Singular supramolecular self-assembling of bis-cyclodextrinyl-bis-lariat hosts with metal bis-aryl-sulfonates as guests†
Florence
Dumarçay-Charbonnier
*,
Jean Pierre
Joly
and
Alain
Marsura
*
UMR CNRS 7565 SRSMC Faculté des Sciences et Technologies, Université de Lorraine, BP70239, 54506 Vandœuvre-lès-Nancy, France. E-mail: alain.marsura@univ-lorraine.fr; Tel: +33683684955
First published on 16th November 2015
Abstract
The self-organization of bis-cyclodextrinyl-lariats (CD-L's) around divalent metal-sulfonates is described. Several new pseudo-rotaxanes were prepared by a template-directed approach involving bis-CD-L synthetic receptors and bis-sulfanilate or bis-tosylate metal salts as anion/cation guests. The non-covalent interactions between hosts and ZnII, CuII, CaII bis-arylsulfonates guests were studied by 1D- and 2D-NMR confirming the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-complexes driven by a dual anion/cation recognition.
1-complexes driven by a dual anion/cation recognition.
Mechanically interlocked molecules (MIAs) as named by Sir Fraser Stoddart1 are a class of molecules in which the components (a macrocycle and a thread-like guest molecule) are linked together by a mechanical bond. The components are connected as a consequence of their topologies.2 The two components of a rotaxane,1 which are MIAs, are kinetically trapped since the ends of the dumbbell are larger than the internal diameter of the ring and prevent the dissociation of the supramolecules.
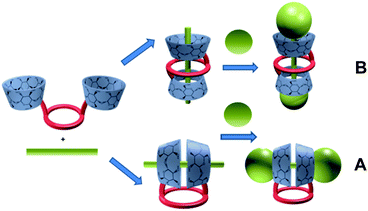
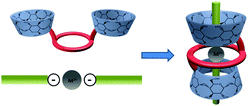
Synthetic macrocyclic polyethers (e.g. crown ethers) have attracted constant interest as models of natural ionophores and as synthetic chiral receptors.3 Among them, lariat ethers can strongly interact with certain cations thanks to their coordinating arms.4 Cyclodextrins as natural macrocycles are cyclic oligosaccharides produced from starch by enzymic conversion with six, seven or eight D-glucose units linked by α-1,4-glucose bonds. They form a “shell” shape with a rather hydrophobic cavity and a hydrophilic outside. This peculiar structure gives them the well-known property to bind hydrophobic molecules in water by inclusion into their cavity. Early, cooperative self-assembly and molecular binding behaviour of cyclodextrin crown-ether hosts mediated by alkali cations has been investigated by Liu group showing increased binding ability and selectivity towards sulfonate fluorescent dyes.4 Our idea was to associate the properties of the crown ethers and cyclodextrins in preparing dimers, studying their complexation properties towards metal coordinated aromatic guests, and looking at if a particular pseudo-[2]rotaxane assembly can take place. In this way, we early reported5 a synthesis of well defined, pseudo[2]rotaxanes by template-directed ‘thermodynamic’ threading reaction between a 1-bis-ureido-β-cyclodextrin chiral crown ether6 or a 1-bis-ureido-β-cyclodextrin achiral crown-ether7 with hydrophobic guests like butanol dimesylate (Busulfan®), 1,12-diaminododecane followed by tetraphenylboron locking or octadecanedioic acid sodium salt, followed by clamping with tetraphenylphosphonium or diphenyl-ethylammonium stoppers.5 It is worth to note that two conformational topologies (Fig. 1) remain possible in presence of a potential threaded guest with such bis-β-cyclodextrin (CD) lariats: an “head-to-head” or an “upstair–downstair” conformation.
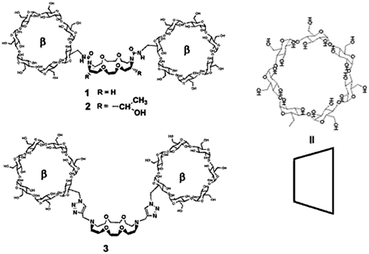
First NMR results were in favour of the “crypt” form, because lack of any cross peaks in 2D-ROESY and no proton chemically induced shifts between crown-ether and guest protons located inside the crown.6,7 In this contribution, we describe the synthesis of pseudo-[2]rotaxanes by threading metal bis-tosylate or bis-sulfanilate8 with three dimers: – a [bis-ureido-β-cyclodextrinyl] [18,6]-achiral-crown 1, – a [bis-ureido-β-cyclodextrinyl]-[18,6]-chiral-crown 2 and the newly synthesized [bis-triazolo-β-cyclodextrinyl]-[18,6]-achiral-crown 3 prepared via the CuI-catalyzed Huisgen dipolar cycloaddition (Fig. 2).9
Taking advantage of Click-chemistry, Joly et al.10 have described the preparation of different new ligands bearing two 1,4-substituted-triazole moieties as potential coordinating side-arms. They used [18,6]-crown and [15,5]-crown ethers and obtained stable mononuclear CuII crystal complexes with different geometries and coordination schemes. Monflier et al.11 have also developed successfully click-chemistry as an efficient tool to access β-cyclodextrins dimers.
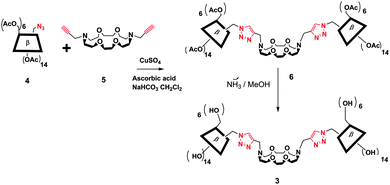
Starting from already known N,N′-dipropargyl diaza-crown-ether 5,4 we synthesized the 3-bis-triazolo-β-cyclodextrinyl crown-ether 3 in 65% yield by click chemistry (Fig. 3) using 6A-azido-6A-deoxy-per-O-acetylated-β-CD 4 as starting material. The target compound 3 was isolated quantitatively from the peracetylated precursor 6 after a deacetylation step using ammoniac in methanol as solvent. Spectroscopic data of 3 and 6 by IR, NMR, ESIMS and elemental analysis are in full accordance with the proposed structures.
A general threading strategy (Fig. 4) was thoroughly used to prepare following pseudo [2]rotaxanes: compound 1 and 2 were stirred for one night with one equivalent of zinc, copper or calcium bis-tosylate adding in water at room temperature in order to obtain 7 to 12 as amorphous solids. Alike pseudo-[2]rotaxanes 13 to 15 were obtained using ZnII or CaII bis-sulfanilates with 1 and 3 in the same conditions (Fig. 5).
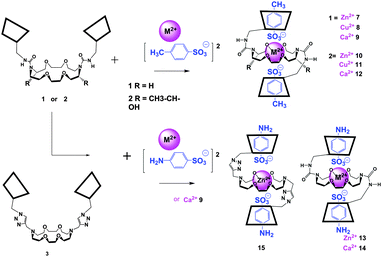 | ||
| Fig. 5 Complexation of zinc, copper or calcium bis-tosylate or bis- sulfanilates with dimers 1 to 3 giving pseudo[2]rotaxanes 7 to 9 and 13 to 15 (non-chiral [18,6]) or 10 to 12 (chiral [18,6]). | ||
In all these cases, one single conformation appeared favored with respect to the strong coordination of the [18,6]-crown with each divalent cation (Fig. 5).
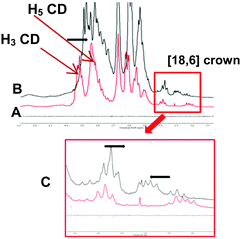
Formation of inclusion complexes 7 to 15 were ascertained by 1D, 2D NMR spectrometries. Inclusion of the phenyl group inside the CD cavity caused upfield chemical induced shift (CIS) of both H3, H5 of the CD and aromatic protons of the guests, one example is being given Fig. 6 and 7 for the CuII complex 8. This phenomenon was observed for each complex. As observed below, when inclusion of tosyl group into the CD core took place, similar up- and downfield shifts of crown-ether protons were also observed when the metal cation coordinates into the crown-ether as illustrated below for 13 in Fig. 8. As expected this result was really in favour of an “upstair–downstair” conformation preference which maintains the face-to-face mode1 of coordination of the metal with sulfonates oxygen of the guest molecule and the coordination to crown oxygen centres to complete a first coordination sphere. Moreover, such an assembly shows a higher level of symmetry in comparison to a “crypt form” one, as proposed in Fig. 1.
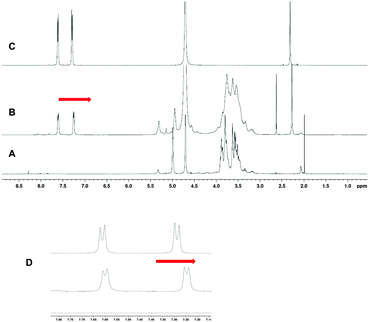 | ||
| Fig. 6 1H-NMR of 8 in D2O (A) free dimer 1 (B) complex 8 (C) copper bis-tosylate and zoom of tosyl protons (D). Red arrows indicate upfield shifts of the tosyl protons. | ||
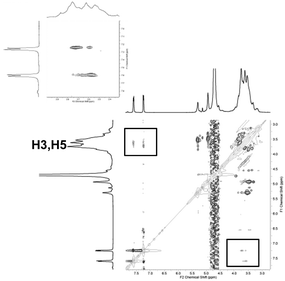 | ||
| Fig. 7 2D-ROESY NMR of 8 in D2O. Characteristic cross peaks of H3 and H5 with phenyl group protons of CuII bis-tosylate. | ||
In summary, NMR results undoubtedly reveal a full inclusion of tosyl or sulfanyl groups of the guests into the two closely interconnected CD cavities along of metal coordination to the [18,6] azamacrocycle. Titration of the dimers 1 and 3 in D2O by ZnII bis-sulfanilate allowed determination of fairly good association constants of 5.4 × 104 M−1 and 1.4 × 104 M−1 respectively (see ESI†).
The stoichiometry of each complex was established with the help of the Job's plot continuous variation method (Fig. 9) which give a R = 0.5 that implies to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry as illustrated below for 7.
1 stoichiometry as illustrated below for 7.
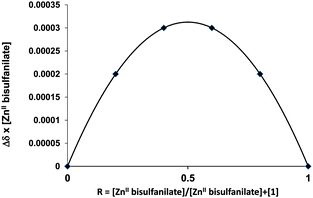 | ||
| Fig. 9 Job's plot corresponding to the chemical shifts of the aromatic protons of ZnII bis-tosylate in D2O for [ZnII bis-tosylate/1]. | ||
In conclusion, new mechanically interlocked molecular systems have been easily obtained from synthetic cyclodextrin dimers by inclusion of aromatic metal sulfonates salts as guest candidates to generate a new spontaneous efficient mode of dual cation/anion molecular recognition. Elsewhere, the NMR results have shown a favoured “upstair–downstair” conformation for the complexes that respects the cation/anion ion pair interaction. Unlike previous bis-ureido dimer, the bis-triazolyl-bis-cyclodextrin new dimer was obtained, in one step, by “click” Huisgen cycloaddition in fairly good yield (65%). Nevertheless, additional experiments should be done in order to ascertain triazole nitrogens participation to the coordination sphere around the metal cation, as described previously with some non-cyclodextrinyl lariats.1 The next steps of this work will be to prepare [2]-rotaxanes by attachments of bulky stoppers at the extremity of aromatic anion guests with the aim to achieve a control on the host–guest equilibrium level. Another, step is presently under progress using hydrophobic ammonium amidinium or guanidinium salts guests in place of previous anionic sulfonates. As mentioned in the field of corresponding literature12 this may be interesting, notably for the development of new pharmaceutical materials as for example new tunable drug delivery molecular devices.
Acknowledgements
Authors are grateful to Mr Tioga Gulon for preparing achiral [bis-ureido-cyclodextrinyl]-[18,6]-crown dimers, to Mrs Brigitte Fernette for recording NMR spectra and to Dr Fabien Lachaud for recording Mass spectra.Notes and references
- J. F. Stoddardt, J. Curr. Chem., 2005, 249, 203–259 Search PubMed; M. Xue, Y. Yang, X. Chi, X. Yan and F. Huang, Chem. Rev., 2015, 115, 7398–7501 CrossRef CAS PubMed; S. Dong, J. Yuan and F. Huang, Chem. Sci., 2014, 5, 247–252 RSC; P. H. Ashton, P. J. Campbell, P. T. Glink, S. Menzer, D. Philp, N. Spencer, J. F. Stoddardt, P. A. Tasker and D. J. Williams, Angew. Chem., Int. Ed. Engl., 1995, 34, 1865–1869 CrossRef , references cited therein.
- G. A. Breault, C. A. Hunter and P. C. Mayers, Tetrahedron, 1999, 55, 5265–5293 CrossRef CAS.
- C. J. Pedersen, J. Am. Chem. Soc., 1967, 89, 7017–7036 CrossRef CAS; C. Zhang, S. Li, J. Zhang, K. Zhu, N. Li and F. Huang, Org. Lett., 2007, 9, 5553–5556 CrossRef PubMed; F. Wang, C. Han, C. He, Q. Zhou, J. Zhang, C. Wang, N. Li and F. Huang, J. Am. Chem. Soc., 2008, 130, 11254–11255 CrossRef PubMed.
- V. J. Gatto, A. M. Arnold, S. R. Viscariello, S. R. Miller, C. R. Morgan and G. Gokel, J. Org. Chem., 1986, 51, 5373–5389 CrossRef CAS; Y. Liu, Z.-Y. Duan, J.-R. Han and L. Cui, Org. Biomol. Chem., 2004, 2, 2359–2364 Search PubMed.
- F. Dumarcay-Charbonnier and A. Marsura, Chem. Commun., 2012, 48, 732–734 RSC.
- S. Menuel, J.-P. Joly, B. Courcot, J. Elysée, N. E. Ghermani and A. Marsura, Tetrahedron, 2007, 63, 1706–1714 CrossRef CAS.
- S. Porvanski, F. Dumarcay-Charbonnier, S. Menuel, V. Bulach, J.-P. Joly and A. Marsura, Tetrahedron, 2009, 65, 6196–6203 CrossRef.
- X.-J. Yang, X. Wang, D.-Y. Chen, J. Yang, L.-D. Lu, X.-Q. Sun, G.-Y. He and J. Chen, J. Appl. Polym. Sci., 2000, 77, 2363–2369 CrossRef CAS.
- R. Huisgen, R. Knorr, L. Möbius and G. Szeimies, Chem. Ber., 1965, 98, 4014–4021 CrossRef CAS.
- J.-P. Joly, M. Beley, K. Selmeczi and E. Wenger, Inorg. Chem. Commun., 2009, 12, 382–384 CrossRef CAS; Z. Benkhellat, M. Allali, M. Beley, E. Wenger, M. Bernard, N. Parizel, K. Selmeczi and J.-P. Joly, New J. Chem., 2014, 38, 419–429 RSC; K. Selmeczi, J.-P. Joly, M. Allali, V. Yeguas, B. Henry and M. Ruiz-Lopez, Eur. J. Inorg. Chem., 2014, 4934–4945 CrossRef.
- M. Mourer, F. Hapiot, E. Monflier and S. Menuel, Tetrahedron, 2008, 64, 7159–7163 CrossRef CAS; M. Mourer, F. Hapiot, S. Tilloy, E. Monflier and S. Menuel, Eur. J. Org. Chem., 2008, 5723–5730 CrossRef.
- D. P. Elder, E. Delaney, A. Teasdale, S. Eley, V. D. Reif, K. Jacq, K. L. Facchine, R. S. Oestrich, P. Sandra and F. David, J. Pharm. Sci., 2010, 99, 2948–2961 Search PubMed; D. A. Haynes, J. A. Chisholm, W. Jones and W. D. S. Motherwell, CrystEngComm, 2004, 6, 584–588 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental section and spectral data. See DOI: 10.1039/c5nj02327k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |