Synthesis, and antitubercular and antimicrobial activity of 1′-(4-chlorophenyl)pyrazole containing 3,5-disubstituted pyrazoline derivatives†
N.
Harikrishna
ab,
Arun M.
Isloor
*ac,
K.
Ananda
d,
Abdulrahman
Obaid
e and
Hoong-Kun
Fun
ef
aMedicinal Chemistry Laboratory, Department of Chemistry, National Institute of Technology Karnataka, Surathkal, Mangalore 575025, India. E-mail: isloor@yahoo.com
bSeQuent Scientific Limited, Baikampady, Mangalore 575011, India
cDepartment of Chemistry, Indian Institute of Information Technology Dharwar, India
dBiological Sciences, Poornaprajna Institute of Scientific Research, Bangalore 562110, India
eDepartment of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh 11451, Kingdom of Saudi Arabia
fX-ray Crystallography Unit, School of Physics, Universiti Sains Malaysia, Penang 11800, Malaysia
First published on 10th November 2015
Abstract
A new series of 1′-(4-chlorophenyl)-5-(substituted aryl)-3′-(substituted aryl)-3,4-dihydro-2H,1′H-[3,4′]bipyrazolyl derivatives (6a–e, 8a–e, 10a–e) have been synthesized, characterized and screened for antimicrobial and antitubercular activity. Among the synthesized compounds, the minimum inhibition concentration of 10e was found to be as low as 1.56 μg ml−1 and that of 10c was 6.25 μg ml−1 as compared to the standard anti-tb drugs pyrazinamide and streptomycin.
Mycobacterium tuberculosis is one of the most dangerous bacteria, which causes infectious diseases and remains out of control in many developing countries.1 It is a remarkable pathogenic bacteria, that latently infects inside the body.2 The control of tuberculosis is the most challenging in the case of the multi-drug resistance strains of Mycobacterium tuberculosis. The spread of multidrug-resistant TB (MDR-TB) and the appearance of extensively drug-resistant TB (XDR-TB) pose new challenges for the prevention, treatment and control of this deadly disease. Fewer new drugs have been approved to treat tuberculosis with very long and complicated therapies.3 Drug-resistant TB can be treated with multidrug combinations over an average span of six months.2
The azole class of drug derivatives and its research have played an important role in medicinal chemistry. Pyrazoles are well known nitrogen containing heterocyclic compounds. As per the literature, pyrazole and its derivatives represent one of the most desirable classes of compounds with a wide variety of pharmacological activities viz., antitubercular,4,5 antifungal,6 antidepressant,7,8 antimicrobial,9 anti-angiogenic,10 analgesic,11 anticancer12 and anticonvulsant.13 Moreover, pyrazoles containing pyrazoline derivatives are important drug molecules and exhibit important pharmacophore activities viz. antioxidant,14 antimicrobial15 and antidiabetic.16 Some of the literature reveals that the presence of substituted phenyl, i.e. 4-chlorophenyl or benzene sulfonamide, at the first position of pyrazole causes enhanced biological activities.17 Based on the above considerations, we hereby report the synthesis of 1-(4-chlorophenyl)pyrazole containing pyrazoline derivatives and their antitubercular activity against Mycobacterial tuberculosis.
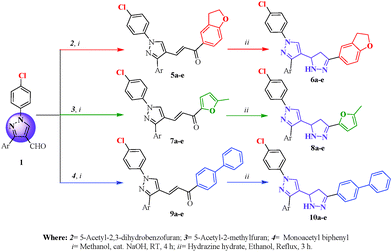
The targeted compounds 1′-(4-chlorophenyl)-5-(2,3-dihydrobenzofuran-5-yl)-3′-(substituted aryl)-3,4-dihydro-2H, 1′H-[3,4′]bipyrazolyl (6a–e), 1′-(4-chlorophenyl)-5-(5-methylfuran-2-yl)-3′-(substituted aryl)-3,4-dihydro-2H,1′H-[3,4′]bipyrazolyl (8a–e) and 5-biphenyl-4-yl-1′-(4-chlorophenyl)-3′-(substituted aryl)-3,4-dihydro-2H,1′H-[3,4′]bipyrazolyl (10a–e) were synthesized according to the steps outlined in Scheme 1.
The basic pyrazole skeleton, i.e. 1,3-disubstituted pyrazole-4-carbaldehydes 1, was synthesized by the Vilsmeier–Haack reaction in moderate to good yields.9 The other key starting materials 5-acetyl-2,3-dihydrobenzofuran 2, 2-acetyl-5-methylfuran 3 and 4-monoacetylbiphenyl 4 were prepared as per the reported literature18 and confirmed using IR and NMR. 1,3-disubstituted pyrazole-4-carbaldehyde 1 reacted with 2, 3 and 4 individually to give chalcone derivatives (5a–e, 7a–e and 9a–e, respectively) which on reacting with hydrazine hydrate in ethanol media under reflux temperature gave 6a–e, 8a–e and 10a–e in reasonable yields.
The formation of 6a–e, 8a–e and 10a–e was confirmed by recording their IR, 1H NMR, 13C NMR and mass spectra. The IR analysis of compound 6a showed a peak at 3322 cm−1 which was due to a NH group. The absorption band at 1607 cm−1 was due to a –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group and –C
N group and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching was observed at 1497 cm−1. The 1H NMR spectrum of 6a in DMSO-d6 solvent shows a triplet at δ 2.90–2.96 which was attributed to the HA proton, and a triplet at δ 3.42–3.49 which was due to the HB proton, of the pyrazoline ring. The characteristic peak of the –NH proton of pyrazoline was observed as a singlet at δ 8.62. The detailed 1H NMR resonances are summarized in the experimental section. The mass spectrum of 6a shows a molecular ion peak at m/z = 441.2 (M+). This in turn confirmed the formation of a compound with the molecular formula of C26H21ClN4O. The other compounds 8a–e and 10a–e are explained in the ESI.† The characterization data of the newly synthesized compounds 6a–e, 8a–e and 10a–e are presented in Table 1.
C stretching was observed at 1497 cm−1. The 1H NMR spectrum of 6a in DMSO-d6 solvent shows a triplet at δ 2.90–2.96 which was attributed to the HA proton, and a triplet at δ 3.42–3.49 which was due to the HB proton, of the pyrazoline ring. The characteristic peak of the –NH proton of pyrazoline was observed as a singlet at δ 8.62. The detailed 1H NMR resonances are summarized in the experimental section. The mass spectrum of 6a shows a molecular ion peak at m/z = 441.2 (M+). This in turn confirmed the formation of a compound with the molecular formula of C26H21ClN4O. The other compounds 8a–e and 10a–e are explained in the ESI.† The characterization data of the newly synthesized compounds 6a–e, 8a–e and 10a–e are presented in Table 1.
| Compounds | Ar1 | Ar2 | Mol. F/mol. wt | M.P. (°C) | Color & nature |
|---|---|---|---|---|---|
| 6a | Phenyl | 5-(2,3-Dihydrobenzofuran) | C26H21ClN4O/440.92 | 156–157 | White solid |
| 6b | 4-Methylphenyl | 5-(2,3-Dihydrobenzofuran) | C27H23ClN4O/454.95 | 151–153 | Off-white solid |
| 6c | 4-Methoxyphenyl | 5-(2,3-Dihydrobenzofuran) | C27H23ClN4O/470.95 | 132–134 | Off-white solid |
| 6d | 4-Chlorophenyl | 5-(2,3-Dihydrobenzofuran) | C26H20Cl2N4O/475.37 | 171–172 | White solid |
| 6e | 2-Thiophene | 5-(2,3-Dihydrobenzofuran) | C24H19ClN4OS/446.95 | 140–141 | White solid |
| 8a | Phenyl | 5-(2-Methylfuran) | C23H19ClN4O/402.88 | 144–145 | White solid |
| 8b | 4-Methylphenyl | 5-(2-Methylfuran) | C24H21ClN4O/416.90 | 206–208 | Off-white solid |
| 8c | 4-Methoxyphenyl | 5-(2-Methylfuran) | C24H21ClN4O2/432.9 | 156–157 | Off-white solid |
| 8d | 4-Chlorophenyl | 5-(2-Methylfuran) | C23H18Cl2N4O/437.32 | 198–200 | Off-white solid |
| 8e | 2-Thiophene | 5-(2-Methylfuran) | C21H17ClN4OS/408.9 | 186–187 | Off-white solid |
| 10a | Phenyl | 4-(Biphenyl) | C30H23ClN4/474.98 | 164–165 | Off-white solid |
