Biogenic silver nanoparticle impregnated hollow mesoporous silicalite-1: an efficient catalyst for p-nitrophenol reduction†
Rituparna
Das
,
Sourav
Ghosh
,
Ipsita Hazra
Chowdhury
and
Milan Kanti
Naskar
*
Sol-Gel Division, CSIR-Central Glass and Ceramic Research Institute, Kolkata 700 032, India. E-mail: milan@cgcri.res.in; Fax: +91 33 24730957
First published on 19th October 2015
Abstract
AgNP impregnated hollow mesoporous silicalite-1 was synthesized using green carambola extract. AgNPs of 10–15 nm size were well dispersed mostly within the shell of hollow silicalite-1 which contained hierarchical porosity. The synthesized product showed enhanced catalytic efficiency for the reduction of p-nitrophenol.
Silver nanoparticles (AgNPs) are currently the focus of intense research interest due to their unique characteristics such as low toxicity, biocompatibility, antimicrobial activity, optoelectronic, cryogenic superconducting, biosensing and catalytic properties.1,2 The intrinsic properties of AgNPs are determined by their size, shape, crystallinity, structure (solid or hollow) and surface functionality.3,4 AgNPs are commonly synthesized by wet-chemical reduction of silver ions in the presence of reducing agents. However, the particles synthesized by the conventional method are agglomerated in the form of clusters or even large particles. The biosynthesis of AgNPs using plant-based extracts has drawn remarkable research interest because they can act as reducing agents as well as capping agents following an environmentally friendly, sustainable and cost effective process. Recently, we have synthesized water dispersible AgNPs using an aqueous extract of Carambola fruit following a simple room temperature procedure.5 For catalytic applications of AgNPs, the catalysts are dispersed in an inorganic matrix (support) to improve their functionality by reducing the aggregation and stabilization of the nanoparticles.6
Zeolite as a catalyst support has attracted interest because of its high thermal and mechanical stability, and unique shape selectivity. However, the presence of microporosity in zeolite could retard the catalytic activity. Introduction of mesoporosity in the zeolite structure can facilitate high dispersion of the catalyst, and also shorten the diffusion path to enhance the catalytic performance. Hollow mesoporous zeolites, in particular, are the most promising candidates as catalytic supports due to their high surface area and high inertness under harsh conditions. One of the existing methods of preparing hollow zeolite spheres is the layer-by-layer assembly technique.7,8 Hollow zeolites are also prepared by the leaching technique with alkali treatment.9–11 Dong et al.12 prepared hollow silicalite-1 spheres by vapour-phase transport (VPT) treatment. Mesoporous zeolites have been synthesized by a soft templating route using cationic or silylated polymers and amphiphilic organosilane as the template,13 the hard templating route,14,15 and the non-templating route involving desilication–dealumination by the alkali leaching technique.16
In the present study, we have synthesized AgNP impregnated hollow mesoporous silicalite-1 using green carambola extract, which we report for the first time, to the best of our knowledge. Catalytic reduction of p-nitrophenol (4-NP) to p-aminophenol (4-AP) has been investigated using the synthesized products. The 4-NP is a water-pollutant present in industrial effluents, while 4-AP is used in the drug industry, as a photographic developer, as a corrosion inhibitor, etc. Here, the AgNP impregnated hollow mesoporous silicalite-1 showed enhanced catalytic efficiency with faster reaction rates (rate constant 5.5 × 10−3 s−1), and can be reused several times without any significant changes in their original catalytic activity.
AgNP impregnated hollow mesoporous silicalite-1 was synthesized hydrothermally at 170 °C/72 h followed by alkali treatment, and impregnation with biogenic AgNPs; the products were characterized by different techniques (Experimental section, ESI†). The catalytic performance of the products for the reduction of 4-NP was also studied (Catalytic performance, ESI†). The parent silicalite-1 (S-1) crystals synthesized hydrothermally at 170 °C/72 h were hexagonal in shape with the size range of 400–800 nm (Fig. S1a and b, ESI†). After alkali treatment with 0.2 M tetrapropyl ammonium hydroxide (TPAOH), a void was created in the interior of the silicalite-1 (HS-1) crystals (Fig. S1c and d, ESI†). The TEM images (Fig. S2a and b, ESI†) show the hexagonally shaped solid and void crystals before and after alkali treatment, respectively. During the desilication process by alkali treatment, the surface Si–OH groups of crystals lose protons rendering negatively charged Si–O−. These Si–O− ions interact with TPA+ ions, which reduces the dissolution of the external surface of silicalite-1. However, silicate oligomers are leached from the interior of the crystal, where crystallization is yet to be completed. The leached silicate oligomers interact with TPA+ ions on the silicalite-1 surface forming a silicate/TPA intermediate state, and recrystallize at 170 °C. Thus, continued desilication and recrystallization resulted in the formation of hollow silicalite-1 crystals. Fig. 1 shows (a and b) the FESEM and (c and d) TEM images of AgNP impregnated hollow silicalite-1 (Ag-HS-1). It is to be noted that the hollowness of all particles was not uniform (TEM image in Fig. S3, ESI† shows the particles with thicker shells compared to the particles shown in Fig. 1c).
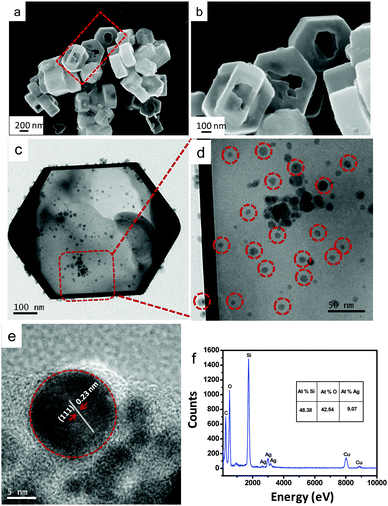 | ||
| Fig. 1 (a and b) FESEM, (c and d) TEM, (e) HRTEM and (f) EDS of AgNP impregnated hollow silicalite-1. | ||
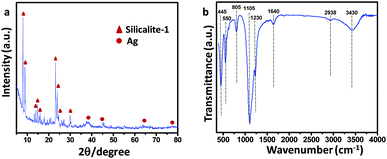
In the impregnation process (at pH 10), the polysaccharide (polyols) and ascorbic acid (C6H8O6) present in the Carambola fruit extract facilitate the formation of AgNPs from Ag+ ions. In alkaline pH, the capping and stabilizing abilities of biomolecules become higher due to the repulsion of electrical charge particles to stabilize AgNPs with smaller sizes. The TEM image (Fig. 1c and d) reveals that AgNPs of size 10–15 nm were dispersed mostly within the shell of hollow silicalite-1. The HRTEM image (Fig. 1e) of the AgNP impregnated sample shows a d-spacing of 0.23 nm corresponding to the (111) plane of Ag, indicating the presence of Ag nanoparticles (shown in dotted circles). Energy dispersive X-ray spectroscopy (EDS) (Fig. 1f) determined the presence of about 9% Ag atoms in the sample. The oxidation state of Ag in AgNP impregnated silicalite-1 was determined by X-ray photoelectron spectroscopy (Fig. S4, ESI†). The spectral profile reveals two peaks at 367.6 and 373.6 eV which corresponded to Ag 3d5/2 and Ag 3d3/2, respectively. This is characteristic of metallic silver (Ag0) in the sample.17Fig. 2 shows the (a) XRD pattern and (b) FTIR of AgNP impregnated hollow silicalite-1.
In the XRD patterns, crystallization of silicalite-1 along with metallic Ag (JCPDS No. 04-0783) was revealed. The characteristic peaks of metallic Ag appeared at 2θ values of around 38.01°, 44.26°, 64.02° and 77.36° corroborating to the crystal planes of (111), (200), (220) and (311), respectively. The characteristic peaks of parent and hollow silicalite-1 are also exhibited in Fig. S5 (ESI†).18 The FTIR spectra (Fig. 2b) of the samples show an intense pentasil vibration at 550 cm−1 of silicalite-1. The appearance of absorption bands at 445 and 805 cm−1 corroborated with Si–O–Si rocking and symmetric stretching and bending vibrations, respectively. The internal and external asymmetric stretching modes of vibrations are observed at 1105 and 1230 cm−1, respectively.19 Interestingly, the presence of an absorption band at 1640 cm−1 indicated that polyols in the carambola extract got oxidized to unsaturated carbonyl groups20 leading to the reduction of Ag+ to metallic Ag. The weak band at 2938 cm−1 was the characteristic C–H stretching vibration of ascorbic acid present in the carambola extract, while O–H stretching vibration of absorbed water shows the band at 3430 cm−1. The characteristic FTIR spectra of parent and hollow silicalite-1 are shown in Fig. S6 (ESI†). Fig. 3 shows (a) N2 adsorption–desorption isotherms, and pore size distributions (PSDs) by (b) DFT and (c) BJH methods for the AgNP impregnated hollow silicalite-1 sample.
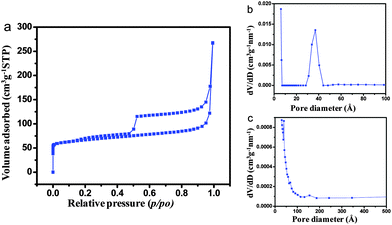 | ||
| Fig. 3 (a) N2 adsorption–desorption isotherms, and pore size distributions (PSD) by (b) DFT and (c) BJH methods of AgNP impregnated hollow silicalite-1. | ||
The N2 adsorption–desorption isotherms show the presence of an H2 hysteresis loop with an abrupt step around p/p0 = 0.5 in the desorption branch. The presence of microporosity and mesoporosity in the sample was confirmed by PSD curves determined by DFT (Fig. 3b) and BJH (Fig. 3c) methods, respectively. The TEM image (Fig. S7, ESI†) also confirmed the presence of mesoporosity (marked as circles) in the sample. The BET isotherms and PSDs of the parent and hollow silicalite-1 are revealed in Fig. S8 and S9 (ESI†), respectively. For the parent silicalite-1 sample, a steep rise in the isotherm occurred at lower relative pressure, around p/p0 = 0.1, which indicated an abundance of microporosity in the samples. However, the isotherms with two hysteresis loops for hollow silicalite-1 show a small loop at around p/p0 = 0.1 and a large loop at around p/p0 = 0.5, reflecting both the microporosity and meso/macro porosity, respectively in the samples. Such isotherms are very unusual, demonstrating the presence of two different types of pore systems with narrow pore size distribution.21 It can be concluded that the hysteresis loops at the p/p0 = 0.5 resulted due to dissolution of silica upon 0.2 M TPAOH treatment. Upon impregnation of AgNPs in hollow silicalite-1, the hollow structure was preserved in the presence of micro and mesoporosity of the samples, which was reflected in the same nature of isotherms in both the hollow silicalite-1 and AgNP impregnated hollow silicalite-1 samples. The textural properties of parent (S-1), hollow silicalite-1 (HS-1) and AgNP impregnated hollow silicalite-1 (Ag-HS-1) are shown in Table 1.
It exhibits that the BET surface area and the microporous surface area decreased in the order of S-1 > HS-1 > Ag-HS-1. It is worth mentioning that the BET surface area is the total specific surface area measured using the BET method. The microporous surface area is obtained by the difference between the BET surface area and the external surface area i.e., the mesoporous surface area (derived from the slope of the t (statistical thickness)-plot).22 For microporous materials like silicalite-1, the linear BET region occurs at p/p0 < 0.1, while the linear t-plot range is obtained at higher p/p0. Significant reduction of the total surface area along with the microporous surface area, and increment of the mesoporous surface area (external surface area) from S-1 to HS-1 (Table 1) could be due to structural distortion.23 It could occur during desilication followed by a second calcination step. The decrease in the microporous surface area from HS-1 to Ag-HS-1 could be attributed to the partial filling of micropores during impregnation of AgNPs. It was evidenced by the closer hysteresis loop at p/p0 < 0.5 (Fig. 3a). Interestingly, the average pore size also significantly increased after desilication of silicalite-1. Furthermore, for the AgNP impregnated hollow silicalite-1 sample, the increase in the average pore diameter and reduction of the surface area were attributed to the development of partial strain generated during the incorporation of AgNPs.24 Compared to S-1, the increase in total pore volume in HS-1 and Ag-HS-1 was due to the presence of higher mesoporosity in the latter samples.
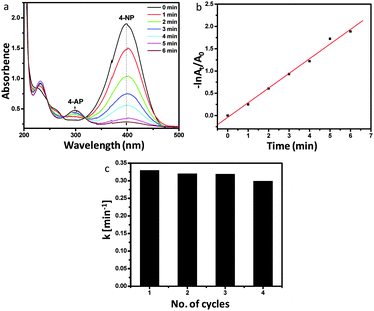
The catalytic performance of AgNP impregnated silicalite-1 was evaluated by the reduction of 4-NP to 4-AP using NaBH4 under ambient conditions. The UV-Vis spectra (Fig. 4a) of the sample reveal that with an increase in the time of catalytic reaction, the absorbance at 400 nm of 4-NP was reduced and the concomitant increase of the 4-AP absorption peak at 296 nm. The reaction was almost completed within 6 min. The reducing activity of NaBH4 was checked in the absence of the Ag-HS-1 catalyst (blank experiment). The UV-Vis absorptions of 4-NP, 4-NP + NaBH4 (at 0 min) and 4-NP + NaBH4 (after 30 min) depict (Fig. S10, ESI†) that the absorption peaks at 317 nm of 4-NP shifted to 400 nm in the presence of NaBH4, which remained almost unchanged even after 30 min of absorption. It is to be noted that in the absence of a catalyst (Ag-HS-1), no characteristic peak at 296 nm appeared for the reduction of 4-NP to 4-AP. It could be explained that the high kinetic barrier between mutually repelling negative ions of 4-NP and BH4− inhibits the further reduction of 4-NP25 in the absence of the Ag-HS-1 catalyst. Fig. 4b shows the logarithm plot of the absorbance (−ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) At/A0) with reaction time. It reveals pseudo first order reaction, and the apparent rate constant (k) was calculated to be 5.5 × 10−3 s−1 (0.33 min−1). The activity parameter κ (rate constant per unit mass of Ag) of the catalyst was calculated to be 65.63 s−1 g−1, which was found to be higher than that reported in the literature (Table S1, ESI†).26–29 This demonstrates that AgNP impregnated hollow silicalite-1 is advantageous for enhanced catalytic efficiency because of good dispersion of AgNPs in hollow architectures with large surface areas.6 The stability of the catalytic efficiency was checked for 4 cycles, indicating the recyclability of the catalyst (Fig. 4c).
At/A0) with reaction time. It reveals pseudo first order reaction, and the apparent rate constant (k) was calculated to be 5.5 × 10−3 s−1 (0.33 min−1). The activity parameter κ (rate constant per unit mass of Ag) of the catalyst was calculated to be 65.63 s−1 g−1, which was found to be higher than that reported in the literature (Table S1, ESI†).26–29 This demonstrates that AgNP impregnated hollow silicalite-1 is advantageous for enhanced catalytic efficiency because of good dispersion of AgNPs in hollow architectures with large surface areas.6 The stability of the catalytic efficiency was checked for 4 cycles, indicating the recyclability of the catalyst (Fig. 4c).
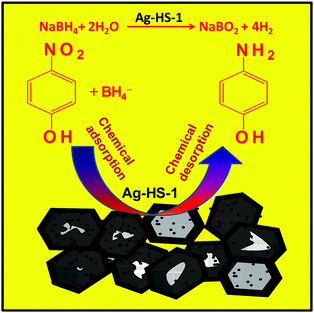
The catalytic reaction mechanism for the reduction of 4-NP to 4-AP in the presence of AgNP impregnated silicalite-1 is shown in Fig. 5. In the reaction mechanism, both the donor BH4− ions are obtained from NaBH4 and the acceptor 4-NP molecules coadsorbed on the catalyst surface (Ag-HS-1) through chemical adsorption.30 Here, NaBH4 could transfer hydrogen species to the catalytic surface (Ag-HS-1) under aqueous conditions.31 In this case, the catalyst acts as a hydrogen shuttle, and simultaneous reduction of 4-NP to 4-AP takes place via the desorption process.
In conclusion, we have firstly reported the synthesis of biogenic AgNP impregnated hollow mesoporous silicalite-1 using green carambola extract. AgNPs of 10–15 nm size were well dispersed mostly within the shell of hollow silicalite-1. The AgNP impregnated hollow silicalite-1 contained hierarchical porosity. The porous product revealed enhanced catalytic efficiency for the reduction of 4-NP to 4-AP. We anticipate that the present method can be applicable for other catalytic activities by tuning the porosity and the size of Ag and/or other noble and transition metal impregnated hollow zeolites.
Acknowledgements
This research was financially supported by the Department of Science and Technology under the DST-SERB sponsored project, GAP 0616 (Grant No. SR/S3/ME/0035/2012), Government of India. RD and IHC are thankful to UGC, and SG is thankful to CSIR for their fellowships.Notes and references
- G. N. R. Tripathi, J. Am. Chem. Soc., 2003, 125, 1178–1179 CrossRef CAS PubMed.
- S. S. Ravi, L. R. Christena, N. Sai Subramanian and S. P. Anthony, Analyst, 2013, 138, 4370–4377 RSC.
- S. Li, Y. Shen, A. Xie, X. Yu, L. Qiu, L. Zhang and Q. Zhang, Green Chem., 2007, 9, 852–858 RSC.
- B. L. Devi and A. B. Mandal, RSC Adv., 2013, 3, 5238–5253 RSC.
- I. H. Chowdhury, S. Ghosh, M. Roy and M. K. Naskar, J. Sol–Gel Sci. Technol., 2015, 73, 199–207 CrossRef CAS.
- Z. Niu, S. Zhang, Y. Sun, S. Gai, F. He, Y. Dai, L. Li and P. Yang, Dalton Trans., 2014, 43, 16911–16918 RSC.
- K. H. Rhodes, S. A. Davis, F. Caruso, B. J. Zhang and S. Mann, Chem. Mater., 2000, 12, 2832–2834 CrossRef CAS.
- V. Valtchev and S. Mintova, Microporous Mesoporous Mater., 2001, 43, 41–49 CrossRef CAS.
- C. S. Mei, Z. C. Liu, P. Y. Wen, Z. K. Xie, W. M. Hua and Z. Gao, J. Mater. Chem., 2008, 18, 3496–3500 RSC.
- C. Dai, A. Zhang, L. Li, K. Hou, F. Ding, J. Li, D. Mu, C. Song, M. Liu and X. Guo, Chem. Mater., 2013, 25, 4197–4205 CrossRef CAS.
- D. Fodor, L. Pacosova, F. Krumeich and J. A. van Bokhoven, Chem. Commun., 2014, 50, 76–78 RSC.
- A. Dong, Y. Wang, Y. Tang, N. Ren, Y. Zhang and Z. Gao, Chem. Mater., 2002, 14, 3217–3219 CrossRef CAS.
- H. Wang and T. J. Pinnavaia, Angew. Chem., Int. Ed., 2006, 45, 7603–7606 CrossRef CAS PubMed.
- W. Fan, M. A. Snyder, S. Kumar, P. S. Lee, W. C. Yoo, A. V. McCormick, R. L. Penn, A. Stein and M. Tsapatsis, Nat. Mater., 2008, 7, 984–991 CrossRef CAS PubMed.
- J.-S. Yu, S. B. Yoon, Y. J. Lee and K. B. Yoon, J. Phys. Chem. B, 2005, 109, 7040–7045 CrossRef CAS PubMed.
- J. Perez-Ramirez, S. Abello, A. Bonilla and J. C. Groen, Adv. Funct. Mater., 2009, 19, 164–172 CrossRef CAS.
- S. Nath, S. K. Ghosh, S. Praharaj, S. Panigrahi, S. Basu and T. Pal, New J. Chem., 2005, 29, 1527–1534 RSC.
- M. M. J. Treacy and J. B. Higgins, Collection of Simulated XRD Powder Patterns for Zeolite, Elsevier, Amsterdam, 2001 Search PubMed.
- J. Brinker and G. W. Scherer, Sol-Gel Sci.: The Physics and Chemistry of Sol-Gel Processing, Academic Press, San Diego, CA, 1990 Search PubMed.
- D. Jain, H. K. Daima, S. Kachhwaha and S. L. Kothari, Dig. J. Nanomater. Biostruct., 2009, 4, 723–727 Search PubMed.
- A.-H. Lu, W. Schmidt, B. Spliethoff and F. Schuth, Adv. Mater., 2003, 15, 1602–1606 CrossRef CAS.
- D. W. Rutherford, C. T. Chiou and D. D. Eberl, Clays Clay Miner., 1997, 45, 534–543 CAS.
- A.-H. Lu, W.-C. Li, W. Schmidt and F. Schuth, Microporous Mesoporous Mater., 2005, 80, 117–128 CrossRef CAS.
- S. M. El-Sheikh, A. A. Ismail and J. F. Al-Sharab, New J. Chem., 2013, 37, 2399–2407 RSC.
- Z. Dong, X. Le, Y. Liu, C. Dong and J. Ma, J. Mater. Chem. A, 2014, 2, 18775–18785 CAS.
- Y. Liu, Y. Zhang, H. Ding, S. Xu, M. Li, F. Kong, Y. Luo and G. Li, J. Mater. Chem. A, 2013, 1, 3362–3371 CAS.
- M. H. Rashid and T. K. Mandal, J. Phys. Chem. C, 2007, 111, 16750–16760 CAS.
- S. Tang, S. Vongehr and X. Meng, J. Mater. Chem., 2010, 20, 5436–5445 RSC.
- A. Gangula, R. Podila, M. Ramakrishna, L. Karanam, C. Janardhana and A. M. Rao, Langmuir, 2011, 27, 15268–15274 CrossRef PubMed.
- Z. Jiang, J. Xie, D. Jiang, X. Wei and M. Chen, CrystEngComm, 2013, 15, 560–569 RSC.
- R. Kaur, C. Giordano, M. Gradzielski and S. Mehta, Chem. – Asian J., 2014, 9, 189–198 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the experimental section, catalytic performance, and characterization. See DOI: 10.1039/c5nj02088c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |