An ultrasensitive sandwich-type electrochemical immunosensor based on δ-MnO2 and palladium nanoparticles covered natural halloysite nanotubes for the detection of hepatitis B surface antigen†
Yueyuan
Li
,
Lihui
Tian
,
Li
Liu
,
Lei
Liu
,
Jingjing
Li
,
Qin
Wei
and
Wei
Cao
*
Key Laboratory of Chemical Sensing & Analysis in Universities of Shandong (University of Jinan), School of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, China. E-mail: jncw88@163.com; jn_chm302@163.com; Fax: +86-53187161600; Tel: +86-53182767890
First published on 6th November 2015
Abstract
A sensitive sandwich-type electrochemical immunosensor for the quantitative detection of hepatitis B surface antigen (HBsAg) was proposed and the nanocomposite of palladium nanoparticles/δ-manganese dioxide/halloysite nanotubes (Pd/δ-MnO2/HNTs) was used as a novel signal amplification strategy. δ-MnO2 could absorb a large amount of palladium nanoparticles (Pd NPs) due to their advantages of large surface area, good adsorption capacity and environmentally friendly. The earth possesses abundant HNTs, which could deter the agglomeration of δ-MnO2 and preserve the advantages of δ-MnO2 once loaded on HNTs. With high electrical catalytic activity of Pd NPs and δ-MnO2, the Pd/δ-MnO2/HNT nanocomposite could improve the analytical signal and achieve high sensitivity. The electroplated gold nanoparticles (Au NPs) as the platform of the immunosensor could immobilize the primary antibodies tightly and enhance the electron transfer efficiently. Under the optimal conditions, the immunosensor exhibited an extremely low detection limit of 0.3 pg mL−1 and a wide linear range from 0.001 ng mL−1 to 20 ng mL−1 for HBsAg. Moreover, it revealed good selectivity, acceptable reproducibility and stability, and exhibited application potentiality in clinical diagnosis.
1. Introduction
As a major health concern worldwide, hepatitis B virus (HBV) infection causes about one million deaths annually.1 Hepatitis B surface antigen (HBsAg) is the cornerstone for the diagnosis of acute or chronic HBV infection, and a predictive biomarker which largely affects the customized therapy and treatment outcomes of individual patients.2In the past few years, various sensing methods have been developed for HBsAg detection, such as radioimmunoassay,3 enzyme-linked immunosorbent assay,4 chemiluminescence5 and so on. In addition, electrochemical immunoassay, based on the principle of the specific binding of the antibody and antigen,6,7 has been developed for the detection of disease in clinical diagnosis. It shows obvious advantages of high sensitivity, low cost and a simple pretreatment procedure.8,9 Meanwhile, this technique has been widely applied in product safety,10 environmental detection,11 clinical medicine,12etc. For a sandwich-type electrochemical immunosensor, many kinds of labels were used to conjugate secondary antibodies (Ab2) for signal amplification.13,14
Nanomaterials for the biosensor fabrication have a lot of advantages including increasing the sensing surface area and amplifying the signal output. Silicate minerals as the widespread compounds are natural and abundant in the earth surface. As a specific kind of silicate mineral, halloysite nanotubes (HNTs) consist of alumina octahedron sheets and silica tetrahedron sheets in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio.15 Compared with carbon nanotubes, HNTs are economically available and have some special characteristics, for instance their different chemical properties outside and inside, and adequate hydroxyl groups on the surface.16,17 Others also utilized this unique characteristic for their research. Cong Chao et al. used dopamine modified HNTs to immobilize enzymes.18 Mingliang Du et al. prepared carboxylated butadiene–styrene rubber nanocomposites with strong mechanical properties.19
1 stoichiometric ratio.15 Compared with carbon nanotubes, HNTs are economically available and have some special characteristics, for instance their different chemical properties outside and inside, and adequate hydroxyl groups on the surface.16,17 Others also utilized this unique characteristic for their research. Cong Chao et al. used dopamine modified HNTs to immobilize enzymes.18 Mingliang Du et al. prepared carboxylated butadiene–styrene rubber nanocomposites with strong mechanical properties.19
Manganese dioxide (MnO2) has attracted broad interest in the recent research, due to the advantages of low cost, environmentally friendly and high electrochemical activity.20,21 δ-Manganese dioxide (δ-MnO2) with a unique layered structure could be conducive to the movement of charged particle transfer in the crystal lattice and to avoid the collapse of the crystal layer structure. However, the high surface energy of δ-MnO2 with a strong self-aggregation tendency could affect the quality of products.22 Interestingly, the thin δ-MnO2 layer could be deposited on the HNT surface (δ-MnO2/HNTs) through the reduction of permanganate, which could preserve the advantages of δ-MnO2 and avoid the agglomeration. It has been found that MnO2 possesses good catalytic activity for the reduction of H2O2.6 It was reported that δ-MnO2 shows well adsorption capacity for the removal of Cd(II) in the solution.23 The large amounts of Pd(II) could be adsorbed and attached on the surface of δ-MnO2/HNTs. As a result, palladium nanoparticles (Pd NPs) could be synthesized by in situ reduction. Pd NPs could increase the catalytic activity towards the reduction of H2O2, leading to a high sensitivity of the designed immunosensor. What is more, the good electrochemical properties and good biocompatibility of Pd NPs are beneficial to the immobilization of Ab2 through the chemical bond of Pd–NH2.24 Hence, the Pd/δ-MnO2/HNTs were the candidate signal label to construct immunosensors.
In this work, we designed a sandwich-type electrochemical immunosensor to achieve the quantitative detection of HBsAg. Electroplated gold nanoparticles (Au NPs) were adsorbed on the electrode as an effective sensing platform to construct the immunosensor. Due to the presence of amino groups (–NH2), the primary antibodies (Ab1) could be conjugated on the surface of the electrode by the chemical binding of Au–NH2. The fabricated immunosensor showed a relatively wide linear range (from 0.001 to 20 ng mL−1) and a low detection limit for the detection of HBsAg (0.3 pg mL−1).
2. Experimental
2.1. Reagents
The HNTs were obtained from clay minerals in Henan, China. The source material was purified and sieved to eliminate aggregates prior to use. The HBsAg and HBsAg antibody (Ab) was obtained from Shanghai Linc-Bio Science Co. Ltd (Shanghai, China). Bovine serum albumin (BSA, 96–99%) was obtained from Sigma (USA). Phosphate buffered saline (PBS) was prepared by mixing the solution of KH2PO4 (1/15 mol L−1) and Na2HPO4 (1/15 mol L−1) to the proper pH values. All the other chemicals were of analytical grade and ultrapure water was used throughout this work.2.2. Apparatus
All electrochemical measurements were performed on a CHI760D electrochemical workstation (Chenhua Instrument Shanghai Co., Ltd, China). A conventional three-electrode configuration was used: a glassy carbon electrode (GCE, 4 mm diameter) as a working electrode, a saturated calomel electrode (SCE) as a reference electrode and a platinum wire (Pt) as a counter electrode. Scanning electron microscopy (SEM) image and Energy Dispersive X-Ray Spectroscopy (EDS) image were obtained using a JEOL JSM-6700F microscope (Japan). Transmission electron microscope (TEM) images were obtained using a JEOL-2100 microscope (Japan). The specific surface area and pore size were measured using an ASAP2020 specific surface area and porosity analyzer (Micromeritics Instrument Corporation, USA). X-Ray Powder Diffraction (XRD) is performed using a D8 advance X-ray diffractometer (Bruker AXS, Germany).2.3. Synthesis of δ-MnO2/HNTs
δ-MnO2/HNTs were synthesized by the redox reaction of MnSO4 and KMnO4 in the solution.25 Firstly, 43.5 mL of aqueous solution containing KMnO4 (1.58 g) and HNTs (2.175 g) was prepared in a flask, and stirred for 20 min at 20 °C. Then, 60 mL of MnSO4·H2O (0.97 g) was added under constant stirring which lasted for another 4 h. After suction filtration, the product was washed many times with water until SO42− was washed off which was verified by the solution of 0.5 mol L−1 BaCl2. At last the product was dried under vacuum. This process could be expressed by the following mechanism:26| 3MnSO4 + 2KMnO4(excess) + 2H2O → 5δ-MnO2 + K2SO4 + 2H2SO4 |
2.4. Preparation of Pd nanoparticles/δ-MnO2/HNTs (Pd/δ-MnO2/HNTs)
In this paper, Pd/δ-MnO2/HNTs were synthesized by an in situ reduction method.27 60 mg δ-MnO2/HNTs was dispersed in 80 mL ethanol water (50 vol%) in a 100 mL beaker, and 13 mL aqueous solution containing Na2PdCl4 (10 mmol L−1) was ultrasonically mixed. After that, excess NaBH4 solution was added dropwise under stirring to reduce the metal cations. The reaction was sustained for 2 h, and Pd/δ-MnO2/HNTs were obtained by centrifugation and dried in a vacuum.2.5. Preparation of the Pd/δ-MnO2/HNT–Ab2 labels
The preparation process of Pd/δ-MnO2/HNT–Ab2 is presented in Fig. 1A. The Ab2 were immobilized onto Pd/δ-MnO2/HNTs through the cross-linking reaction between the amino group of Ab2 and Pd NPs. 1.2 mg of the Pd/δ-MnO2/HNT nanocomposite was dispersed into 1 mL of ultrapure water for 30 min ultrasonic treatment. Then Ab2 dispersion (10 μg mL−1, 1 mL) was added to the solution of Pd/δ-MnO2/HNTs (1.2 mg mL−1, 1 mL). The mixture was shaken mildly for 12 h at 4 °C. After centrifugation, 1 mL of PBS (pH = 7.4) was added to the precipitation. The Ab2 could be anchored by Pd NPs and loaded on the surface of the Pd/δ-MnO2/HNTs. Then the prepared Pd/δ-MnO2/HNTs–Ab2 was stored at 4 °C for further usage. | ||
| Fig. 1 (A) Preparation process of Pd/δ-MnO2/HNTs–Ab2 and (B) schematic presentation of the immunosensor fabrication. | ||
2.6. Fabrication of an immunosensor
The illustration of the sandwich-type electrochemical immunosensor fabrication procedure is shown in Fig. 1B. GCE was polished to a mirror-like finished with 0.05 μm alumina powder and then cleaned by ultrapure water before using. The electrodeposition of Au NPs on GCE was obtained using HAuCl4 (1 wt%, 2 mL) aqueous solution at a constant potential (−0.2 V) for 30 s.28 After that, Ab1 (10 μg mL−1, 6 μL) was added onto the electrode and dried at room temperature. After washing, 3 μL BSA (1 wt%) solution was dropped onto the electrode to eliminate nonspecific binding sites. Then 6 μL of the HBsAg with various concentrations was modified onto the electrode and the modified electrode was washed with the PBS (pH = 7.4) to remove unbound HBsAg molecules. Finally, the prepared Pd/δ-MnO2/HNTs–Ab2 (6 μL) was dropped onto the electrode surface at room temperature, and the electrode was washed thoroughly for measurements.2.7. Electrochemical measurements
The potential scanning of the amperometric i–t curve method was selected at −0.4 V, which was used for all amperometric measurements of the immunosensors.29 Until the background current of the amperometric measurement of the immunosensor was stabilized, 10 μL 5 mol L−1 H2O2 was injected into the PBS (10 mL) to form 5 mmol L−1 H2O2 solutions under mild stirring, and the current change was recorded.3. Results and discussion
3.1. Characterization of HNTs and Pd/δ-MnO2/HNTs
The morphologies of the HNTs were confirmed by SEM and TEM. In Fig. 2A, the majority of the HNTs consist of cylindrical tubes. And Fig. 2B shows that the HNTs exhibit clearly hollow tubular structures. Brunauer–Emmett–Teller (BET) characterization of the HNTs and δ-MnO2/HNTs was carried out. The specific surface area and the adsorption average pore width of HNTs is 43.99 m2 g−1 and 8.24 nm, respectively (Fig. 2C). And the specific surface area of δ-MnO2/HNTs is 72.3585 m2 g−1 (Fig. 2D), the results indicate that the specific surface area increases significantly with the loading of δ-MnO2 on HNTs. To determine the crystal structure of the deposited δ-MnO2, XRD patterns of δ-MnO2 (curve a), δ-MnO2/HNTs (curve b) and HNTs (curve c) were obtained, and shown in Fig. 2E. In curve a, the diffraction peaks at around 12°, 24°, 37° and 66° are in agreement with the standard cards of δ-MnO2 (JCPDS 18-0802).26 In comparison with curves b and c, the results showed that δ-MnO2 was deposited on HNTs successfully. The SEM image of Pd/δ-MnO2/HNTs is shown in Fig. 2F, from which it could be clearly observed that the crystal layer structure of δ-MnO2 (point a and b) is loaded on the surface of HNTs, and this result is in good agreement with the BET. It could also be found that Pd NPs (point c and d) were absorbed on δ-MnO2/HNTs. The EDS measurement of the Pd/δ-MnO2/HNT nanocomposite further confirms the existence of Pd NPs and the successful synthesis of a Pd/δ-MnO2/HNT nanocomposite, Fig. S1 (ESI†).3.2. Characterization of the electrocatalytic activity
To demonstrate the significance of Pd/δ-MnO2/HNTs and electroplating Au NPs in the designed immunosensor for signal amplification, experiments for the H2O2 reduction of electroplated Au NPs, HNTs, δ-MnO2/HNTs and Pd/δ-MnO2/HNTs were respectively carried out. The results are shown in Fig. 3. | ||
| Fig. 3 Amperometric response of the immunosensors with different labels: (a) electroplating Au NPs, (b) HNTs, (c) δ-MnO2/HNTs, (d) Pd/δ-MnO2/HNTs. | ||
Testing by the amperometric i–t curve, weak signals are detected when the electroplated Au NPs (curve a) and HNTs (curve b) are modified on the electrode, which indicates that electroplated Au NPs and HNTs exhibited a little catalytic activity to H2O2. After δ-MnO2/HNTs are applied as the label (curve c), there is a litter larger current response. The result suggest that δ-MnO2 could catalyze the redox reaction of H2O2. As is expected, the electrode using Pd/δ-MnO2/HNTs as a label exhibited the biggest current change (curve d). It could be inferred that the Pd NPs could further catalyze the redox procedure of H2O2. As a result, the analytical signal Pd/δ-MnO2/HNTs with Pd NPs and δ-MnO2 could promote the multiple signal amplification toward the reduction of H2O2.
3.3. Optimization of the experimental conditions
The properties of the immunosensor are influenced by many factors. For the sake of obtaining best analytical performance of HBsAg, experimental conditions of pH and the concentration of Pd/δ-MnO2/HNTs were optimized.The pH of the PBS is an important factor to the performance of immunosensors for that the immobilized protein could be damaged by the highly acidic or alkaline of surroundings.30 And the pH optimized experiment was investigated with same concentration of Pd/δ-MnO2/HNTs (1 mg mL−1) and HBsAg (5 ng mL−1) modified on the surface of the electrode. Under this condition, various pHs of the PBS (pH = 4.2–8.7) have been prepared in advance. As shown in Fig. 4A, with the increase of pH from 4.2 to 6.8, the current signal increases significantly, and decreases with the further increase of pH from 6.8 to 8.7. As a result, the pH of PBS for all electrochemical measurements is chosen at 6.8.
 | ||
| Fig. 4 The optimization of experiment conditions with pH (A) and the concentration Pd/δ-MnO2/HNTs (B). Error bar = SD (n = 5). | ||
The concentration of Pd/δ-MnO2/HNTs would affect the catalytic performance and the quantity of Ab2, which are bound up with specific HBsAg (5 ng mL−1). As shown in Fig. 4B, with the concentration increasing (0.4–1.2 mg mL−1), the current signal increases quickly. But with further increasing of the concentration from 1.2 to 1.8 mg mL−1, it decreases slowly. To conclude from this situation, the increase of the Pd/δ-MnO2/HNT film thickness might increase the electron transfer resistance. With this circumstance, the electron transfer became more difficult. Therefore, the optimal concentration of Pd/δ-MnO2/HNTs in this study was 1.2 mg mL−1.
3.4. Characterization of the immunosensor
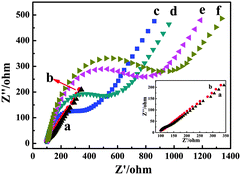
The impedance of the electrode consists of the real (Z′) and imaginary (Z′′) parts.| Z = Z′ + jZ′′ |
The Nyquist plot is a plot of −Z′′ versus Z′, and it is the most common data representation method.31 The impedance spectra consist of a semicircle portion and a linear portion. The semicircle portion at a high frequency region is associated with the electrochemical process subjecting to electron transfer, where the diameter corresponds to the resistance.32 As a result, the resistance change could be judged by the diameter change of the semicircle portion. Fig. 5 shows the Nyquist plots of the electrochemical impedance spectroscopy (EIS) in the process of modification of the electrode. And the EIS was scanned in a solution containing 2.5 mmol L−1 K3[Fe(CN)6] and 0.1 mol L−1 KCl. In this work, it could be observed that the bare GCE exhibits a very small resistance (curve a). After Au NPs are electrodeposited on the surface of bare GCE, a smaller resistance (curve b) is observed attributing to the good electron transfer ability of Au NPs. Additionally, when the Ab1 (curve c), BSA (curve d), HBsAg (curve e) and Pd/δ-MnO2/HNTs–Ab2 (curve f) are modified onto the electrode surface, the resistance further increases indicating the successful modification of the non-conductive bioactive substances. As a result, the immunosensor is fabricated successfully.
3.5. Assay performance
In this work, the fabricated immunosensor was used for detecting different concentrations of HBsAg under the optimum conditions. And the current responses of the immunosensor increase proportionally with the increasing concentrations of HBsAg in the range from 0.001 to 20 ng mL−1. The equation of the calibration plot is shown in Fig. 6. The regression equation was ΔI (μA) = 102.495 + 8.647 × lg(cHBsAg) (ng mL−1) and with a statistically correlation coefficient of 0.996. A low detection limit of the immunosensor was estimated to be 0.3 pg mL−1. The low detection is ascribed to several factors. Firstly, electroplating Au NPs possess good biocompatibility, perfect conductivity and the ability to link Ab1 strongly by the chemical bond of Au–NH2. In addition, the composite Pd/δ-MnO2/HNTs have the advantages of δ-MnO2 which have large surface area, high electrochemical activity and good adsorption capacity. With these merits δ-MnO2/HNTs could absorb a large amount of Pd NPs. And Pd/δ-MnO2/HNTs with Pd NPs and δ-MnO2 could multiply amplify the current response to the catalytic activity of H2O2. Consequently, the immunosensor could increase the sensitivity and improve the electrical signal. | ||
| Fig. 6 Calibration curve of the immunosensor toward different concentrations of HBsAg. Error bar = SD (n = 5). | ||
3.6. Selectivity, reproducibility and stability
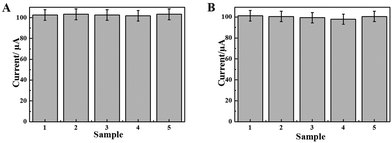
The selectivity, reproducibility and stability of the immunosensors are important parameters for their practical applications.The selectivity of the immunosensor was examined utilizing the major elements of human serum, such as, BSA, glucose, human immunoglobulin G (HIgG) and vitamin C (Vc) as interference substances. The HBsAg solution (1.0 ng mL−1) containing 100 ng mL−1 of different interference substances was used to evaluate the cross-reactivity of the immunosensor, and the results are depicted in Fig. 7A. The relative standard deviations (RSDs) of these current changes are 0.61%, assuming that the influence of interference on the immunosensor is negligible. The immunosensor exhibited good selectivity.
Simultaneously, the reproducibility of the immunosensor was estimated with the detection of 0.5 ng mL−1 HBsAg by a series of five electrodes. The RSD value of the measured five electrodes is 1.30% (Fig. 7B). The results show that the precision and reproducibility of the fabricated immunosensor are quite good. In addition, the immunosensors were stored at 4 °C and the current response of the immunosensor has no apparent change after one week storage. After two weeks, the current response, compared with the immunosensors without being stored, decreased 5.30%, indicating that the stability of the immunosensor is at the acceptable range.
3.7. Real sample analysis
To demonstrate the application of the developed sandwich immunosensor, we detected three samples of the healthy human serum (Table S1, ESI†). The RSD values of the three samples were less than 5.0%. And added the different concentrations of HBsAg (0.5, 2.5, and 5.0 ng mL−1) in the sample 1 for the spike recovery test. The results are shown in Table 1, the average recoveries and the RSD values are in the range of 100.38–101.98% and 3.68–4.88% respectively. Consequently, the novel immunosensor could be clinically acceptable to detect HBsAg in serum samples.| Added (ng mL−1) | Found (ng mL−1) | Average (n = 5, ng mL−1) | RSD (n = 5, %) | Recovery (%) |
|---|---|---|---|---|
| 0 | 0.1772, 0.1640, 0.1707, 0.1805, 0.1610 | 0.1707 | 4.88 | — |
| 0.5 | 0.6818, 0.6891, 0.6586, 0.7021, 0.6311 | 0.6726 | 4.17 | 100.38 |
| 2.5 | 2.7230, 2.6656, 2.7965, 2.6444, 2.5409 | 2.674 | 3.55 | 101.98 |
| 5.0 | 5.1185, 5.1458, 4.9181, 5.2847, 5.4273 | 5.179 | 3.68 | 100.16 |
4. Conclusion
In this work, we designed an immunosensor based on a novel signal amplification system by employing the electroplated gold nanoparticles as the platform and the palladium nanoparticles/δ-manganese dioxide/halloysite nanotubes were used as the signal label for the quantitative detection of hepatitis B surface antigen. The designed immunosensor exhibited an extremely low detection limit and a wide detection range. With the acceptable selectivity, reproducibility and stability, the immunosensor has promising applications in clinical diagnosis.Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 21375047 and 21377046), the Science and Technology Development Plan of Shandong Province (No. 2014GSF120004), the Science and Technology Plan Project of Jinan (No. 201307010), the Natural Science Foundation of Shandong Province (No. ZR2011BL008).References
- A. J. Thompson, T. Nguyen, D. Iser, A. Ayres, K. Jackson, M. Littlejohn, J. Slavin, S. Bowden, E. J. Gane and W. Abbott, Hepatology, 2010, 51, 1933–1944 CrossRef CAS PubMed.
- S. Chevaliez, C. Hézode, S. Bahrami, M. Grare and J.-M. Pawlotsky, J. Hepatol., 2013, 58, 676–683 CrossRef CAS PubMed.
- A. Neurath and N. Strick, Intervirology, 1979, 11, 128–132 CrossRef CAS PubMed.
- C. Kendall, I. Ionescu-Matiu and G. R. Dreesman, J. Immunol. Methods, 1983, 56, 329–339 CrossRef CAS PubMed.
- M. Deguchi, N. Yamashita, M. Kagita, S. Asari, Y. Iwatani, T. Tsuchida, K. Iinuma and I. K. Mushahwar, J. Virol. Methods, 2004, 115, 217–222 CrossRef CAS PubMed.
- L. Ji, Z. Guo, T. Yan, H. Ma, B. Du, Y. Li and Q. Wei, Biosens. Bioelectron., 2015, 68, 757–762 CrossRef CAS PubMed.
- H. Fan, Z. Guo, L. Gao, Y. Zhang, D. Fan, G. Ji, B. Du and Q. Wei, Biosens. Bioelectron., 2015, 64, 51–56 CrossRef CAS PubMed.
- Y. Li, J. He, C. Xia, L. Gao and C. Yu, Biosens. Bioelectron., 2015, 70, 392–397 CrossRef CAS PubMed.
- Y. Liu, Q. Zhang, H. Wang, Y. Yuan, Y. Chai and R. Yuan, Biosens. Bioelectron., 2015, 71, 164–170 CrossRef CAS PubMed.
- R. Chauhan, P. R. Solanki, J. Singh, I. Mukherjee, T. Basu and B. Malhotra, Food Control, 2015, 52, 60–70 CrossRef CAS.
- L. Liu, D. Xu, Y. Hu, S. Liu, H. Wei, J. Zheng, G. Wang, X. Hu and C. Wang, Food Control, 2015, 53, 72–80 CrossRef CAS.
- L. Zhao, S. Li, J. He, G. Tian, Q. Wei and H. Li, Biosens. Bioelectron., 2013, 49, 222–225 CrossRef CAS PubMed.
- Y. Wang, Y. Wang, X. Pang, B. Du, H. Li, D. Wu and Q. Wei, Sens. Actuators, B, 2015, 214, 124–131 CrossRef CAS.
- L. Jiang, J. Han, F. Li, J. Gao, Y. Li, Y. Dong and Q. Wei, Electrochim. Acta, 2015, 160, 7–14 CrossRef CAS.
- W. O. Yah, A. Takahara and Y. M. Lvov, J. Am. Chem. Soc., 2012, 134, 1853–1859 CrossRef CAS PubMed.
- C. Chao, B. Zhang, R. Zhai, X. Xiang, J. Liu and R. Chen, ACS Sustainable Chem. Eng., 2013, 2, 396–403 CrossRef.
- P. M. Ajayan and O. Z. Zhou, Carbon nanotubes, Springer, 2001, pp. 391–425 Search PubMed.
- C. Chao, J. Liu, J. Wang, Y. Zhang, B. Zhang, Y. Zhang, X. Xiang and R. Chen, ACS Appl. Mater. Interfaces, 2013, 5, 10559–10564 CAS.
- M. Du, B. Guo, Y. Lei, M. Liu and D. Jia, Polymer, 2008, 49, 4871–4876 CrossRef CAS.
- X. Zhang, X. Chang, N. Chen, K. Wang, L. Kang and Z.-h. Liu, J. Mater. Sci., 2012, 47, 999–1003 CrossRef CAS.
- A. Jokic, M. Wang, C. Liu, A. Frenkel and P. Huang, Org. Geochem., 2004, 35, 747–762 CrossRef CAS.
- S. S. Tripathy and S. B. Kanungo, J. Colloid Interface Sci., 2005, 284, 30–38 CrossRef CAS PubMed.
- L. Dong, Z. Zhu, H. Ma, Y. Qiu and J. Zhao, J. Environ. Sci., 2010, 22, 225–229 CrossRef CAS.
- S. Mandal, D. Roy, R. V. Chaudhari and M. Sastry, Chem. Mater., 2004, 16, 3714–3724 CrossRef CAS.
- X. Li, G. Pan, Y. Qin, T. Hu, Z. Wu and Y. Xie, J. Colloid Interface Sci., 2004, 271, 35–40 CrossRef CAS PubMed.
- S. Devaraj and N. Munichandraiah, J. Phys. Chem. C, 2008, 112, 4406–4417 CAS.
- T. Sun, Z. Zhang, J. Xiao, C. Chen, F. Xiao, S. Wang and Y. Liu, Sci. Rep., 2013, 3, 2527–2532 Search PubMed.
- Y. Wu, W. Xu, L. Bai, Y. Yuan, H. Yi, Y. Chai and R. Yuan, Biosens. Bioelectron., 2013, 50, 50–56 CrossRef CAS PubMed.
- X. Ren, T. Yan, Y. Zhang, D. Wu, H. Ma, H. Li, B. Du and Q. Wei, Biosens. Bioelectron., 2014, 58, 345–350 CrossRef CAS PubMed.
- Z. Cui, Y. Cai, D. Wu, H. Yu, Y. Li, K. Mao, H. Wang, H. Fan, Q. Wei and B. Du, Electrochim. Acta, 2012, 69, 79–85 CrossRef CAS.
- A. Lasia, Modern aspects of electrochemistry, Springer, 2002, pp. 143–248 Search PubMed.
- W. Lu, J. Ge, L. Tao, X. Cao, J. Dong and W. Qian, Electrochim. Acta, 2014, 130, 335–343 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nj01251a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |