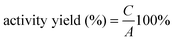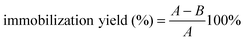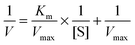Facile one-pot assembly of adhesive phenol/FeIII/PEI complexes for preparing magnetic hybrid microcapsules
Yang
Wang
,
Yun
Zhang
*,
Chen
Hou
,
Fu
He
and
Mingzhu
Liu
*
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, College of Chemistry and Chemical Engineering, Institute of Biochemical Engineering and Environmental Technology, Lanzhou University, Lanzhou 730000, China. E-mail: zhangyun@lzu.edu.cn; mzliu@lzu.edu.cn; Fax: +86-931-8912113; Fax: +86-931-8912582
First published on 16th November 2015
Abstract
Magnetic organic–inorganic hybrid microcapsules consisting of plant phenols, polyethylenimine (PEI) and FeIII ion complexes were prepared in a facile one-pot way. Porous CaCO3 microparticles were used as the hard template for the adsorption of negatively charged Fe3O4 nanoparticles, which acted as the source of magnetism for recycling. Moreover, the coated Fe3O4 nanoparticles also helped to improve the rigidness of the microcapsules away from rupturing during multiple reuses. Upon addition of tannic acid (TA), PEI and FeIII ions, the magnetic CaCO3 microparticles were coated with the adhesive complexes through chemical chelation and covalent bonding. Then the template was removed using EDTA to construct the target microcapsules. During the CaCO3 formation step, Candida rugosa lipase (CRL) was used as the biomolecule which was encapsulated in the CaCO3 microparticles. Characterizations demonstrated that the as-prepared magnetic microcapsules showed a robust structure, so that the enzyme inside could be protected physically. As a result, the magnetic hybrid microcapsules exhibited high efficiency in enzyme catalysis and stability against the environment, due to the high biocompatibility and robust structure.
1. Introduction
Naturally occurring phenols and polyphenols, which can assemble into functional materials for organic–inorganic hybrid construction, are leading advances in materials design and application.1–3 A salient feature of naturally joined hybrid materials is the inheritance of the essential properties of the organic and inorganic components, and the creation of hierarchical structures and functionality with synergistic merits via various building blocks.4 As ideal ingredients to form naturally joined hybrid materials, families of plant phenols such as epigallocatechin gallate (EGCG), epicatechin gallate (ECG), epigallocatechin (EGC), and tannic acid (TA)5 which display antioxidant, antibacterial, antimicrobial, antimutagenic, and anticarcinogenic properties have been developed for scientific applications, due to their strong solid–liquid interfacial activity. For example, the high dihydroxyphenyl (catechol) and trihydroxyphenyl (gallic acid, GA) content of TA has a strong affinity towards surfaces.2,6 Although the mussel inspired protein, polydopamine (PDA), has attracted interest in the same field because it is simple for substrate coating and modification,7 the high costs and the characteristically dark color of PDA coatings may be impediments for some practical applications. Thus, TA has been a prominent constituent for organic–inorganic film construction due to its desirable properties, such as high mechanical and thermal stability, pH-responsive disassembly, nontoxicity, and being a hundredfold less costly than dopamine.2,6,8Due to its unique structural properties, TA can make familiar interactions with a variety of materials to form metal–organic films via multiple reaction pathways, including electrostatic interactions, hydrogen bonding, hydrophobic interactions, and like many other polyphenols, metal chelation.9–11 Recently, Guo et al. reported the engineering of metal–phenolic networks to introduce a library of metal–phenolic motif materials, which provided an extensive field for the study and application of metal–organic films.8 As has been proved, the coordination between TA and FeIII ions is fast (in seconds), structurally rigid and highly biocompatible, forming a TA–FeIII film with desired properties, such as high biocompatibility, facile degradability, cyto-protectability and second-step functionality.2
Enzymes are superior over chemical catalysts because of their high effectiveness, high specificity, and green reaction conditions.12 Among the enzymes applied in biocatalysis, lipases [E.C. 3.1.1.3] have been widely studied due to their “interfacial activation” feature when catalyzing the hydrolysis of carboxylic acid esters to carboxylic acids and alcohols, and the reactions of chemo-, regio- and stereoselective esterification or trans-esterification under micro/non-aqueous conditions in an efficient and specific way.13,14 However, the industrial application of enzymes still has many challenges due to the low stability, high cost, and difficult recycling and regeneration. As a very promising strategy, the immobilization of enzymes shows lots of advantages involving enhancing the catalytic stability, feasibility for continuous operations, recycling the enzyme and a significant reduction of costs and so on.15 As a result, we decided to utilize natural phenols (TA) and inorganic materials (FeIII ions and magnetic Fe3O4 nanoparticles) to explore a high efficiency and time-saving method with a novel and much simpler route for the construction of enzyme reactors.
Herein, efforts were made to construct enzyme microcapsules using low-cost plant polyphenols (TA), polyethylenimine (PEI) and inorganic materials (FeIII ions and magnetic Fe3O4 nanoparticles) as precursors for the formation of Fe3O4/TA–FeIII–PEI hybrid microcapsules. More specifically, Fe3O4 nanoparticles were adsorbed on porous CaCO3 microparticles, then the organic–inorganic hybrid layer was formed in a one-pot step through chelation of TA and FeIII ions and the Schiff base reaction between TA and PEI. Lastly, CaCO3 templates were removed and the Fe3O4/TA–FeIII–PEI hybrid microcapsules were prepared. During the film formation process, covalent binding between PEI and TA could improve the toughness of the hybrid film more than sole chelation of TA and metal ions. The magnetic hybrid microcapsules could respond to external magnetic field stimuli, which is important for practical applications with enzyme recycling and reuse. Furthermore, the incorporated Fe3O4 nanoparticles also helped to retain an intact and rigid structure. In particular, compared to the wide application of polydopamine films, plant polyphenol-inspired coatings not only retain many of the advantages of polydopamine and deposit under similar conditions, but are also colorless and derived, in some cases, from reagents a hundredfold less costly than dopamine. Candida rugosa lipase (CRL) was immobilized in the microcapsules, accompanied with CaCO3 template formation, and the catalytic activity and stability were then investigated in detail.
2. Materials and methods
2.1 Materials
FeCl3·6H2O and FeCl2·4H2O were purchased from the AiHua Fine Chemicals Co., Ltd (China); polyethylenimine (PEI, MW ca. 800), Candida rugosa lipase (CRL, Type VII) and Bovine serum albumin (BSA) were purchased from the Sigma Chemical Co.; ethylenediamine tetraacetic acid disodium (EDTA), hydrochloric acid (HCl), and other chemicals and reagents were analytical grade, and obtained from the Tianjing Chemical Reagent Company (China).2.2 Preparation of Fe3O4/TA–FeIII–PEI hybrid microcapsules
Citric acid coated Fe3O4 nanoparticles and Fe3O4–CaCO3 microparticles were prepared according to our previous report.16 For the adhesive coating, Fe3O4 doped CaCO3 microparticles (10 mg ml−1) were suspended in deionized water which consisted of a mixture of FeIII ions (0.2 or 0.4 mg ml−1), TA (0.8 or 1.6 mg ml−1) and PEI (0.4, 0.8, or 1.6 mg ml−1) under gentle stirring. Twenty seconds later, the microparticles were collected by an external magnetic field, and washed with deionized water. Lastly, the Fe3O4/TA–FeIII–PEI hybrid microcapsules were obtained after removal of CaCO3 templates with a 0.1 M EDTA solution at room temperature. The Fe3O4/TA–FeIII hybrid microcapsules were also prepared under the same conditions without PEI addition.2.3 Assay of CRL immobilization
The efficiency of immobilization was evaluated in terms of activity yield and immobilization yield as follows:
where A is the activity of lipase added in the initial immobilization solution, B is the total activity of the residual lipase in the immobilization and washing solution after the immobilization procedure, and C is the activity of the immobilized lipase.
The relative activity (%) is the ratio between the activity of every sample and the maximum activity of the sample.
The residual activity (%) is the ratio between the activity of each sample and the initial activity of the sample.
All data used in these formulas are the averages of triplicate experiments.
The effect of temperature on the catalytic activities of free and immobilized CRL were measured by the hydrolysis of olive oil in a water bath at 37 °C for 30 min, after they were first incubated in phosphate buffer (0.1 M, pH = 7.0) among the temperature range of 20–90 °C for 30 min. The relative activity was compared.
Thermal stabilities of the free and immobilized CRL were determined by measuring the activities after incubation in phosphate buffer (0.1 M, pH = 7.0) at 50 °C for 240 min with continuous stirring. A sample was removed after a 30 min interval and tested for enzymatic activity. The residual activity was calculated as above.
where V (U mg−1) was the initial reaction rate, [S] (ml mg−1) was the initial substrate concentration, Vmax (U mg−1) was the maximum reaction rate obtained at an infinite initial substrate concentration, and Km (mg ml−1) was the Michaelis–Menten constant.
2.4 Characterization
Fourier transform infrared (FTIR) spectra were obtained in transmission mode on a FTIR spectrometer (American Nicolet Corp. Model 170-SX) using the KBr pellet technique. The morphologies of the samples were characterized by a field-emission scanning electron microscope (SEM, Hitachi S-4800, Japan) and transmission electron microscope (TEM, FEI Tecnal G2F30) equipped with energy-dispersive X-ray spectroscopy (EDX, Oxford Instrument). Magnetization measurements were performed on a vibrating sample magnetometer (LAKESHORE-7304, USA) at room temperature. The surface composition and oxidation state of the samples were determined by X-ray photoelectron spectroscopy (XPS, ESCALAB210).3. Results and discussion
3.1 Preparation and characterization of hybrid microcapsules
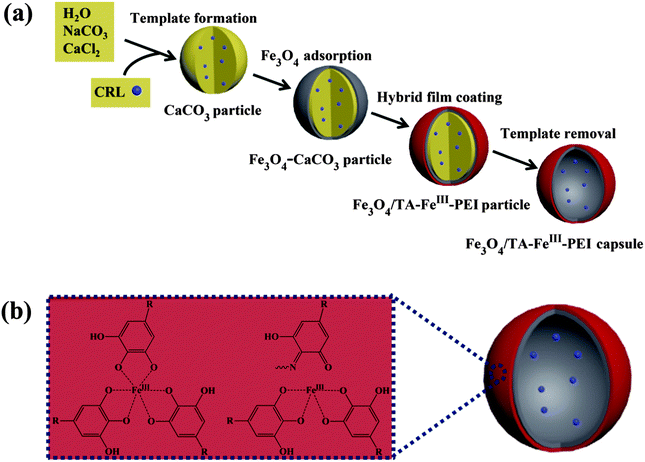
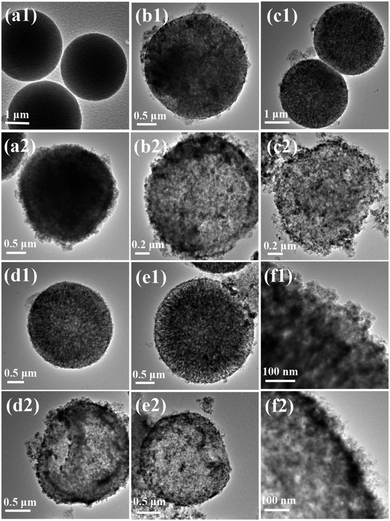
Fig. 1(a) shows the synthesis process of Fe3O4/TA–FeIII–PEI hybrid microcapsules, which can be divided into four steps: (1) preparation of CaCO3 microparticles using CaCl2 and NaCO3 aqueous solutions, thus, biomolecules (such as enzymes) can be encapsulated into the CaCO3 templates; (2) adsorption of citric acid coated Fe3O4 nanoparticles by electrical and physical interactions. Our previous work has reported the mechanism of preparing the magnetic CaCO3 template, where negatively charged Fe3O4 nanoparticles with a diameter of about 10–15 nm can be adsorbed into the lumen or onto the surface of CaCO3 microparticles. (3) Coating of organic–inorganic hybrid film onto magnetic CaCO3 microparticles with plant phenol (TA), metal ions (FeIII) and PEI via a biomimetic route; (4) removal of the CaCO3 sacrificial templates through EDTA treatment. Compared with many other related works for the preparation of organic–inorganic hybrid microcapsules,18–20 our work aims at designing an easily recyclable, low cost and time-saving method for the fabrication of biological capsules. During the outer layer formation process, the galloyl groups from TA can react with FeIII ions to form an octahedral complex, and the catechol from TA can be cross-linked with PEI to form hydrogels (Fig. 1(b)),21 thus the adhesive film can be produced.Fig. 2 demonstrates morphologies of the prepared microparticles and microcapsules. As can be seen from Fig. 2(a1), the as-prepared CaCO3 microparticles possessed a uniform, spherical shape with a diameter of about 3 μm. After adsorption of Fe3O4 nanoparticles, many tiny nanoparticles (with a diameter of about 10–15 nm) were assembled on CaCO3 microparticles to cover the original smooth surface (Fig. 2(a2)), indicating the successful preparation of Fe3O4–CaCO3 microparticles. Several batches of Fe3O4/TA–FeIII–PEI microcapsules were prepared to vary the concentration of TA, FeIII ions and PEI in the reaction mixtures as follows: TA 0.2 mg ml−1, FeIII ions 0.8 mg ml−1 and PEI 0.4 mg ml−1 (Fe3O4/TA0.2–FeIII0.8–PEI0.4), TA 0.2 mg ml−1, FeIII ions 0.8 mg ml−1 and PEI 0.8 mg ml−1 (Fe3O4/TA0.2–FeIII0.8–PEI0.8), and TA 0.4 mg ml−1, FeIII ions 1.6 mg ml−1 and PEI 1.6 mg ml−1 (Fe3O4/TA0.4–FeIII1.6–PEI1.6). For comparison, TA 0.2 mg ml−1 and FeIII ions 0.8 mg ml−1 repeated coating was performed 3 times (Fe3O4/(TA0.2–FeIII0.8)3). As can be seen from Fig. 2(b1), (c1), (d1) and (e1), after coating with the hybrid layer, the microparticles held a uniform surface and spherical structure, which were not affected by the interactions among TA, FeIII ions and PEI. After template removal, the hollowed microcapsules were formed and no obvious collapse appeared. The slight creases of the wall made it have a pisiform appearance (Fig. 2(d2) and (e2)). The wall of the hybrid microparticles became thicker and rougher with the increase of the coating concentration, and the spherical morphologies of the hybrid microparticles became distinct and intact (Fig. 2(b2), (c2), (d2) and (e2)). Besides, it can be clearly observed that Fe3O4 nanoparticles were wrapped in the hybrid layer after the CaCO3 microparticle dissolution (Fig. 2(f1) and (f2)). As for the microcapsule Fe3O4/(TA0.2–FeIII0.8)3, we also obtained the expected result (Fig. 2(b2)), though it would not be the optimized option for enzyme immobilization due to the weaker wall structure without PEI doping. Thus, it can be verified that the hollow and robust Fe3O4/TA–FeIII–PEI microcapsules were successfully achieved.
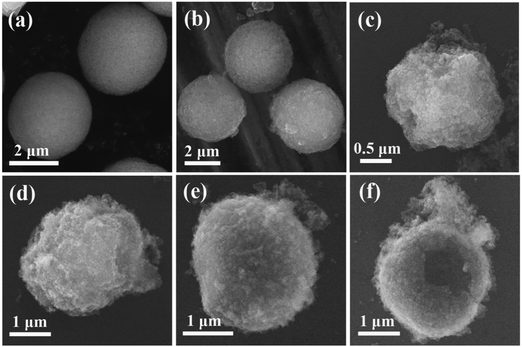
To further observe the surface features of the microparticles and microcapsules, SEM images were conducted. As shown in Fig. 3, after adsorption of the negatively charged Fe3O4 nanoparticles, the surface of the CaCO3 microparticles became rough and coarse (Fig. 3(a) and (b)), which was consistent with the TEM images. It further indicated that the Fe3O4 nanoparticles were distributed uniformly on the surface of the CaCO3 microparticles. In the cases of the as-prepared hybrid microcapsules, all of them possessed a plump structure due to the inlaid Fe3O4 nanoparticles (Fig. 3(c)–(f)). Moreover, an absence of or decrease in the concentration of PEI would lead to an incompact wall structure (Fig. 3(c) and (d)). When the concentration of TA, FeIII ions and PEI doubled, the superfluous complex was adhered to the side of the microcapsules (Fig. 3(f)). Uniform and tidy microcapsules were obtained with the premium conditions for Fe3O4/TA0.2–FeIII0.8–PEI0.8 (Fig. 3(e)). In addition, compared with the PDA–Fe3O4 microcapsules we made,16 the newly prepared Fe3O4/TA–FeIII–PEI microcapsules had a more micromesh and homogeneous wall structure, attributed to the rapid coating process.
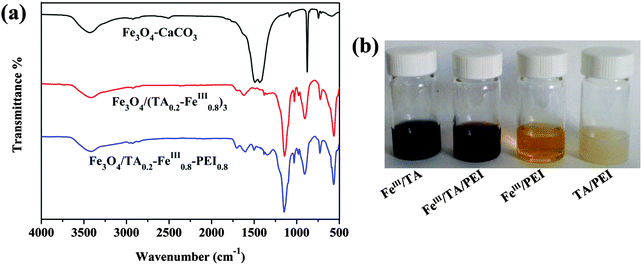
FTIR was conducted to examine the functional groups of the prepared magnetic hybrid materials. As shown in Fig. 4(a), the characteristic peak at 580 cm−1 of Fe3O4–CaCO3 can be attributed to the lattice absorption of the Fe3O4 nanoparticles, the absorption bands appearing at 1491/1434 cm−1, 1087 cm−1, and 876 cm−1 can be assigned to the vibrations of the carbonate group in CaCO3. In the spectrum of Fe3O4/(TA0.2–FeIII0.8)3, the decreased intensity of the C–OH stretching peak of TA at around 1250 cm−1 shows evidence that the phenolic groups are coordinated with FeIII ions.8 The same situation is also observed in the Fe3O4/TA0.2–FeIII0.8–PEI0.8 microcapsule. The absorption peak at 1622 cm−1 is attributed to the o-benzoquinone derivative arising from the oxidation of TA22 which evidenced the rationality of the step reaction with PEI. After reaction with PEI, the old peak of Fe3O4/(TA0.2–FeIII0.8)3 at 1622 cm−1 disappeared and the new peak of Fe3O4/TA0.2–FeIII0.8–PEI0.8 at 1601 cm−1 represented the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, which successfully confirmed the reaction between TA and PEI.
N, which successfully confirmed the reaction between TA and PEI.
To further confirm the reaction phenomenon of the compounds, images of the different reacting mixtures are displayed (Fig. 4(b)). Under neutral conditions, the mixture of FeIII ions and TA solution transformed into a dark blue solution due to the formation of tris-pyrogallato iron complexes. After PEI was added into the above mixture, it turned into a prunosus sticky liquid immediately. The mixture of TA and PEI became milky white hydrogels immediately as they were mixed. Thus the reactions among FeIII ions, TA and PEI were proven to be fast, visually.
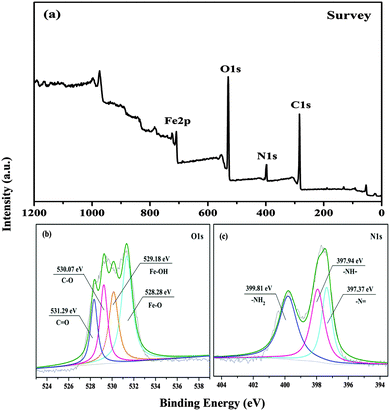
XPS was performed to identify the presence of metal ions and PEI in the microcapsule shells (Fig. 5). As displayed in Fig. 5, C1s, O1s, N1s, and Fe2p peaks were detected in the survey spectra, and this is in agreement with the hybrid wall compositions. From the O1s photoelectron spectrum (Fig. 5(b)), the peaks at ∼531.29, ∼530.07, ∼529.18, and ∼528.28 eV can be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O, Fe–OH, and Fe–O species, respectively. C
O, C–O, Fe–OH, and Fe–O species, respectively. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O corresponded to the o-benzoquinone derived from TA and Fe–O/Fe–OH arose from the coordination between TA and FeIII ions. From the N1s spectra (Fig. 5(c)), the peaks appearing at ∼399.81, ∼397.94, and ∼397.37 eV can be attributed to –NH2, –NH–, and –N
O corresponded to the o-benzoquinone derived from TA and Fe–O/Fe–OH arose from the coordination between TA and FeIII ions. From the N1s spectra (Fig. 5(c)), the peaks appearing at ∼399.81, ∼397.94, and ∼397.37 eV can be attributed to –NH2, –NH–, and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) , respectively, which suggested successful chemical crosslinking between TA and PEI.
, respectively, which suggested successful chemical crosslinking between TA and PEI.
 | ||
| Fig. 5 XPS of Fe3O4/TA0.2–FeIII0.8–PEI0.8 microcapsules: (a) survey spectrum, (b) O1s core-level spectrum, and (c) N1s core-level spectrum. | ||
The hysteresis loops of the prepared magnetic nanoparticles are shown in Fig. 6. From Fig. 6 we can see that the saturation magnetization (MS) values are about 66.67 emu g−1 for the CA–Fe3O4 nanoparticles, and 34.69 emu g−1 for the Fe3O4/TA0.2–FeIII0.8–PEI0.8 microcapsules, respectively. As a result, the microcapsules used for the CRL immobilization could be separated quickly and easily from the reaction medium with an external field. Compared to the magnetic PDA microcapsules we previously made,16 the as-prepared microcapsules possessed a significantly higher saturation magnetization, and they would obtain an improved efficiency for the immobilized enzyme recycling and reuse. Furthermore, there is no hysteresis in the magnetization, with both remanence and coercivity being zero, indicating that the as-prepared Fe3O4/TA0.2–FeIII0.8–PEI0.8 microcapsules are superparamagnetic at room temperature.23
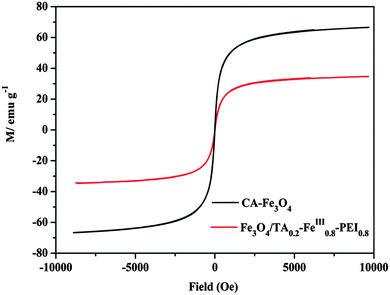 | ||
| Fig. 6 Magnetic hysteresis loops of CA–Fe3O4 nanoparticles, and Fe3O4/TA0.2–FeIII0.8–PEI0.8 microcapsules. | ||
3.2 Application of hybrid microcapsules for enzyme immobilization and enzyme catalysis
For the CRL immobilization, Fe3O4/TA0.2–FeIII0.8–PEI0.8 microcapsules were used. As shown in Fig. 7, with the increase of CRL concentration, the encapsulation efficiency decreased monotonically and the immobilized enzyme exhibited an increased activity, simultaneously. At an enzyme concentration between 1.0 and 1.5 mg ml−1, the relative activity of immobilized CRL reached up to 97%. In addition, to evaluate the enzymatic properties, the kinetics of the immobilized CRL with a concentration of 1.0 mg ml−1 in Fe3O4/TA0.2–FeIII0.8–PEI0.8 microcapsules were calculated from an enzymatic assay by the Michaelis–Menten enzyme kinetics model (Table 1). As displayed in Table 1, in contrast to free CRL, the higher Km for CRL–Fe3O4/TA0.2–FeIII0.8–PEI0.8 indicated a lower affinity of the CRL towards the substrates because of additional diffusion resistance after encapsulation. The lowered Vmax for the immobilized CRL indicated that the microencapsulation restricted the activity of the enzyme, which was possibly due to the inner diffusion lowering the accessibility of substrates to the active sites on CRL.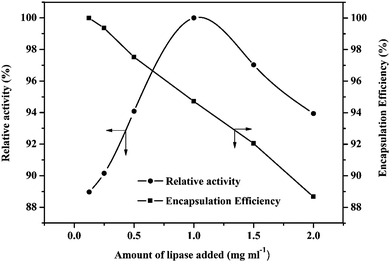 | ||
| Fig. 7 Effect of enzyme amount and encapsulation efficiency of Fe3O4/TA0.2–FeIII0.8–PEI0.8 microcapsules. | ||
| K m (mg ml−1) | V max (U mg−1) | |
|---|---|---|
| Free CRL | 0.43 | 6.05 |
| CRL–Fe3O4/TA0.2–FeIII0.8–PEI0.8 | 0.54 | 5.10 |
The pH stabilities of CRL–Fe3O4/TA0.2–FeIII0.8–PEI0.8 microcapsules and free CRL are compared in Fig. 8(a). The CRL–Fe3O4/TA0.2–FeIII0.8–PEI0.8 kept >71% of its initial activity at pH 5.0–8.0, and showed a decline below pH 4.0 and above 9.0. In comparison, the free CRL retained 29% of the relative activity at pH 3.0 and 42% of the relative activity at pH 9.0. In addition, CRL–Fe3O4/TA0.2–FeIII0.8–PEI0.8 showed a broader pH scope. As a result, the Fe3O4/TA0.2–FeIII0.8–PEI0.8 used for CRL immobilization exhibited a markedly improved adaptability in a wide pH range, which can greatly expand the applications of lipase in chemical and biocatalytic industries. This phenomenon can be explained by the buffering effect of the hybrid layer of the microcapsules. The abundant –OH/–O− pairs on TA and the –NH2/–NH3+ pairs on PEI could tune the local pH value under basic or acidic conditions. Therefore, the CRL near the hybrid walls would stay in the buffer region, against environmental mutation, to maintain the activity of the CRL due to the positive influence of the TA and PEI ingredients.
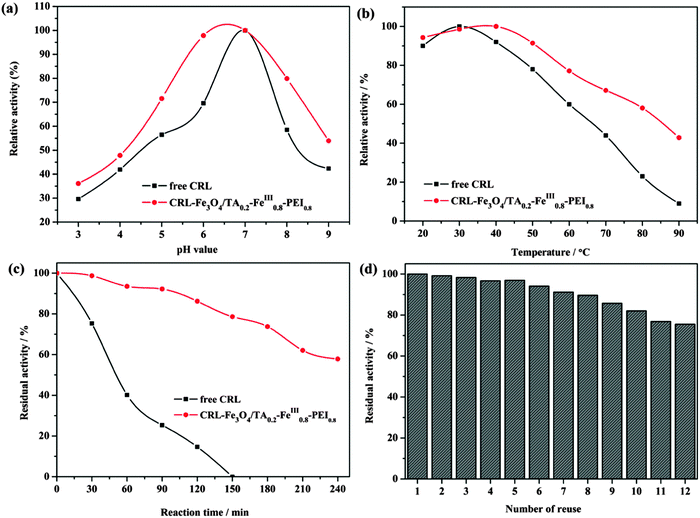 | ||
| Fig. 8 Effect of (a) pH value, (b) temperature, (c) stability, and (d) number of reuses of free and immobilized CRL. | ||
When the hydrolysis for olive oil emulsion was operated at a series of temperature ranges, the immobilized CRL showed enhanced relative activities compared to the free CRL (Fig. 8(b)). Compared with free lipase, the immobilized CRL kept its relative activity up to 80% in the temperature range of 20–60 °C and exhibited more than 60% of its relative activity at 90 °C, revealing a more superb heat endurance than that of the free lipase. It seemed that the interaction between the positively charged PEI and negatively charged CRL molecules would prevent enzymes from denaturing at high temperatures.24
Strong thermal stability is one of the critical factors in industrial applications. Fig. 8(c) shows the residual activity of the free and immobilized lipase at 50 °C for the hydrolysis reaction of olive oil. From Fig. 8(c) we can see that after being incubated for 150 min, free CRL lost its activity while immobilized CRL retained its residual activity as high as 58%, until the incubation time reached 240 min. This phenomenon probably resulted from the excellent thermal stability, good mechanical hardness and high biocompatibility of the prepared organic–inorganic hybrid microcapsules, which protected the CRL from unfolding and conformational transitions.
A high reusability of lipase is critical for potential applications in industry. As presented in Fig. 8(d), the immobilized enzyme kept a high activity at 75% after reuse 12 times, due to the sturdy stability of the hybrid microcapsules which effectively ameliorated the denaturation and leakage of enzyme under multiple reaction circles. Moreover, the loss of microcapsules during each recycle cannot be ignored. As a result, the layer assembled by Fe3O4 and TA–FeIII–PEI had a high biocompatibility and strong mechanical properties, which could effectively mitigate the deactivation, leaching and embedding of the encapsulated enzymes.
4. Conclusion
A facile and easy method was developed to prepare magnetic metal–polyphenol–polyethylenimine hybrid microcapsules by combining plant phenol chelation with covalent bonding. The hybrid wall with a negligible cytotoxicity provided an appropriate environment for the enzyme inside. The plant phenol (tannic acid) constructed microcapsule walls exhibited excellent characteristics such as a high biocompatibility, second-step functionality, being colorless, low cost and time-saving (in seconds). Meanwhile, the polyethylenimine motifs in the hybrid layer are in charge of enhancing the toughness of the hybrid layer. Significantly, the incorporated Fe3O4 nanoparticles had a practical dual role in the microcapsule formation and application; both as the recyclable ingredient and the powerful skeleton to retain the intact, rigid, hollowed structure during the multiple reuse. The formulated hybrid magnetic microcapsules exhibited a high encapsulation efficiency for Candida rugosa lipase and improved activity in catalysis compared with the free lipase. Therefore, the method we introduced may be extended to prepare many other hybrid materials and applied in bio-fields.Acknowledgements
The authors thank the financial supports from the National Natural Science Foundation of China (No. 21374045, 21074049). This paper is dedicated to the memory of Prof. Yanfeng Li, who passed away recently.References
- H. Lee, N. F. Scherer and P. B. Messersmith, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12999–13003 CrossRef CAS PubMed.
- T. S. Sileika, D. G. Barrett, R. Zhang, K. H. A. Lau and P. B. Messersmith, Angew. Chem., Int. Ed., 2013, 52, 10766–10770 CrossRef CAS PubMed.
- E. Haslam, Practical polyphenolics: from structure to molecular recognition and physiological action, Cambridge University Press, 1998 Search PubMed.
- C. Y. Tian, C. H. Zhang, H. Wu, Y. X. Song, J. F. Shi, X. L. Wang, X. K. Song, C. Yang and Z. Y. Jiang, J. Mater. Chem. B, 2014, 2, 4346–4355 RSC.
- T. J. Kim, J. L. Silva, M. K. Kim and Y. S. Jung, Food Chem., 2010, 118, 740–746 CrossRef CAS.
- H. Ejima, J. J. Richardson, K. Liang, J. P. Best, M. P. Koeverden, G. K. Such, J. W. Cui and F. Caruso, Science, 2013, 341, 154–157 CrossRef CAS PubMed.
- Y. L. Liu, K. L. Ai and L. H. Lu, Chem. Rev., 2014, 114, 5057–5115 CrossRef CAS PubMed.
- J. L. Guo, Y. Ping, H. Ejima, K. Alt, M. Meissner, J. J. Richardson, K. Peter, D. Elverfeldt, C. E. Hagemeyer and F. Caruso, Angew. Chem., Int. Ed., 2014, 53, 5546–5551 CrossRef CAS PubMed.
- H. I. Oh, J. E. Hoff, G. S. Armstrong and L. A. Haff, J. Agric. Food Chem., 1980, 28, 394–398 CrossRef CAS.
- E. Costa, M. Coelho, L. M. Ilharco, A. Aguiar-Ricardo and P. T. Hammond, Macromolecules, 2011, 44, 612–621 CrossRef CAS.
- T. Shutava, M. Prouty, D. Kommireddy and Y. Lvov, Macromolecules, 2005, 38, 2850–2858 CrossRef CAS.
- J. Garcia, Y. Zhang, H. Taylor, O. Cespedes, M. E. Webb and D. J. Zhou, Nanoscale, 2011, 3, 3721–3730 RSC.
- K. Jaeger and M. Reetz, Trends Biotechnol., 1998, 16, 396–403 CrossRef CAS PubMed.
- Y. Omprakash and I. Toyoko, Biomacromolecules, 2005, 6, 2809–2814 CrossRef PubMed.
- X. Liu, X. Chen, Y. F. Li, X. Y. Wang, X. M. Peng and W. W. Zhu, ACS Appl. Mater. Interfaces, 2012, 4, 5169–5178 CAS.
- C. Hou, Y. Wang, H. Zhu and L. C. Zhou, J. Mater. Chem. B, 2015, 3, 2883–2891 RSC.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- J. F. Shi, W. Y. Zhang, S. H. Zhang, X. L. Wang and Z. Y. Jiang, J. Mater. Chem. B, 2015, 3, 465–474 RSC.
- W. Y. Zhang, J. F. Shi, X. L. Wang, Z. Y. Jiang, X. K. Song and Q. H. Ai, J. Mater. Chem. B, 2014, 2, 1371–1378 RSC.
- X. L. Wang, Z. Y. Jiang, J. F. Shi, Y. P. Liang, C. H. Zhang and H. Wu, ACS Appl. Mater. Interfaces, 2012, 4, 3476–3483 CAS.
- M. Krogsgaard, A. Andersen and H. Birkedal, Chem. Commun., 2014, 50, 13278–13281 RSC.
- A. Dutta and S. K. Dolui, Appl. Surf. Sci., 2011, 257, 6889–6896 CrossRef CAS.
- K. E. Mooney, J. A. Nelson and M. Wagner, Chem. Mater., 2004, 16, 3155–3161 CrossRef CAS.
- X. L. Wang, J. F. Shi, Z. Li, S. H. Zhang, H. Wu, Z. Y. Jiang, C. Yang and C. Y. Tian, ACS Appl. Mater. Interfaces, 2014, 6, 14522–14532 CAS.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |