Modification of condensed tannins: from polyphenol chemistry to materials engineering
Danny E.
García
*a,
Wolfgang G.
Glasser
b,
Antonio
Pizzi
cd,
Sebastian P.
Paczkowski
ef and
Marie-Pierre
Laborie
ef
aÁrea Productos Químicos, Unidad de Desarrollo Tecnológico (UDT), Universidad de Concepción, Bio-Bio, Chile. E-mail: d.garcia@udt.cl
bDepartment of Sustainable Biomaterials, Virginia Polytechnic Institute and State University, Blacksburg, Virginia, USA
cLaboratoire d'Etudes et de Recherche sur le Matériau Bois (LERMAB), University of Lorraine, 27 rue du Merle Blanc, Epinal, France
dDepartment of Physics, King Abdulaziz University, Jeddah, Saudi Arabia
eFreiburger Materialforschungszentrum–FMF, Albert-Ludwigs University of Freiburg, Freiburg, Germany
fChair of Forest Biomaterials, University of Freiburg, Freiburg, Germany
First published on 6th November 2015
Abstract
Condensed tannins (CTs) are high molar mass polyphenolic bio-polymers based on flavonol units. CTs have been well-studied due to their respective biological activity. However, the application of CTs in several areas is limited because of their physicochemical properties. The objective of this review is to investigate the state of the art regarding the chemical modification of CTs, and to outline recent and potential applications of tannin derivatives. An overview of the most important reactions is given, and a comprehensive summary of the experimental parameters for modification (chemicals, time, temperature, solvent type, and yield) is presented. The impact of the modification on the physicochemical properties of derivatives in comparison to native tannin behavior is discussed. Finally, the applicability of modified or unmodified CTs is described, referring to academic articles and patents. Future research in terms of modification reaction type, as well as derivatizing agents, is discussed.
1. Condensed tannins (CTs): renewable building-block polymers
1.1. CTs as abundant polyphenols
Metabolites produced by living organisms are classified as either primary or secondary. Primary metabolites are essential, and their concentration within the organism depends only on their physiological state.1 Proteins, nucleic acids, carbohydrates, and lipids are prominent examples for primary metabolic products. In contrast, secondary metabolites are not strictly essential for the survival of the organism, but they play an important ecological role in nature.2 There are five major classes of secondary metabolites defined by their chemical structure: nitrogen compounds (alkaloids, non-proteinogenic aminoacids, lectins, and cyanogens), isoprenoids (terpenes and steroids), polyketides, fatty acids, and phenols are the main groups found in nature.3Phenolic structures with more than one –OH group, which are called polyphenols, are the largest class of secondary metabolites in vascular plants and they are mainly constituted from phenyl propanoids (coumarins, flavonoids, tannins, and lignin) from the shikimate–cinnamate pathway.4 Polyphenols are synthesized exclusively in vascular plants and in a wide variety of species (e.g. monocotyledons, dicotyledons, ferns, and conifers), and plant tissues (e.g. leaves, cone, seedpot, wood, blade, stipe, and bark).5 However, this review will focus on CTs as the most studied polyphenols nowadays.
In vascular plants, tannins are the third most abundant group of secondary metabolites after the two main wood constituent carbohydrates (in the form of cellulose and hemicellulose) and lignin. Despite that lignin is the widest distributed aromatic biopolymer in nature, the peculiar reactivity, diverse biological activity, high –OH/monomer content, and versatility of CTs in terms of physicochemical properties are recognized advantages for developing CT-based biomaterials. In addition, in soft tissues of woody plants such as leaves, needles, and bark, tannins can exceed lignin in abundance.5
Tannins are divided into two different classes: hydrolizable tannins (HT) and CTs. The former are found in chestnut (Castanea sp.), myrabolans (Terminalia and Phyllantus sp.), dividivi (Caesalpinia sp.), oak (Quercus sp.), eucalyptus (Eucalyptus sp.), and extracts of Asian Myrica sp. The limited distribution of HTs comprising only some dicotyledonian species, as well as their molecular structure and their low nucleophilicity decreased the interest in their utilization beyond traditional uses.7 CTs constitute more than 90% of the total world production of commercial tannins (>220.000 tons per year), and are chemically and economically more interesting as a bio-polymer.6,7 CTs and their flavonoid precursors are substantially concentrated in some barks and woods. They are highly abundant in barks of legumes (Acacia sp. (wattle or mimosa), Lotus sp., and Sericea sp.), conifers (Tsuga sp. and Pinus sp.), birch (Betula sp.), gambier (Uncaria sp.), and mangrove (Rhizophora sp.) species, and in the wood of Schinopsis sp. (quebracho). Tannin extracts mainly from barks of Pinus pinaster or Acacia sp.,2–4 and from Schinopsis sp. wood are commercially exploited. The main use of CTs comprise alternative medicine, the leather industry, and for adhesive preparation. Table 1 shows the CT content of selected tanniferous species.
| Species | Extractives (%) | CTs (%) | Ref. |
|---|---|---|---|
| a From wood. | |||
| Larix leptolepis | 2.9–11.0 | 41.8–55.6 | 3 |
| Rhizophora apiculata | 12.8–20.2 | 43.5–50.8 | |
| Acacia mangium | 18.4–37.9 | 46.7–52.9 | |
| Acacia auriculiformis | 18.9–28.6 | 38.1–52.6 | |
| Acacia nilotica | 49.5–55.0 | 11.5–13.0 | 5 |
| Schinopsis balansae | 23.0 | 21.0 | 6 |
| Pinus radiata | 16.0–20.0 | 12.0–16.0 | |
| Acacia mearnsii | 42.0–51.0 | 35.0 | |
| Rhizophora mangle | 45.0 | 36.0 | |
| Pinus pinaster | 35.8–50.0 | 15–25 | 7 |
| Pinus caribaea | 17.6 | 9.2–15.4 | 9 |
| Picea abies | 33.0 | 8.0–11.0 | 11 |
1.2. CT chemistry
The monomer unit of CTs consists of flavonoid units (C15: flavan-3-ols or flavan-3,4-diols), which are condensed by 4 → 8 or 4 → 6 linkages. Their structure is a derivative of the basic C6–C3–C6 flavan unit, being two aromatic rings separated by three carbon atoms.12,13 The flavan rings are labelled A, C, and B, respectively, with a systematic numbering of carbon atoms (Fig. 1).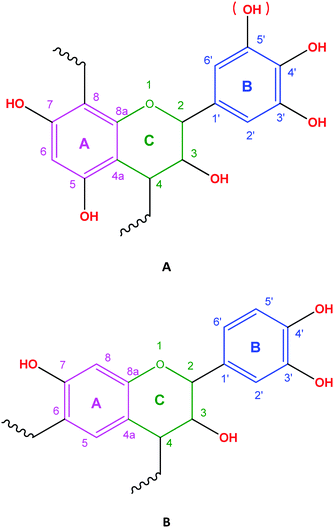 | ||
| Fig. 1 Chemical structure of the C15 unit in condensed tannins. C → C linkage between C15 units: C4 → C8 (A), and/or C4 → C6 (B). | ||
The CT chemistry is dominated by the presence of multiple –OH groups with narrow but varying dissociation constants (pKa catechin-base CTs: pKa C-3′–OH: 9.02; pKa C-4′–OH: 9.12; pKa C-5–OH: 9.43; pKa C-7–OH: 9.58). In consequence, acid and base catalyzed rearrangements, nucleophilic substitutions, and electrophilic aromatic substitutions are common reactions.14,15
Nucleophilic substitution occurs at the –OH, introducing either acyl- or alkyl-groups. Acylation is commonly achieved by a reaction with acid chlorides or anhydrides,16 whereas alkylation is achieved with alkyl halides or epoxides.15 These reactions are the focus of this review and are described in detail in the sections below.
Activation towards electrophilic aromatic substitution is due to multiple phenol groups. The hydroxyl is a powerful electron donor (+M) especially under basic conditions (pH: 9–12) where the phenoxide ion is formed, which pushes electrons onto the A- and B-ring, thereby enhancing the nucleophilic potential in specific C15 reactive sites.
Under acidic conditions the reactivity of the rings towards electrophilic compounds (e.g. aldehydes) increases. Phenol groups are ortho and para directing. Hence, the meta phenol groups, which are common on the A-ring, both activate the C-6 and C-8 positions. In contrast, the vicinal di-phenol groups (catechol- or pyrogallol-type B-ring) cause a general activation on the B-ring rather than a specific activation of their corresponding C-ring atoms. Therefore, the B-ring is relatively unreactive during electrophilic substitutions in comparison to the A-ring. It's reactivity can be enhanced by selecting specific pH and catalyst type. However, from a practical point of view this is not efficient as the reactivity of the A-ring is increased as well, which increases the polymerization kinetics dramatically.
Further studies on the reactivity of CTs based on model compounds (resorcinol, phloroglucinol, and flavonoids) have been undertaken by selective reactions.15 These studies showed that a derivatization of the C-8 site or a linkage to another C15 unit at this position is ubiquitous for a derivatization or an advancing polymerization at the C-6 position. These model studies allowed an understanding of the general order of reactivity of the CT-monomer unit carbon atoms (8 > 6 ≫ 3′ > 4′ > 5′). However, the molar mass (Mw) and the steric hindrances conditioned by the conformation of CTs are factors that must be considered to explain the varied and sometimes unpredictable reactivity.17
Under acidic conditions two competing reactions occur: (1) the polymeric or oligomeric chain can be degraded to their C15 monomers and (2) the flavonoid units can condense. The first reaction is thought to be induced by the hydrolysis of the heterocyclic C-ring and its electrophilic attack upon a nucleophilic center of the A-ring.15
Under alkaline conditions, epimerization and rearrangements occur. Epimerization of catechin to epicatechin occurs in hot water or diluted alkaline solutions. This epimerization involves only a change in stereochemistry at the C-2 of the C-ring. Under strong basic conditions this epimerization reaches equilibrium in several hours.15–18 Rearrangement and loss of aromaticity of the C15 unit in alkaline medium is also reported.19
1.3. Physicochemical properties of CTs
There are few studies on the physical properties of CTs. In general, CTs are low-density amorphous non-crystalline pale yellow-slightly brown solid oligomers/polymers. Numerous tannins are optically active, while their stereochemistry is highly influenced by the C15 sequence. Dried tannin extracts are hygroscopic and exhibit a strong absorption in the UV-Vis region with several local maxima (λmax: 250 and 290 nm). At room temperature, CT-extracts are mainly soluble in water, short-chain alcohols (methanol, ethanol), and acetone aqueous solutions, and yield an acid environment with a sharp “puckering” and “astringent” flavor.They yield colloids in water where they form viscous solutions (e.g. 40–45 wt%, 5 mPa s−1 at 20 °C) due to the strong hydrogen bridge formed between the –OH groups and the water molecules.7,20 The viscosity depends on the CT concentration, the dispersity, the topology and the ratio of hydrophilic/hydrophobic domains. The viscosity of some tannin extracts is increasing the difficulty to formulate CT-based materials or to spray CT-based resins, especially in the wood-based composites industry.7,10
In addition, CTs are thermo- and UV-labile compounds prone to oxidation. The residual weight at 600 °C oscillates between 40–50% as a consequence of decomposition processes between 220–350 °C.22 However, the thermal behavior is strongly associated with the Mw and the chemical structure.23,24 In general, CTs decompose in three main degradation steps according to their specific chemical structure and their linkages. The B-ring stripping process at temperatures between 200–250 °C opens up the C15 structure, which enables sequential degradations.26
Considering that CTs are bio-polymers, the glass transition temperature (Tg) is an important variable to be considered for CT-applications in materials engineering. Their Tg is strongly associated with Mw, the purity grade, and the moisture content. The Tg of native tannin oscillates between 120 and 180 °C. In general, it is difficult to detect Tg in CT samples. In addition, the reproducibility of the values is very low, regardless of the experimental parameter variation using DSC (Differential Scanning Calorimetry).37
2. Chemical modification of CTs
2.1. Main derivatization pathways
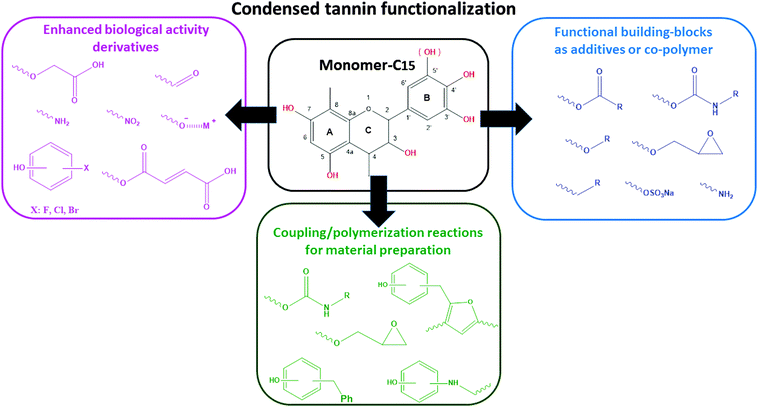
Derivatization is the process of chemically modifying a compound to produce a new structure. The new properties of these structures can be suitable for selected purposes. Thus, by selectively changing the properties through chemical modification, the range of potential utilization of the original compound can be increased.8,15 In case of CTs, modification of the native tannin extracts can overcome drawbacks like low solubility, high viscosity, and too high or too low reactivity. Modification allows further enhancement of these properties under consideration of the requirements for the selected application. According to the recent literature, derivatization seems to be an appropriate way to use this renewable product as a building-block for bio-polymer engineering and chemical synthesis.15,25–27Several CTs have been subjected to a large variety of derivatization reactions. This section has been restricted to the identification and the discussion of the most relevant work according to the impact on physicochemical properties.
Tables 2 and 3 show the main modification reactions reported in polyphenols and the common derivatizing agents used during modification, respectively. According to the reactivity of the CT monomers discussed above, functionalization on the –OH groups, as well as the nucleophilic positions on the rings, are the most important sites for derivatization (see Fig. 1).
| Reaction-type | Derivatizing agent | Ref. |
|---|---|---|
| Functionalization of the –OH group(s) | ||
| Esterification | Acid chlorides, anhydrides, or carboxylic acids | 15 |
| Alkylation | Acid chlorides or alkylene oxides | 32 and 33 |
| Carboxymethylation | Chloroacetic acid | 46 |
| Carbamate formation | Isocyanates | 38 and 39 |
| Dealkylation | HCl | 37 |
| Oxidation/reduction | Redox agent (K2Cr2O7, KMnO4), enzymes | 40 |
| Chelate formation | Transitional metals (Fe3+, Cu2+, Zn2+) | 41 |
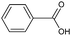
| Benzoylation | Benzoic acid/H+ | 7 |
| H/deuterium exchange | Deutherated solvents (D2O, EtOD) | 42 |
| Functionalization of the aromatic ring(s) | ||
| Alkylation | Alkyl halides/K2CO3 or OH− | 43 |
| Acetylation | CH3COO− | 25 |
| Methylolation/condensation | Aldehydes/H+ or OH− | 44 |
| Halogenation | Halogens (Cl2, Br2, I2) | 45 |
| Sulphonation or sulphitation | H2SO4 or Na2SO3 | 30 |
| Amination | NH3/H2O | 47 |
| Nitration | HNO3/H2O or HNO3/AcOH | 48 |
| Nitrosation | NaNO2/H+ | 49 |
| Phenolation | Phenol | 50 |
| Depolymerization | Mercaptan/H+ | 34 |
The degree of chemical modification (DS) depends on the polyphenol type, the derivatizing agent, and the experimental conditions. A wide range of strategies to get tailored derivatives as additives,28 fiber reinforcements,29 biologically-active compounds,30 thermosetting systems,31 foams,32 and copolymers33 are documented based on either one-step synthesis, or multiple-stage reactions.
In CT monomers there are three sites for derivatization. There are reactions that are selective towards one of these three derivatization sites.34 The A-ring can be derivatized at the C-5 or C-7 –OH group or at the C-6 position (see Fig. 1). On the B-ring, derivatization takes place mainly at the C-3′ or C-4′ –OH group (or additionally at the C-5′ –OH group in case of delphinidins or gallo-pyrogallols). The C-ring can be modified at the C-3 –OH and at the C-4 inter-C15 linkage. In C15 terminal units of CTs, derivatization may occur at the C-8 position.35
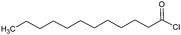
The synthesis of CT-ester derivatives can be achieved by several methods such as Fischer esterification, alcoholysis of acid halides or anhydrides,15 novel approaches with catalysts,51,52 microwave irradiation,53 and replacing halides by less toxic organic moieties are also reported.54 Fischer esterification is the classic route to prepare CT-esters. In the course of this reaction an acid reacts with –OH groups via nucleophilic acyl substitution. Aliphatic alcohols are relatively reactive in Fischer esterification, while in the case of hydroxylated phenols the reaction has to be enhanced by a dehydrating agent or by other strategies.
For instance, acid halides offer a higher reactivity in comparison to their corresponding carboxylic acids or anhydrides. Therefore, CTs can be easily acylated this way.55
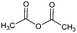
The reaction is often carried out in the presence of hydroxides or organic (pyridine, ionic liquids, and amines) bases. The base-catalysis assists the activation of the –OH for an electrophilic attack (alcoholysis) of anhydrides in a comparable way as acid halides. Anhydrides are less reactive than acid halides, but the first one is often used for modification in order to gain structural information of products and intermediates.25,56 In comparison to low Mw polyphenols, CT derivatives are often synthesized under less drastic conditions according to temperature and reaction time and this is independent of the derivatizing agent.57
This is caused by the fact that alcoholysis reactions are often affected by steric hindrances. Bulky groups slow down the reaction, resulting in a defined –OH reactivity order, where the A- and B-ring aromatic –OH groups show a higher reactivity than the C-ring aliphatic –OH groups.15
However, beyond the noticeable differences between –OH reactivities, the conformational structure of CTs influences reactivity even at high temperatures and pressures. Tannin from mimosa and quebracho tannin are considered less-reactive CTs upon certain modification reactions (oxybutylation and oxypropylation) than pine tannin.82,83 The C4 → C6 linkages confer a peculiar “angular” configuration, which maximizes the steric hindrance upon alkylation. Such results are in contrast to the high reactivity of pine tannins (PRBT and PPBT), described a so-called “linear” conformational oligomer-chain. Anyway, it has to be taken into account that apart from steric hindrances, all the mentioned tannins exhibit very similar pKa values of their OH-groups.
On the other hand, specific CT reactivity is conditioned by the reaction type. For instance, hydroxypropylation reaction takes place on the A-ring at low derivatizing agent content, while isocyanates react preferably on the B-ring. So, the selectivity seems not to be related exclusively to the acidity values of –OH groups.15,27
Direct acylation of CTs with short-chain chemicals is strongly dependent on the experimental conditions. Pyridine and aliphatic amines are frequently used as catalysts, and chloroform, acetone, or aqueous solutions are the best choices as solvents, considering the low solubility of native CTs in organic liquids. O-Acylation of CTs with alkyl halide and long-chain esters in chloroform and polar solvents, respectively, provided low yields regardless of the stoichiometry of the derivatizing agent. Therefore, a high Mw of CTs requires a higher reactivity of the reaction agents in order to favor high yields (Table 5).49
While ionic liquids have been used for the derivatization of flavonoids and other chemically-related polyphenols15,29,59 the utilization of catalysts with polar functionalities such as carbodiimides, triazoles, and organic salts (chloroformates) has not been used in CT derivatizations. Table 4 shows a wide range of experimental conditions used for modification via O-acylation.60–67
| CT | DA | T (°C) | t (h) | Solv. | Cat. | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| CT: condensed tannin, DA: derivatizing agent, T: temperature, t: time, Solv.: solvent, Cat.: catalyst, PRBT: radiata pine bark tannin, QT: quebracho tannin, MEI: methyl imidazole, n.i.: no information, VA: vinyl acetate, AH: anhydrides, Hld.: halides, THF: tetrahydrofuran, DMSO: dimethyl sulfoxide, Pen: pentanone, Acet: acetone, Chlor.: chloroform, Py: pyridine, DOX: dioxane, TEA: trimethylamine. | |||||||
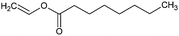
| PRBT, QT | VA | 20 and 70 | 48 | H2O, THF, DMSO | KOH, TEA | n.i. | 16 |
| PRBT | AH | 65 | 16 | — | MEI | n.i. | 29 |
| 65 | 4 and 6 | — | MEI | n.i. | |||
| 70 | 4 | 2-Pen/Acet. | MEI | 0–134 | 59 | ||
| PRBT, QT | Hld. | 75 | 24 | Chlor. | Py | 8–24 | 15 |
| Nutgall tannin | 22 | 2 | Acet./Py | TEA | n.i | 66 | |
| Wattle tannin | 20 | n.i. | 1,4-DOX | Py | n.i | 67 | |
| CT | DA (equiv.) | T (°C) | t (h) | Solv. | Cat. | Yield (%) |
|---|---|---|---|---|---|---|
| CT: condensed tannin, PRBT: radiata pine bark tannin, QT: quebracho tannin, DA: derivatizing agent, T: temperature, t: time, Solv.: solvent, Cat.: catalyst, Chlor.: chloroform, THF: tetrahydrofuran, DMSO: dimethylsulfoxide, TEA: trimethylamine, Py: pyridine.a According to Hadley.15 | ||||||
| Reaction with lauroylCl (esterification) | ||||||
| PRBT | 3 | 75 | 24 | Chlor. | Py | 10 |
| Excess | 24 | |||||
| QT | 3 | 19 | ||||
| Excess | — | 8 | ||||
| Reaction with vinyl laureate (trans-esterification) | ||||||
| PRBT | Excess | 20 | 48 | THF | KOH | 7 |
| QT | 12 | |||||
| PRBT | Excess | 70 | DMSO | TEA | 21 | |
| QT | 8 | |||||
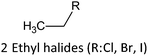
Alkyl bromides are more reactive than alkyl chlorides in CT-etherification reactions, which lead to higher yields. As the reaction is induced by an SN2 mechanism, the primary alkyl halides are ideal for supporting E2 elimination on secondary substrates.15
Asymmetric ethers should be synthesized by reactions between sterically hindered alkoxides and the less sterically hindered halides.43,70 Other strategies comprising alkylation with carbonates using phosphines71 and nitrogenous buffers72 instead of alkali hydroxides and amines73 led to reasonable yields. However, the above-mentioned strategies have not been used for high Mw polyphenol modifications.
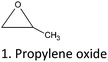
On the other hand, studies on the selective/non-selective alkylation of CTs described the use of ethylene oxide, alkyl sulphates, and halides as derivatizing agents.15 Other commercial CTs have been alkylated using agents like oxides, organic sulphates, acid chlorides, and halides. These alkylation strategies led to mono-alkylation of the C15 unit as the major reaction product.
Double-functionalization using mixtures of mercaptans and electrophilic agents have been used to increase the biological activity of the derivatives.75 This strategy yields a highly homogeneous product, because of the multiple reactive sites in polyphenols where bulky-halides induce the O-acylation instead of the alkylation.43 Therefore, short-chain derivatizing agents are preferable in comparison to bulky derivatizing agents.
Experimental conditions for the alkylation of CTs are listed in Table 6.
| CT | DA | T (°C) | t (h) | Solv. | Cat. | Ref. |
|---|---|---|---|---|---|---|
| CT: condensed tannin, PRBT: radiata pine bark tannin, MBT: mimosa bark tannin, *: unspecified, DA: derivatizing agent, T: temperature, t: time, Solv.: solvent, Cat.: catalyst, DGD: diglycidyl di-epoxide, PGDE: polyglycidyl di-epoxide, TCP-TMA: N-(3-chloro-2-hydroxypropyl) trimethyl ammonium chloride, BO: 1-bromo octane, TCAA: trichloroacetic acid, C/F: condensation/formaldehyde, Resorc.: resorcinol, Catech.: catechol, GT: gelation time, OA: 1-octyl alcohol, MDF: dimethylformamide, n.u.: not utilized. | ||||||
| PRBT | DGD and PGDE | 80–90 | GT | H2O | NaOH | 74 |
| Tannin* | 1-BO | 60 | 2 | DMF | K2CO3 | 66 |
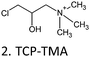
| Tannin* | TCP-TMA C/F | 48 | 1.5 | H2O | NaOH | 75 |
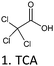
| MBT | TCAA, Resorc., Catech., Na2SO3 | 86 and 90 | 2, 1.5 and 1.7 | H2O, OA, H2O | n.u. | 30 |
Epoxides are used for the alkylation of CTs in a wide range of temperatures (20–200 °C). High pressure and the combination of less polar aprotic solvents (toluene and acetone) or aqueous solutions are used to enhance the derivatization reaction with alkylene oxides.15,37,38,76,77 The chemical structure of CTs, the Mw distribution, and the –OH pattern significantly affect the degree of substitution (DS).25–27,37,38
2.2. Alkylation of CTs with alkylene oxides
Alcoholysis of epoxides is a simple method to prepare hydroxyalkyl ethers. CT-ethers are used as chain-extenders in polyurethanes or as a co-monomer for polyester co-polymerization.77,78 The ring opening reaction of an epoxide is an efficient route to prepare ethers from high Mw polyphenols.79 On the other hand, alkylene oxides can be used for the preparation of simple phenol- and flavonoid-derivatives.15The “oxypropylation” reaction and related short-chain oxyalkylation is an alternative pathway for the functionalization of CTs and lignin. This reaction has been extensively studied also for the modification of non-phenolic biopolymers and is one of the most attractive etherification alternatives considering the dramatic impact on properties.80,81
The general reaction mechanism of short-chain alkylene oxides, like propylene-, ethylene-, and butylene oxides, is determined by the high strain within the epoxide rings. This leads to their high reactivity compared to larger cyclic ethers. Epoxide ring scission can occur under neutral, basic, or acidic conditions, at any of the ring carbons. This high reactivity and flexibility in ring scission are the two major reasons for the feasibility of these short chain alkylene oxides for high Mw polyphenol modification.
Under basic or neutral conditions monoalkyl-substituted epoxides react predominantly at the less substituted carbon, due to steric effects (SN2 type mechanism). Under acidic conditions the phenate attack at the more substituted propylene oxide-carbon increases, but the attack at the less substituted carbon is still predominant.15,81
Acid-catalyzed ring opening occurs with a partial carbenium ion character on the reaction center. This is more in agreement with a SN1 type mechanism. The rate constant of alkoxylation (k) highly depends on the pKa of the –OH group.15 The order of reactivity is as follows: kphenolic(A-![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) and
and![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ring) –OH > kprimary –OH > ksecondary (C-ring) –OH > ktertiary –OH. However, CT-modification via acid-catalysis has not been reported yet.
ring) –OH > kprimary –OH > ksecondary (C-ring) –OH > ktertiary –OH. However, CT-modification via acid-catalysis has not been reported yet.
Base-catalyzed ring opening reaction is the most common pathway in order to modify CTs with alkylene oxides. Typical catalysts are KOH or NaOH. Polyphosphazenium, aluminum tetraphenyl porphine, caesium hydroxide, and tertiary amines show excellent results for simple phenol and flavonoid modification, as well,50 while mineral bases still being the most useful catalysts for CTs modification.
The oxyalkylation of CTs has been used recently.15,81–83 Modification was achieved mainly under drastic experimental conditions (pressure and temperature),82 as well as at room temperature.81 The most comprehensive research concerning CT derivatization with PO and butylene oxide using a Parr reactor was conducted by Hadley15 and Arbenz and Avérous,82,83 respectively (Table 7). A positive linear relation between the PO molarity and the yield of oxypropylated CTs with an optimum ratio at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (OH
3 (OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO) was reported.
PO) was reported.
| Polyphenol | PO (equiv.) | Yield (%) | Conditions |
|---|---|---|---|
| CT: condensed tannin, PRBT: radiata pine bark tannin, PPBT: pinaster pine bark tannin, QT: quebracho tannin, DA: derivatizing agent, PO: propylene oxide, BO: butylene oxide, MBT: mimosa bark tannin, *(CT/alkylene oxide ratio, w/w), CE: chain extended derivatives, THF: tetrahydrofuran, DMSO: dimethylsulfoxide, TEA: trimethylamine, n.i.: no information. | |||
| QT15 | 1 | 93 |
T: 110 °C t: 24 h
Catalyst: TEA Solvent: toluene, alcohols DA: propylene oxide (PO) Parr reactor |
| 3 | 93 | ||
| 5 | 79 | ||
| 10 | 47 (CE) | ||
| 20 | 28 (CE) | ||
| PRBT15 | 1 | 99 | |
| 3 | 100 | ||
| 5 | 96 | ||
| 10 | 84 (CE) | ||
| 20 | 58 (CE) | ||
| PPBT81 | 1 | 91 |
T: 22 °C t: 24 h
Catalyst: NaOH Solvent: water DA: PO (p: 1 atm) |
| 2 | 86 | ||
| 3 | 89 | ||
| 4 | 92 | ||
| 5 | 94 | ||
| 6 | 85 | ||
| Gambier CT,82,83 MBT,83 QT,83 PPBT83 | 40/60* | n.i. (CE) |
T: ≥150 °C t: 1–24 h
Catalyst: KOH Solvent: water |
| 30/70* | |||
| 20/80* | |||
| 10/90* | |||
In addition, a strong relationship between the DS, and selected physico-chemical properties was established when CT-monomer units (catechin) were used.
Alternative strategies based on less drastic conditions (22 °C, 1 atm) have recently been described in the literature.25–27P. pinaster tannin oxypropylation improves the solubility of derivatives in several organic solvents, as well as the thermal resistance. Under the mentioned conditions the derivatives show non-oligomerization of the chain,81 while pressure conditions yield chain extended CT-derivatives.82,83
More complex epoxides such as di-epoxides of diglycidyl ether and polyglycidyl ether type have been used to cross-link bark tannins. The variation of the pH showed that the opening of the epoxide moiety was favored in basic media.74
The oxypropylation reaction is a common strategy to modify the physicochemical properties of natural polyol sources and this is particularly noticeable in the case of PPBT.81 The effect of internal plasticization of PO-modified CTs was extensively studied.32–36 The functionalization or chain-grafting degree improves the thermal properties and solubility.77,81
The solubility in non-polar solvents increases due to the derivatization and this extends the utilization potential of CTs in materials science, mainly for thermoplastic engineering. The type of alkylene influences the solubility of the derivatives, e.g. propoxylation leads to a better solubility than butoxylation.15,82
Some systematic assays have been performed regarding the effect of chemical modification on CT-solubility trends. Hydroxypropylation of highly purified P. pinaster bark tannin improved the solubility in short-chain alcohols, as well as THF, dioxane, and acetic acid, while in consequence the solubility in polar solvents (H2O, acetone, and acetonitrile) was reduced. The solubility trend of modified CTs follows a non-linear behavior for protic and a linear trend for aprotic solvents at the entire range of experimental modification degree (DS: 0.1–4.7). The trend suggests a cross-over point associated with specific interaction of the solvents proton with specific groups (–OH, grafting) as a function of the aromatic ring-type and conformation.
However the influence of the extraction method of the neat CT on CT-derivative solubility behavior has not been well-clarified. Hydroxypropyl derivatives from PRBT, obtained under pilot-plant conditions, exhibited a peculiar behavior. As expected, the modification changed the derivatives' solubility in several solvents significantly. However, the difference in solubility as a function of the DS was negligible. Concomitants such as carbohydrate moieties and terpene traces in the extract might affect the solubility trends in a high extent.
On the other hand, the Tg of CTs decreases due to oxyalkylation. The Tg-reduction is determined by two factors: (i) the intramolecular hydrogen bond rupture and (ii) the increasing free volume.56 Because of the advantage in solubility and thermal resistance CT-hydroxyalkyl derivatives were used for polyurethane foams,77 and for resin formulations.73,81
Hadley15 reported a significant decrease of the Tg value (ΔTg: ∼70 °C) when catechin was oxypropylated. This decrease followed a linear trend with increasing DS (1 → 5). However, it was difficult to establish a correlation between DS and Tg values when PRBT and QT were used.
In addition, PPBT modification decreased the Tg by 0.9 °C per wt% upon oxypropylation, while PRBT derivatives showed a reduction of 0.7–0.8 °C per wt%. The Tg rate reduction in both cases was similar to the plasticization rate reported for oxypropyl-lignin (1.0 °C per wt%). The aromatic content, the rigidity, as well as the Mw of the oligomers seemed to be the main factors that affect the Tg reduction in modified CTs.
2.3. Other derivatization reactions
Besides acylation and alkylation as traditional strategies to modify CTs, several other derivatization methods were used. Nucleophilic depolymerization,84 chelate formation,85 sulphitation86 and the Mannich reaction87 are four routes for CT functionalization that are described here (Table 8).| CT | DA | T (°C) | t (h) | Solv. | Cat. | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| CT: condensed tannin, DA: derivatizing agent, T: temperature, t: time, Solv.: solvent, Cat.: catalyst, *min, PAs: procyanidins, PRBT: radiata pine bark tannin, MBT: mimosa bark tannin, BMC: benzylmepcantan, PG: phloroglucinolysis, F: formaldehyde, n.i.: not informed. n.u.: not utilized. | |||||||
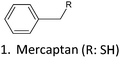
| PAs | BMC | 90 | 2* | MeOH | HCl | 88 | 90 |
| BMC and PG | 80 and 20 | 50 and 48 | EtOH and DOX/H2O | AcOH and HCl | 25–38 | 91 | |
| MBT | CuCl2/NH3 | 20 | n.i. | H2O | n.u. | n.i. | 92 |
| PRBT | F | 80 | 0.3 | HNO3 | 93 | ||
Despite the lack of systematic studies providing valuable insight into the influence of chemical modification in a wide range of experimental conditions, Fig. 2 illustrates the most common applications of modified CTs as a function of the chemical structure of the grafted-chain.
The physicochemical properties of CT-derivatives are correlated to the DS, the type of the derivatized polyphenol, the derivatizing agent, and the specific sites where the derivatization occurs. In general, the derivatives exhibit considerable changes in the physicochemical properties in comparison to the corresponding native polyphenols.21,77
2.4. Solubility and viscoelastic properties
Acetylation and alkylation of CTs can alter the solubility,81 reactivity,81 the Mw distribution,56 and the thermal, and rheological properties15 to a high extent. Hydrophobic modifications improve the interactions of CT with less polar solvents, bio-polymers,28,29,59 and membranes,94 and reduce the water binding capacity of processed bio-polymers.25,26 In the context of CT-based polyurethane resin synthesis,33 partially benzoylated CTs alter the solubility of the highly polar tannins in order to match the solubility of the diisocyanates and thus to allow the polymerization reaction.382.5. Reactivity
CT reactivity was severely affected by modification. The influence of the grafting on the electronic configuration of aromatic rings (specifically the nucleophilic centers) was the reason for the dramatic reduction in reactivity due to the replacement of the aromatic –OH by secondary aliphatic moieties.2.6. Thermal properties and reaction kinetics
A higher DS usually leads to an increased thermal stability and is associated with a higher degradation onset temperature.16 Long-chain grafting on P. radiata bark tannin and quebracho tannin retarded the decomposition.16 In detail, the substitution pattern on both the B-ring and the C15 → C15 linkage type affects the stability and the degradation onset. By benzoylating tannins that were used in polyurethane chemistry, the –OH/C15 ratio was reduced, which decreased the kinetics of the reaction with the isocyanates and led, in consequence, to a better network formation and enhanced thermal stability. While mechanisms that dominate the thermal decomposition of certain derivatives are known, the thermo-protective role of derivatives used as a block co-polymer in materials such as polyurethane foams and selected thermoplastics is unclear.2.7. Biological properties
The effects of chemical modifications on the biological properties of flavonoid derivatives have been studied by antioxidant and anticancerogenic screening.17 Derivatization patterns in specific sites improved the therapeutic activity50 and the derivatives' feasibility for wood preservation against termites and fungi.81 CT derivatization by sulphonation, catecholation, and phloroglucinolation enhanced the bacteriostatic power and the biodegradability of CT-based rigid foams.303. Utilization of CTs with an emphasis on materials science
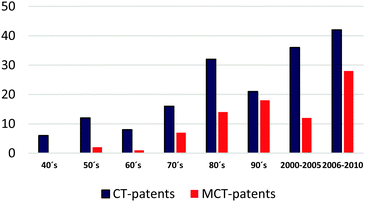
The most representative patents according to the impact of the content on databases (United States Patent and Trademark Office and the European Patent Office) and additional bibliographic sources were surveyed. This overview shows several patents (granted or pending) concerning applications of CT. Fig. 3 shows the evolution of published patents related to CT- and modified CT (MCT)-applications (Tables 9 and 10).| Patent (year) | Assignee | CTs-type/key word |
|---|---|---|
| CT: condensed tannin, MCT: modified condensed tannin, PU: polyurethane. | ||
| US2354672 (1944) | Resinous Prod & Chem. Co. | CT/resin |
| US2590760 (1952) | Da Veiga Vinicio | |
| US3254038 (1966) | Borden Co. | CT/adhesive |
| US3515556 (1970) | Eastman Kodak Co. | CT/photography film |
| US3856845 (1974) | ITT | MCT/bark etherification |
| US3886066 (1975) | E. I. Du Pont De Nemours & Co. | CT/non-porous material |
| US4090919 (1978) | Tanabe Seiyaku Co., Ltd | CT/protein immobilization |
| US4558080 (1985) | Dearborn Chemical Co. | CT/polymer |
| US4595736 (1986) | Betzdearborn Inc. | MCT/aqueous systems |
| US4944812 (1988) | Henkel Corporation | MCT/corrosion resistance |
| US5134215 (1992) | Nalco Chemical Co. | MCT/modified cement |
| US5158711 (1992) | Mitsubishi Nuclear Fuel Co. | MCT/adsorption |
| US5270083 (1993) | Cecco Trading, Inc. | MCT/preservative |
| US5659002 (1997) | Nalco Chemical Co. | MCT/Mannich polymers |
| US5698601 (1997) | Bayer AG | CT/PU foams |
| US5830315 (1998) | Betzdearborn Inc. | MCT/water purification |
| US5912037 (1999) | W. R. Grace & Co., Conn. | MCT/beverages |
| US5977287 (1999) | Betzdearborn Inc. | MCT/aqueous systems |
| Patent (year) | Assignee | CTs-type/key word |
|---|---|---|
| CT: condensed tannin, MCT: modified condensed tannin. | ||
| US6478986 (2002) | Tanac S.A. | MCT/coagulant |
| WO2004058843 (2004) | Borden Chemical Australia Pty | CT/binding agent |
| EP1437419A1 (2004) | Tanac S.A. | CT/bioactive |
| US20060084718 (2006) | Stancliffe Mark R. | CT/binder |
| WO2008079652A1 (2008) | Gen Electric Co. | CT/polymeric coagulants |
| US7611632 (2009) | CT/co-polymer | |
| US7998351 (2011) | Vinod Kumar Rai | CT/coagulants |
| US7998351B2 (2011) | ||
| US20120148740 (2012) | Yang Chia-Wei | CT/epoxy resins |
| WO2012162684A2 (2013) | E.I. Du Pont De Nemours & Co. | CT/rigid foam |
| WO2013010668 (2013) | Silvachimica S.R.L., Universite' De Lorraine – Institut Enstib-Lermab | |
| WO2012162653A3 (2013) | E.I. Du Pont De Nemours & Co. | |
| US20130252909A1 (2013) | University of Iowa Research Foundation | CT/HIV inhibitors |
| EP2805 943 A1 (2013) | Albert-Ludwigs-Universität, Freiburg | MCT/propylene oxide |
| US8642088 (2013) | Wisconsin Alumni Res. Foundation | CT/chitosan-composites |
| US20140093720 (2014) | E.I. Du Pont de Nemours & Co. | CT/rigid foam |
| EP 2617792 A4 (2015) | Korea Advanced Inst Sci & Tech, Inno Therapy Inc. | CT/adhesives |
Before ca. 1980, CTs and CT-derivatives were used for several commercial applications like traditional leather tanning, adhesive additives, vinification, for pharmaceutical purposes, asphalt additives, dispersants, in medicine, as well as additives for tiles, floorings, and drilling muds.15 However, the most cited patents between 1940 and 1980 describe the use of native tannin for conventional applications.
As dispersants, CTs can alter the viscosity of various solids. It reduces the viscosity in clays to improve the manufacturing of bricks and pottery.18 A tannin based concrete additive provides improved plasticity, uniformity, flowability, and reduced shrinkage of the concrete.
The CT capacity to immobilize metals and macromolecules has been used for the design of tannin-based products (Tanfloc® and Silvafloc®) to clean water by flocculation,95–98 and to restore ecosystems. Collagen-tannin fiber membranes, which are selective to UO22+,99 tannin hydrogels,100 and tannin-rigid foams101 have been utilized to remove harmful chemicals from aqueous solutions, as well. CTs were also immobilized on substrates for the selective complexation with proteins. Such immobilization on inert and/or active matrices was also used to capture chemical compounds for further analysis,102–104 and in general to manufacture absorbent composites.105 Complexed CTs are used to inhibit fouling on vessel surfaces,106 and as polyphenol containing algae they are an efficient bio-based strategy to control harmful marine populations. In addition, applications were invented for purification technologies,40,75,92,93 antioxidant films,107 and cosmetics.55 Although the pharmaceutical use of CTs is well-known and applied, several investigations reported new properties and applications in alternative medicine in the last 60 years. Potential applications include prevention of chronic diseases, antimicrobial uses, antitumor activity,108 and cardio-protective effects.109
On the other hand, CT-derivatives were often used after 1980. Modified CTs play a key role in application areas where properties such as high miscibility and solubility in a non-polar medium, enhanced/suppressed reactivity, biological activity, and high thermal resistance are critical factors to consider. Derivatives instead of native CTs are useful in order to improve physicochemical properties and durability of thermoplastics. Functionalization enables high miscibility in biodegradable polymer blends such as poly(lactic) acid (PLA),16,18 and improves flexibility in polyurethane-based materials.81 Despite this specific chemical modification, oxyalkylation and partial acetylation are strategies to slow down the kinetics of complex reactions. There are no reports describing the understanding of the kinetics of the polymerization mechanisms, the polymerization stages, and the rheological behavior of these modified CTs. In addition, CT-modification might diversify properties of CT-based foams. Grafting of CT with aliphatic moieties may affect the ratio of hard/soft segments, which confers a dramatic impact on properties.
4. Concluding remarks and perspectives for the future
Considering the wide distribution of condensed tannins in vascular plants, these renewable natural products exhibit greater prospects in materials science beyond their traditional use in the leather industry and as components of adhesive resins.The applicability of condensed tannins is restricted by high or low reactivity and poor solubility in organic solvents. A strategy to overcome this limitation is to modify the chemical structure by derivatization reactions. Among many possibilities, acetylation and alkylation, and here especially O-acylation using acid chlorides or anhydrides and alkyl halide, are the most frequent reactions. They change the physicochemical properties of the derivatives for their effective use in the industry and for purification technologies. Condensed tannin modification with conventional chemicals (e.g. anhydrides and halides) and propylene oxide affects drastically the physicochemical properties of the derivatives and provides high performance as macro building-blocks for materials engineering. The utilization of derivatives instead of native tannins has been described in several scientific journal articles and patents. The new trend points to the use of native and modified tannins as components of novel materials that are essentially based on: (i) the condensed tannin's properties as phenolic biopolymer with high molar mass, and (ii) the versatility conferred by the grafting functionalization. In the future, it is expected that tannin will be used in other research fields beyond medicine, adhesives, and the leather industry. Modified tannin as an additive and a block co-polymer for thermoplastics (ultraviolet-filter), tannin-based co-polymer with enhanced biological activity, and as macro building-blocks for hybrid resins preparation are promising research areas. Acetylation using specific derivatizing agents (non-linear alkyl halide and cyclic anhydrides) might yield useful alternative chemicals in order to tailor properties and diversify applications.
In the future, it is expected that tannins and tannin-derivatives may play a key role as nutraceutical products, as well as controlled release promoters, volatile organic compounds (VOCs) sequesters, and moisture retainers in promising research areas such as food science and agriculture.
Acknowledgements
D. E. García thanks the Basal Project (PFB-27) from the Concepción University, Chile.References
- D. E. García, Pastos y Forrajes, 2004, 27, 1 Search PubMed.
- R. W. Hemingway, in Organic Chemicals and Biomass, ed. I. S. Goldstein, CRC, Boca Raton, Florida, 1981, p. 190 Search PubMed.
- R. Makino, S. Ohara and K. Hashida, J. Trop. Agric. Sci., 2009, 21, 45 Search PubMed.
- E. Haslam, The shikimate pathway, John Wiley & Sons, New York, 1974, p. 316 Search PubMed.
- P. J. Hernes and J. I. Hedges, Geochim. Cosmochim. Acta, 2004, 68, 1293 CrossRef CAS.
- E. Roffael, B. Dix and J. Okum, Holz- und Werktoff, 2000, 58, 301 CrossRef CAS.
- A. Pizzi, J. Macromol. Sci., Polym. Rev., 1980, 18, 247 CrossRef.
- A. Pizzi, in Monomers, polymers and composites from renewable resources, ed. N. Belgacem and A. Gandini, Elsevier Ltd., 2008, p. 179 Search PubMed.
- N. S. A. Derkyi, D. Sekyere, N. A. Darkwa and N. B. Boakye, Int. J. Chem. React. Eng., 2011, 9, 1 Search PubMed.
- M. C. Vieira, R. C. C. Lelis, B. C. D. Silva and G. D. L. Oliveira, Floresta e Ambiente, 2011, 18, 1 CrossRef.
- K. Kemppainen, M. Siika-aho, S. Pattathil, S. Giovando and K. Kruus, Ind. Crops Prod., 2014, 52, 158 CrossRef CAS.
- H. A. Stafford, Flavanoid Metabolism, CRC Press, Boca Raton, Florida, USA, 1990, p. 63 Search PubMed.
- M. N. Nurulhuda, L. T. Chew, N. M. Y. Mohd and R. M. A. Abdul, Holz Roh- Werkst., 1990, 48, 381 CrossRef CAS.
- C. Cren-Olivé, J. M. Wieruszeski, E. Maesc and C. Rolando, Tetrahedron Lett., 2002, 43, 4545 CrossRef.
- J. Hadley, Derivatisation of polyphenols, MSc thesis, The University of Waikato, N.Z. Hamilton, 2007, p. 157 Search PubMed.
- J. H. Bridson, W. J. Grigsby and L. Main, J. Appl. Polym. Sci., 2013, 129, 181 CrossRef CAS.
- S. Burda and W. Oleszek, J. Agric. Food Chem., 2001, 49, 2774 CrossRef CAS PubMed.
- K. Hashida, R. Makino and S. Ohara, Holzforschung, 2009, 63, 319 CrossRef CAS.
- K. Hashida and S. Ohara, J. Wood Chem. Technol., 2002, 22, 11 CrossRef CAS.
- L. J. Porter, in Plant Polyphenols, ed. R. W. Hemingway and P. E. Laks, Plenum Press, New York, 1992, p. 245 Search PubMed.
- I. Volf, I. Ignat, M. Neamtu and V. I. Popa, Chem. Pap., 2014, 68, 121 CAS.
- M. Pantoja-Castro and H. González-Rodríguez, Rev. Latinoam. Quím., 2011, 39, 107 CAS.
- R. W. Hemingway and J. J. Karchesy, Chemistry and significance of condensed tannins, Springer, US, 1989, p. 553 Search PubMed.
- M. Gaugler and W. J. Grigsby, J. Wood Chem. Technol., 2009, 29, 305 CrossRef CAS.
- D. E. García, W. G. Glasser, A. Pizzi, A. Osorio-Madrazo and M.-P. Laborie, Ind. Crops Prod., 2013, 49, 730 CrossRef.
- D. E. García, W. G. Glasser, A. Pizzi, A. Osorio-Madrazo and M.-P. Laborie, Holzforschung, 2014, 68, 411 CrossRef.
- D. E. García, W. G. Glasser, A. Pizzi, S. Paczkowski and M.-P. Laborie, J. Mass Spectrom., 2014, 49, 1050 CrossRef PubMed.
- C. Luo, W. Grigsby, N. Edmonds, A. Easteal and J. Al-Hakkak, J. Appl. Polym. Sci., 2010, 117, 352 CAS.
- W. J. Grigsby and J. F. Kadla, Macromol. Mater. Eng., 2014, 299, 368 CrossRef CAS.
- J. Ge, X. Shi, M. Cai, R. Wu and M. Wang, J. Appl. Polym. Sci., 2003, 90, 2756 CrossRef CAS.
- J.-M. Raquez, M. Deléglise, M.-F. Lacrampe and P. Krawczak, Prog. Polym. Sci., 2010, 35, 487 CrossRef CAS.
- J.-J. Ge, Bull. Kyushu Univ. For., 1998, 79, 21 Search PubMed.
- K. Hofmann and W. G. Glasser, J. Wood Chem. Technol., 1993, 13, 73 CrossRef CAS.
- P. Schofield, D. M. Mbugua and A. N. Pell, Anim. Feed Sci. Technol., 2001, 91, 21 CrossRef CAS.
- D. E. García, W. G. Glasser, A. Pizzi, C. Lacoste and M.-P. Laborie, Ind. Crops Prod., 2014, 62, 84 CrossRef.
- G. Sartori and R. Maggi, Advances in Friedel–Crafts acylation reactions: catalytic and green processes, CRC Press, 2010, p. 216 Search PubMed.
- M. Kessler, G. Ubeaud and L. Jung, J. Pharm. Pharmacol., 2003, 55, 131 CrossRef CAS PubMed.
- J.-J. Ge, CN98110709, 1998.
- J.-J. Ge, CN98100914, 1998.
- G. Alexandru, T. Lupascu, N. Timbaliuc and A. Meghea, Cent. Eur. J. Chem., 2013, 18, 1 Search PubMed.
- N. Bellotti, B. del Amo and R. Romagnoli, Procedia Mater. Sci., 2012, 1, 259 CrossRef CAS.
- E. Obreque-Slier, C. Mateluna, Á. Peña-Neira and R. López-Solís, J. Agric. Food Chem., 2010, 58, 8375 CrossRef CAS PubMed.
- K. S. Babu, T. H. Babu, P. V. Srinivas, B. S. Sastry, K. H. Kishore, U. S. N. Murty and J. M. Rao, Bioorg. Med. Chem. Lett., 2005, 15, 3953 CrossRef CAS PubMed.
- N. E. El Mansouri, Q. Yuan and F. Huang, BioResources, 2011, 6, 2647 CAS.
- W. R. Lotz, US5270083, 1993.
- Z. H. Huang, G. Z. Fang and B. Zhang, Chem. Ind. Forest Prod., 2007, 27, 27 CAS.
- F. Braghiroli, V. Fierro, A. Pizzi, K. Rode, W. Radke, L. Delmotte, J. Parmentier and A. Celzard, Ind. Crops Prod., 2013, 44, 330 CrossRef CAS.
- A. R. Pourali and A. Goli, J. Chem. Sci., 2011, 123, 63 CrossRef CAS.
- S. González-Mancebo, M. P. García-Santos, J. Hernández-Benito, E. Calle and J. Casado, J. Agric. Food Chem., 1999, 47, 2235 CrossRef.
- S. Laurichesse and L. Avérous, Prog. Polym. Sci., 2014, 39, 1266 CrossRef CAS.
- M.-Y. Chen and A. S.-Y. Lee, J. Chin. Chem. Soc., 2003, 50, 103 CrossRef CAS.
- M. Ikejiri, K. Miyashita, T. Tsunemi and T. Imanishi, Tetrahedron Lett., 2004, 45, 1243 CrossRef CAS.
- Z. Duan, Y. Gu and Y. Deng, J. Mol. Catal. A: Chem., 2006, 246, 70 CrossRef CAS.
- X. Yu, R. Zhao and G. Q. Liu, Se Pu, 2000, 18, 25 CAS.
- E. Perrier, A. Mariotte, A. Boumendjel and D. Bresson-Rival, Fr. Pat., 6235294, 2001 Search PubMed.
- S. S. Kelley, W. G. Glasser and T. C. Ward, J. Wood Chem. Technol., 1988, 8, 341 CrossRef CAS.
- J. Barry, G. Bram and A. Petit, Tetrahedron Lett., 1988, 29, 4567 CrossRef CAS.
- S.-C. Lin and M.-H. Rei, China Taipei Pat.,5136084, 1992 Search PubMed.
- W. J. Grigsby, J. H. Bridson, C. Lomas and J.-A. Elliot, Polymers, 1013, 5, 344 CrossRef.
- C. Wu, Transesterification, phase transition, rheology, and mechanical properties of blends of thermoplastic polyester and thermotropic polyester, PhD thesis, University of Akron, Department of Polymer Engineering, 2006, p. 330 Search PubMed.
- T. Weidlich, L. Dušek, B. Vystrčilová, A. Eisner, P. Švec and A. Růžička, Appl. Organomet. Chem., 2012, 26, 293 CrossRef CAS.
- B. Freedman, R. O. Butterfield and E. H. Pryde, J. Am. Oil Chem. Soc., 1986, 63, 1375 CrossRef CAS.
- M. I. Martins, R. F. Pires, M. J. Alves, C. E. Hori, M. H. M. Reis and V. L. Cardoso, Chem. Eng. Trans., 2013, 32, 817 Search PubMed.
- J. Sherma and B. Fried, Handbook of Thin-Layer Chromatography, CRC Press, 2003, p. 1048 Search PubMed.
- M. Sakai, M. Suzuki, N. Fumio and H. Yukihiko, Jp. Pat., 0618203A1, 2005 Search PubMed.
- E. Nomura, A. Hosoda, T. Hashizume, K. Kimura, K. Asahi, M. Yamazaki and H. Taniguchi, Jp. Pat., 2004307362, 2004 Search PubMed.
- A. P. Barbosa, E. B. Mano and C. T. Andrade, For. Prod. J., 2000, 50, 89 CAS.
- R. M. Roberts and A. A. Khalaf, Friedel–Crafts alkylation chemistry: a century of discovery, M. Dekker, 1984, p. 790 Search PubMed.
- L. G. Wade, Organic chemistry, Pearson Prentice Hall, 2010, p. 1262 Search PubMed.
- C. L. Wang, X. Zheng, W. D. Meng, H. Q. Li and F. L. Qing, Tetrahedron Lett., 2005, 46, 5399 CrossRef CAS.
- C. E. Summer, Jr., B. J. Hitch and B. L. Bernard, WO Pat., 1991016292A1, 1991 Search PubMed.
- A. Gissot, A. Wagner and C. Mioskowski, Tetrahedron, 2004, 60, 6807 CrossRef CAS.
- H. Dressler, Hydroxyalkylation of phenols or thiophenols with cyclic organic carbonates using triorganophosphine catalysts, US Pat., 5059723A, 1991 Search PubMed.
- R. Soto, J. Freer and J. Baeza, Bioresour. Technol., 2005, 96, 95 CrossRef CAS PubMed.
- D. B. Mitchell, R. L. Minnis, T. P. Curran, S. M. Deboo, J. A. Kelly, R. Patwardhan and W. T. Tai, US Pat., 5843337, 1998 Search PubMed.
- W. G. Glasser, C. Barnett, T. Rials and V. P. Saraf, J. Appl. Polym. Sci., 1984, 29, 1815 CrossRef CAS.
- W. G. Glasser, V. P. Saraf and W. H. Newman, J. Adhes., 1982, 14, 233 CrossRef CAS.
- W. G. Glasser, O. H.-H. Hsu, D. L. Reed, R. C. Forte and L. C.-F. Wu, in Urethane chemistry and applications, ed. K. Edwards, R. S. Graff, F. E. Bailey, S. Stinson, D. Klempner and W. G. Glasser, ACS Symposium No. 172, 1981, pp. 311–338 Search PubMed.
- G. Constantinescu, G. Cazacu and V. I. Popa, Cellul. Chem. Technol., 2005, 39, 201 CAS.
- A. de Menezes, D. Pasquini, A. Curvelo and A. Gandini, Cellulose, 2009, 16, 239 CrossRef CAS.
- D. E. García, Pinus pinaster (Ait.) bark tannin and its hydroxypropyl derivatives as building-blocks for bio-material design, PhD thesis, Freiburg University, Freiburg, Germany, 2014, p. 215 Search PubMed.
- A. Arbenz and L. Avérous, Ind. Crops Prod., 2015, 67, 295 CrossRef CAS.
- A. Arbenz and L. Avérous, RSC Adv., 2014, 4, 61564 RSC.
- S. Matthews, I. Mila, A. Scalbert, B. Pollet, C. Lapierre, C. L. M. Hervé du Penhoat, C. Rolando and D. M. X. Donnelly, J. Agric. Food Chem., 1997, 45, 1195 CrossRef CAS.
- M. Pérez, M. García, G. Blustein and M. Stupak, Biofouling, 2007, 23, 151 CrossRef PubMed.
- C. Amen-Chen, H. Pakdel and C. Roy, Bioresour. Technol., 2001, 79, 277 CrossRef CAS PubMed.
- M. R. Finck and P. E. Reed, US Pat., 5659002, 1997 Search PubMed.
- E. M. Kuskoski, J. J. Rios, J. M. Bueno, R. Fett, A. M. Troncoso and A. G. Asuero, Open Anal. Chem. J., 2012, 6, 1 CrossRef CAS.
- N. Bellotti, C. Deyá, B. del Amo and R. Romagnoli, Ind. Eng. Chem. Res., 2010, 49, 3386 CrossRef CAS.
- B. Labarbe, V. Cheynier, F. Brossaud, J.-M. Souquet and M. Moutounet, J. Agric. Food Chem., 1999, 47, 2719 CrossRef CAS PubMed.
- R. K. Gupta and E. Haslam, J. Chem. Soc., Perkins Trans. 1, 1978, 3, 892 RSC.
- H. Yamaguchi, M. Higuchi and I. Sakata, Mokuzai Gakkaishi, 1991, 37, 942 CAS.
- G. Palma, J. Freer and J. Baeza, Water Res., 2003, 37, 4974 CrossRef CAS PubMed.
- J. Li, Y. He and Y. Inoue, Polym. Int., 2003, 52, 949 CrossRef CAS.
- J. Sánchez-Martín, J. Bertrán-Heredia and G. Frutos-Blanco, Sep. Purif. Technol., 2009, 67, 295 CrossRef.
- J. Sánchez-Martín and J. Bertrán-Heredia, Appl. Water Sci., 2012, 2, 199 CrossRef.
- J. Beltrán-Heredia, J. Sánchez-Martín and M. C. Gómez-Munoz, Chem. Eng. J., 2010, 162, 1019 CrossRef.
- J. Beltrán-Heredia, J. Sánchez-Martín and M. Jiménez-Giles, Water, Air, Soil Pollut., 2011, 222, 53 CrossRef.
- L. Xuepin, M. Hewei, W. Ru and S. Bi, J. Membr. Sci., 2004, 243, 235 CrossRef.
- I. A. Sengil and M. Özacar, J. Hazard. Mater., 2008, 157, 277 CrossRef CAS PubMed.
- G. Tondi, C. W. Oo, A. Pizzi, A. Trosa and M.-F. Thevenon, Ind. Crops Prod., 2009, 29, 336 CrossRef CAS.
- T. Watanabe, T. Mori, T. Tosa and I. Chibata, J. Chromatogr. A, 1981, 207, 13 CrossRef CAS.
- T. Watanabe, Y. Matuo, T. Mori, R. Sano, T. Tosa and I. Chibata, J. Solid-Phase Biochem., 1978, 3, 161 CAS.
- J. Nkiliza, US Pat., 5808119, 1998 Search PubMed.
- S. González-Mancebo, M. P. García-Santos, J. Hernández-Benito, E. Calle and J. Casado, J. Agric. Food Chem., 1999, 47, 2235 CrossRef.
- A. Silkina, A. Bazes, J.-L. Mouget and N. Bourgougnon, Mar. Pollut. Bull., 2012, 64, 2039 CrossRef CAS PubMed.
- M. del M. Castro López, C. López de Dicastillo, J. M. López Vilariño and M. V. González Rodríguez, J. Agric. Food Chem., 2013, 61, 8462 CrossRef PubMed.
- S. Uesato, Y. Kitagawa, Y. Hara, H. Tokuda, M. Okuda, X. Y. Mou, T. Mukainaka and H. Nishino, Bioorg. Med. Chem. Lett., 2000, 10, 1673 CrossRef CAS PubMed.
- S. S.-M. Kim, S.-W. Kang, J.-S. Jeon and B.-H. Um, J. Med. Plants Res., 2012, 6, 2839 Search PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |