Self-limited self-assembly of nanoparticles into supraparticles: towards supramolecular colloidal materials by design
Esteban
Piccinini
a,
Diego
Pallarola
ab,
Fernando
Battaglini
c and
Omar
Azzaroni
*ab
aInstituto de Investigaciones Fisicoquímicas Teóricas y Aplicadas (INIFTA), Departamento de Química, Facultad de Ciencias Exactas, Universidad Nacional de La Plata (UNLP), CONICET, La Plata, Argentina. E-mail: azzaroni@inifta.unlp.edu.ar; Web: http://softmatter.quimica.unlp.edu.ar
bConsejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Buenos Aires, Argentina
cINQUIMAE, Departamento de Química Inorgánica, Analítica y Química Física, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Ciudad Universitaria, Pabellón 2, C1428EHA Buenos Aires, Argentina
First published on 6th April 2016
Abstract
For quite a while, scientists have resorted to colloidal synthesis to mimic complex structural and functional materials found in Nature. In particular, within the past few years, the synthesis of suprastructures with novel properties that emerge from the coupling of diverse nanoscale functional units has defined new boundaries in materials science. In this mini-review, we survey the most recent and outstanding achievements on the rational design of supraparticles based on the self-limited self-assembly of nanoparticles, and their application in fields like biology, medicine and energy.
Introduction
Supramolecular science and self-assembly are the hallmark of contemporary materials science as they provide versatile routes for the development of novel functional materials with molecular- and nanoscale-level feature control.1–3 Looking at Nature's technological design, we observe that self-assembly is a powerful process of spontaneous organization capable of simplifying and promoting the formation of superstructures with suitable size and predetermined functions.4In recent years, there has been tremendous progress in the self-assembly of highly organized nanoscale architectures in three dimensions,5,6 in which the harmony between repulsive interactions and attractive noncovalent forces mediates the self-organization of structural motifs and stabilizes discrete self-assembling mesoscopic suprastructures that consist of predesigned nanoparticles.7,8 The vision to create supraparticles using nanoparticles as elementary functional and structural units has been around for more than a decade.9 Indeed, the combination of supramolecular principles and nanoscopic structures has been the core concept behind the emerging field of “nanoarchitectonics”, a term popularized by Ariga and co-workers.10
Within this framework, Kotov and workers11 gave an important thrust to the rational design of supraparticles based on the self-limited self-assembly of nanoparticles. These authors elegantly demonstrated that in the presence of competing forces (attractive versus repulsive forces), self-limiting self-assembly processes involving non-uniform inorganic nanoparticles can lead to the formation of highly ordered 3D mesostructures. This marked a profound departure from previous practices and notions of self-assembly in materials science. We should bear in mind that until that moment, the generation of self-limiting nanostructures was almost an exclusive domain of biological systems. Since then, the formation of supraparticles through the self-limited self-organization of inorganic nanocrystals has opened a new and challenging area in nanotechnology and has given rise to a new trend in materials science. We already know that nanomaterials represent a new generation of advanced materials that exhibit unusual chemical and physical properties, different from those of bulk materials. However, as we go forward into the use of self-limited supraparticles, the horizon expands: we can manipulate their collective properties by combining multiple components individually tailored for specific functions. As a result, this strategy not only offers mesoscale compositional and structural control of self-limited colloidal materials, in a reproducible fashion, with enormous potential for scalability, but also holds promise to catalyze the development of new functional colloidal materials for advanced technologies and specific applications.
Over the years, several research groups have made very important contributions to today's rich collection of functional self-limited supraparticles displaying attractive material properties. The aim of this mini-review is twofold: first, to discuss the major efforts made by the research community to design and construct supraparticles, and second, to show the enormous potential of colloidal supraparticles in a wide variety of fields of advanced nanotechnology. Many new developments in the area of supramolecular colloidal materials have recently occurred, and important new applications are planned, particularly in catalysis, sensing, electrochemistry and photochemistry. It is therefore hoped that this work will serve as an attractive starting point for someone, regardless of background, who is keen on obtaining some appreciation of the origins of this field, of the current state of knowledge and of the breadth of approaches that have been successful in bringing this field to a new level of maturity.
Assembly mechanism and synthesis
Self-limited self-assembly is the ideal structural and synthetic paradigm for turning a variety of nanoparticles into superstructures with low polydispersity and controlled composition, dimensions and morphology by harnessing spontaneous non-covalent interactions.9,12 However, effective control over these parameters requires a careful assessment of the forces underlying the assembly process.13,14 Previous studies have experimentally and theoretically described in depth the formation mechanism of self-limited colloidal systems.11,15–21 As a general description, the assembly process is governed by a delicate interplay between repulsive and attractive forces; supraparticle growth ceases when the repulsive forces between them and their nanoprecursors equal the attractive forces. The assemblies reach a terminal size only when they achieve an equilibrium state, that is, the system is thermodynamically controlled (unlike template-assisted methods where templates exert kinetic control).22 The fact that the assembly mechanism is thermodynamically controlled allows tuning of the properties (e.g., composition, dimensions and morphology) of the supraparticles by simply modifying the environmental conditions such as temperature,23 ionic strength,17 pH,24 and the presence of specific ligands.25,26 Although the assembly mechanism has been mainly described for dispersive and electrostatic interactions, similar approaches can be extended to other supramolecular interactions.25–27Supraparticles prepared using diverse materials such as metal or semiconductor nanoparticles, proteins, polymers and DNA have been reported. For example, Kotov and coworkers have studied the assembly mechanism of a variety of polydisperse nanoparticles (CdSe, CdS, ZnSe, PbS and Au/CdSe stabilized with citrate anions) into monodisperse supraparticles (Fig. 1a).11 van der Waals attractions were found to be the driving force of the spontaneous assembly. Because of the negative charge of the nanoparticles, the absolute zeta potential increased substantially as the supraparticles grew until they achieved their terminal size. Next, the authors extended their studies to the self-assembly of both positively charged CdTe nanoparticles and cytochrome C (CytC).17 In both studies, experimental results showed strong agreement with molecular dynamic simulations, in which the Lennard-Jones (12–6) potential was used to model the van der Waals attractions, while a screened Coulomb potential was used for electrostatic interactions. Interestingly, the assembly process exhibited intermediate stages; individual nanoparticles assembled into loose elongated aggregates, which later evolved into more uniform clusters comprising 15–25 nanoparticles. Then, supraparticles compressed leading to a decrease in polydispersity.11,20 In the case of CdSe supraparticles, the reduction of the particles' polydispersity was from a standard deviation (s.d.) of 25–30% (nanoparticles) to a s.d. of 8–10% (supraparticles). Other studies implemented a variation of the Kotov approach. Negatively charged gold nanoparticles (AuNPs) (capped with citrate anions) and positively charged proteins were mixed in aqueous media to obtain terminal and stable nanoclusters.19,29 Because of the presence of oppositely charged components, it would be expected that only attractive (van der Waals and electrostatic) forces participate. This fact would render self limited growth impossible. Nevertheless, the authors found that the extent of agglomeration strongly depends on the concentration ratio [protein]/[AuNPs] (Fig. 1b). Full agglomeration and precipitation occurred at [protein]/[AuNPs] corresponding to a protein monolayer on the gold nanoparticles. On the other hand, stable nanoclusters could be obtained above and below this value because of the supraparticle zeta potential being significantly different from zero. Intriguingly, despite having an opposite charge, protein multilayers on AuNPs could stabilize and self-limit the cluster growth.19,30
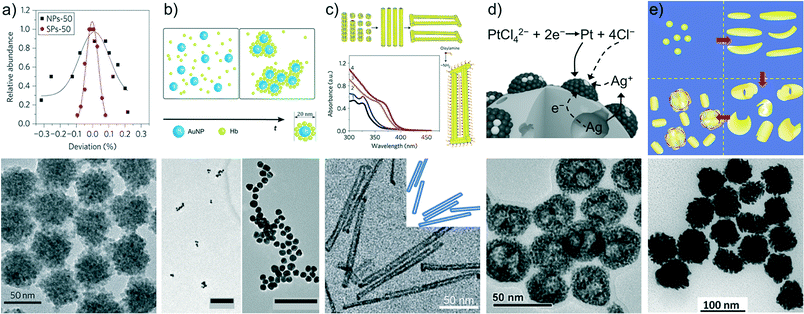 | ||
| Fig. 1 a) Self-assembly of CdTe nanoparticles into spherical and uniform supraparticles exhibiting a significant reduction in polydispersity. b) Agglomeration of gold nanoparticles at high (left) and low (right) hemoglobin concentrations. c) Growth stages of the high-temperature assembly of ZnSe nanoparticles (top) leading to couples of colloidal nanorods (bottom). d) Mesoporous surfactant-free colloids made of platinum-group nanocrystals obtained through coprecipitation on silver particles as sacrificial substrates. e) Growth mechanism of “meatball”-like gold supraparticles synthesized by a seed-mediated approach. Figures reproduced with permission from ref. 11, 19, 23, 28 and 18. Copyright 2012 and 2014 Nature Publishing Group; Copyright 2015 American Chemical Society; Copyright 2015 Wiley-VCH Verlag GmbH & Co. KGaA. | ||
Self-limited supraparticles have also been synthesized through more complex synthetic routes: seed-mediated,18,31,32 sacrificial substrate,28 thermal,23 and other methods.33,34 Among them, Banin and colleagues found that semiconductor ZnSe nanoparticles could assemble spontaneously via a high-temperature synthetic route, which resulted in stable and monodisperse nanorod couples connected by twinning structures (Fig. 1c).23 The authors demonstrated that the nanorods assembled from individual ZnSe nanoparticles when the temperature of the system was increased to 230 °C. Furthermore, the nanorods could fuse at both edges forming nanorod couples only when the temperature was ramped up to 280 °C. This synthesis was extended to CdSe and PbSe through a simple cation-exchange approach. On the other hand, mesoporous supraparticles with a surfactant-free surface were synthesized through simultaneous precipitation of silver halides and platinum-group nanocrystals in the course of the oxidative etching of Ag particles (Fig. 1d).28 Finally, seed-mediated synthesis for the formation of gold “meatball”-like supraparticles has been reported by Fu et al. (Fig. 1e).18 Small gold nanoparticle seeds (∼4 nm) were mixed with a HAuCl4 gold precursor, a CTAC surfactant and ascorbic acid as a reducing agent. To capture the “short-lived” intermediates, the seed-mediated growth mechanism was slowed down utilizing an ingenious flow-based microfluidic chip. Experimental results and Monte Carlo simulations confirmed a four-step synthetic mechanism: i) seed-mediated formation of ultrathin gold nanoplates, ii) folding and rolling up of ultrathin nanoplates, iii) self-assembly of folded and rolled gold nanoplates to form core–shell intermediate supraparticles and iv) growth of supraparticles until the electrostatic repulsion balanced the van der Waals attraction.
A toolbox of intermolecular interactions
A comprehensive understanding of the interactions involved in supramolecular architectures is a pre-requisite to design and control the assembly processes and consequently the properties of the resulting assemblies. A broad number of interactions such as van der Waals,11,17,18,21,35 electrostatic,32,36,37 metal–ligand coordination,24,26,27 host–guest,38,39 biomolecular recognition,25,40 nucleic acid base pairing,41,42 and protein–protein interactions43,44 make up the repertoire of intermolecular forces between nanomaterials that lead the fate of supraparticle assembly. As such, a wide and diverse variety of nanoassemblies based on these molecular interactions have been built using various nanomaterials. These assemblies range from all-inorganic11,28,34,37 to soft-inorganic (hybrid),17,19,30 and all-organic systems.25,38,45 Among them, a number of studies have focused on the construction of assemblies through van der Waals and electrostatic interactions due to the simplicity of this approach. For example, van der Waals and electrostatic forces mediate the synthesis of hybrid supraparticles of sphere shape and narrow size distribution made of cytochrome C (CytC) and CdTe nanoparticles. Taking advantage of the positive charge of the CdTe/CytC supraparticles, nitrate reductase (NRed), a negatively charged enzyme, was integrated into the supramolecular construct (Fig. 2a).17 Similarly, Hu et al. reported that gold nanorods, stabilized by cetyltrimethylammonium bromide (CTAB), and chiral CdTe nanoparticles, stabilized by D- or L-cysteine, spontaneously assembled through electrostatic interactions. The geometry of these inorganic supraparticles was found to depend on the nanoparticle![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nanorod molar ratio and could be tuned from a dimer scissor-like geometry to single nanorods. Interestingly, the chiral supraparticles showed an enantiomeric preference associated with chiral interactions between the nanoparticles situated around the nanorods.35 The authors found that the difference in chiroptical activity between D- and L-supraparticles came not from the differences in chiroptical activity of individual D- and L-nanoparticles, but rather from the chirality of the nanorod supraparticle as a whole. Steric repulsive forces can also play an important role in the size-limitation of supraparticles. For example, the aggregation of ZnSe nanoparticles was controlled by adjusting the amount of stabilizing agent (oleic acid). These ZnSe supraparticles can be doped with Fe3O4, endowing them with magnetic properties, or can be prepared with CdS, resulting in light-emissive supraparticles.34
nanorod molar ratio and could be tuned from a dimer scissor-like geometry to single nanorods. Interestingly, the chiral supraparticles showed an enantiomeric preference associated with chiral interactions between the nanoparticles situated around the nanorods.35 The authors found that the difference in chiroptical activity between D- and L-supraparticles came not from the differences in chiroptical activity of individual D- and L-nanoparticles, but rather from the chirality of the nanorod supraparticle as a whole. Steric repulsive forces can also play an important role in the size-limitation of supraparticles. For example, the aggregation of ZnSe nanoparticles was controlled by adjusting the amount of stabilizing agent (oleic acid). These ZnSe supraparticles can be doped with Fe3O4, endowing them with magnetic properties, or can be prepared with CdS, resulting in light-emissive supraparticles.34
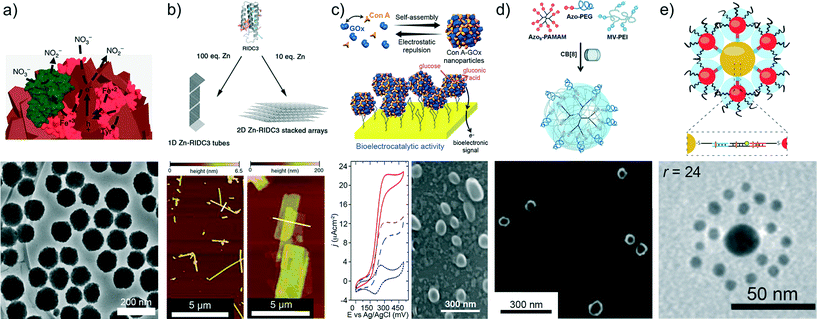 | ||
| Fig. 2 a) Bionic supraparticles made of CdTe nanoparticles, cytochrome C and nitrate reductase displaying enhanced enzyme activity when the supraparticles are illuminated at the maximum CdTe absorption. b) RIDC3 self-assembles through Zn2+ coordination into helical nanotubes or multilayered 2D arrays with crystalline order. c) Protein supraparticles self-assembled from concanavalin A and glucose oxidase through carbohydrate–lectin molecular recognition. The as-obtained supraparticles display carbohydrate-recognition properties and retain the enzyme activity. d) Supramolecular ternary nanoparticles based on host–guest interactions between cucurbit[8]uril, methyl viologen-functionalized PEI and azobenzene moieties. e) Core–satellite superstructure self-assembled from gold nanoparticles coated with core DNA or satellite DNA, and using a linker of complementary DNA. Figures reproduced with permission from ref. 17, 24, 25, 46 and 42. Copyright 2014 Nature Publishing Group; Copyright 2014 National Academy of Science; Copyright 2015 Royal Society of Chemistry; Copyright 2014 Wiley-VCH Verlag GmbH & Co. KGaA. | ||
The rational design of assemblies bearing well-ordered internal structures is a great challenge. Toward this goal, the location of motifs on the surface of the building blocks able to achieve specific interactions has been proven to be a powerful approach to control the orientation and order of the components within the superstructure. In this context, ligand–metal coordination has recently attracted considerable attention due to its features of strength, directionality and reversibility.24,26,27,47,48 For example, a designed variant of cytochrome cb562 (RIDC3) with coordination motifs on its surface can arrange through Zn2+ metal binding into one-dimensional helical nanotubes and two- or three-dimensional crystalline assemblies (Fig. 2b).24 As metal coordination interactions are dependent on external stimuli, the size and morphology of the metal-directed assemblies could be reversibly tuned by varying the pH or the metal concentration. Metal coordination can also lead to the formation of assemblies with non-random distribution of nanoscale building blocks. FeS2 pyrite nanodiscoids stabilized with carboxylated end-groups spontaneously assembled in the presence of a wide variety of metal ions (Zn2+, Na+, Ca2+, Mn2+, Cu2+ and Ru2+) forming supraparticles exhibiting liquid crystal-like structures. Small-angle X-ray scattering (SAXS) measurements evidenced that the planes of the discoids are oriented parallel to the planes of nanosheets that were stacked on top of each other.26
Recognition-driven assembly is another molecular strategy that allows the binding of specific components with control over the composition and size of the resulting supraparticles. Recently, stable enzymatic colloids were synthesized by lectin–carbohydrate recognition-directed assembly of a ligand-binding protein (concanavalin A, Con A) and a ligand-presenting enzyme (glucose oxidase, GOx) (Fig. 2c).25 It was shown that multiple lectin–carbohydrate interactions within the supramolecular construct were responsible for conferring remarkable structural integrity and improved recognition properties. Similarly, other protein–protein interactions (e.g., biotin–streptavidin) have been used to mediate the construction of self-assembled superstructures.40,49 On the other hand, the inclusion of amines or hydrophobic guests into the cavity of a macrocyclic host (i.e., host–guest interactions) has also received considerable attention.38,46 Stoffelen and Huskens prepared soft size-tunable supraparticles using cucurbit[8]uril to link methyl viologen-functionalized PEI with naphthol-functionalized PEG (monovalent) and naphthol-functionalized PAMAM (multivalent), resulting in host–guest ternary complexes with micromolar affinity (Fig. 2d).38,46 As a consequence of the interplay between multivalent and monovalent host–guest interactions, the size of the supraparticles could be controlled by varying the ratio of naphthol–PEG to naphthol–PAMAM. Furthermore, replacing naphthol-functionalized guests with azobenzene-functionalized ones leads to the formation of supraparticles whose assembly and disassembly can be reversibly switched by alternating UV and visible light.46
The use of DNA for directing the arrangement of nanomaterials is also a very attractive and promising approach for designing self-assembled self-limited superstructures. Complementary nucleic acid sequences bound to different building blocks can self-assemble through DNA hybridization. The striking feature of this approach is the precise and programmable control over the spatial assembly of nanoparticles.41,42 For example, gold nanoparticles coated with core DNA or satellite DNA self-assembled into a core–satellite superstructure using a linker to achieve DNA hybridization (Fig. 2e).42 A broad variety of superparticle arrangements was obtained by adjusting the ratio between satellite and core nanoparticles, or increasing the number of satellite nanoparticle layers.
Properties and applications
Advances in colloidal functional supraparticle synthesis with designed nanoscopic properties have given rise to novel and fascinating applications in several major fields like energy, medicine and biology.9,12–14,50,51 The modular structure of supraparticles leads to materials with properties that emerge from the coupling of diverse nanoscale building blocks, and/or from the combination of their functionalities. As such, the integration of enzymes with nanoparticles into ordered superstructures can induce synergistic effects arising not only from the intrinsic properties of the selected components, but fundamentally from the controlled spatial arrangement of the environment that hosts the functional biomolecules. Metal nanoparticle/enzyme hybrid supraparticles have been successfully applied to the photoenzymatic reduction of NO3− by incorporating nitrate reductase (NRed) to supraparticle assemblies made of cadmium telluride (CdTe) nanoparticles and cytochrome C (CytC).17 The spontaneous self-organization of the components into tightly packed nanoscale assemblies facilitates all the electron transfer reactions, yielding a fourfold increase in NO2− production compared with unassembled entities. The self-assembled supramolecular architectures can be further engineered in such a way to improve the functional attributes of enzymes.39,52 For example, self-assembled protein nanowires designed by utilizing specific cucurbit[8]uril (CB[8])-based supramolecular interactions with Phe-Gly-Gly (FGG)-tagged glutathione S-transferase (GST) were used as scaffolds to incorporate an artificial antioxidative glutathione peroxidase (GPx).39 The resulting supraparticle assemblies exhibited enhanced antioxidative activity compared to the monomers in a lipid peroxidation reaction. Similarly, highly-ordered protein nanowires synthesized by electrostatic self-assembly between stable protein one (SP1) homododecameric cricoid nanorings and poly(amino amine) (PAMAM) dendrimers incorporating GPx and superoxide dismutase (SOD) centers were employed as dual-enzyme-cooperative antioxidative systems (Fig. 3a).52 Remarkably, protein nanowires catalyze the reduction of both O2˙− and H2O2 far more efficiently than the uncooperative enzymes, resembling the antioxidative defense mechanism present in living organisms. Metal coordination-driven self-assembly of proteins into uniform nanostructures has also been used to create suitable environments for hosting enzymes.24,47 RIDC3, a designed variant of cytochrome cb562 could self-assemble through Zn2+ coordination into uniform 1D or 2D arrays.24 Due to their metal-mediated frameworks, the RIDC3 arrays display very high thermal stability (∼90 °C), maintaining their structural order in several polar organic solvents, in contrast with what is observed with monomers. The markedly stabilized electron transfer properties of the RIDC3 arrays were successfully exploited to spatiotemporally control the growth of dense PtNP arrays.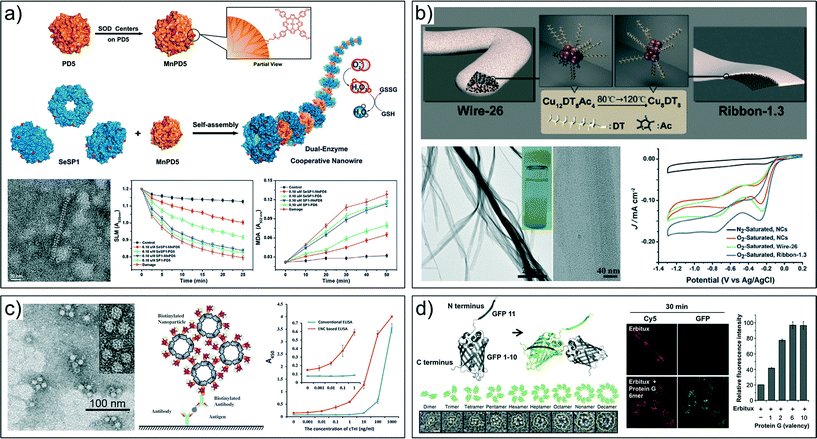 | ||
| Fig. 3 a) Dual-enzyme cooperative nanowire systems with both GPx and SOD activities exhibiting enhanced antioxidative capacity according to the inhibition of mitochondria damage to the swelling level of mitochondria (SLM) and the level of lipid peroxidation (MDA). b) CuNC self-assembling architectures from wire-26 to ribbon-1.3 and their electrocatalytic properties as cathode catalysts for oxygen reduction reactions (ORR). c) Self-assembly of biotinylated ferritin nanoparticles (bFNPs) and streptavidin-labeled horseradish peroxidases (SA-HRPs) into enzyme nanocomposites (ENCs) and their use for detection of cardiac troponin I (cTnI). d) Discrete GFP (nano)polygons labeled with protein G (antibody-binding protein) employed to control the level of antibody-EGFR (epidermal growth factor receptor) clusters and subsequently receptor internalization. Reproduced with permission from ref. 52, 53, 40 and 44. Copyright 2015 American Chemical Society; Copyright 2014 Wiley-VCH Verlag GmbH & Co. KGaA; Copyright 2015 Nature Publishing Group. | ||
Many strategies based on the self-limiting growth of nanoparticle materials have been explored for the design of geometrically sophisticated superstructures for photo- and electrocatalytic applications.26,50 From the perspective of functionality, these hierarchical nanostructures benefit from the emergent collective properties induced by the interaction of their components.54 Surfactant-free mesoporous colloidal superparticles with controlled size and composition, consisting of Pt and AgCl nanocrystals, were synthesized through coprecipitation of Pt and AgCl on sacrificial colloidal Ag particles.28 The as-synthesized hemispherical colloidal superparticles exhibited significantly improved catalytic activity and recycling performance in catalyzing the reduction of hexacyanoferrate(III) (Fe(CN)63−) with thiosulfate ions (S2O32−) in comparison with a conventional platinum nanoparticle catalyst. The enhanced catalytic performance of colloidal superparticles was attributed to the fully accessible ligand-free large active surface area and their great stability in polar solvents. Effective electrocatalysts for oxygen reduction reactions (ORR) were also developed based on controllable self-organization of nanometer-sized building blocks. Copper nanoclusters (CuNCs) capped by 1-dodecanethiol (DT) were self-assembled into ultrathin ribbons by tuning the cooperation between dipolar and van der Waals interactions within the assembly (Fig. 3b).53 This approach proved efficient for overcoming the practical limitations of ultra-small clusters associated with aggregation and fusion,55 yielding supramolecular assemblies that significantly enhance the structural stability without lowering the catalytic activity.
Monodisperse supramolecular nanoparticle assemblies rendering multiple functional molecules are highly appealing for various biosensing and biochemical applications. For example, enzyme nanocomposites (ENCs) constructed through the assembly of biotinylated ferritin nanoparticles (bFNPs) and streptavidin-labeled horseradish peroxidases (SA-HRPs) were used for detection of cardiac troponin I (cTnI), an important biomarker for the diagnosis of acute myocardial infarction, in an ELISA-type assay (Fig. 3c).40 ENCs allowed the recruitment of hundreds of HRP molecules to each antibody–antigen complex providing a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold sensitivity improvement compared with typically used enzymatic conjugates. Other approaches based on protein-induced nanoparticle agglomeration are envisioned to have positive impact on this field. The controlled agglomeration of gold nanoparticles with proteins provides sensitive means for detecting specific molecular binding events by localized surface plasmon resonance (LSPR),19 fluorescence quenching,56 surface-enhanced Raman spectroscopy (SERS),57 or combinations of them. Furthermore, self-assembly of proteins into supramolecular architectures offers the possibility of creating nano-assemblies with defined structures and specific multivalent molecular interactions.25,58,59 Multivalency can provide simultaneous increases in binding affinity and specificity, and signal amplification to biosensing and biochemical tools.60,61 Recently, fully protein-based and monodisperse assemblies with precisely controlled valency were created by the spontaneous assembly of an engineered green fluorescent protein (GFP) inside cells (Fig. 3d).44 GFP polygons labeled with binding protein G (antibody-binding protein) were employed to control antibody-mediated clustering of epidermal growth factor receptor (EGFR) on cell surfaces by regulating the internalization of Erbitux, an antibody drug that induces EGFR downregulation. Erbitux clustered by protein G polygons showed significantly faster internalization compared with free Erbitux. The internalization rate was augmented as the valency of protein G polygons increased, providing experimental evidence for the contributions of multivalency to protein binding affinities. Another example of the exquisite interplay within the designed nanoarchitectures is the appearance of unusual chirality.35,41 Gold nanorods were assembled by the polymerase chain reaction into DNA-bridged systems with strong chiroplasmonic activity.41 The strong polarization rotation achieved by the side-by-side assemblies allowed detection of DNA at very low concentrations (3.7 aM), substantially more sensitive than typical PCR with nanoparticles.
000-fold sensitivity improvement compared with typically used enzymatic conjugates. Other approaches based on protein-induced nanoparticle agglomeration are envisioned to have positive impact on this field. The controlled agglomeration of gold nanoparticles with proteins provides sensitive means for detecting specific molecular binding events by localized surface plasmon resonance (LSPR),19 fluorescence quenching,56 surface-enhanced Raman spectroscopy (SERS),57 or combinations of them. Furthermore, self-assembly of proteins into supramolecular architectures offers the possibility of creating nano-assemblies with defined structures and specific multivalent molecular interactions.25,58,59 Multivalency can provide simultaneous increases in binding affinity and specificity, and signal amplification to biosensing and biochemical tools.60,61 Recently, fully protein-based and monodisperse assemblies with precisely controlled valency were created by the spontaneous assembly of an engineered green fluorescent protein (GFP) inside cells (Fig. 3d).44 GFP polygons labeled with binding protein G (antibody-binding protein) were employed to control antibody-mediated clustering of epidermal growth factor receptor (EGFR) on cell surfaces by regulating the internalization of Erbitux, an antibody drug that induces EGFR downregulation. Erbitux clustered by protein G polygons showed significantly faster internalization compared with free Erbitux. The internalization rate was augmented as the valency of protein G polygons increased, providing experimental evidence for the contributions of multivalency to protein binding affinities. Another example of the exquisite interplay within the designed nanoarchitectures is the appearance of unusual chirality.35,41 Gold nanorods were assembled by the polymerase chain reaction into DNA-bridged systems with strong chiroplasmonic activity.41 The strong polarization rotation achieved by the side-by-side assemblies allowed detection of DNA at very low concentrations (3.7 aM), substantially more sensitive than typical PCR with nanoparticles.
The past few years have also witnessed cutting-edge advances in other fields of applications, such as protein purification,62 drug delivery,42,46 biomedical imaging,16 and light harvesting.36 The integration of inorganic nanoparticles, such as quantum dots, with protein nanostructures with well-defined morphology constitutes a remarkable example of the potential of rational self-assembled nanostructure design. Recently, CdTe quantum dots with tuned optical properties were employed as linkers to direct the electrostatic-driven assembly of SP1 nanorings into highly-ordered CdTe–SP1 hybrid nanostructures.36 Sequential assembly of different-sized quantum dots with SP1 nanorings allowed the distance between adjacent donors and acceptors to be spatially organized. The highly-ordered assemblies separate quantum dots enough to prevent fluorescence self-quenching, while at the same time holding them in close enough proximity to allow efficient energy transfer among the quantum dots, thus yielding efficient light-harvesting scaffolds.
Conclusions and outlook
Since ancient times, mankind has utilized colloids as sources of functional materials. During the past decades, the ingenuity of chemists and materials scientists provided the means for extending our capabilities to master the physical world on its molecular scale. For instance, as we move further into the new century, materials science seems indeed to offer almost unlimited opportunities for achieving full control over particle interactions. This ultimately permits, in close resemblance to Nature, modulation of assembly pathways in order to precisely direct the structure and properties of highly functional supraparticles. We are far from being able to reproduce the complexity of biological, self-limited, self-assembled systems, but in recent years, a growing scientific community has been concerned with creating self-assembled supraparticle systems that allow us, at the molecular/nanoscale level, to control the collective and structural properties of 2D and 3D architectures.The underlying idea behind the formation of colloidal supraparticles is to use a bottom-up approach for assembling individually characterized nanoscale constructs, which together execute a controllable function. The beauty of such systems comes into light when nanobuilding blocks of very diverse characteristics are rationally chosen and subsequently assembled so as to yield self-limited mesoscopic structures with tailored physical, chemical, or even biological properties. Our ability to construct supraparticles from scratch, coupled with a better understanding of their structure–property relationships, is the cornerstone of the design of sophisticated functional nanoarchitectures. It seems evident that certain classes of nanomaterials will play different roles in the creation of colloidal supraparticles – some will be structural, while others will be more functional, just like their biological counterparts.
It is worth noting that although self-limited, self-assembled biological systems are invariably characterized by their high functionality in terms of their intrinsic biochemical roles, the functionality of the majority of synthetic supraparticles so far investigated is somewhat limited. We thus believe that the incorporation of designed functionality into supraparticular colloidal materials will undoubtedly continue to attract increased attention in future studies. Over the past several years, we have witnessed the appearance of a vast repertoire of synthetic schemes at our disposal enabling the formation of nanomaterials with tailorable physical, chemical and even biological properties. Concomitantly, more and more nanomaterials become eligible for self-assembling into supraparticles, thus adding more possible functions into these colloidal materials. These functions encompass redox, biochemical, catalytic, electrical, and optical properties that ultimately can give rise to interesting synergistic effects.
In the light of the above discussion, it is clear that the formation of supraparticles has provided an entirely new way of rationalizing colloidal materials in terms of two essential processes: self-assembly and self-limited growth. Our understanding of these processes and our ability to manipulate them granted access to the programmable colloidal assembly of hierarchically structured supramolecular materials with potential applications that could lead to interesting technologies. In this respect, the reproducibility of self-assembly protocols will be essential as well as the necessity of gathering expertise from engineers in order to integrate these superstructures into real-world devices.
In summary, this mini-review has attempted to illustrate the concepts, ideas and potential applications of self-limited, self-assembled supraparticles. It is hoped that the developments discussed above can be used as starting points for more detailed investigations as well as thought starters and idea triggers to generate new concepts in hierarchically structured supramolecular materials.
Acknowledgements
This work was financially supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET – Argentina), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT-Argentina; PICT-163/08, PICT-2010-2554, PICT-2013-0905), the Austrian Institute of Technology GmbH (AIT–CONICET Partner Group: “Exploratory Research for Advanced Technologies in Supramolecular Materials Science” – Exp. 4947/11, Res. No. 3911, 28-12-2011) and Universidad Nacional de La Plata. E. P. gratefully acknowledges a doctoral fellowship from CONICET. D. P., F. B. and O. A. are staff members of CONICET.References
- Supramolecular Materials and Technologies, ed. D. N. Reinhoudt, John Wiley & Sons Ltd., Chichester, 1999 Search PubMed.
- Supramolecular Design for Biological Applications, ed. N. Yui, CRC Press, Boca Raton, 2002 Search PubMed.
- Supramolecular Organization and Materials Design, ed. W. Jones and C. N. R. Rao, Cambridge University Press, Cambridge, 2002 Search PubMed.
- Nanocrystals Forming Mesoscopic Structures, ed. M. P. Pileni, Wiley-VSH, Weinheim, 2005 Search PubMed.
- Nanoparticle Assemblies and Superstructures, ed. N. A. Kotov, CRC Press, Boca Raton, 2006 Search PubMed.
- S. Srivastava and N. A. Kotov, Soft Matter, 2009, 5, 1146–1156 RSC.
- N. A. Kotov, J. Mater. Chem., 2011, 21, 16673–16674 RSC.
- M. P. Pileni, J. Mater. Chem., 2011, 21, 16748–16758 RSC.
- Y. Xia and Z. Tang, Chem. Commun., 2012, 48, 6320–6336 RSC.
- K. Ariga, Q. Ji, W. Nakanishi, J. P. Hill and M. Aono, Mater. Horiz., 2015, 2, 406–413 RSC.
- Y. Xia, N. Trung Dac, M. Yang, B. Lee, A. Santos, P. Podsiadlo, Z. Tang, S. C. Glotzer and N. A. Kotov, Nat. Nanotechnol., 2011, 6, 580–587 CrossRef CAS PubMed.
- T. Wang, D. LaMontagne, J. Lynch, J. Zhuang and Y. C. Cao, Chem. Soc. Rev., 2013, 42, 2804–2823 RSC.
- L. Wang, L. Xu, H. Kuang, C. Xu and N. A. Kotov, Acc. Chem. Res., 2012, 45, 1916–1926 CrossRef CAS PubMed.
- D. Luo, C. Yan and T. Wang, Small, 2015, 11, 5984–6008 CrossRef CAS PubMed.
- K. P. Johnston, J. A. Maynard, T. M. Truskett, A. U. Borwankar, M. A. Miller, B. K. Wilson, A. K. Dinin, T. A. Khan and K. J. Kaczorowski, ACS Nano, 2012, 6, 1357–1369 CrossRef CAS PubMed.
- A. K. Murthy, R. J. Stover, A. U. Borwankar, G. D. Nie, S. Gourisankar, T. M. Truskett, K. V. Sokolov and K. P. Johnston, ACS Nano, 2013, 7, 239–251 CrossRef CAS PubMed.
- J. I. Park, T. D. Nguyen, G. de Queiros Silveira, J. H. Bahng, S. Srivastava, G. Zhao, K. Sun, P. Zhang, S. C. Glotzer and N. A. Kotov, Nat. Commun., 2014, 5, 3593–3601 CAS.
- Q. Fu, Y. Sheng, H. Tang, Z. Zhu, M. Ruan, W. Xu, Y. Zhu and Z. Tang, ACS Nano, 2015, 9, 172–179 CrossRef CAS PubMed.
- S. T. Moerz, A. Kraegeloh, M. Chanana and T. Kraus, ACS Nano, 2015, 9, 6696–6705 CrossRef CAS PubMed.
- N. Trung Dac, B. A. Schultz, N. A. Kotov and S. C. Glotzer, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, E3161–E3168 CrossRef PubMed.
- Z. Wu, J. Liu, Y. Li, Z. Cheng, T. Li, H. Zhang, Z. Lu and B. Yang, ACS Nano, 2015, 9, 6315–6323 CrossRef CAS PubMed.
- Y. Wang, J. He, C. Liu, W. H. Chong and H. Chen, Angew. Chem., Int. Ed., 2015, 54, 2022–2051 CrossRef CAS PubMed.
- G. Jia, A. Sitt, G. B. Hitin, I. Hadar, Y. Bekenstein, Y. Amit, I. Popov and U. Banin, Nat. Mater., 2014, 13, 302–308 Search PubMed.
- J. D. Brodin, J. R. Carr, P. A. Sontz and F. A. Tezcan, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 2897–2902 CrossRef CAS PubMed.
- E. Piccinini, D. Pallarola, F. Battaglini and O. Azzaroni, Chem. Commun., 2015, 51, 14754–14757 RSC.
- K. Hirai, B. Yeom, S.-H. Chang, H. Chi, J. F. Mansfield, B. Lee, S. Lee, C. Uher and N. A. Kotov, Angew. Chem., Int. Ed., 2015, 54, 8966–8970 CrossRef CAS PubMed.
- Y. Bai, Q. Luo, W. Zhang, L. Miao, J. Xu, H. Li and J. Liu, J. Am. Chem. Soc., 2013, 135, 10966–10969 CrossRef CAS PubMed.
- Y. Hu, Y. Liu and Y. Sun, Adv. Funct. Mater., 2015, 25, 1638–1647 CrossRef CAS.
- P. Sevilla, S. Sanchez-Cortes, J. V. Garcia-Ramos and A. Feis, J. Phys. Chem. B, 2014, 118, 5082–5092 CrossRef CAS PubMed.
- M. S. Strozyk, M. Chanana, I. Pastoriza-Santos, J. Perez-Juste and L. M. Liz-Marzan, Adv. Funct. Mater., 2012, 22, 1436–1444 CrossRef CAS.
- Q. Fu, G. Ran and W. Xu, RSC Adv., 2015, 5, 37512–37516 RSC.
- H. Zhou, J.-P. Kim, J. H. Bahng, N. A. Kotov and J. Lee, Adv. Funct. Mater., 2014, 24, 1439–1448 CrossRef CAS.
- T. Wang, X. Wang, D. LaMontagne, Z. Wang, Z. Wang and Y. C. Cao, J. Am. Chem. Soc., 2012, 134, 18225–18228 CrossRef CAS PubMed.
- G. Yang, H. Zhong, R. Liu, Y. Li and B. Zou, Langmuir, 2013, 29, 1970–1976 CrossRef CAS PubMed.
- T. Hu, B. P. Isaacoff, J. H. Bahng, C. Hao, Y. Zhou, J. Zhu, X. Li, Z. Wang, S. Liu, C. Xu, J. S. Biteen and N. A. Kotov, Nano Lett., 2014, 14, 6799–6810 CrossRef CAS PubMed.
- L. Miao, J. Han, H. Zhang, L. Zhao, C. Si, X. Zhang, C. Hou, Q. Luo, J. Xu and J. Liu, ACS Nano, 2014, 8, 3743–3751 CrossRef CAS PubMed.
- W. Zhang, J. Zheng, C. Tan, X. Lin, S. Hu, J. Chen, X. You and S. Li, J. Mater. Chem. B, 2015, 3, 217–224 RSC.
- C. Stoffelen and J. Huskens, Chem. Commun., 2013, 49, 6740–6742 RSC.
- C. Hou, J. Li, L. Zhao, W. Zhang, Q. Luo, Z. Dong, J. Xu and J. Liu, Angew. Chem., Int. Ed., 2013, 52, 5590–5593 CrossRef CAS PubMed.
- D. Men, T.-T. Zhang, L.-W. Hou, J. Zhou, Z.-P. Zhang, Y.-Y. Shi, J.-L. Zhang, Z.-Q. Cui, J.-Y. Deng, D.-B. Wang and X.-E. Zhang, ACS Nano, 2015, 9, 10852–10860 CrossRef CAS PubMed.
- W. Ma, H. Kuang, L. Xu, L. Ding, C. Xu, L. Wang and N. A. Kotov, Nat. Commun., 2013, 4, 2689–2696 Search PubMed.
- L. Y. T. Chou, K. Zagorovsky and W. C. W. Chan, Nat. Nanotechnol., 2014, 9, 148–155 CrossRef CAS PubMed.
- Y.-T. Lai, E. Reading, G. L. Hura, K.-L. Tsai, A. Laganowsky, F. J. Asturias, J. A. Tainer, C. V. Robinson and T. O. Yeates, Nat. Chem., 2014, 6, 1065–1071 CrossRef CAS PubMed.
- Y. E. Kim, Y.-N. Kim, J. A. Kim, H. M. Kim and Y. Jung, Nat. Commun., 2015, 6, 7134–7142 CrossRef CAS PubMed.
- V. Liljestrom, J. Seitsonen and M. A. Kostiainen, ACS Nano, 2015, 9, 11278–11285 CrossRef CAS PubMed.
- C. Stoffelen, J. Voskuhl, P. Jonkheijm and J. Huskens, Angew. Chem., Int. Ed., 2014, 53, 3400–3404 CrossRef CAS PubMed.
- J. D. Brodin, X. I. Ambroggio, C. Tang, K. N. Parent, T. S. Baker and F. A. Tezcan, Nat. Chem., 2012, 4, 375–382 CrossRef CAS PubMed.
- J. D. Brodin, S. J. Smith, J. R. Carr and F. A. Tezcan, J. Am. Chem. Soc., 2015, 137, 10468–10471 CrossRef CAS PubMed.
- F. Ennen, P. Fenner, S. Boye, A. Lederer, H. Komber, B. Voit and D. Appelhans, Biomacromolecules, 2016, 17, 32–45 CrossRef CAS PubMed.
- B. Pelaz, S. Jaber, D. J. de Aberasturi, V. Wulf, T. Aida, J. M. de la Fuente, J. Feldmann, H. E. Gaub, L. Josephson, C. R. Kagan, N. A. Kotov, L. M. Liz-Marzan, H. Mattoussi, P. Mulvaney, C. B. Murray, A. L. Rogach, P. S. Weiss, I. Willner and W. J. Parak, ACS Nano, 2012, 6, 8468–8483 CrossRef CAS PubMed.
- D. J. Irvine, M. C. Hanson, K. Rakhra and T. Tokatlian, Chem. Rev., 2015, 115, 11109–11146 CrossRef CAS PubMed.
- H. Sun, L. Miao, J. Li, S. Fu, G. An, C. Si, Z. Dong, Q. Luo, S. Yu, J. Xu and J. Liu, ACS Nano, 2015, 9, 5461–5469 CrossRef CAS PubMed.
- Z. Wu, Y. Li, J. Liu, Z. Lu, H. Zhang and B. Yang, Angew. Chem., Int. Ed., 2014, 53, 12196–12200 CrossRef CAS PubMed.
- D. J. Milliron, R. Buonsanti, A. Llordes and B. A. Helms, Acc. Chem. Res., 2014, 47, 236–246 CrossRef CAS PubMed.
- H. Yin, H. Tang, D. Wang, Y. Gao and Z. Tang, ACS Nano, 2012, 6, 8288–8297 CrossRef CAS PubMed.
- A. Schreiber, M. C. Huber, H. Coelfen and S. M. Schiller, Nat. Commun., 2015, 6, 6705–6715 CrossRef CAS PubMed.
- L. A. Lane, X. Qian and S. Nie, Chem. Rev., 2015, 115, 10489–10529 CrossRef CAS PubMed.
- D. Pallarola, C. von Bildering, L. I. Pietrasanta, N. Queralto, W. Knoll, F. Battaglini and O. Azzaroni, Phys. Chem. Chem. Phys., 2012, 14, 11027–11039 RSC.
- D. Pallarola, N. Queralto, F. Battaglini and O. Azzaroni, Phys. Chem. Chem. Phys., 2010, 12, 8071–8083 RSC.
- D. Pallarola, N. Queralto, W. Knoll, O. Azzaroni and F. Battaglini, Chem. – Eur. J., 2010, 16, 13970–13975 CrossRef CAS PubMed.
- D. Pallarola, N. Queralto, W. Knoll, M. Ceolin, O. Azzaroni and F. Battaglini, Langmuir, 2010, 26, 13684–13696 CrossRef CAS PubMed.
- K. Zhang, J. Yi and D. Chen, J. Mater. Chem. A, 2013, 1, 14649–14657 CAS.
| This journal is © The Royal Society of Chemistry 2016 |