| 10b | 4-Methylphenyl | 4-(Biphenyl) | C31H25ClN4/489.01 | 157–158 | Off-white solid |
| 10c | 4-Methoxyphenyl | 4-(Biphenyl) | C31H25ClN4O/505.01 | 160–162 | Off-white solid |
| 10d | 4-Chlorophenyl | 4-(Biphenyl) | C30H22Cl2N4/509.43 | 184–186 | White solid |
| 10e | 2-Thiophene | 4-(Biphenyl) | C28H21ClN4S/481.01 | 217–219 | Off-white solid |
The antimicrobial activity of all the synthesized compounds was screened against Staphylococcus aureus (Gram positive bacteria), Mycobacterium smegmatis (tubercular variant), and Candida albicans (fungi). The minimum inhibitory concentration (MIC) of all the organisms tested at different concentrations ranged from 500 to 3.9 μg ml−1. Most of the compounds exhibited activity with the MIC ranging from 62.5 to 7.8 μg ml−1. The structure activity relationship of the synthesized compounds was explained based on the MIC. Compound 10e showed the lowest MIC against all the tested organisms due to the presence of a 2-thiophene group at the third position of the pyrazole ring and biphenyl at the third position of the pyrazoline ring. The second lowest MIC was obtained for the following compounds: 6a due to the presence of 2,3-dihydrobenzofuran at the third position of the pyrazoline ring and a phenyl group at the third position of pyrazole, compound 10c due to biphenyl at the third position of pyrazoline and 4-methoxyphenyl at the third position of pyrazole and compound 10d due to biphenyl substitution on pyrazoline at the third position and p-chlorophenyl at the third position of the pyrazole ring. Other compounds 6d, 6e, 8b, 8c and 8d showed moderate activity with the MIC values ranging from 62.5 to 125 μg ml−1 against the tested organisms. The antimicrobial results of the final compounds 6a–e, 8a–e and 10a–e are tabulated in Table 2.
| Synthesized compound | MIC in μg ml−1 | |||
|---|---|---|---|---|
| M. smegmatis | S. aureus | C. albicans | M. tuberculosis | |
| ABS; antibacterial standard ciprofloxacin; AFS; antifungal standard fluconazole; PZA; anti-tb standard pyrazinamide; —: no inhibition detected; control; dimethylsulfoxide. | ||||
| 6a | 15.6 | 15.6 | 31.25 | 12.5 |
| 6b | 500 | 500 | 500 | 50 |
| 6c | 500 | 500 | 500 | 50 |
| 6d | 62.5 | 62.5 | 62.5 | 25 |
| 6e | 62.5 | 125 | 62.5 | 25 |
| 8a | 250 | 125 | 125 | 25 |
| 8b | 62.5 | 125 | 62.5 | 25 |
| 8c | 62.5 | 62.5 | 62.5 | 25 |
| 8d | 62.5 | 62.5 | 62.5 | 25 |
| 8e | 125 | 31.25 | 125 | 50 |
| 10a | 125 | 125 | 125 | 50 |
| 10b | 500 | 500 | 500 | 50 |
| 10c | 15.6 | 15.6 | 31.25 | 6.25 |
| 10d | 15.6 | 62.5 | 31.25 | 50 |
| 10e | 7.8 | 15.6 | 31.25 | 1.56 |
| ABS | <5 | <5 | — | 3.12 |
| AFS | — | — | <10 | — |
| PZA | — | — | — | 3.12 |
| Streptomycin | — | — | — | 6.25 |
The MIC against the pathogenic bacteria Mycobacterium tuberculosis H37Rv at different concentrations ranging from 100 to 0.8 μg ml−1 is also represented in Table 2. Most of the compounds exhibited activity with the MIC ranging from 50 μg ml−1 to 1.56 μg ml−1. Compound 10e showed the lowest MIC (1.56 μg ml−1) among all other compounds and it was more active than the standard first-line anti-tb drug pyrazinamide (MIC value of 3.12 μg ml−1). The second lowest MIC against the tested microorganisms (6.25 μg ml−1) was obtained for compound 10c and it was similarly active to the standard anti-tb drug streptomycin (MIC value of 6.25 μg ml−1). Compound 6a showed the third lowest MIC (12.5 μg ml−1). Other compounds 6d, 6e, 8a, 8b, 8c and 8d showed moderate activity with an MIC value of 25 μg ml−1 against tested Mycobacterium tuberculosis. This indicates that most of the pyrazole containing pyrazoline compounds with 5-methylfuran substitution at the third position are able to show moderate activity against M. Tuberculosis. Compounds 6b, 6c, 8e, 10a, 10b and 10d show less activity against the tested organisms with an MIC value of 50 μg ml−1.
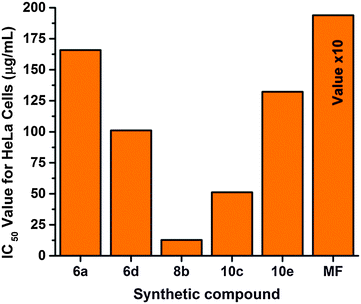
An in vitro cytotoxicity study was carried out using HeLa cells at Stellixir Biotech Pvt. Ltd, Bangalore. The five compounds 6a, 6d, 8b, 10c and 10e which had the highest to moderate antimicrobial and antituberculosis activity were tested for cytotoxicity with HeLa cells, represented in Fig. 1. The IC50 values of the synthetic compounds were found to be moderately effective for the HeLa cells. The control cells which are not treated with any compound show 100% viability. One of the synthetic compounds, 8b, shows the highest cytotoxicity (12.83 μg ml−1) among the tested compounds. The compounds which have cytotoxicity below a 50 μg ml−1 IC50 value are usually considered as toxic compounds.
In conclusion, compounds 6a–e, 8a–e and 10a–e were synthesized, characterized and investigated for their in vitro antimicrobial activity by the Resazurin reduction method. These compounds were used for determining the MIC in 96 well microplates,19 and antitubercular activity using a microplate alamar blue assay (MABA) method,20 and proved to be very good antimicrobial and antitubercular agents. The results are consistent with specific substitution utilised in tuberculosis chemotherapy and antimicrobial agents. Compounds 10e and 10c showed the best screening results among all the synthesized compounds. This indicates that the newly synthesized pyrazole containing pyrazoline compounds might emerge as antituberculosis drugs.
Experimental
All the chemicals were purchased from Sigma Aldrich and Spectrochem-India. Melting points were determined using the open capillary method and are uncorrected. The IR spectra (in KBr pellet) were recorded using a Perkin-Elmer FTIR-4000–400 cm−1 spectrophotometer. The NMR spectra were obtained using a Bruker Avance-400 spectrometer (400 MHz) for 1H NMR and 13C NMR using tetramethylsilane (TMS) as the internal standard. The mass spectrum was recorded using a LC-MS Applied biosystems MDS SCIEX-API 4000 spectrometer. Elemental analysis was performed using a Flash EA 1112 series CHNS-O analyzer. The completion of the reaction was checked using thin layer chromatography (TLC) with ready-made aluminium sheets (Merck F254). The names of the structures were mentioned as per chemdraw.A mixture of 1,3-disubstituted pyrazole-4-carbaldehyde (1a–e) (0.01 mol) and an acetyl derivative (2, 3 and 4) (0.91 g, 0.01 mol) in methanol (10 ml) was stirred in the presence of 10% sodium hydroxide solution (2 ml) at ambient temperature for 5 h. The resultant yellow color reaction mass was filtered and washed with 5 ml of ethanol to get a chalcone intermediate (5a–e, 7a–e and 9a–e) in reasonably good yields (80–95%). The compound (5a–e, 7a–e and 9a–e) (0.005 mol) was taken in ethanol (10 ml) and an excess amount of hydrazine hydrate (1.3 g, 0.015 mol) was added. The reaction mixture was heated at reflux temperature for 2 h and the reaction was monitored using TLC [hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetate (4
ethylacetate (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)]. The reaction mass was cooled to room temperature and stirred for 0.5 h. The solid product was filtered and washed with ethanol to get the final compound (6a–e, 8a–e and 10a–e).
1)]. The reaction mass was cooled to room temperature and stirred for 0.5 h. The solid product was filtered and washed with ethanol to get the final compound (6a–e, 8a–e and 10a–e).
1′-(4-Chlorophenyl)-5-(2,3-dihydrobenzofuran-5-yl)-3′-phenyl-3,4-dihydro-2H,1′H-[3,4′]bipyrazolyl (6a)
IR (KBr νmax cm−1): 3322 (N–H str), 3064 (Ar–H str), 2919 (C–H aliphatic str), 1607 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1497 (C
N str), 1497 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 824 (C–Cl str); 1H NMR (400 MHz, DMSO-d6, ppm): δ 2.90–2.96 (t, 1H, HA, J = 12.8 Hz), 3.19 (t, 2H, –CH2), 3.42–3.49 (t, 1H, HB, J = 13.6 Hz), 4.56 (t, 2H, –CH2), 4.89–4.92 (t, 1H, HX, J = 10.4 Hz), 6.77 (m, 1H, Ar–H), 7.33–7.36 (m, 2H, Ar–H), 7.43–7.56 (m, 6H, Ar–H), 7.77–7.95 (m, 4H, Ar–H), 8.62 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6, ppm): δ 160.5, 151.2, 138.8, 133.2, 130.7, 130.1, 129.9, 129.7, 129.1, 128.6, 128.4, 128.2, 128.0, 126.3, 122.9, 121.0, 120.2, 109.2, 71.7, 55.5, 29.3; MS: m/z = 441.2 (M+), anal. calcd for C26H21ClN4O; calcd: C, 70.82; H, 4.80; N, 12.71; found: C, 70.83; H, 4.80; N, 12.72.
C str), 824 (C–Cl str); 1H NMR (400 MHz, DMSO-d6, ppm): δ 2.90–2.96 (t, 1H, HA, J = 12.8 Hz), 3.19 (t, 2H, –CH2), 3.42–3.49 (t, 1H, HB, J = 13.6 Hz), 4.56 (t, 2H, –CH2), 4.89–4.92 (t, 1H, HX, J = 10.4 Hz), 6.77 (m, 1H, Ar–H), 7.33–7.36 (m, 2H, Ar–H), 7.43–7.56 (m, 6H, Ar–H), 7.77–7.95 (m, 4H, Ar–H), 8.62 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6, ppm): δ 160.5, 151.2, 138.8, 133.2, 130.7, 130.1, 129.9, 129.7, 129.1, 128.6, 128.4, 128.2, 128.0, 126.3, 122.9, 121.0, 120.2, 109.2, 71.7, 55.5, 29.3; MS: m/z = 441.2 (M+), anal. calcd for C26H21ClN4O; calcd: C, 70.82; H, 4.80; N, 12.71; found: C, 70.83; H, 4.80; N, 12.72.
1′-(4-Chlorophenyl)-5-(5-methylfuran-2-yl)-3′-phenyl-3,4-dihydro-2H,1′H-[3,4′]bipyrazolyl (8a)
IR (KBr νmax cm−1): 3316 (N–H str), 3117 (Ar–H str), 2923 (C–H aliphatic str), 1594 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1499 (C
N str), 1499 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 829 (C–Cl str); 1H NMR (400 MHz, DMSO-d6, ppm): δ 2.31 (s, 3H, –CH3), 2.84–2.90 (t, 1H, HA, J = 13.0 Hz), 3.31 (m, 1H, HB), 4.89–4.95 (t, 1H, HX, J = 10.6 Hz), 6.17 (s, 1H, Ar–H), 6.50 (s, 1H, Ar–H), 7.44–7.76 (m, 8H, Ar–H), 7.94–7.95 (d, 2H, Ar–H, J = 6.0 Hz), 8.59 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6, ppm): δ 152.9, 151.1, 147.3, 142.1, 138.7, 133.2, 130.7, 129.9, 129.1, 128.6, 128.4, 128.0, 123.7, 120.2, 110.9, 108.3, 55.2, 13.9; MS: m/z = 403.2 (M+), anal. calcd for C23H19ClN4O; calcd: C, 68.57; H, 4.75; N, 13.91; found: C, 68.60; H, 4.76; N, 13.92.
C str), 829 (C–Cl str); 1H NMR (400 MHz, DMSO-d6, ppm): δ 2.31 (s, 3H, –CH3), 2.84–2.90 (t, 1H, HA, J = 13.0 Hz), 3.31 (m, 1H, HB), 4.89–4.95 (t, 1H, HX, J = 10.6 Hz), 6.17 (s, 1H, Ar–H), 6.50 (s, 1H, Ar–H), 7.44–7.76 (m, 8H, Ar–H), 7.94–7.95 (d, 2H, Ar–H, J = 6.0 Hz), 8.59 (s, 1H, –NH); 13C NMR (100 MHz, DMSO-d6, ppm): δ 152.9, 151.1, 147.3, 142.1, 138.7, 133.2, 130.7, 129.9, 129.1, 128.6, 128.4, 128.0, 123.7, 120.2, 110.9, 108.3, 55.2, 13.9; MS: m/z = 403.2 (M+), anal. calcd for C23H19ClN4O; calcd: C, 68.57; H, 4.75; N, 13.91; found: C, 68.60; H, 4.76; N, 13.92.
5-Biphenyl-4-yl-1′-(4-chlorophenyl)-3′-phenyl-3,4-dihydro-2H,1′H-[3,4′]bipyrazolyl (10a)
IR (KBr νmax cm−1): 3312 (N–H str), 3073 (Ar–H str), 2921 (C–H aliphatic str), 1593 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1497 (C
N str), 1497 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 830 (C–Cl str); 1H NMR (400 MHz, DMSO-d6, ppm): δ 2.97–3.03 (dd, 1H, HA, J = 10.9 Hz), 3.49–3.56 (dd, 1H, HB, J = 10.7 Hz), 4.97–5.03 (dt, 1H, Hx), 7.30–7.38 (m, 2H, Ar–H), 7.45–7.55 (m, 6H, Ar–H), 7.64–7.65 (d, 1H, Ar–H, J = 3.3 Hz), 7.68 (s, 1H, pyrazole-5H), 7.70–7.71 (dd, 5H, Ar–H, J = 1.4 Hz), 7.80–7.82 (d, 2H, Ar–H, J = 8.5 Hz), 7.89–7.91 (d, 2H, Ar–H, J = 7.7 Hz), 8.61 (s, 1H, –NH); MS: m/z = 475.1 (M+), anal. calcd for C30H23ClN4; calcd: C, 75.86; H, 4.88; N, 11.80; found: C, 75.90; H, 4.90; N, 11.90.
C str), 830 (C–Cl str); 1H NMR (400 MHz, DMSO-d6, ppm): δ 2.97–3.03 (dd, 1H, HA, J = 10.9 Hz), 3.49–3.56 (dd, 1H, HB, J = 10.7 Hz), 4.97–5.03 (dt, 1H, Hx), 7.30–7.38 (m, 2H, Ar–H), 7.45–7.55 (m, 6H, Ar–H), 7.64–7.65 (d, 1H, Ar–H, J = 3.3 Hz), 7.68 (s, 1H, pyrazole-5H), 7.70–7.71 (dd, 5H, Ar–H, J = 1.4 Hz), 7.80–7.82 (d, 2H, Ar–H, J = 8.5 Hz), 7.89–7.91 (d, 2H, Ar–H, J = 7.7 Hz), 8.61 (s, 1H, –NH); MS: m/z = 475.1 (M+), anal. calcd for C30H23ClN4; calcd: C, 75.86; H, 4.88; N, 11.80; found: C, 75.90; H, 4.90; N, 11.90.
Acknowledgements
AMI thanks the Director of the National Institute of Technology Karnataka, India for providing the research facilities. The authors extend their appreciation to The Deanship of Scientific Research at King Saud University for funding the work through research group project no. RGP-VPP-207.Notes and references
- A. Konstantinos, Aust. Prescr., 2010, 33, 12–18 Search PubMed.
- Y. Zhang, K. Post-Martens and S. Denkin, Drug Discovery Today, 2006, 11, 21–27 CrossRef CAS PubMed.
- V. Bhowruth, L. G. Dover and G. S. Besra, Prog. Med. Chem., 2007, 45, 169–203 CAS.
- N. K. Piyush, P. S. Shailesh and K. R. Dipak, New J. Chem., 2014, 38, 2902–2910 RSC.
- R. A. Gupta and S. G. Kaskhedikar, Med. Chem. Res., 2013, 22, 3863–3880 CrossRef CAS.
- P. Horrocks, M. R. Pickard, H. H. Parekh, S. P. Patel and R. B. Pathak, Org. Biomol. Chem., 2013, 11, 4891–4898 CAS.
- M. A. Abdel, G. R. Abou and A. A. Hassan, Eur. J. Med. Chem., 2009, 40, 3480–3487 CrossRef PubMed.
- E. Palaska, M. Aytemir, I. T. Uzbay and D. Erol, Eur. J. Med. Chem., 2001, 36, 539–543 CrossRef CAS PubMed.
- N. Harikrishna, A. M. Isloor, K. Ananda, A. Obaid and H. K. Fun, RSC Adv., 2015, 5, 43648–43659 RSC.
- M. S. Christodoulou, S. Liekens, K. M. Kasiotis and S. A. Haroutounian, Bioorg. Med. Chem., 2010, 18, 4338–4350 CrossRef CAS PubMed.
- A. M. Isloor, B. Kalluraya and M. Rao, J. Saudi Chem. Soc., 2000, 4, 265–270 CAS.
- I. Koca, A. Ozgur, K. A. Coskun and Y. Tutar, Bioorg. Med. Chem., 2013, 21, 3859–3865 CrossRef CAS PubMed.
- Z. ZuhalOzdemir, H. B. Kandilci, B. Gumusel, U. Calis and A. Bilgin, Eur. J. Med. Chem., 2007, 42, 373–379 CrossRef PubMed.
- V. H. S. Jois, B. Kalluraya and K. S. Girisha, J. Serb. Chem. Soc., 2014, 79, 1469–1475 CrossRef CAS.
- P. T. Chovatia, J. D. Akabari, P. K. Kachhadia, P. D. Zalavadia and H. S. Joshi, J. Serb. Chem. Soc., 2007, 71, 713–720 CrossRef.
- H. G. Garg and P. P. Singh, J. Chem. Soc. C, 1969, 1141–1143 RSC.
- F. A. Ragab, N. M. A. Gawab, H. H. Georgey and M. F. Said, Eur. J. Med. Chem., 2013, 63, 645–654 CrossRef CAS PubMed.
- R. J. Alabaster, I. F. Cottrell, H. Marley and S. H. B. Wright, Synthesis, 1988, 950–952 CrossRef CAS.
- F. V. Driessche, P. Rigole, G. Brackman and T. Coenye, J. Microbiol. Methods, 2014, 98, 31–34 CrossRef PubMed.
- M. C. S. Lourenco, M. V. N. deSouza, A. C. Pinheiro, M. L. Ferreira, R. S. B. Goncalves, T. C. M. Nogueira and M. A. Peralta, ARKIVOC, 2007, XV, 181–191 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nj02237a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |