d–g). In addition, the MoV2O8 nanorods have a large specific surface area of 18.26 m2 g−1, which was determined using N2 adsorption–desorption isotherms (Fig. 4h). The value is far larger than those of the MoV2O8 nanobelts (2.48 m2 g−1) and bulk MoV2O8 (1.65 m2 g−1) (Fig. S3†). The larger specific surface area of the MoV2O8 nanorods may result from their small particle size, while the small particle size can dramatically reduce the diffusion time of lithium ions and improve the lithium insertion–extraction kinetics because of a shorter Li+ diffusion pathway. Therefore, this feature of the MoV2O8 nanorods may be expected to be beneficial for their electrochemical performance.
As discussed above, although a higher calcining temperature was used, the MoV2O8 nanorods still maintained the morphology of the Mo–V-based precursor, which is very common in many reported works. In view of this fact, we performed time-dependent experiments to investigate the formation process of the precursor nanorods. As shown in Fig. S5a,† the sample obtained with a solvothermal reaction time as short as 3 h, was composed of small-size nanoparticles. When prolonging the reaction time to 12 h, it is clear that besides nanoparticles, some irregular nanorods started to form (Fig. S5b†). With further extending the reaction time to 24 h, more nanorods were formed (Fig. S5c†) and when the reaction time was as long as 36 h, uniform nanorods were obtained (Fig. S5d†). Based on the experimental results, it can be deduced that the formation process of the nanorods may involve the following several steps. In the initial stage, smaller nanoparticles were generated in the reaction system via a homogeneous nucleation process. In the following step, nanoparticles with larger sizes are able to form at the expense of smaller ones through Ostwald ripening. In the ripening process stage, larger particles can be directed to grow into nanorods with a uniform structure.39–41
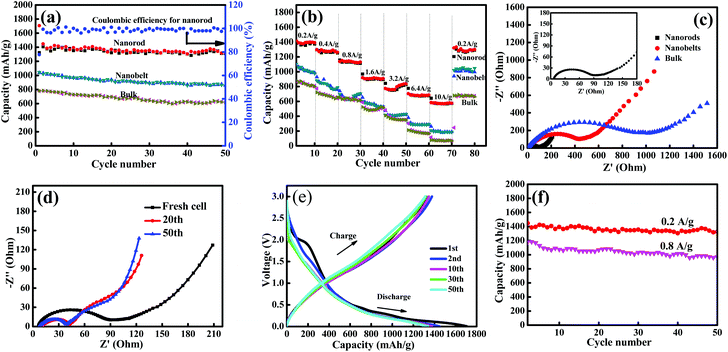
The Li-storage properties of the resultant samples as anode materials were evaluated with Li foil as a counter electrode. Fig. 5a shows the cycling performance of the MoV2O8 nanorods, MoV2O8 nanobelts and bulk MoV2O8 at a current density of 0.2 A g−1 in the voltage range of 0.005–3 V vs. Li+/Li. It can be clearly seen that the cycling stability of the MoV2O8 nanorod electrode is much higher than those of the nanobelts and the bulk. The initial discharge capacity is as high as 1706 mA h g−1. In the subsequent charge process, a charge capacity of 1327 mA h g−1 is achieved, leading to a Coulombic efficiency of 77%. The irreversible capacity loss in the first discharge–charge process can be attributed to the inevitable decomposition of electrolyte and the formation of a solid electrolyte interphase (SEI) film on the surface of the electrode, which is commonly observed in metal oxide-based anode materials.42 However, for the MoV2O8 nanobelt and bulk MoV2O8 electrodes, their initial discharge capacities are 1046 and 780 mA h g−1, respectively, which are far lower than that of the MoV2O8 nanorods. After 50 cycles, the MoV2O8 nanorods still delivered a high reversible capacity of 1325 m Ah g−1, which is 77.7% of the initial discharge capacity. The delivered capacity of the MoV2O8 nanorods is much higher than those of the MoO3 nanowire arrays (∼630 mA h g−1) and V2O5 nanorods (∼460 mA h g−1) at the same current density.43,44 This capacity is also higher than other anode materials, such as ZnO (∼600 mA h g−1), Fe2O3 (∼900 mA h g−1), CuO (∼600 mA h g−1) and carbonaceous materials (theoretical capacity of 372 mA h g−1).45–47 Considering that the MoV2O8 nanorods display high capacity and good cycling stability, the material could be a good candidate as an anode material for LIBs. As for the nanobelts and the bulk, their reversible capacities decrease to 854 mA h g−1 and 617 mA h g−1 after 50 cycles but with slightly higher Coulombic efficiencies of 81.6% and 79.1%, respectively. According to previous reports,48–50 the anion (i.e. oxygen) plays an important role during the lithiation reaction and it can act as a redox center, thus leading to possible V–“O–Li” and Mo–“O–Li” interactions. Compared to the MoV2O8 nanobelts and the bulk, the MoV2O8 nanorods have a smaller size, and thus they have many more redox centers to interact with Li than the nanobelts and the bulk. These redox centers can result in an additional enhancement in the specific capacity. In addition, the average Coulombic efficiency of the MoV2O8 nanorods is calculated to be above 98%.
Besides the high capacity and good cycling stability, the MoV2O8 nanorod electrode also exhibited an excellent rate performance, as shown in Fig. 5b. The electrode was further tested at step-wise current densities from 0.2 to 10 A g−1 and then reversed to 0.2 A g−1. As the current densities were increased from 0.2 to 0.4, 0.8, 1.6, 3.2, 6.4, and 10.0 A g−1, the reversible capacities of the MoV2O8 nanorods were 1360, 1270, 1130, 900, 800, 680 and 570 mA h g−1, respectively, all of which are higher than those of the nanobelts and the bulk. The reversible capacity (570 mA h g−1) obtained at a current density as high as 10.0 A g−1 is even higher than the theoretical capacity of graphite. Furthermore, the rate capability of the as-prepared MoV2O8 nanorods is also comparable to those of other reported anode materials.51–53 The excellent rate performance of the MoV2O8 nanorods can be attributed to their small-sized one-dimensional nanorod structure, which can result in fast kinetics for redox reactions involving lithium ions and electron diffusion to/from the electrolyte/particle interface. Meanwhile, they can also accommodate the local volume change during repeated Li+ insertion/extraction, and thus improve the cycling stability. As is well known, high rate capability materials are highly desirable for LIBs, which can greatly reduce the charge/discharge time in practical applications. The fast kinetics of the redox reactions of the MoV2O8 nanorods were further confirmed through electrochemical impedance spectroscopy (EIS) measurements, which were performed from 100 KHz to 0.01 Hz. As shown in Fig. 5c, the high frequency region of the semicircle represents the SEI resistance (RSEI) and contact resistance (Rf), and the high-medium frequency region represents the charge transfer resistance (Rct) on the electrode/electrolyte interface, whereas the inclined line at the low frequency after the semicircle corresponds to the diffusion barrier layer impedance (ZT) and semi-infinite diffusion impedance (Zw), respectively.54–57 Obviously, the semicircle diameter of the MoV2O8 nanorods in the high-medium frequency is far smaller than those of the nanobelts and the bulk, implying their fast kinetics process. Furthermore, the EIS spectra of the MoV2O8 nanorods after different numbers of cycles at the discharge state at a current density of 0.2 A g−1 were also performed (Fig. 5d). Different from most reports the cell impedance increases with the increase in the number of cycles. The diameter of the semicircle in the high-middle frequency range decreases obviously with the increasing number of cycles, indicating lower contact and charge-transfer impedances, which is consistent with some reports.58–60 The possible reason for this result may be a gradual stabilization of the SEI layer during cycling.
The galvanostatic charge–discharge curves for the 1st, 2nd, 10th, 30th, and 50th cycles of the MoV2O8 nanorods at a current density of 0.2 A g−1 are shown in Fig. 5e. The 1st discharge and charge capacities of the MoV2O8 nanorods are as high as 1706 and 1327 mA h g−1, respectively. After 50 cycles, 77.7% of the initial discharge capacity and 99.8% of the initial charge capacity can be retained. Furthermore, the initial discharge curve shows a pronounced plateau at about 3.0–1.5 V, and the capacity fading is so rapid that only 200 mA h g−1 of the specific capacity can be used. However most of the capacities could be used at a voltage range of 0.7–0.005 V. The first plateau at a higher voltage can be assigned to a Faradic capacitance of the material,61 and the capacity lower than 0.5 V is associated with a gradual lithium storage process. This phenomenon is very common in most metal oxides with higher theoretical specific capacity. Recently, Lian et al.62 demonstrated that high-rate pre-lithiation and reactivation is an effective strategy to restructure active materials and optimize the SEI layer ex situ to obtain high-performance electrodes. By pre-lithiation and pre-reactivation, electrode materials with an optimized microstructure can be packaged into battery cells for practical applications with exceptional rate performance and cyclic stability as well as high energy density. Therefore, in future work, we will use this prospective strategy to design other high-performance electrodes.
Moreover, the cycling performance of the MoV2O8 nanorod electrode with a current density of 0.8 A g−1 was also tested (Fig. 5f). It is obvious from the results that even at higher current density, the electrode still exhibits good cycling stability and the capacity fading is only 18% after 50 cycles. The long cycling performance was also performed at the current density of 0.8 A g−1. As shown in Fig. S6,† an excellent cycling stability with a highly stable discharge capacity of about 887 mA h g−1 after 200 cycles, was observed. To investigate the structural stability of the MoV2O8 nanorods after cycling, SEM analysis was performed (Fig. S7†). The SEM image of the MoV2O8 nanorod electrode after 50 cycles at a current density of 0.2 A g−1 shows that the morphology of MoV2O8 was well preserved after the Li+ insertion/extraction processes. Certainly, some residual organic electrolyte or related species are also observed on the electrode surface, which can form a protective film on the surface. This result further verifies that the small-sized one-dimensional nanorods can accommodate the local volume change during repeated Li+ insertion/extraction.
4. Electrochemical reaction mechanism
To gain insight into the lithium storage mechanism of the MoV2O8 nanorods, we first performed ex situ XRD analyses during the lithiation/delithiation processes. Fig. S8† shows ex situ XRD patterns of the MoV2O8 nanorod electrode with the specified discharge and charge voltages during the 10th cycle between 0.005 and 3 V, which exhibit notable differences under different charge and discharge voltages. When the discharge voltage is 2.2 V, the electrode material has changed from MoV2O8 to Li2MoO4. However, from the enlarged XRD patterns (Fig. 6a), it can be observed that the main peaks corresponding to the Li2MoO4 phase shift to lower angles decreasing the discharge voltage, and return to their initial scattering angles during the increase of the charge voltage.To further clarify the electrochemical processes during Li intercalation, XPS measurements were also performed at different stages. For the electrode discharged to 0.005 V (Fig. 6b) at 0.2 A g−1 (after 10 cycles), two strong peaks, corresponding to Mo 3d5/2 and Mo 3d3/2, can be deconvoluted into three components. Besides Mo6+ (235.8 and 232.6 eV), other weak peaks with binding energies of 233.3 eV (Mo5/2), 229.8 eV (Mo3/2) and 232.5 eV (Mo5/2), 228.9 eV (Mo3/2) can be assigned to Mo4+ and metallic Mo,32,63 respectively, indicating that Mo exists partially in a low oxidation state after the insertion of Li+. The peak at 55.2 eV in the Li 1s XPS pattern indicates the formation of Li2O (Fig. S9†).64 On the basis of the above discussions, it can be concluded that Li2MoO4 can partially undergo a redox reaction and then decompose into Mo and Li2O during the discharge process. However, the characteristic peaks of metal Mo and Li2O were not detected through XRD, which can be attributed to the amorphous nature of a small amount of metallic Mo and Li2O.65,66 When the electrode is charged to 3.0 V, low oxidation state Mo is partially re-oxidized to a high oxidation state again during the subsequent delithiation process. Furthermore, the high-resolution V 2p spectrum at the end of the first discharge state confirms the existence of two components (Fig. 6c). The peaks with the binding energies of 516.6 eV and 523.8 eV can be assigned to V3+, while the other peaks with binding energies of 515.5 eV and 522.7 eV can be indexed to V2+. This result implies that V5+ has been reduced into V3+ and V2+ during the discharge process. However, when the same electrode is charged to 3.0 V, V3+ and V2+ are not fully converted to V5+, as shown in Fig. 6c.26,67–70 Based on the XRD and XPS results, we deduce that the electrochemical reaction mechanism of the MoV2O8 nanorods as an anode in LIBs should involve three processes, a phase transformation, a Li intercalation–deintercalation reaction and a partial redox reaction.
In order to get more in-depth information on the electrochemical performance of the MoV2O8 nanorods, the phase and microstructure evolution of the MoV2O8 nanorod electrode at the 10th discharged–charged state were also investigated using TEM. Fig. 6d presents a low-magnification TEM image of the lithiated electrode (discharged to 0.005 V), which clearly shows the rod-like morphology of the active material. The HRTEM image (Fig. 6e) shows that the interplanar spacing of 0.237 nm is larger than that of the (122) planes of Li2MoO4 (0.23 nm),71 which can be ascribed to the Li intercalation into Li2MoO4. Besides, as the electrode is charged to 3.0 V, the morphology of the nanorods can also be observed (Fig. 6f). The HRTEM image in Fig. 6g shows that the interplanar spacing of 0.233 nm is smaller than the interplanar spacing of 0.237 nm, but is larger than that of the pure Li2MoO4 phase. The TEM result further verifies the Li intercalation–deintercalation mechanism. Due to the amorphous characteristic of metallic Mo, LiV2O5 and Li1+xV2O5, the related XRD and TEM parameters cannot be measured. This phenomenon of amorphization during the intercalation has also been reported previously in vanadates.72
CV is a powerful technique for studying redox couples and structural transformations for both cathode and anode materials during the charge–discharge process. CV curves of the MoV2O8 nanorods were carried out at a scan rate of 0.3 mV s−1 in the voltage window of 0.005–3.0 V vs. Li/Li+. As shown in Fig. 6h, it can be clearly seen that four cathodic peaks and two anodic peaks in the first cycle are observed at 0.20, 0.75, 1.75, 2.20, and 1.25, 2.50 V, respectively. On the basis of ex situ XRD, TEM, and XPS studies, it can be concluded that the first-discharge reaction is a phase transformation process, forming a mixture of amorphous “LiV2O5” and an electrochemically active “Li2MoO4” phase (Table 1, eqn (1)).73 The peaks at 1.75 and 0.2 V can be assigned to the irreversible formation of an SEI layer and these peaks disappear in the following cycles. The peak at 0.75 V can be attributed to the consecutive intercalation of lithium ions to form lithium-rich Li1+xV2O5 (Table 1, eqn (3)). The peak at 1.25 V is caused by the Li deintercalation from lithium-rich Li1+xV2O5 to LiV2O5 (Table 1, eqn (4)).26,27,68,69 The peak at 2.2 V is responsible for the Li intercalation into Li2MoO4 causing the formation of partially lithium-rich Li2+yMoO4 (including metallic Mo) (Table 1, eqn (2)). The peak at 2.5 V is associated with Li deintercalation from partially lithium-rich Li2+yMoO4 to Li2MoO4 (Table 1, eqn (5)).74,75
| Number | Reaction equations | Voltage |
|---|---|---|
| 1 | MoV2O8 → Li2MoO4 + LiV2O5 | |
| 2 | Li2MoO4 + yLi+ + ye− → Li2+yMoO4 | Discharge to 2.2 V |
| 3 | LiV2O5 + xLi+ + xe− → Li1+xV2O5 | Discharge to 0.75 V |
| 4 | Li1+xV2O5 → LiV2O5 + xLi+ + xe− | Charge to 1.25 V |
| 5 | Li2+yMoO4 → Li2MoO4 + yLi+ + ye− | Charge to 2.5 V |
5. Conclusions
In summary, we have reported the successful preparation of MoV2O8 nanorods via a simple solvothermal method followed by a calcining treatment in air at 500 °C for 3 h. The nanorods have lengths of about 150–300 nm and thicknesses of about 20–30 nm. The resultant MoV2O8 nanorods were first evaluated as an anode material for LIBs, and excellent lithium storage performance was achieved. At a current density of 0.2 A g−1, the MoV2O8 nanorod electrode could deliver a specific capacity of over 1325 mA h g−1 after 50 cycles, which was much higher than those of the nanobelts and bulk. When the current density was increased to 0.8 A g−1, the specific capacity of 969 mA h g−1 could be retained after 50 cycles. Even when cycled at 5 and 10 A g−1, comparable capacities of 680 and 570 mA h g−1 could still be achieved, indicating a superior rate capability. Using ex situ XRD, XPS and TEM measurements, one plausible mechanism has been proposed, which involves phase transformation, intercalation–deintercalation and partial redox processes. We believed that the findings in the current work represent a significant step forward in the development of high-performance LIBs for large-scale energy storage systems.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 21471016 and 21271023) and the 111 Project (B07012).References
- Z. Yang, J. Zhang, M. C. W. Kintner-Meyer, X. Lu, D. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 111, 3577–3613 CrossRef CAS PubMed.
- B. Scrosati, J. Hassoun and Y. K. Sun, Energy Environ. Sci., 2011, 4, 3287–3295 Search PubMed.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2009, 22, 587–603 CrossRef.
- H. Li, Z. X. Wang, L. Q. Chen and X. J. Huang, Adv. Mater., 2009, 21, 4593–4607 CrossRef CAS.
- N. Naoki and Y. Gleb, Part. Part. Syst. Charact., 2013, 1–20 Search PubMed.
- B. Y. Sharma, N. Sharma and G. V. S. Rao, Adv. Funct. Mater., 2007, 17, 2855–2861 CrossRef.
- M. V. Reddy, B. L. W. Wen, K. P. Loh and B. V. R. Chowdari, ACS Appl. Mater. Interfaces, 2013, 5, 7777–7785 Search PubMed.
- M. V. Reddy, G. V. Rao and B. V. R. Chowdari, Chem. Rev., 2013, 113, 5364–5457 CrossRef CAS PubMed.
- B. L. Ellis, K. T. Lee and L. F. Nazar, Chem. Mater., 2010, 22, 691–714 CrossRef CAS.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- X. L. Xiao, X. F. Liu and H. Zhao, Adv. Mater., 2012, 24, 5762–5766 CrossRef CAS PubMed.
- S. Ko, J. I. Lee and H. S. Yang, Adv. Mater., 2012, 24, 4451–4456 CrossRef CAS PubMed.
- L. Yu, L. Zhang, H. B. Wu, G. Q. Zhang and X. W. Lou, Energy Environ. Sci., 2013, 6, 2664–2671 Search PubMed.
- G. Z. Yang, H. W. Song, H. Cui and C. X. Wang, Nano Energy, 2014, 5, 9–19 CrossRef CAS.
- S. X. Jin and C. X. Wang, Nano Energy, 2014, 7, 63–71 CrossRef CAS.
- T. Christie, M. V. Cherian, C. H. Sow and B. V. R. Chowdari, ACS Appl. Mater. Interfaces, 2013, 5, 918–923 Search PubMed.
- Y. L. Xiao, J. T. Zai, L. Q. Tao, B. Li, Q. Y. Han, C. Yu and X. F. Qian, Phys. Chem. Chem. Phys., 2013, 15, 3939–3945 RSC.
- J. Bai, X. G. Li, G. Z. Liu, Y. T. Qian and S. L. Xiong, Adv. Funct. Mater., 2014, 24, 3012–3020 CrossRef CAS.
- G. Q. Zhang, L. Yu, H. B. Wu, H. E. Hoster and X. W. Lou, Adv. Mater., 2012, 24, 4609–4613 CrossRef CAS PubMed.
- C. T. Cherian, J. Sundaramurthy, M. V. Reddy, P. S. Kumar, K. Mani, D. Pliszka, C. H. Sow, S. Ramakrishna and B. V. R. Chowdari, ACS Appl. Mater. Interfaces, 2013, 5, 9957–9963 Search PubMed.
- C. Z. Yuan, J. Y. Li, L. R. Hou, X. G. Zhang, L. F. Shen and X. W. Lou, Adv. Funct. Mater., 2012, 22, 4592–4597 CrossRef CAS.
- J. F. Li, S. L. Xiong, Y. R. Liu, Z. C. Ju and Y. T. Qian, ACS Appl. Mater. Interfaces, 2013, 5, 981–988 Search PubMed.
- S. K. Kim, H. W. Lee, P. Muralidharan, D. H. Seo, W. S. Yoon, D. K. Kim and K. Kang, Nano Res., 2011, 4, 505–510 CrossRef CAS.
- L. Zhou, H. B. Wu, T. Zhu and X. W. Lou, J. Mater. Chem., 2012, 22, 827–829 RSC.
- J. Zhao, F. Q. Wang, P. P. Su, M. R. Li, J. Chen, Q. H. Yang and C. Li, J. Mater. Chem., 2012, 22, 13328–13333 RSC.
- G. Z. Yang, H. Cui, G. W. Yang and C. X. Wang, ACS Nano, 2014, 8, 4474–4487 CrossRef CAS PubMed.
- L. H. Gan, D. R. Deng, Y. J. Zhang, G. Li, X. Y. Wang, L. Jiang and C. R. Wang, J. Mater. Chem. A, 2014, 2, 2461–2466 RSC.
- M. V. Reddy, B. L. W. Wen, K. P. Loh and B. V. R. Chowdari, ACS Appl. Mater. Interfaces, 2013, 5, 7777–7785 Search PubMed.
- T. Yang, D. G. Xia, Z. L. Wang and Y. A. Chen, Mater. Lett., 2009, 63, 5–7 CrossRef CAS.
- S. Y. Zhang and L. J. Ci, Micro Nano Lett., 2012, 7, 1101–1104 Search PubMed.
- X. Y. Zhao, M. H. Cao and C. W. Hu, Mater. Res. Bull., 2013, 48, 2289–2295 CrossRef CAS.
- H. C. Zeng, W. Ng, K. L. H. Cheong, F. Xie and R. Xu, J. Phys. Chem. B, 2001, 105, 7178–7181 CrossRef CAS.
- J. M. Song, Y. Z. Lin, H. B. Yao, F. J. Fan, X. G. Li and S. H. Yu, ACS Nano, 2009, 3, 653–660 CrossRef CAS PubMed.
- J. F. Marco, J. R. Gancedo, M. Gracia, J. L. Gautier, E. I. Ríos and F. J. J. Berry, Solid State Chem., 2000, 153, 74–81 CrossRef CAS.
- Y. E. Roginskaya, O. V. Morozova, E. N. Lubnin, Y. E. Ulitina, G. V. Lopukhova and S. Trasatti, Langmuir, 1997, 13, 4621–4627 CrossRef CAS.
- A. Michailovski, J. D. Grunwaldt, A. Baiker, R. Kiebach, W. Bensch and G. R. Patzke, Angew. Chem., Int. Ed., 2005, 44, 5643–5647 CrossRef CAS PubMed.
- G. R. Patzke, A. Michailovski, F. Krumeich, R. Nesper, J. D. Grunwaldt and A. Baiker, Chem. Mater., 2004, 16, 1126–1134 CrossRef CAS.
- M. Shahida, J. L. Liu, Z. Ali, I. Shakir and M. F. Warsi, J. Power Sources, 2013, 230, 277–281 CrossRef.
- Y. G. Sun, B. Mayers, T. Herricks and Y. N. Xia, Nano Lett., 2003, 7, 956–960 Search PubMed.
- H. E. Wang, Z. G. Lu, D. Qian, Y. J. Li and W. Zhang, Nanotechnology, 2007, 18, 1–5 Search PubMed.
- S. Mahesh, A. Gopal, R. Thirumalai and A. Ajayaghosh, J. Am. Chem. Soc., 2012, 134, 7227–7230 CrossRef CAS PubMed.
- W. J. Kwak, K. C. Lau, C. D. Shin, K. Amine, L. A. Curtiss and Y. K. Sun, ACS Nano, 2015, 9, 4129–4137 CrossRef CAS PubMed.
- P. Meduri, E. Clark, J. H. Kim, E. Dayalan, G. U. Sumanasekera and M. K. Sunkara, Nano Lett., 2012, 12, 1784–1788 CrossRef CAS PubMed.
- A.M. Glushenkov, M. F. Hassan, V. I. Stukachev, Z. P. Guo, H. K. Liu, G. G. Kuvshin-ov and Y. Chen, J. Solid State Electrochem., 2010, 14, 1841–1846 CrossRef CAS.
- Q. S. Xie, X. Q. Zhang, X. B. Wu, H. Y. Wu, X. Liu, G. H. Yue, Y. Yang and D. L. Peng, Electrochim. Acta, 2014, 125, 659–665 CrossRef CAS.
- J. X. Zhu, Z. Y. Yin, D. Yang, T. Sun, H. Yu, H. E. Hoster, H. H. Hng, H. Zhang and Q. Y. Yan, Energy Environ. Sci., 2013, 6, 987–993 Search PubMed.
- B. Wang, X. L. Wu, C. Y. Shu, Y. G. Guo and C. R. Wang, J. Mater. Chem., 2010, 20, 10661–10664 RSC.
- S. Denis, R. Dedryvere, E. Baudrin, S. Laruelle, M. Touboul, J. OlivierFourcade, J. C. Jumas and J. M. Tarascon, Chem. Mater., 2000, 12, 3733–3739 CrossRef CAS.
- F. Leroux, G. R. Goward, W. P. Power and L. F. Nazar, Electrochem. Solid-State Lett., 1998, 1, 255–258 CrossRef CAS.
- D. H. Sim, X. H. Rui, J. Chen, H. T. Tan, T. M. Lim, R. Yazami, H. H. Hng and Q. Y. Yan, RSC Adv., 2012, 2, 3630–3633 RSC.
- J. Bai, X. G. Li, G. Z. Liu, Y. T. Qian and S. L. Xiong, Adv. Funct. Mater., 2014, 24, 3012–3020 CrossRef CAS.
- W. P. Kang, Y. B. Tang, W. Y. Li, X. Yang, H. T. Xue, Q. D. Yang and C. S. Lee, Nanoscale, 2015, 7, 225–231 RSC.
- Y. Zhao, Y. Huang, Q. F. Wang, K. Wang, M. Zong, L. Wang, W. G. Zhan and X. Sun, RSC Adv., 2013, 3, 14480–14485 RSC.
- X. K. Huang, S. M. Cui, J. B. Chang, P. B. Hallac, C. R. Fell, Y. T. Luo, B. Metz, J. W. Jiang, P. T. Hurley and J. H. Chen, Angew. Chem., Int. Ed., 2015, 54, 1490–1493 CrossRef CAS PubMed.
- J. F. Li, S. L. Xiong, Y. R. Liu, Z. C. Ju and Y. T. Qian, ACS Appl. Mater. Interfaces, 2013, 5, 981–988 Search PubMed.
- J. Y. Jang, Y. W. Lee, Y. J. Kim, J. Lee, S. M. Lee, K. T. Lee and N. S. Choi, J. Mater. Chem. A, 2015, 3, 8332–8338 RSC.
- J. S. Cho, Y. J. Hong and Y. C. Kang, ACS Nano, 2015, 9, 4026–4035 CrossRef CAS PubMed.
- Y. Liu, C. H. Mi, L. H. Su and X. G. Zhang, Electrochim. Acta, 2008, 53, 2507–2513 CrossRef CAS.
- Y. Liu and X. G. Zhang, Electrochim. Acta, 2009, 54, 4180–4185 CrossRef CAS.
- D. Su, H. Kim, W. Kim and G. Wang, Chem. – Eur. J., 2012, 18, 8224–8229 CrossRef CAS PubMed.
- E. J. Yoo, J. Kim and I. Honma, Nano Lett., 2008, 8, 2277–2282 CrossRef CAS PubMed.
- H. T. Sun, G. Q. Xin, T. Hu, M. P. Yu, D. L. Shao, X. Sun and J. Lian, Nat. Commun., 2014, 5, 4526 CAS.
- R. H. Wang, J. Yang, K. Y. Shi, B. Wang, L. Wang, G. H. Tian, B. Bateer, C. G. Tian, P. K. Shen and H. G. Fu, RSC Adv., 2013, 3, 4771–4777 RSC.
- A. L. M. Reddy, M. M. Shaijumon, S. R. Gowda and P. M. Ajayan, Nano Lett., 2009, 9, 1002–1006 CrossRef CAS PubMed.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J.-M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS PubMed.
- Y. P. Liu, G. T. Yu, G. D. Li, Y. H. Sun, T. Asefa, W. Chen and X. X. Zou, Angew. Chem., Int. Ed., 2015, 54, 1–6 CrossRef.
- D. H. Sim, X. H. Rui, J. Chen, H. T. Tan, T. T. M. Lim, R. Yazami, H. H. Hng and Q. Y. Yan, RSC Adv., 2012, 2, 3630–3633 RSC.
- Y. L. Cheah, V. Aravindan and S. Madhavi, ACS Appl. Mater. Interfaces, 2012, 4, 3270–3277 Search PubMed.
- Y. Xu, M. Dunwell, L. Fei, E. Fu, Q. L. Lin, B. Patterson, B. Yuan, S. G. Deng, P. Andersen, H. M. Luo and G. F. Zou, ACS Appl. Mater. Interfaces, 2014, 6, 20408–20413 Search PubMed.
- S. S. Kim, S. Ogura, H. Ikuta, Y. Uchimoto and M. Wakihara, Solid State Ionics, 2002, 146, 249–256 CrossRef CAS.
- D. Hara, J. Shirakawa, H. Ikuta, Y. Uchimoto, M. Wakihara, T. Miyanaga and I. Watanabe, J. Mater. Chem., 2002, 12, 3717–3722 RSC.
- N. Sharma, K. Shaju, M. G. V. Subbarao, B. V. R. Chowdari, Z. L. Dong and T. J. White, Chem. Mater., 2004, 16, 504–512 CrossRef CAS.
- D. Mikhailova, A. Voss, S. Oswald, A. A. Tsirlin, M. Schmidt, A. Senyshyn, J. Eckert and H. Ehrenberg, Chem. Mater., 2015, 27, 4485–4492 CrossRef CAS.
- X. D. Liu, Y. C. Lyu, Z. H. Zhang, H. Li, Y. S. Hu, Z. X. Wang, Y. M. Zhao, Q. Kuang, Y. Z. Dong, Z. Y. Liang, Q. H. Fan and L. Q. Chen, Nanoscale, 2014, 6, 13660–13667 RSC.
- N. N. Leyzerovich, K. G. Bramnik, T. Buhrmester, H. Ehrenberg and H. Fuess, J. Power Sources, 2004, 127, 76–84 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nr05602k |
| This journal is © The Royal Society of Chemistry 2016 |