Synthesis of D–D–A-type small organic molecules with an enlarged linker system towards organic solar cells and the effect of co-adsorbents on cell performance†
Priyanka P.
Kumavat
a,
Prashant K.
Baviskar
b,
Babasaheb R.
Sankapal
c and
Dipak S.
Dalal
*a
aSchool of Chemical Sciences, North Maharashtra University, Jalgaon – 425 001, M.S., India. E-mail: dsdalal2007@gmail.com; Fax: +91-257-2258403; Tel: +91-257-2257432
bDepartment of Physics, School of Physical Sciences, North Maharashtra University, Jalgaon – 425 001, M.S., India
cNano Material and Device Laboratory, Department of Applied Physics, Visvesvaraya National Institute of Technology, Nagpur – 440010, M.S., India
First published on 13th November 2015
Abstract
The synthesis of two D–D–A type novel small molecules bearing N,N-dimethyl/ethyl aniline as a donor and a methine unit connected with cyano and carboxyl groups as an acceptor linked with conjugated π-linkers has been performed. The obtained compounds were characterized by using FT-IR, 1H-NMR, 13C-NMR, LC-HR-MS, TGA, FE-SEM and UV-Vis spectroscopic techniques. These designed small organic molecules have been utilized for the fabrication of novel organic solar cells on high surface area titanium dioxide films consisting of interconnected nanoparticles with the device configuration FTO/TiO2/organic compound/electrolyte/platinum. The effect of cholic acid and dodecylamine as co-adsorbents with an organic compound has been studied towards the device performances and the results are incorporated herein.
1. Introduction
Over the past couple of decades, the search for better and clean renewable energy resources has been intensified due to the increase in energy consumption. Solar energy is one of the several promising renewable energy resources that could contribute to a sustainable energy supply.1,2 Moreover, organic solar cells (OSCs) has gained considerable interest as future renewable alternative energy resources due to their added advantages such as low-cost fabrication, lightweight, and good mechanical flexibility.3–6 Hence, a broad range of distinct device technologies based on organic molecules are being developed very rapidly.Two novel organic dyes containing julolidine as the electron donor and cyanoacetic acid or rhodamine-3-acetic acid as the electron acceptor bridged by the bithiophene unit were synthesized which resulted in 2.6–6.7% efficiencies.7 Benzimidazole based metal free organic dyes with and without cyano vinyl units have been designed and synthesized via one step processes and tested as sensitizers in dye sensitized solar cells.8 A significant enhancement of ternary blend organic solar cell efficiency using poly[[4,8-bis[(2-ethylhexyl)oxy]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl][3-fluoro-2-[(2-ethylhexyl)carbonyl]thieno[3,4-b]thiophenediyl]] (PTB7) as a sensitizer has also been reported.9 A systematic analysis of relevant parameters for the production of blade coated solar cells using a low band gap/fullerene blend was conducted and efficiencies of upto 3.6% for coated and 1.6% for printed cells on a flexible substrate was obtained.10 Inverted solar cells consisted of a blade-coated poly(N-9′-heptadecanyl-2,7-carbazole-alt-5,5-(4′,7′-di-2-thienyl-2′,1′,3′-benzothiadiazole)) (PCDTBT) and [6,6]-phenyl C71-butyric acid methyl ester (PC71BM) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 by weight) active layer sandwiched between a ZnO electron extraction layer and a MoO3/Ag anode has been reported with a power conversion efficiency (PCE) of around 5%.11 High open-circuit voltage (1.03 V) has been achieved by solution-processed organic solar cells based on benzothiadiazole-triphenylamine small molecules incorporating π-linkage.12 A series of solution-processable, p-type, low-optical-gap semiconductors based on penta- and hexa-thiophenes asymmetrically endcapped with a triarylamine or triarylamino-substituted dendron and dicyanovinyl groups has been reported for a PCE ranging from 0.47 to 1.72%.13 Bulk heterojunction solar cells have been prepared by using donor materials 2,5-bis[4-(N,N-diphenylamino)styryl]thiophene and 2,5-bis[4-(N,N-diphenylamino)styryl]-2,2′-bithiophene and resulted in PCE values of 0.23 and 0.34%, respectively.14 A PCE of 1.42% has been achieved by oligomer FPTTPA as an efficient electron donor in bulk heterojunction organic solar cells.15 A series of oligomers constituting alternating thiophene and fluorenone residues, designed to obtain efficiency of around 0.7%.16 A new family of soluble extended arylacetylene optoelectronic materials has been reported with a highest PCE of around 1.3%.17 Also a new series of thiophene-based monodispersed molecular materials has shown how the photovoltaic properties of a bulk heterojunction device can be controlled by suitably tailoring the properties of the donor molecule and efficiencies ranging from 0.2 to 1.0%.18 Improved PCE with 3% of cyanine solar cells with polyaniline anodes has also been reported.19 A novel molecule (D(CATBTzT)BDT) has been synthesized with a maximum PCE of 3.61%.20 Oligomers containing dithieno[3,2-b:2,3-d]pyrrole and thieno[2,3-c]pyrrole-4,6-dione units for solution-processed OSCs with PCEs ranging from 0.3 to 2.2% have been reported.21 Efficiencies of 0.62 and 0.64% with improved stability has been achieved by inserting an ultra-thin layer of gold at the PEDOT:PSS/CuPc interface in a planar CuPc/C60 based organic solar cell.22
4 by weight) active layer sandwiched between a ZnO electron extraction layer and a MoO3/Ag anode has been reported with a power conversion efficiency (PCE) of around 5%.11 High open-circuit voltage (1.03 V) has been achieved by solution-processed organic solar cells based on benzothiadiazole-triphenylamine small molecules incorporating π-linkage.12 A series of solution-processable, p-type, low-optical-gap semiconductors based on penta- and hexa-thiophenes asymmetrically endcapped with a triarylamine or triarylamino-substituted dendron and dicyanovinyl groups has been reported for a PCE ranging from 0.47 to 1.72%.13 Bulk heterojunction solar cells have been prepared by using donor materials 2,5-bis[4-(N,N-diphenylamino)styryl]thiophene and 2,5-bis[4-(N,N-diphenylamino)styryl]-2,2′-bithiophene and resulted in PCE values of 0.23 and 0.34%, respectively.14 A PCE of 1.42% has been achieved by oligomer FPTTPA as an efficient electron donor in bulk heterojunction organic solar cells.15 A series of oligomers constituting alternating thiophene and fluorenone residues, designed to obtain efficiency of around 0.7%.16 A new family of soluble extended arylacetylene optoelectronic materials has been reported with a highest PCE of around 1.3%.17 Also a new series of thiophene-based monodispersed molecular materials has shown how the photovoltaic properties of a bulk heterojunction device can be controlled by suitably tailoring the properties of the donor molecule and efficiencies ranging from 0.2 to 1.0%.18 Improved PCE with 3% of cyanine solar cells with polyaniline anodes has also been reported.19 A novel molecule (D(CATBTzT)BDT) has been synthesized with a maximum PCE of 3.61%.20 Oligomers containing dithieno[3,2-b:2,3-d]pyrrole and thieno[2,3-c]pyrrole-4,6-dione units for solution-processed OSCs with PCEs ranging from 0.3 to 2.2% have been reported.21 Efficiencies of 0.62 and 0.64% with improved stability has been achieved by inserting an ultra-thin layer of gold at the PEDOT:PSS/CuPc interface in a planar CuPc/C60 based organic solar cell.22
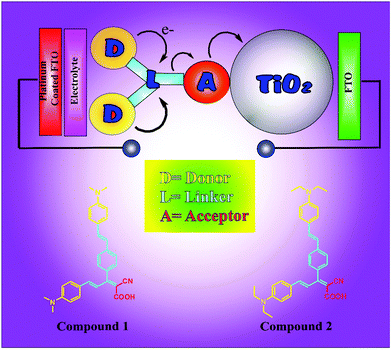
In this work, an attempt has been made to synthesize N,N-dimethylaniline as a donor and a methine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) unit connecting with cyano (–CN) and carboxyl (–COOH) groups as an acceptor linked with conjugated π-linkers. These materials are used as active materials on a high surface area TiO2 layer coated on a fluorine doped tin oxide coated glass substrate and used to fabricate solar cells. The influence of cholic acid and dodecylamine in combination with formed materials has been studied towards the improvement of the device performance.
CH–) unit connecting with cyano (–CN) and carboxyl (–COOH) groups as an acceptor linked with conjugated π-linkers. These materials are used as active materials on a high surface area TiO2 layer coated on a fluorine doped tin oxide coated glass substrate and used to fabricate solar cells. The influence of cholic acid and dodecylamine in combination with formed materials has been studied towards the improvement of the device performance.
2. Experimental
2.1 Chemicals and Instrumentations
4-(Dimethylamino)benzaldehyde, cyanoacetic acid, ammonium acetate, tetra-n-butyl ammonium iodide, and propylene carbonate were purchased from sd-fine chem limited, India. 4-(Diethylamino)benzaldehyde was purchased from Sigma Aldrich and 4-methylacetophenone from Spectrochem chemicals. Chromatographic separations were carried out on silica gel (60–120 mesh). All chemicals used were of AR grade.1H-NMR and 13C-NMR spectra were recorded on a Bruker Advance II spectrometer operating at 400 and 100 MHz, respectively. Liquid Chromatography-High resolution mass (LC-HR-MS) spectra were recorded on a 6550 iFunnel QTOF LC-MS/MS Make-Agilent Technologies 1290 Infinity Binary Pump. The thermogravimetric analyses (TGA) were carried out on a Perkin Elmer 4000 Instrument under purified nitrogen gas flow with a 10 °C min−1 heating rate. Surface roughness and morphology of thin films were characterized by Field Emission-Scanning Electron Microscopy (FE-SEM) on an S-4800 instrument from Hitachi, Japan, operated at 10 kV (Kilo Volt) and elemental analysis was performed by the EDS unit coupled with the FE-SEM unit. The optical absorption spectra were recorded (i.e. in between 300 and 800 nm wavelength ranges) using a UV-Vis spectrophotometer (Shimadzu 2450) with a 1.0 cm quartz cell. Film thicknesses were measured by using a thickness profiler DEKTAK-150 profilometer for area 1000 nm. The current density–voltage (J–V) characteristics of the organic solar cells (OSCs) were measured under AM 1.5G (100 mW cm−2) illumination which was provided by a 3A grade solar simulator (Newport, USA, 94043A, calibrated with a standard crystalline silicon solar cell).
2.2 Synthesis of organic compounds
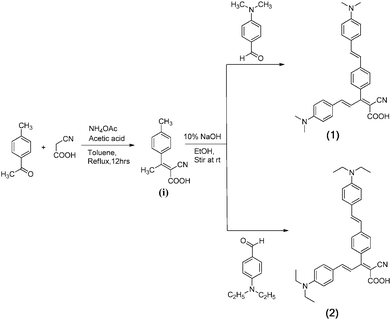
Scheme 1 presents the synthetic route for the preparation of compound 1 and compound 2.First, the starting material 2-cyano-3-p-tolybut-2enolic acid (i) was synthesized from 1.34 mL of 4-methylacetophenone (10 mmol) and 0.85 g of cyanoacetic acid (10 mmol), 0.192 g of ammonium acetate (5 mmol) and 1.71 mL of acetic acid (5 mmol), stirred well for 12 h at 100 °C in toluene. This reaction mixture was poured in water and extracted in ethyl acetate. The obtained starting material (i) was purified by hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (9
ethyl acetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Then 1 g of 2-cyano-3-p-tolybut-2enolic acid (i) (5 mmol), 0.74 g of 4-(dimethylamino)benzaldehyde (5 mmol) for compound 1 and 0.88 g of 4-(diethylamino)benzaldehyde (5 mmol) for compound 2, respectively, with 10% NaOH were stirred at room temperature for 8–10 h in ethanol. This reaction mixture was poured in water and neutralized with 1
1). Then 1 g of 2-cyano-3-p-tolybut-2enolic acid (i) (5 mmol), 0.74 g of 4-(dimethylamino)benzaldehyde (5 mmol) for compound 1 and 0.88 g of 4-(diethylamino)benzaldehyde (5 mmol) for compound 2, respectively, with 10% NaOH were stirred at room temperature for 8–10 h in ethanol. This reaction mixture was poured in water and neutralized with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 HCl for compound 1 and compound 2. Obtained precipitates were filtered and washed with distilled water, the yellow coloured compounds were obtained as crude products. These crude products were purified by silica-gel column chromatography with ethyl acetate
1 HCl for compound 1 and compound 2. Obtained precipitates were filtered and washed with distilled water, the yellow coloured compounds were obtained as crude products. These crude products were purified by silica-gel column chromatography with ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene (1
toluene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) and then purified products were recrystallized with aqueous ethanol.
9) and then purified products were recrystallized with aqueous ethanol.
2.3 Characterization data (detailed spectra are given in S1, ESI†)
IR (cm−1): 3097, 2990, 2231, 1695, 1566, 1271, 914.
1H NMR (400 MHz, CDCl3): 2.36 (s, 3H), 2.60 (s, 3H), 7.29 (d, 2H, J = 7.9), 7.42 (d, 2H, J = 8.0).
13C NMR (100 MHz, CDCl3): 20.84, 22.80, 105.05, 116.90, 125.50, 126.87, 127.25, 128.59, 129.03, 129.10, 137.48, 140.05, 163.53, 171.03.
MS: calculated for C12H11NO2 201; found for ([C12H11NO2] + H) 202.1.
IR (cm−1): 2886, 2811, 2218, 1654, 1537, 1179, 936, 813.
1H NMR (400 MHz, CDCl3): 2.98 (brs, 6H), 3.04 (brs, 6H), 6.66 (d, 2H, J = 8.8 Hz), 6.69 (d, 2H, J = 9.0 Hz), 7.27 (d, 2H, J = 8.0 Hz), 7.44 (d, 2H, J = 15.36 Hz), 7.56 (d, 2H, J = 8.8 Hz), 7.64 (d, 2H, J = 15.36 Hz), 7.85 (d, 2H, J = 8.0 Hz), 7.92 (d, 2H, J = 8.1 Hz), 7.97 (s, 1H).
13C NMR (100 MHz, CDCl3): 21.15, 39.91, 40.12, 93.96, 111.27, 111.49, 115.95, 117.61, 118.50, 121.92, 128.06, 128.91, 130.27, 133.29, 135.76, 142.45, 144.66, 151.66, 153.14, 153.51, 164.84, 188.27.
LC-HR-MS: calculated for C30H29N3O2 463.2279; found for ([C30H29N3O2]–H) 462.2206.
IR (cm−1): 3074, 2975, 2222, 1686, 1528, 1176, 935, 814.
1H NMR (400 MHz, CDCl3): 1.18–1.25 (m, 12H), 3.39–3.49 (m, 8H), 6.65–6.69 (m, 4H), 7.28 (d, 4H, J = 16.04 Hz), 7.52 (d, 4H, J = 8.88 Hz), 7.93 (dd, 4H, J = 8.48 & 4.32 Hz), 8.0858 (s, 1H).
13C NMR (100 MHz, CDCl3): 12.16, 43.98, 44.16, 93.18, 110.83, 117.64, 118.0, 127.93, 128.84, 130.52, 133.67, 145.35, 151.06, 153.35, 165.01.
LC-HR-MS: C34H37N3O2 519.2656; found for ([C34H37N3O2] + H) 520.2713.
2.4 Fabrication of solar cell devices
Solar cells were fabricated on fluorine doped tin oxide (FTO) coated glass substrates. The FTO substrates were cleaned ultrasonically with acetone, toluene, methanol, and isopropyl alcohol subsequently. On the FTO substrate, a layer of TiO2 film with a maximum thickness of 3.5 μm and a roughness of 310 nm was coated by the dip coating method using acetone dispersion. For this, TiO2 powder was dispersed in acetone (4.0% w/v) and dispersed conditions were maintained by sonication. To this dispersion pre-cleaned FTO substrate was dipped for 10 seconds and removed for drying. This dipping and drying cycle were repeated 10 times. After 10 successive cycles these TiO2 coated FTO films were first dried at 100 °C for 30 min and then annealed at 500 °C for 1 h in air.23 Then different solutions containing different combinations of synthesised organic compounds (10 mM compound 1 and compound 2), 5 mM cholic acid and 5 mM dodecylamine, were prepared separately in ethanol and coated on FTO/TiO2 films by a simple dipping method at 60 °C for 10 minutes. Cholic acid24–28 and dodecylamine29,30 were added to organic compounds as co-adsorbents in order to study their effect on cell efficiency. Fig. 1 shows the graphical representation of prepared OSCs.The typical maximum thickness of the fabricated device layers in the FTO/TiO2/organic compound photoanode was 4.9 μm along with a roughness of 336 nm. This photoanode was sandwiched together with platinum coated FTO as the counter electrode (cathode). Then the electrolyte was introduced into the photoanode and cathode by using capillary action, which was composed of 0.5 M tetra-n-propyl ammonium iodide, 0.1 M I2 in ethylene carbonate/acetonitrile (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 volumes).31 The apparent area of OSCs was 0.2 cm2 and performances of OSCs were studied by J–V measurements.
8 volumes).31 The apparent area of OSCs was 0.2 cm2 and performances of OSCs were studied by J–V measurements.
3. Results and discussion
3.1 Synthetic route of organic compounds
In the recent past, researchers have been concentrating on the development of newer small organic molecules containing donor–acceptor moieties linked with π-linkers because such small molecules have shown good applications in photovoltaic cells. In literature, the synthesis of two D–A–D donors involving isoindigo (IsI) as a central acceptor block and benzofuran (BF) or dithienopyrrole (DTP) as side donor units and their preliminary evaluation in organic photovoltaic cells has been reported.32 Moreover, four A–D–A type small molecules using 4,4,9,9-tetrakis(4-hexylphenyl)-indaceno[1,2-b:5,6-b]dithiophene as central building blocks, bithiophene or terthiophene as π-bridges, alkyl cyanoacetate or rhodanine as end acceptor groups were synthesized and investigated as electron donors in solution-processed OSCs;33 furan-bridged thiazolo[5,4-d]thiazole based D–π–A–π–D type linear chromophores for solution-processed bulk-heterojunction organic solar cells;34 indole and triisopropyl phenyl as capping units for a diketopyrrolopyrrole (DPP) acceptor central unit: an efficient D–A–D type small molecule for organic solar cells.35In continuation of our work on the synthesis of organic molecules36–40 and their application in organic photovoltaic cells,41–44 herewith we are reporting the synthesis of two small organic molecules namely 2-cyano-5-(4-(dimethylamino)phenyl)-3-(4-(4-(dimethylamino)styryl)phenyl)penta-2,4-dienoic acid (compound 1) and 2-cyano-5-(4-(diethylamino)phenyl)-3-(4-(4-(diethylamino)styryl)phenyl)penta-2,4-dienoic acid (Compound 2) (the synthesis route is shown in Scheme 1). Initially, the starting material 2-cyano-3-p-tolybut-2enolic acid (i) was synthesized by the knoevenagel condensation of 4-methylacetophenone with cyanoacetic acid by reflux in toluene using ammonium acetate and acetic acid. Then the product (i) was subjected to condensation with 4-(dimethylamino)benzaldehyde or 4-(diethylamino)benzaldehyde with the removal of a water molecule in the presence of 10% NaOH using ethanol as a solvent to give compound 1 and compound 2 respectively. The synthesis of both these compounds has been confirmed by different characterisation techniques (detailed spectra are given in S2, ESI†).
3.2 Thermal stability of organic compounds
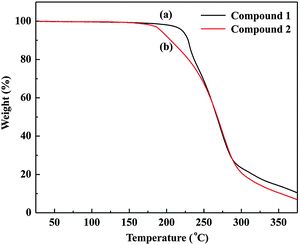
The thermal properties of both compounds are investigated by thermogravimetric analysis (TGA). Both compounds exhibit good thermal stability with a decomposition temperature (5% weight loss) at 221 °C and 192 °C under nitrogen (Fig. 2). The high thermal stability of the compounds prevents the degradation of the active layer in cells which ultimately results in high stability of the cells.3.3 Optical properties
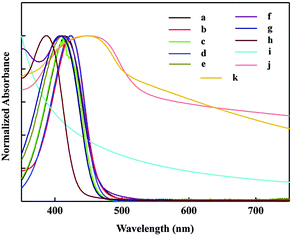
Fig. 3 shows the normalized UV-Vis absorption spectra under different conditions. The UV-Vis optical spectrum of compound 1 and compound 2 in ethanol without a co-adsorbent shows absorption peaks at 415 and 424 nm respectively, whereas with cholic acid the absorption peaks shifted to 452 and 429 nm, respectively. The addition of cholic acid to both compounds resulted in a slight shift in the maximum wavelength towards the more visible range. Moreover the addition of dodecylamine to both compounds resulted in a shift of the wavelength towards the UV region. TiO2 coated on the FTO plate shows lower absorbance in the visible region as compared to absorbance for OSCs. Thin films of both compounds with cholic acid coated on FTO/TiO2 showed a broad absorption band throughout the visible region (400–800 nm). Compared to that in solution, the absorption spectrum of both films suggests that strong intermolecular interaction and aggregation exist in the solid state. The optical band gap of TiO2 is estimated to be 3.26 eV by the absorption onset at 324 nm of the thin film (the detailed plot is given in S2, ESI†).3.4 Electrochemical properties
In order to determine the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of the synthesized molecules, cyclic voltammetry (CV) was employed in on a glassy carbon working electrode in 1000 ppm tetra-n-butylammonium-hexafluorophosphate (TBAPF6) in acetonitrile solution as the supporting electrolyte at a scan rate of 100 mV s−1 under a nitrogen atmosphere at room temperature. The potential of the Ag/AgCl reference electrode was calibrated by using a ferrocene/ferrocenium redox couple which has the known oxidation potential of +4.8 eV. The HOMO and LUMO energy levels and the electrochemical band gaps (Eecg) of both molecules were calculated from the onset oxidation potentials (Eoxi) and onset reduction potentials (Ered) according to the following equations:45,46| HOMO = (Eoxi − Eferrocene1/2 + 4.8) eV |
| LUMO = (Ered − Eferrocene1/2 + 4.8) eV |
| E ecg = LUMO − HOMO |
The onset oxidation and reduction potential of compound 1 is 1.37 V and −1.38 V respectively, thus the corresponding HOMO and LUMO energy level is −5.77 eV and −3.02 eV. For compound 2, onset oxidation and reduction potential of Compound 1 is 1.35 V and −1.38 V respectively, and the corresponding HOMO and LUMO energy level is −5.75 eV and −3.02 eV. Band gaps calculated from the HOMO and LUMO are 2.75 eV and 2.73 eV for compound 1 and compound 2. respectively, which are consistent with the optical band gaps (Eoptg) 2.76 eV and 2.74 eV calculated from the UV-Vis absorption onset (the detailed plot is given in S3, ESI†). The CV curves of these materials are shown in Fig. 4 and the electrochemical energy levels are summarized in Table 1. The HOMO and LUMO energy levels of the two synthesised organic molecules showed that they were suitable as donors in organic solar cells with TiO2 as the acceptor.
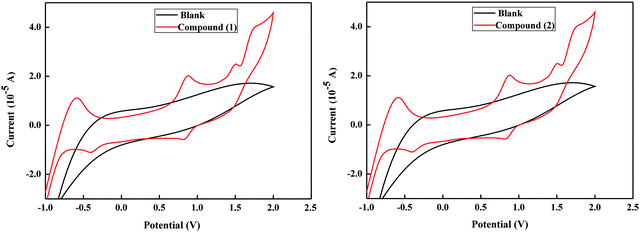 | ||
| Fig. 4 Cyclic voltammetry of compound 1 and 2 in 1000 ppm tetra-n-butylammonium-hexafluorophosphate (TBAPF6) in acetonitrile solution at a scan rate of 100 mV s−1. | ||
| Material | E oxi (V) | E red (V) | HOMO (eV) | LUMO (eV) | E ecg (eV) | E optg (eV) |
|---|---|---|---|---|---|---|
| Compound 1 | 1.37 | −1.38 | −5.77 | −3.02 | 2.75 | 2.76 |
| Compound 2 | 1.35 | −1.38 | −5.75 | −3.02 | 2.73 | 2.74 |
3.5 Surface characterization
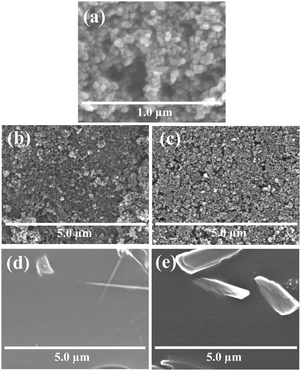
FE-SEM was utilized to study the morphology of TiO2 and TiO2/organic compound films. For this purpose, active layers were coated on glass plates by using the fabrication process described in the ‘Fabrication of solar cell devices’ section and the morphology is shown in Fig. 5 (detailed reports are given in S4, ESI†).FE-SEM images revealed that the addition of cholic acid in organic compounds increases the smoothness and homogeneity of the surface over TiO2 particles showing an amorphous pattern which minimizes the chance of void formation in OSCs and ultimately results in moderate efficiency as compared to organic compounds without cholic acid. EDAX analysis (detailed spectra are given in S5, ESI†) shows the elemental composition of respective films and EDAX spectra confirm the presence of respective elements in films.
3.6 Photovoltaic properties
| Device | Cell structure | Max. wavelengtha (nm) | V oc (V) | J sc (mA cm−2) | FF | Efficiency (%) |
|---|---|---|---|---|---|---|
| a Absorption spectra recorded in ethanol. | ||||||
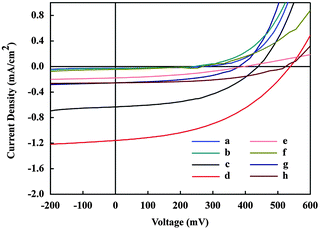
| a | FTO/TiO2/compound 1/electrolyte/Pt electrode | 415 | 0.272 | 0.265 | 0.0435 | 0.003 |
| b | FTO/TiO2/compound 2/electrolyte/Pt electrode | 424 | 0.233 | 0.029 | 0.3194 | 0.002 |
| c | FTO/TiO2/compound 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cholic acid (2 cholic acid (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/electrolyte/Pt electrode 1)/electrolyte/Pt electrode |
452 | 0.439 | 0.632 | 0.4688 | 0.130 |
| d | FTO/TiO2/compound 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cholic acid (2 cholic acid (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/electrolyte/Pt electrode 1)/electrolyte/Pt electrode |
429 | 0.538 | 1.158 | 0.4438 | 0.277 |
| e | FTO/TiO2/compound 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dodecylamine (2 dodecylamine (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/electrolyte/Pt electrode 1)/electrolyte/Pt electrode |
411 | 0.398 | 0.178 | 0.372 | 0.026 |
| f | FTO/TiO2/compound 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dodecylamine (2 dodecylamine (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/electrolyte/Pt electrode 1)/electrolyte/Pt electrode |
411 | 0.272 | 0.045 | 0.2836 | 0.003 |
| g | FTO/TiO2/compound 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cholic acid cholic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dodecylamine (2 dodecylamine (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/electrolyte/Pt electrode 1)/electrolyte/Pt electrode |
408 | 0.394 | 0.257 | 0.4452 | 0.045 |
| h | FTO/TiO2/compound 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cholic acid cholic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dodecylamine (2 dodecylamine (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/electrolyte/Pt electrode 1)/electrolyte/Pt electrode |
389 | 0.535 | 0.261 | 0.4685 | 0.065 |
The corresponding photovoltaic parameters i.e., short circuit photocurrent (Jsc), open circuit voltage (Voc), fill factor (FF) and power conversion efficiency (η) are compiled in Table 2. The efficiency of 0.277% for compound 2 with cholic acid is obtained with an open-circuit voltage (Voc) of 0.538 V, a short-circuit current density (Jsc) of 1.158 mA cm−2, and a fill factor (FF) of 0.4438 (device d in Table 2). Therefore, it was concluded that cholic acid has resulted in an increase in cell efficiency, whereas dodecylamine has shown adverse effects on cell efficiency.
| Dipping cycles | Thickness (μm) | Roughness (nm) | V oc (V) | J sc (mA cm−2) | FF | Efficiency (%) |
|---|---|---|---|---|---|---|
| 10 | 3.5 | 310 | 0.538 | 1.158 | 0.4438 | 0.277 |
| 20 | 5.9 | 316 | 0.716 | 0.892 | 0.3963 | 0.253 |
| 30 | 11.3 | 289 | 0.803 | 0.618 | 0.4461 | 0.223 |
4. Conclusions
In conclusion, we have developed and characterized two small organic compounds as novel electron transporting chromophores towards photovoltaic applications. The study of the bilayer heterojunction organic solar cells with the incorporation of two N,N-dimethyl/ethylaniline as a donor groups and a methine unit connecting with cyano and carboxyl as accepting groups and titanium dioxide as an acceptor material showed an efficiency up to 0.277% with an open circuit voltage of 0.538 V. The study of co-adsorbents revealed that the presence of cholic acid increases cell efficiency, whereas dodecylamine has shown adverse effects on cell efficiency.Acknowledgements
One of the authors (PPK) acknowledges UGC, New Delhi, for SAP (DSA-I) fellowship under the scheme ‘Research Fellowship in Sciences for Meritorious Students’.References
- L. Yu, W. Shi, L. Lin, Y. Liu, R. Li, T. Peng and X. Lib, Dalton Trans., 2014, 43, 8421 RSC.
- T. Higashinoa and H. Imahori, Dalton Trans., 2015, 44, 448 RSC.
- B. Kippelen and J. L. Brédas, Energy Environ. Sci., 2009, 2, 251 CAS.
- M. Helgesen, R. Sondergaard and F. C. Krebs, J. Mater. Chem., 2010, 20, 36 RSC.
- C. J. Brabec, S. Gowrisanker, J. J. M. Halls, D. Laird, S. J. Jia and S. P. Williams, Adv. Mater., 2010, 22, 3839 CrossRef CAS PubMed.
- T. Kuwabara, C. Iwata, T. Yamaguchi and K. Takahashi, ACS Appl. Mater. Interfaces, 2010, 2, 2254 CAS.
- G. Wu, F. Kong, J. Li, W. Chen, X. Fang, C. Zhang, Q. Chen, X. Zhang and S. Dai, Dyes Pigm., 2013, 99, 653 CrossRef CAS.
- S. Manoharan and S. Anandan, Dyes Pigm., 2014, 105, 223 CrossRef CAS.
- R. Lin, M. Wright, B. P. Veettil and A. Uddin, Synth. Met., 2014, 192, 113 CrossRef CAS.
- A. Schneider, N. Traut and M. Hamburger, Sol. Energy Mater. Sol. Cells, 2014, 126, 149 CrossRef CAS.
- S. Alem, J. Lu, R. Movileanu, T. Kololuoma, A. Dadvand and Y. Tao, Org. Electron., 2014, 15, 1035 CrossRef CAS.
- L. Wanga, L. Yin, C. Ji, Y. Zhang, H. Gao and Y. Li, Org. Electron., 2014, 15, 1138 CrossRef.
- W. Zhang, S. C. Tse, J. Lu, Y. Tao and M. S. Wong, J. Mater. Chem., 2010, 20, 2182 RSC.
- J. Kwon, W. Lee, J. Kim, S. Noh, C. Leeb and J. Hong, New J. Chem., 2010, 34, 744 RSC.
- H. Choi, S. Paek, J. Song, C. Kim, N. Cho and J. Ko, Chem. Commun., 2011, 47, 5509 RSC.
- W. Porzio, S. Destri, M. Pasini, U. Giovanella, M. Ragazzi, G. Scavia, D. Kotowski, G. Zotti and B. Vercelli, New J. Chem., 2010, 34, 1961 RSC.
- F. Silvestri, A. Marrocchi, M. Seri, C. Kim, T. J. Marks, A. Facchetti and A. Taticchi, J. Am. Chem. Soc., 2010, 132, 6108 CrossRef CAS PubMed.
- S. Colella, M. Mazzeo, R. Grisorio, E. Fabiano, G. Melcarne, S. Carallo, M. D. Angione, L. Torsi, D. Suranna, F. Mastrorillic and G. Gigli, Chem. Commun., 2010, 46, 6273 RSC.
- B. Fan, F. A. de Castro, B. Tsu-Te Chu, J. Heier, D. Opris, R. Hany and F. Nuesch, J. Mater. Chem., 2010, 20, 2952 RSC.
- Y. Chen, Z. Du, W. Chen, Q. Liu, L. Sun, M. Sun and R. Yang, Org. Electron., 2014, 15, 405 CrossRef CAS.
- L. G. Mercier, A. Mishra, Y. Ishigaki, F. Henne, G. Schulz and P. Bäuerle, Org. Lett., 2014, 16, 2642 CrossRef CAS PubMed.
- S. S. Ardestani, R. Ajeian, M. N. Badrabadi and M. Tavakkoli, Sol. Energy Mater. Sol. Cells, 2013, 111, 107 CrossRef.
- B. R. Sankapal, M. Ch. Lux-Steiner and A. Ennaoui, Appl. Surf. Sci., 2005, 239, 165 CrossRef CAS.
- C. Magne, M. Urien, I. Ciofini, T. Tugsuza and T. Pauporte, RSC Adv., 2012, 2, 11836 RSC.
- M. Matsui, M. Ono, Y. K. K. Funabiki, T. Yoshida, H. Jin Kim, C. K. Hong, S. Higashijima and H. Miura, Dyes Pigm., 2013, 99, 829 CrossRef CAS.
- M. Hosseinnezhad, K. Gharanjig and S. Moradian, Mater. Technol., 2015, 30, 189 CrossRef CAS.
- S. K. S. Lightbourne, H. B. Gobeze, N. K. Subbaiyan and F. D'Souza, J. Photonics Energy, 2015, 5, 530891 Search PubMed.
- M. Hosseinnezhad, S. Moradian and K. Gharanjig, Opto-Electron. Rev., 2015, 23, 126 CAS.
- X. X. Zhao, J. J. Song, Y. Z. Yang and X. G. Liu, J. Funct. Mater., 2013, 44, 1332 CAS.
- L. Yan, Y. Li, Y. Yang, X. Liu, Y. Chen and B. Xu, Fullerenes, Nanotubes, Carbon Nanostruct., 2014, 23, 549 CrossRef.
- P. Baviskar, R. Gore, A. Ennaoui and B. Sankapal, Mater. Lett., 2014, 116, 91 CrossRef CAS.
- A. Yassin, P. Leriche, M. Allain and J. Roncali, New J. Chem., 2013, 37, 502 RSC.
- H. Bai, Y. Wang, P. Cheng, Y. Li, D. Zhu and X. Zhan, ACS Appl. Mater. Interfaces, 2014, 6, 8426 CAS.
- M. Nazim, S. Ameen, M. S. Akhtar, H. Seo and H. Shin, RSC Adv., 2015, 5, 6286 RSC.
- G. D. Sharma, M. A. Reddy, K. Ganesh, S. P. Singh and M. Chandrasekharam, RSC Adv., 2014, 4, 732 RSC.
- P. P. Kumavat, A. D. Jangale, D. R. Patil, K. S. Dalal, J. S. Meshram and D. S. Dalal, Environ. Chem. Lett., 2013, 11, 177 CrossRef CAS.
- D. R. Patil, Y. B. Wagh, P. G. Ingole, K. Singh and D. S. Dalal, New J. Chem., 2013, 37, 3261 RSC.
- Y. B. Wagh, A. Kuwar, S. K. Sahoo, J. Gallucci and D. S. Dalal, RSC Adv., 2015, 5, 45528 RSC.
- Y. A. Tayade, D. R. Patil, Y. B. Wagh, A. D. Jangale and D. S. Dalal, Tetrahedron Lett., 2015, 56, 666 CrossRef CAS.
- Y. A. Tayade, S. A. Padvi, Y. B. Wagh and D. S. Dalal, Tetrahedron Lett., 2015, 56, 2441 CrossRef CAS.
- P. K. Baviskar, W. Tan, J. Zhang and B. R. Sankapal, J. Phys. D: Appl. Phys., 2009, 42, 125108 CrossRef.
- X. Yin, W. Tan, J. Zhang, Y. Lin, X. Xiao, X. Zhou, X. Li and B. R. Sankapal, J. Appl. Electrochem., 2009, 39, 147 CrossRef CAS.
- P. K. Baviskar, J. B. Zhang, V. Gupta, S. Chand and B. R. Sankapal, J. Alloys Compd., 2012, 510, 33 CrossRef CAS.
- P. Baviskar, A. Ennaoui and B. Sankapal, Sol. Energy, 2014, 105, 445 CrossRef CAS.
- L. Zhang, S. Zeng, L. Yin, C. Ji, K. Li, Y. Li and Y. Wang, New J. Chem., 2013, 37, 632 RSC.
- Y. Chen, Z. Du, W. Chen, S. Wen, L. Sun, Q. Liu, M. Sun and R. Yang, New J. Chem., 2014, 38, 1559 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nj02270c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |