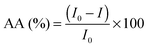Antioxidant activity of phytoestrogen type isoflavones in biomimetic environments
Mariana
Voicescu
*a,
Petra
Hellwig
b and
Aurelia
Meghea
c
aInstitute of Physical Chemistry “Ilie Murgulescu” of the Romanian Academy, Splaiul Independentei 202, 060021 Bucharest, Romania. E-mail: voicescu@icf.ro
bLaboratoire de Bioelectrochimie et Spectroscopie, UMR 7140, CNRS, Université de Strasbourg, 1, rue Blaise Pascal, 67070, Strasbourg, France
cUniversity POLITEHNICA of Bucharest, Faculty of Applied Chemistry and Materials Sciences, Polizu 1, 78126 Bucharest, Romania
First published on 19th November 2015
Abstract
The work aims to simulate in vitro the antioxidant activity of four phytoestrogen type isoflavones, whose effects are closer to those of estradiol (daidzein, formononetin, genistein, biochanin A) in biomimetic environments (in lecithin lipidic bi-layers and on silver nanoparticles), using the chemiluminescent system luminol–hydrogen peroxide, in phosphate buffer, pH 7.4. The contribution of a carrier protein, human serum albumin (HSA), to the antioxidant activity of these isoflavones has also been investigated. The rate of the fluorescence quenching of HSA by isoflavone as well as the binding constant of isoflavone to HSA, in both lecithin lipidic bi-layers and on silver nanoparticles, has also been investigated by fluorescence spectroscopy. The results are discussed with relevance to the oxidative stress process.
1. Introduction
Isoflavones, oestrogen-like molecules with a similar structure to 17β-oestradiol, function as antioxidants in plants and offer alternative therapies for a range of hormone dependent conditions, such as cancer, menopausal symptoms, cardiovascular disease, and osteoporosis.1–3 In these lines, evaluation of the antioxidant activity of isoflavones and their biological metabolites in different conditions and by different methods has been reported.4–8Phosphatidylcholine is one of the essential phospholipids of all cell membranes, forming lipidic bi-layers, so that the functioning of the membrane systems depends on the composition and integrity of the phospholipidic structure. Phosphatidylcholine plays an important role in cell proliferation and regeneration as well as in the transmembranar transport of different molecules, transport that depends on membrane fluidity and implicitly on phospholipid composition. Liposomes (that are formed by hydration of a thin lipid film generating thus fluid liquid-crystalline bi-layers) represent important vesicles for the transport and the controlled release of medicines and biomolecules in living systems,9,10 studies connected with the binding and antioxidant activity of some flavonoid type compounds in different lipid cell membranes being recently undertaken.11–13
Isoflavone – serum protein interactions have been reported,14–17 with a special interest in genistein (4′,5,7-trihydroxy isoflavone), a phytoestrogen with a large domain of physiological and pharmacological functions used especially as an inhibitor in cell proliferation. Genistein has estrogen-modulating properties, one of the pharmaco-dynamic actions being that of preventing estrogen-dependent cancers, especially mammary cancer.18
Metal nanoparticles (NPs) have been of recent interest due to their use in biotechnology and clinical medicine, especially as carriers of biomolecules (proteins, enzymes, drugs).19–23 In these lines, studies on silver nanoparticles (SNPs)–serum albumin and SNPs–serum protein–antitumor drug interaction were reported.23–25
Due to its high sensitivity and rapidity, the chemiluminescence (CL) method is reported as a tool for the evaluation of antioxidant activity.26,27 The CL is based on the generation of reactive oxygen species (ROS) (HO˙, O2˙−, 1O2, ROO˙ radicals) in a luminescent system and on studying the antioxidant effect of several molecules one is interested in, with the reduction of the CL intensity being considered a measure of the antioxidant activity.26–31
This work is an extension of the previous paper32 and aims to simulate in vitro the antioxidant activity of four phytoestrogen type isoflavones, whose effects are closer to those of estradiol (daidzein, formononetin, genistein, biochanin A) in biomimetic environments (in lecithin lipidic bi-layers and on silver nanoparticles), using the chemiluminescent system luminol–hydrogen peroxide, in phosphate buffer, pH 7.4. The contribution of a carrier protein, human serum albumin (HSA), to the antioxidant activity of these isoflavones has also been investigated. The rate of the fluorescence quenching of HSA by isoflavone as well as the binding constant of isoflavone to HSA, in both lipidic bi-layers and on silver nanoparticles, has also been investigated by fluorescence spectroscopy. The results are relevant to understanding the role of antioxidants in human health.
2. Materials and methods
2.1. Chemicals
Phytoestrogen type isoflavones used (biochanin A (BCH) (product No. D2016), genistein (GS) (≥98%) (product No. G6649), daidzein (DZ) (≥98%) (product No. D7802) and formononetin (≥99%) (product No. 47752)) were purchased from Sigma and their chemical structures are presented in Table 1.The stock solutions of isoflavones were prepared as follows: BCH, 5.48 mM in ethanol/water mixture 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, v/v; GS, 2 mM in dimethyl sulfoxide (DMSO); DZ, 2.12 mM in ethanol; formononetin, 3.05 mM in methanol. Ethanol and DMSO were purchased from Merck; methanol was purchased from Sigma.
25, v/v; GS, 2 mM in dimethyl sulfoxide (DMSO); DZ, 2.12 mM in ethanol; formononetin, 3.05 mM in methanol. Ethanol and DMSO were purchased from Merck; methanol was purchased from Sigma.
Human serum albumin (HSA) (product No. A9511) was purchased from Merck.
L-α-Phosphatidylcholine (PC) from egg yolk (∼60%; product No. 61755) was purchased from Sigma and the stock solution, 8 mg ml−1, was prepared in ethanol.
Isoflavones, PC and HSA were used without further purification and all other solvents used were of analytical grade.
The luminol (LH2) (purchased from Fluka) – hydrogen peroxide (H2O2) (purchased from Sigma) with the concentration of LH2 = 2.5 × 10−5 M and H2O2 = 30 mM in phosphate buffer (purchased from Sigma-Aldrich), 100 mM, pH = 7.4, was considered as the reference system.
The silver nanoparticles (SNPs) were prepared according to the literature.33 Silver nitrate (AgNO3, purity 99.99%) and the reducing agent sodium borohydride (NaBH4) were purchased from Sigma-Aldrich. Briefly, aliquots of 1 mM AgNO3 solution were added under vigorous stirring to a solution containing NaBH4 so that their final concentrations were 0.1 mM AgNO3 and 7 mM NaBH4, respectively. No capping agent was needed as the resulting nanoparticles were electrostatically stabilized.
2.2. Methods
The chemiluminescence (CL) measurements were performed using a TD 20/20 chemiluminometer (Turner Designs). The CL method, data acquisition and the chemiluminescent system used, luminol–hydrogen peroxide, have been previously described.28–31The in vitro antioxidant activity, AA (%), of the studied isoflavones and isoflavones/PC/SNPs systems, by means of free radical scavenging, was calculated according to the following equation:
The fluorescence emission spectra of HSA protein were recorded using a Jasco FP-6500 spectrofluorometer equipped with a 150 W xenon lamp, using 3 nm bandpass for the excitation and emission monochromators, a detector response of 1 s, and data pitch of 0.5 nm. The excitation wavelength for tryptophan (Trp) emission was 280 nm.
The FAR-IR spectra, 50–600 cm−1, were recorded using a Vertex 70 spectrometer from Bruker. A deuterated triglyceryl sulfate detector and a Hg source were used, at a scan velocity of 2.5 kHz. For one sample five spectra with a resolution of 4 cm−1 (128 scans) were averaged. The contribution of the polyethylene window and from humidity was interactively subtracted.
3. Results and discussion
3.1. Structural aspects and antioxidant activity of isoflavones in lecithin lipidic bi-layers
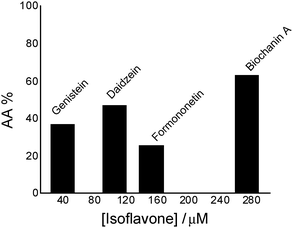 | ||
| Fig. 1 Antioxidant activity (AA%) of isoflavones in the chemiluminescent system luminol (2.5 × 10−5 M) – hydrogen peroxide (30 mM), in phosphate buffer, 0.1 M, pH 7.4. | ||
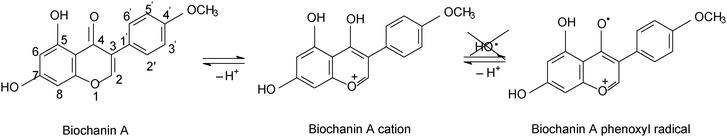
Comparing genistein and daidzein, no significant difference in their antioxidant activity was observed; thus the –OH group at C-5 position of the genistein’s structure leads to a slight decrease in its antioxidant activity. In contrast, formononetin and biochanin A showed that their antioxidant activity depends on the absence and the presence of the –OH group at C-5 position of their structure. It was observed that the methoxy group (–OCH3) strongly reduced the antioxidant activity of formononetin and significantly improved the antioxidant activity of biochanin A. It is well known that the antioxidant activity of flavonoids and phenolic acid compounds is related to the structure, mainly to electron delocalization in the aromatic ring.34,35 It was found that these compounds upon formation of a phenoxyl radical during oxidation are stabilized by the resonance effect of the aromatic ring when H atoms are substituted by any electron donating group.34,35 Thus, in the case of biochanin A, it can be expected that the presence of the –OCH3 group inhibits the HO˙ radical formation and forms less-toxic phenoxyl radicals (Scheme 1).
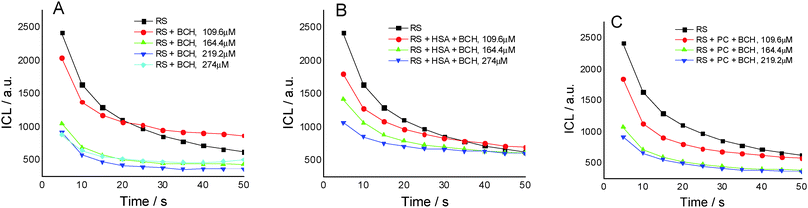
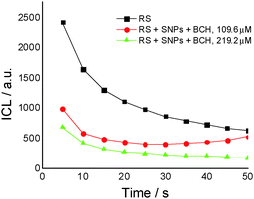
Fig. 2A presents the effect of biochanin A concentration on the CL intensity of the LH2–H2O2 system, in phosphate buffer, pH 7.4, as a function of time. It is observed that at high concentrations of biochanin A, between 164.4 and 274 μM, the CL intensity decreases due to its scavenging performance of the reactive oxygen species (HO˙, O2˙−, 1O2, ROO˙ radicals) involved in the chemiluminescent emission. For a lower biochanin A concentration, 109.6 μM, at ∼20 s at the beginning of the CL reaction, the CL intensity increases as a result of an increase in ROS. As a consequence it can be suggested that biochanin A presents antioxidant activity at higher concentrations; at lower concentrations, 109.6 μM, it offers protection against ROS as a function of the reaction time.
In the presence of HSA (Fig. 2B) the antioxidant activity of biochanin A is slightly improved (CL intensity decreases) when biochanin A is used in low concentrations, while at higher concentrations an increase of CL intensity is noticed. This behavior which does not appear in the absence of HSA (Fig. 2A) can be due to the oxidation of the Cis34 residue from sulfenic acid, HSA–SOH. One has to also notice that HSA captures the free radicals 20 seconds after the CL reaction was initiated; in the first moments of the CL reaction there are more free radicals in the presence of HSA than in the presence of the luminol–hydrogen peroxide reference system.32 In lecithin lipidic bi-layers (Fig. 2C), biochanin A at 109.6 μM concentration leads to a decrease of CL intensity which is maintained with time, 5–50 seconds from the initiation of the CL reaction, so that ROS generation, especially HO˙ and ROO˙ radicals, is more reduced and thus leads to the inhibition of lipid peroxidation.
The ability of genistein and daidzein to reduce free radical formation and to scavenge free radicals is well known.36,37 According to Arora et al.,4 genistein, daidzein and their glycosylated and methoxylated derivatives inhibit lipid peroxidation in a liposomal system, and they appeared to be more potent inhibitors of metal-ion-induced peroxidation than of the peroxidation induced by peroxyl radicals.4
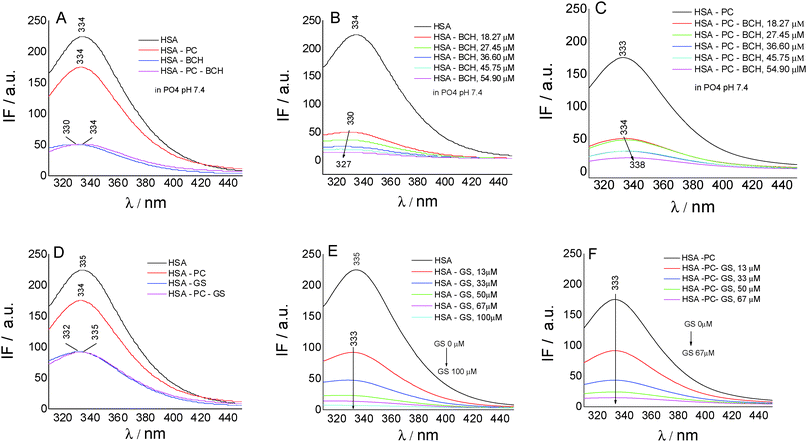
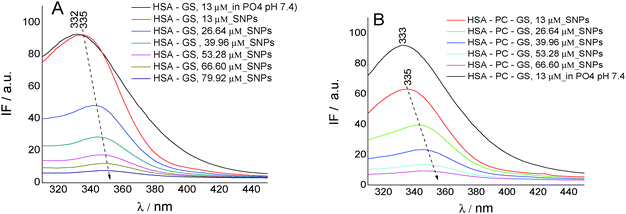
To gain insights into the biochanin A–HSA interaction in lecithin lipidic bi-layers, the quenching of HSA fluorescence (intrinsic fluorescence from the Trp214 residue) (λem = 334 nm) in the presence of biochanin A (BCH) (λem = 330 nm), PC (λem = 334 nm), and PC-BCH (λem = 334 nm), in phosphate buffer, 0.1 M, pH 7.4, is shown in Fig. 3A. The quenching of HSA fluorescence with the increase of biochanin A concentration in the absence (Fig. 3B) and in the presence of PC (Fig. 3C) was attributed to a static process, analyzed with the Stern–Volmer equation:
| F0/F = 1 + Ksv[Q] |
Complementary information on the affinity of BCH binding to HSA in lecithin lipidic bi-layers was obtained using the modified Benesi–Hildebrand equation:40
| 1/ΔF = 1/ΔFmaxK[HSA] + 1/ΔFmax |
Fig. 3D–F present by comparison the quenching of HSA fluorescence with the increase of the genistein concentration in the absence (Fig. 3E) and in the presence of PC (Fig. 3F). In this case the value of the HSA fluorescence quenching constant by genistein is kq = 3.49 × 1013 M−1 s−1 and by incorporating GS in lecithin lipidic bi-layers the value of the rate constant of HSA fluorescence quenching is kq = 1.80 × 1013 M−1 s−1. Thus the quenching process of HSA fluorescence by BCH is faster than in the case of GS. Regarding the binding affinity of GS to HSA in lipidic bilayers, one can say that it is weaker, K = 0.93 × 10−5 M−1 (SE = 0.0017; r2 = 0.999), than in the phosphate buffer, K = 2.22 × 10−5 M−1 (SE = 0.98208; r2 = 0.997). Thus, in lecithin lipidic bi-layers, the binding affinity of BCH to HSA is higher than the binding affinity of GS to HSA.
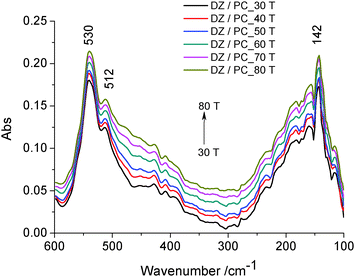
Two absorption bands can be noticed: the bands at about 530 cm−1 and 512 cm−1 attributed to wagging and rocking vibrations of the O–P–O group as well as of the hydrophilic groups of the lipidic bilayer43 directed to the outside, and the band at about 142 cm−1, due to the contribution of intra-molecular H-bond (ν(H⋯O)) vibrations. In this range of temperature, no thermal degradation of the –OH groups at C-7 and C-4′ positions is considered. It was shown that the number and position of the –OH groups, especially at the C-4′ position, are important factors regarding the antioxidant activity of isoflavone.4 Therefore, daidzein incorporated in lecithin lipidic bi-layers has a well ordered structure which is stabilized by intramolecular H-bonds.
3.2. Structural aspects and antioxidant activity of isoflavones on silver nanoparticles (SNPs)
For the same biochanin A concentration (109.6 μM) but in a complex of silver nanoparticles (SNPs) (Fig. 5), CL intensity decreases about 2 times, the induction of the oxidation process being dependent on time: 25 seconds after the beginning of the CL reaction, a slight increase of CL intensity takes place, due to ROS generation.
Therefore, based on the CL results it seems that biochanin A acts as a scavenger of the ROS most efficiently in the SNP complexes.
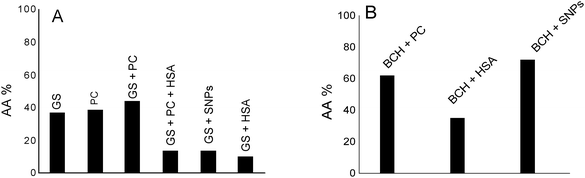
Fig. 6A shows the antioxidant activity of genistein (GS) in lecithin lipidic bi-layers (PC) in the presence of human serum albumin (HSA) and on a silver nanoparticles support (SNPs).
GS presents the best antioxidant activity of trapping ROS (∼45%) when it is encapsulated in lecithin lipidic bi-layers while on the SNPs support its antioxidant activity is reduced (∼15%); it was suggested that in the presence of HSA, the GS tested concentration, respectively 40 μM, does not represent the optimum concentration at which GS has a pronounced antioxidant activity.
Overall, the system chemiluminescence percentage that remained was ∼40% in the presence of GS and PC. This means that the lecithin lipidic bi-layers without GS were capable of trapping ROS of the luminol–hydrogen peroxide system similarly to GS. On the other hand, in GS + PC + HSA, GS + SNPs and GS + HSA, the percentage of chemiluminescence that remained was lower than in GS, GS + PC and PC. This means that GS + PC + HSA, GS + SNPs and GS + HSA were able to trap the ROS of the hydrogen peroxide/luminol system reducing the chemiluminescence generation by the system. Then, SNPs and HSA would be able to trap ROS.
Fig. 6B shows by comparison the antioxidant activity of biochanin A in PC, HSA as well as on SNPs. As can be observed, the antioxidant activity of biochanin A increases as follows: SNPs > PC > HSA.
On SNPs, the fluorescence intensity of HSA decreases as genistein concentration increases, the fluorescence emission being 4 nm red-shifted from λem = 335 nm to λem = 349 nm. The same behavior is observed in lecithin lipidic bi-layers but the fluorescence quenching of HSA is weaker.
The value of the rate constant of HSA fluorescence quenching for the GS/HSA/SNPs system is kq = 1.71 × 1013 M−1 s−1 and for the GS/HSA/PC/SNPs system it is kq = 1.66 × 1013 M−1 s−1. Regarding the binding constant, we observed that the ability of HSA to bind GS is better when Trp214 is included in lecithin lipidic bi-layers (K = 0.30 × 10−5 M−1; SE = 0.00681; r2 = 0.998) and when adsorbed on the SNP surface (K = 0.41 × 10−5 M−1; SE = 0.0422; r2 = 0.959). This feature suggests no perturbation of HSA secondary structure when included in lecithin lipidic bi-layers and than adsorbed on SNPs.
4. Conclusion
By the chemiluminescence method, the antioxidant activity of the studied estrogen-mimetic isoflavones is found to decrease as follows: biochanin A > daidzein > genistein > formononetin.Daidzein included in lecithin lipidic bi-layers has a well ordered structure which is stabilized by intra-molecular H-bonds.
In lecithin lipidic bi-layers as well as in silver nanoparticle complexes the antioxidant activity of biochanin A increases. For tested concentrations, biochanin A presents antioxidant activity at higher concentrations (274 μM) while at lower concentrations (109.6 μM) it does not offer protection against reactive oxygen species; the greatest efficiency with which biochanin A traps reactive oxygen species is found in silver nanoparticle complexes.
The protective effect of phytoestrogen type isoflavones, as free molecules and when bound to serum albumins, in lecithin lipidic bi-layers and on silver nanoparticles, showed a good antioxidant activity that may be useful as further systems in the oxidative stress process, to better understand the role of antioxidants in human health.
Acknowledgements
This work was supported by a grant from the Romanian National Authority for Scientific Research, CNCS – UEFISCDI, project number PN-II-RU-TE-2012-3-0055 and by the Fondation de la recherche medicale (to PH).References
- R. J. Miksicek, Mol. Pharmacol., 1993, 44, 37–43 CAS.
- K. D. R. Setchell and A. Cassidy, J. Nutr., 1999, 129, 758S–767S CAS.
- J. J. B. Anderson, M. Anthony, M. Messina and S. C. Garner, Nutr. Res. Rev., 1999, 12, 75–116 CrossRef CAS PubMed.
- A. Arora, M. G. Nair and G. M. Strasburg, Arch. Biochem. Biophys., 1998, 356, 133–141 CrossRef CAS PubMed.
- C. H. Lee, L. Yang, J. Z. Xu, S. Y. V. Yeung, Y. Huang and Z.-Y. Chen, Food Chem., 2005, 90, 735–741 CrossRef CAS.
- C. Niamnuy, M. Nachaisin, J. Laohavanich and S. Devahastin, Food Chem., 2011, 129, 899–906 CrossRef CAS PubMed.
- A. K. Ghimeray, P. Sharma, W. Hu, C. W. Jin, C. H. Park, H. S. Rho and D. Ha Cho, J. Med. Plants Res., 2013, 7, 1129–1137 CAS.
- B. M. Popović, D. Štajner, R. Ždero, S. Orlović and Z. Galić, Sci. World J., 2013, 134656, DOI:10.1155/2013/134656.
- G. Gregoriadis and D. E. Neerunjun, Eur. J. Biochem., 1974, 47, 179–185 CrossRef CAS PubMed.
- J. P. Sculier, A. Coune, F. Meunier, C. Brassinne, C. Laduron, C. Hollaert, N. Collette, C. Heymans and J. Klastersky, Eur. J. Cancer Clin. Oncol., 1988, 24, 527–538 CrossRef CAS PubMed.
- S. Chaudhuri, A. Banerjee, K. Basu, B. Sengupta and P. K. Sengupta, Int. J. Biol. Macromol., 2007, 41, 42–48 CrossRef CAS PubMed.
- S. Chaudhuri, K. Basu, B. Sengupta, A. Banerjee and P. K. Sengupta, Luminescence, 2008, 23, 397–403 CrossRef CAS PubMed.
- P. K. Sengupta and S. Chaudhuri, J. Indian Chem. Soc., 2010, 87, 213–220 CAS.
- Q. Bian, J. Liu, J. Tian and Z. Hu, Int. J. Biol. Macromol., 2004, 34, 275–279 CrossRef PubMed.
- H. G. Mahesha, S. A. Singh, N. Srinivasan and A. G. Appu Rao, FEBS J., 2006, 273, 451–467 CrossRef CAS PubMed.
- J.-S. Mandeville, E. Froehlich and H. A. Tajmir-Riahi, J. Pharm. Biomed. Anal., 2009, 49, 468–474 CrossRef CAS PubMed.
- M. Kuzdzal, O. Wesolowska, J. Strancar and K. Michalak, Chem. Phys. Lipids, 2011, 164, 283–291 CrossRef CAS PubMed.
- C. A. Lamartiniere, Am. J. Clin. Nutr., 2000, 71, 1705S–1707S CAS.
- P. K. Jain, X. Huang, I. H. El-Sayed and M. A. El-Sayed, Acc. Chem. Res., 2008, 41, 1578–1586 CrossRef CAS PubMed.
- N. J. Halas, Nanomedicine, 2009, 4, 369–371 CrossRef PubMed.
- N. R. Panyala, E. M. Pena-Mendez and J. Havel, J. Appl. Biomed., 2009, 7, 75–91 CAS.
- M. Homberger and U. Simon, Philos. Trans. R. Soc., A, 2010, 368, 1405–1453 CrossRef CAS PubMed.
- R. De-Llanos, S. Sanchez-Cortes, C. Domingo, J. Garcia-Ramos and P. Sevilla, J. Phys. Chem. C, 2011, 115, 12419–12429 CAS.
- M. Voicescu, S. Ionescu and D. G. Angelescu, J. Nanopart. Res., 2012, 14(10), 1174, DOI:10.1007/s11051-012-1174-0.
- M. Voicescu, D. G. Angelescu, S. Ionescu and V. S. Teodorescu, J. Nanopart. Res., 2013, 15, 1555, DOI:10.1007/s11051-013-1555-z.
- O. Hirayama, M. Takagi, K. Hukumoto and S. Katoh, Anal. Biochem., 1997, 247, 237–241 CrossRef CAS PubMed.
- C. Dodeigene, L. Thunus and R. Lejeune, Talanta, 2000, 51, 415–439 CrossRef.
- M. Voicescu, G. Ionita, T. Constantinescu and M. Vasilescu, Rev. Roum. Chim., 2006, 51, 683–690 CAS.
- M. Voicescu, G. Ionita, M. Vasilescu and A. Meghea, J. Inclusion Phenom. Macrocyclic Chem., 2006, 54, 217–219 CrossRef CAS.
- M. Voicescu, G. Ionita, A. Beteringhe, M. Vasilescu and A. Meghea, J. Fluoresc., 2008, 18, 953–959 CrossRef CAS PubMed.
- M. Voicescu, R. Ion and A. Meghea, J. Serb. Chem. Soc., 2010, 75, 333–341 CrossRef CAS.
- M. Voicescu, C. L. Nistor and A. Meghea, J. Lumin., 2015, 157, 243–248 CrossRef CAS.
- D. G. Angelescu, M. Vasilescu, R. Somoghi, D. Donescu and V. S. Teodorescu, Colloids Surf., A, 2010, 366, 155–162 CrossRef CAS.
- M. E. Couvelier, H. Richard and C. Berset, Biosci., Biotechnol., Biochem., 1992, 56, 324–325 CrossRef.
- C. A. Rice-Evans, N. J. Miller and G. Paganga, Free Radical Biol. Med., 1996, 20, 933–956 CrossRef CAS PubMed.
- P. G. Pietta, J. Nat. Prod., 2000, 63, 1035–1042 CrossRef CAS.
- Z. Djuric, G. Chen, D. R. Doerge, L. K. Heilbrun and O. Kucuk, Cancer Lett., 2001, 172, 1–6 CrossRef CAS PubMed.
- K. D. R. Setchell and H. Adlercreutz, Role of gut flora in toxicity and cancer, Academic Press, New York, 1988 Search PubMed.
- J. R. Lakowicz and G. Weber, Biochemistry, 1973, 12, 4161–4170 CrossRef CAS PubMed.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS.
- R. Hielscher and P. Hellwig, ChemPhysChem, 2010, 11, 435–441 CrossRef CAS PubMed.
- R. Hielscher and P. Hellwig, Specific far infrared spectroscopic properties of phospholipids, in Spectroscopy of Biological Molecules, ed. M. P. Marques, L. A. E. Batista de Carvalho and P. I. Haris, IOS Press, 2013 Search PubMed.
- K. Leberle, I. Kempf and G. Zundel, Biophys. J., 1989, 55, 637–648 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |