Tribology of surface-grafted polymer brushes
Piotr
Mocny
and
Harm-Anton
Klok
*
École Polytechnique Fédérale de Lausanne (EPFL), Institut des Matériaux et Institut des Sciences et Ingénierie Chimiques, Laboratoire des Polymères, Bâtiment MXD, Station 12, CH-1015 Lausanne, Switzerland. E-mail: piotr.mocny@epfl.ch; harm-anton.klok@epfl.ch; Fax: +41 21 693 5650; Tel: +41 21 693 4866
First published on 5th April 2016
Abstract
Surface-grafted polymer brushes are a very attractive class of boundary lubricants. This article presents an overview of the tribological properties of surface-attached synthetic polymers. After a brief review of some mechanistic considerations and a discussion of tribological characterization tools, the lubrication properties of the three main classes of surface-attached polymer brushes, viz. (i) hydrophilic, (ii) hydrophobic and (iii) fluorinated brushes, will be presented. The article concludes with a brief discussion of possible applications of polymer brush-based lubricants.
Introduction
Friction is a ubiquitous phenomenon that occurs between two elements sliding against each other in opposite directions, resulting in a resistive force. Friction can be quantitatively expressed as a coefficient of friction (μ or COF) and defined as the ratio of the force F required to initiate or sustain relative tangential motion to the normal force N, i.e. μ = F/N.1 Strategies that overcome or reduce friction are key to the functioning of many mechanical, electromechanical and biological systems.2 One way to decrease friction at solid–solid interfaces is to incorporate a thin layer of a viscous fluid between the contacting surfaces, which supports the normal load and prevents direct contact of solid surfaces (lubrication).3,4 Inefficient lubrication can lead to wear of the material in the frictional area and the generation of debris particles, which can cause further damage.2,5–10Over the last few decades, the field of tribology, which includes the study of friction between interacting surfaces, has received great attention, because of its great contribution to energy losses in industry.4,11 Worldwide, ca. 100 million terajoules are used annually to overcome friction, which represents one fifth of all the energy produced.12,13 Notably, the effective implementation of new tribology solutions over the last 5–9 years has been proposed to allow 17.5% of the energy used in road transport to be saved.13 Tribology is not only important for industrial and technological systems, but is also critical to the functioning of many biological systems.14–20 Articular cartilage layers, for example, cover the major synovial joints such as hips or knees. They support a wide range of stresses and impacts and maintain extremely low levels of friction. These biological constructs are able to operate without disturbance for an 80 year lifespan, undergoing more than 108 loading cycles.21 This indicates their enormous robustness. This performance is the result of exceptional hydration lubrication, which is provided most importantly by hyaluronan (linear polysaccharide), lubricin (proteoglycan) and phospholipids (mainly phosphatidylcholine).14,15,22 Lubricin, which is present in the superficial layer of articular cartilage, immobilizes hyaluronan, which in turn complexes with phosphatidylcholine to create a brush-like boundary lubrication layer.15 Each component in this layer is charged, which allows strong binding of water molecules. Consequently, a tenacious hydration layer is formed, which can support the load and reduce shear forces. Importantly, the components of the self-assembled structures are acting together and none of them alone can provide efficient lubrication at high pressures that is found in major synovial joints.15
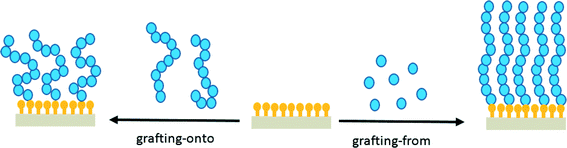
An interesting class of thin, polymer-based lubrication layers, which emulate some of the structural features of the brush-like macromolecules that provide lubrication at articular cartilage surfaces, are polymer brushes.23,24 The term polymer brush refers to a class of thin polymer coatings in which each of the chains is tethered to an underlying substrate (Fig. 1).25–31 At high grafting densities, i.e. when the distance between neighboring grafting points is small, steric repulsion leads to chain stretching and a brush-type conformation of the surface-tethered polymer chains. At lower grafting densities, tethered chains can assume different conformations, referred to as mushroom or pancake structures. Polymer brushes can be prepared via two principal strategies (Fig. 1). The grafting-onto method involves physi- or chemisorption of pre-synthesized polymers that contain appropriate chain-end functional groups, which either have a high affinity to or can react with a complementary reactive group on the substrate of interest. The grafting-from strategy is a bottom-up approach in which polymer chains are grown via surface-initiated polymerization (SIP) from a substrate modified with functional groups that can initiate a polymerization reaction. Generally, the grafting-from strategy is considered the preferred method to synthesize well-defined and densely-grafted polymer brushes. Using controlled/“living” surface-initiated polymerization techniques, such as atom transfer radical polymerization (ATRP), reversible addition–fragmentation chain-transfer (RAFT) polymerization, nitroxide-mediated polymerization (NMP) or photoiniferter mediated polymerization (PIMP), polymer brushes can be prepared with accurate control over film thickness, composition and architecture.30 The highly efficient lubrication properties of synthetic polymer brushes were first reported in 1993/1994.32–34 The earliest coatings that were analyzed consisted of polystyrene brushes grafted onto mica surfaces by zwitterionic chain ends. These thin films were found to reduce the effective friction coefficient (measured in toluene) to below 0.001 under contact pressures up to 1 MPa.
This review article will present an overview of the tribological properties of surface-grafted polymer brushes. In the next section, the mechanistic aspects of the lubrication properties of polymer brushes will be discussed. After that various tribological characterization methods, which can be used to study the lubrication properties of polymer brushes, will be presented. The following three sections will discuss the tribological properties of the three main classes of polymer brushes, viz. hydrophilic, hydrophobic and fluorinated brushes. The article will conclude with a brief discussion of possible applications of polymer brush-based lubricants.
Mechanistic considerations
The friction between two sliding surfaces depends in general on three parameters: the viscosity of the lubricant η, the sliding speed v and the applied load N. Three lubrication regimes can be distinguished on the basis of the so-called Stribeck curve35,36 (Fig. 2), which represents the friction coefficient as a function of a dimensionless parameter, given by B = ηv/N. A small B number usually leads to the formation of a very thin lubricant layer, whereas a high B reflects a thick film. The highest friction occurs in the boundary lubrication regime, which represents a complete asperity contact between the two surfaces. In the mixed lubrication regime, the partial load is supported by the lubricating fluid. Finally, at minimum friction begins hydrodynamic lubrication, where the surfaces are no longer touching and the load is supported entirely by the fluid. As the film thickness increases more frictional force is necessary to shear the fluid, which results in an increase in friction coefficient. Stribeck curves for polymer brush boundary lubricants are sometimes reported.9,36–38 They very often reveal a weak dependence of shearing velocity on frictional forces, which resembles boundary lubrication. On the other hand, only hydrodynamic effects can explain remarkable lubrication performance. Therefore, this brush-specific regime is sometimes referred to as “polymer brush-enhanced elastohydrodynamic lubrication”36 or simply “brush-lubrication regime” replacing mixed and boundary regimes, typical for conventional lubricants.38 Recently, Bielecki et al. were able to identify both brush- and hydrodynamic lubrication regimes of hydrophobic methacrylate-based polymer brushes with oils of different viscosities on Stribeck plots (Fig. 3).38 The results were compared with friction between bare surfaces, i.e. bare-bare SiO2-borosilicate glass ball, and showed a similar straight-line hydrodynamic behavior in both cases. For lower speed × viscosity numbers, brush-modified surfaces exhibited a much lower friction coefficient plateau. The nearly constant friction force in this region is usually explained by the velocity-dependent interpenetration of opposing polymer chains, which acts as a self-regulating mechanism.39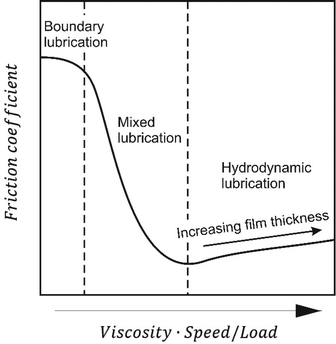 | ||
| Fig. 2 Stribeck curve illustrating fluid film lubrication and boundary film lubrication regimes. Reproduced from ref. 89 with permission from The Royal Society of Chemistry. | ||
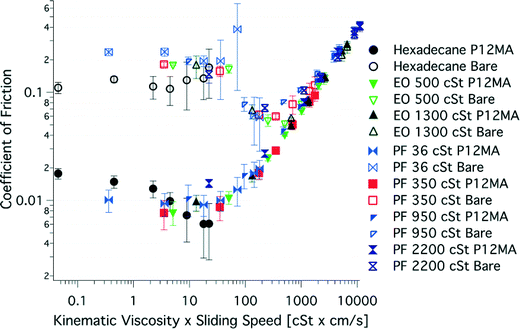 | ||
| Fig. 3 Stribeck-like curves obtained by Bielecki et al. displaying lubrication of 250 nm poly(dodecylmethacrylate) (P12MA) brushes measured at 20 mN load in several fluids with different kinematic viscosities. Empty and filled markers denote friction between bare (bare-bare SiO2-borosilicate glass ball) and brush-functionalized surfaces. Reproduced from ref. 38 with permission from Springer Science + Business Media. | ||
The outstanding lubrication properties of surface-grafted polymer brushes originate from their unique architecture. In a good solvent environment, solvation/hydration repulsion effects result in swelling and chain stretching of the surface-tethered polymer grafts normal to the surface. Two compressed, sliding surfaces bearing such polymer brush coatings present very low friction, which has been attributed to entropically suppressed interpenetration on compression, which was in fact weakly observed only at the periphery of the polymer brush layer. This results in a relatively fluid shared interfacial zone, as there are no chain entanglements within this interface.33,40
In aqueous environments, the lubrication properties of polymer brushes can be further improved by taking advantage of polyelectrolyte effects. Mobile counterions induce high osmotic pressures that resist compression and interpenetration even further at low to moderate pressures.41–43 At high pressures, lubrication proceeds via the hydration lubrication mechanism provided by sub-nanometer hydration shells surrounding charged monomer units.41,44–46 These shells withstand high pressures without being squeezed out, but at the same time remain mobile due to rapid exchange with free water molecules, and thus fluid under shear. The most recent class of surface-attached boundary lubricants are polyzwitterionic polymer brushes.17–20,47 Because they have no net charge and therefore are not associated with mobile counterions, they are believed to support lubrication entirely by the hydration lubrication mechanism (Fig. 4). Each zwitterionic monomer is capable of strongly binding to around 15 water molecules.40 This high hydration is the reason for their exceptional performance.
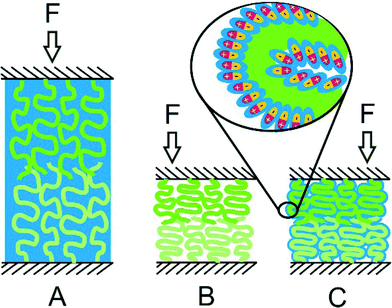 | ||
| Fig. 4 Cartoons illustrating the compression of hydrated polymer brushes under increasing load F:57,90 (A) a swollen brush at low compression; (B) neutral polymer brushes with expelled water; (C) polyzwitterionic brushes that maintained hydration under high compression. Blue color denotes water; the inset presents the enlarged area from brush C to show zwitterionic groups surrounded by a sub-nanometer water layer. | ||
Tribological characterization methods
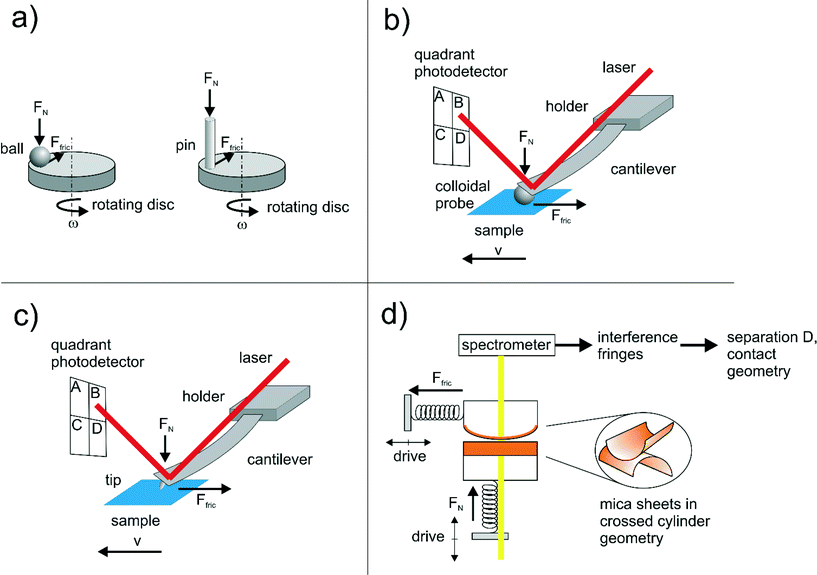
Friction is generally a consequence of different energy dissipation pathways. For rough surfaces, pressure is distributed non-uniformly and high local pressures at asperity contacts can cause high-energy-loss plastic deformations. Specifically for polymer brushes, interpenetration, although very weak, leads to frictional dissipation. Finally, one can consider molecular-level friction, in which bonds between sliding surfaces are continuously being created and broken. In the case of hydration lubrication, the energy is dissipated during shear of sub-nanometer hydration shells.48 Depending on the length scale that a particular tribological characterization test probes, different effects can dominate.49–51 Macrotribological measurements are usually done in a pin-on-disk or ball-on-disk contacting geometry in the sliding or rolling regime, while nanotribological tests are performed using colloidal probe atomic force microscopy (AFM),52,53 lateral force microscopy (LFM)54 or surface force apparatus (SFA)41,47,55 (Fig. 5). The forces are in the range of Newtons and milli-/nano-Newtons in macro- and nanotribology, respectively.49 Contact areas in AFM, LFM and SFA measurements can be treated as a single asperity contact, so the effect of roughness can be excluded.51 High local contact pressures on asperities at macroscopic measurements can lead to wear of surfaces and increase of friction caused by debris, the so-called “third body”.49,50 On the other hand, a sharp AFM/LFM tip can plough through the boundary layer and cause damage as well.47 Therefore, the aforementioned categories of measurement are difficult to correlate, as they involve different dissipation mechanisms.49–51 The surface friction force data is acquired in several ways depending on the measurement. AFM is run in contact mode and lateral voltage signal data points are collected in trace and retrace directions with the slow scan axis disabled. Both curves form a friction loop, from which one-half of the separation between trace and retrace frictions is a measure of the friction force.56 SFA uses lateral and shear springs of certain spring constants Kn and Ks connected to the sample stage. As bending of the springs responds to changes in surface separation and applied lateral motion, friction data can be readily obtained.57 The separation (D) and geometry of the contact can be obtained by looking into interference fringes, which are generated by light passing through mica sheets mounted on two crossed cylindrical lenses (Fig. 5).Hydrophilic polymer brushes
The majority of polymer brush-based boundary lubricants are hydrophilic. The design of many of these hydrophilic polymer brushes is inspired by the material interfaces that provide lubrication in joints. Hydrophilic polymer brush-based boundary lubricants can be subdivided into three categories, viz. (i) neutral, (ii) polyanionic and polycationic and (iii) polyzwitterionic brushes, each of which will be discussed in more detail below.Neutral polymer brushes
Neutral hydrophilic polymer brushes are composed of polar monomers, which interact with water molecules by hydrogen bonding, e.g. oligo(ethylene glycol) methacrylate (OEGMA),20 ethylene oxide (EO)/ethylene glycol (EG),4–10,58–65 hydroxyethyl methacrylate (HEMA)56 or acrylamide (AM).66 The bound water molecules provide a lubricative hydration layer on the surfaces under moderate pressures.47 The conformation of neutral chains tethered to the substrate is weakly affected by the presence of salts in aqueous solution, because of their nonionic character.62Table 1 provides a comprehensive overview of all hydrophilic polymer brush-based boundary lubricants that have been reported. For each entry, Table 1 gives the dry film thickness and grafting density as well as the friction coefficients and the specific conditions that are used to determine this number.| Polymer | Example structure | Grafting method and substrate | d dry [nm] | σ [chains per nm2] | μ | Pressure or normal load | Conditions | Remarks | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a Dry thickness calculated from reported adsorbed mass onto surfaces using density values for the polymers (1.12 g cm−3 for PEG/PEO polymers and copolymers). b Grafting density calculated using the dry thickness and molecular weight of the adsorbed polymer. c Mini traction machine (MTM). d Pin-on-disk tribometer. e Ball-on-plate tribometer. f AFM. g Colloidal probe LFM. h Surface force apparatus (SFA). i Measured with a polymer solution as a lubricant. j Measured with a polymer-free solution as a lubricant; SRR – slide-to-roll ratio, defined as SRR = (Vball − Vdisk)/U, where U = (Vball + Vdisk)/2 and Vball and Vdisk are the surface velocities of the ball and disk surface, respectively; vs – sliding speed. k Numbers in round brackets indicate molecular weight in kDa, while numbers in square brackets define grafting ratio (number of polymer backbone mers per PEG side chain); SDS: sodium dodecyl sulfate; CTAB: cetyltrimethylammonium bromide. | |||||||||
| PAAm-g-PEG | PAAm(14)-[6.5]-PEG(5)k | Grafted onto glass | 0.8–1.4a (84–154 ng cm−2) | PEG chains: 0.10–178 | 0.0001–0.03 (0.005 on average)c | — | SRR = 10%; v up to 2500 mm s−1 | Tribo-pair: glass/steeli | 58 |
| PAAm(70)-g[3.5]-PEG(5)k | 0.2–0.3d | 0.5–5.0 N | v s from 1–19 mm s−1 | ||||||
| PLL-g-PEG | PLL(10)-g[2.9]-PEG(2)kPLL(20)-g[2.9]-PEG(5)k PLL(20)-g[3.5]-PEG(5)k |
Grafted onto glass, silicon oxide (SiOx), steel (FeOx surface) | 1.4 nma (glass); 0.5–1.8a nm (SiOx);0.5a (FeOx) | PEG chains: 0.33b (FeOx); 0.17–0.22b (SiOx); 0.15 (FeOx) | 0.0002–0.002c | — | SRR = 10%; v up to 2500 mm s−1 | Tribo-pair: glass/steeli | 58 |
| 0.0001–0.001c | 10 N; 410 MPa | SRR = 10%; v up to 3000 mm s−1 | Tribo-pair: glass/steeli | 5 | |||||
| 0.26–0.09c | 3.0 N; 460 MPa | SRR = 50% or 100%; mean v up to 900 mm s−1 | Tribo-pair: steel/steeli | 4 | |||||
| 0.15–0.09d | 0.5–5.0 N; 320–700 MPa | v s = 0.2–0.8 mm s−1 | Tribo-pair: glass/steeli | ||||||
| 0.1d | 2 N; 510 MPa | v s = 5 mm s−1 | Tribo-pair: glass/steeli | 8 | |||||
| Grafted onto mica and silicon oxide (SiOx) | 2.6a | PEG chains: 0.33b | <0.001h | Up to 60 mN; 20 MPa | v s = 5 μm s−1 | Tribo-pair: mica/micai | 10 | ||
| 0.20–0.55g | Up to 30 nN | v s = 1400 nm s−1 | Tribo-pair SiO2/SiO2 (grafted/non-grafted);j friction increased in 2-propanol | 6 | |||||
| 0.022–0.57g | Up to 35 nN | v s = 1400 nm s−1 | Tribo-pairs: Si/Si (grafted/non-grafted and grafted/grafted);j friction increased in bad solvent | 7 | |||||
| Grafted onto PDMS | 1.3a | PEG chains: 0.16b | 0.02–0.04d | 0.5–5 N | v s = 0.1–15 mm s−1 | Tribo-pair: PDMS/PDMSi | 9 | ||
| PLL(20)-g[2.9]-PEG(5)k PLL(20)-g[3.6]-PEG(5)k | Grafted onto silicon nitride Si3N4, silicon carbide SiC | 15.7 (Si3N4), 16.8 (SiC) | PEG chains: 1.93–2.07b | 0.003–0.2d | 2–5 N | v s =1.4–185 mm s−1 | Tribo-pairs: Si3N4/Si3N4 and SiC/SiC; high friction for low vs = 1.4–11 mm s−1i | 64 | |
| ((CH3)3N+)-PEG-NHS | ((CH3)3N+)-PEG(3.4)-NHSk | Grafted onto mica | 1.34a | 0.25b | 0.030–0.3h | From 0.01 MPa; | v s = 300 nm s−1 | Tribology between two grafted mica surfaces; friction increased at higher compressionsj | 62 |
| Sil-PEG | Sil-PEG(5)k | Grafted onto glass | 0.49–2.21 | 0.06–0.29b | 0.08–0.58d | 2 N; 510 MPa | v s = 5 mm s−1 | Tribo-pair: glass/steel; high friction was due to wear during measurementj | 8 |
| PEO-PPO-PEO | EO37-PO56-EO37 | Grafted onto PDMS | 1.5–3.0a | PEO chains: 0.15–1.06b | 0.02–0.15d | Up to 5 N | v s = 1–100 mm s−1 | Tribo-pair: PDMS/PDMSi | 59 |
| 1.46a | PEO chains: 0.30b | 0.032–0.5d | 0.5–5 N | v s = 0.1–15 mm s−1 | Tribo-pair: PDMS/PDMS;i high friction for low vs < 5 mm s−1 | 9 | |||
| PO15-EO26-PO15PO15-EO10-PO15 PO22-EO14-PO22 |
Grafted onto silicon, titanium | 0.5–6.0a (Si); 1.1–11.7a (Ti) | PPO chains: 0.25–2.61b (Si); 0.55–5.09b (Ti); | 0.01–0.24 (Si);d 0.1–0.3 (Ti)d | 4–8 N (895–1127 MPa for Si/steel, and 798–1000 MPa for Ti/steel) | v s = 10–100 mm s−1 | Tribo-pairs: Si/steel, and Ti/steeli | 65 | |
| POEGMA | — | Grafted from silicon by SI-ATRP | 60 | — | 0.02–0.04d | 0.5 N; 0.32 MPa | v s = 2 mm s−1 | Tribo-pair: Si/PDMS;j measured with surfactant (SDS, CTAB) solutions | 20 |
| — | Grafted from cross-linked polyethylene by SI-PMP | 100–150 | — | 0.03e | 0.98 N (29 MPa) | v s =50 mm s−1 | Tribo-pair: crosslinked PE/Co–Cr–Mo alloy;j lubricants: pure water, acellular simulated body fluid, 25 vol% bovine serum | 67 | |
| PHEMA | — | Grafted from silicon by SI-ATRP | 2–7 | 0.26 | 0.04–0.18f | Up to 100 nN | v s = 8.0 μm s−1 | Tribo-pair: Si/Si3N4j | 56 |
| P(AM-co-MAPTAC) | Copolymer consisting of 99% AM (acrylamide) and 1% MAPTAC | Grafted onto gold coated silicon | — | — | 0.26–0.69g | Up to 900 nN | v s = 10–100 μm s−1 | Tribo-pair: Au coated Si/SiO2j | 66 |
One of the first studies on the lubrication properties of neutral polymer brushes investigated PEG (poly(ethylene glycol)) grafted onto mica surfaces via trimethylammonium chain-ends.62 Unfortunately, the relatively small molecular weight (3400 Da) and low grafting density (σ = 0.29 ± 0.09 chains per nm2, assuming hexagonal packing) of the film did not prevent interpenetration of the opposing polymer chains during shear force measurements. Therefore, these coatings could only support quite low pressures 0.1–10 atm while maintaining low friction, μ = 0.030 ± 0.015. There are several other reports on PEG-type polymer brush lubricants, which are synthesized by the grafting-onto method. Such brushes are in general more prone to wear, as compared to brushes grafted from surfaces. Nevertheless, unlike their more stable cousins, grafted-onto films can provide self-healing by reversible re-adsorption. Thus, they can also withstand similar pressures when a measurement is done in a liquid of sufficient concentration of the free polymer. This effect has been observed for brush films prepared from polymers that show fast adsorption kinetics, such as PLL-g-PEG.8 Methoxy-poly(ethylene glycol)-trimethylsilylether (Sil-PEG), on the other hand, is an example of a polymer that possesses slow adsorption kinetics and is not capable of efficient self-healing. Other examples of lubricative neutral polymer brushes that have been reported are poly(oligo(ethylene glycol) methacrylate) (POEGMA)20,67 and poly(2-hydroxyethyl methacrylate) (PHEMA).56 Interestingly, POEGMA brushes have been tested on cross-linked polyethylene bearings mimicking conditions of natural joint lubrication and have been shown to possess a coefficient of friction as low as 0.03 at ca. 29 MPa.67
Polyanionic and polycationic brushes
Table 2 lists the polyanionic- and polycationic-based boundary lubricants that have been reported so far and summarizes the friction properties of the different systems. These brushes are more robust than their neutral analogues. For instance, covalently attached polyelectrolyte brushes, such as poly(acrylic acid) (PAA) grafted from silicon wafers, can maintain their lubrication properties at pressures as high as 45 MPa,53 when slid against a bare silica surface. The robustness of the lubrication properties of polyelectrolyte brush films is due to a variety of reasons. On the one hand, the hydration layer in polyelectrolyte brushes is more robust, because of stronger ion–dipole interactions between charged polymer chains and water molecules. Furthermore, chain stretching is more pronounced due to the presence of both inter- and intramolecular repulsions. These electrostatic repulsions depend on and can be varied by tuning the pH, the nature of the counterion as well as the chemical composition of the brush. Polyanionic and polycationic brushes can be composed of either weak monomers, such as anionic acrylic acid (AA),53 methacrylic acid (MAA),18,53,54,68–70 or strong electrolyte monomers, such as cationic 2-(methacryloyloxy)ethyl trimethylammonium chloride (METAC),18,20,71 3-(methacroylamino)propyl trimethylammonium chloride (MAPTAC),66,72 2-(dimethylamino)ethyl methacrylate (DMAEMA),67 anionic 3-sulfopropyl methacrylate potassium salt (SPMA),18,20,21,71 sulfonated glycidyl methacrylate (SGMA)41 or 2-methacryloyloxyethyl phosphate (MPA).67 The friction properties of polyelectrolyte brushes depend on pH as well as the presence of counterions. Dunér et al. have observed that weak polyelectrolyte PAA brushes crosslink and collapse in the presence of Ca2+.53 However, it did not result in a change of friction at pH = 3 and μ increased drastically only at pH = 7.5. This behavior was explained by strong interactions between Ca2+ and PAA functional groups. During shearing, the carboxylate–calcium ion–carboxylate bridges are continuously disrupted and restored, which dissipates energy. On the other hand, Wei et al. have shown that the friction coefficients of PMAA brushes can be reversibly changed from 0.0068 at pH 12 to 1.1 at pH 2, respectively.18 The friction properties of strong polyelectrolyte-based PMETAC and PSPMA brushes can be tuned from superior lubrication (μ = ca. 10−3) to ultrahigh friction (μ > 1) by counterion exchange or enclosure of surfactants within the brushes, but are found to be insensitive to changes in pH.18,20 DMAEMA and PMPA brushes grafted from crosslinked polyethylene have been tested for applications in prosthetics using a ball-on-plate tribometer with a Co–Cr–Mo alloy ball.67 The coefficients of friction of these surfaces were 0.05 and 0.15, respectively, in an acellular simulated body fluid (SBF). On the other hand, in 25 vol% bovine serum (BS), the coefficients were 0.13 for PDMAEMA and 0.06 for PMPA brushes. The authors suggested that the positively charged –NH+(CH3)2 groups of the PDMAEMA brushes attract negatively charged proteins, such as albumin, that are present in BS. Their adsorption makes the sliding difficult. Another interesting example is the work carried out by Banquy et al.55 who have prepared a lubricin-like polymer consisting of a bottle-brush domain decorated with PMPC, and two cationic domains with P(DMAEMA-co-MMA) chains. This macromolecule was grafted onto mica surfaces by electrostatic interactions and showed coefficients of fiction as low as 0.0025 at 2.1 MPa load in pure water. This is an unusual performance of grafted-onto assemblies, which show the potential of bio-inspired synthetic bottle-brush polymers.| Polymer | Substrate | d dry [nm] | μ | Pressure or normal load | Conditions | Remarks | Ref. |
|---|---|---|---|---|---|---|---|
|
The coefficient of friction was measured by:
a Pin-on-disk tribometer.
b Ball-on-plate tribometer.
c Ball-on-block tribometer.
d Colloidal probe AFM.
e SFA.
All measurements were done with a polymer-free solution as a lubricant unless stated otherwise. PNIPAAm: poly(N-isopropylacrylamide); TMAB: tetramethylammonium bromide; counterions: TEAB: tetraethylammonium bromide, TBAB: tetrabutylammonium bromide, TFSI: bis(trifluoromethanesulfonimide); surfactants: DTAB: dodecyltrimethylammonium bromide, CTAB: cetyltrimethylammonium bromide, SDS: sodium dodecyl sulfate, SL: sodium laurate. |
|||||||
| PMAA | Grafted from silicon by SI-ATRP | 35 | 0.006–1.6a | 0.5 N (0.23 MPa) | v s = 2 mm s−1 | Tribo-pair: Si/PDMS; friction increases in the following order: Na+ < TBAB, DTAB, CTAB (rapidly); Na+ < NH+ < TMAB < TEAB (gradually); Na+/K+ < Cu2+, Fe3+ (rapidly) | 18 |
| Grafted from silicon by SI-PIMP | 15–240 | 0.017–0.035a | 1 N | v s = 0.25–10 mm s−1 | Tribo-pair: Si/PDMS; thin brushes (30 nm) are not stable; their long-term μ can be up to 1 | 69 | |
| PAA | Grafted from silicon by SI-PIMP | 17 | <0.018–0.25d | Up to 55 nN (45 MPa) | v s = 1 mm s−1 | Tribo-pair: Si/Si (non-grafted); μ increased to 0.25 in 10 mM CaCl2 at pH = 3.5 | 53 |
| PMETAC | Grafted from silicon by SI-ATRP | 20 | 0.006–0.828a | 0.5 N (0.23 MPa) | v s = 2 mm s−1 | Tribo-pair: Si/PDMS; friction increases in the following order: Cl− < ClO4− < PF6− < TFSI− (gradually) | 18 |
| 40 | 0.001-1a | 0.5 N (0.32 MPa) | Tribo-pair: Si/PDMS; friction increases in the following order: Cl− < SL < SDS | 20 | |||
| — | 0.08a | 0.49 N; 139 MPa | v s = 150 mm s−1 | Tribo-pair: Si/glass (both surfaces were grafted) | 71 | ||
| PMAPTAC | Grafted onto mica | — | Below detection limitd | <0.2 mN m−1 (ca. 1 nN) | v s = 2 μm s−1 | Tribo-pair: mica/Si (both surfaces were grafted) | 72 |
| 0.06d | 0.2–4 mN m−1 (ca. 1–20 nN) | ||||||
| 0.37d | Above 4 mN m−1 (ca. 20 nN) | ||||||
| Below detection limitd | Up to 9 mN m−1 (ca. 45 nN, around 20 MPa) | v s = 2 μm s−1 | Tribo-pair: mica/Si (both surfaces were grafted); measured with SDS surfactant solution | ||||
| 0.05d | 10–12 mN m−1 (ca. 50–60 nN) | ||||||
| Grafted onto gold coated silicon | — | 0.24–0.35d | 0–900 nN | v s = 10–100 μm s−1 | Tribo-pair: Au coated Si/Si | 66 | |
| PMPA | Grafted from cross-linked polyethylene by SI-PIMP | 100–150 | 0.02–0.15b | 0.98 N (29 MPa) | v s = 50 mm s−1 | Tribo-pair: crosslinked PE/Co–Cr–Mo alloy; lubricants: pure water, acellular simulated body fluid, 25 vol% bovine serum | 67 |
| PDMAEMA | Grafted from cross-linked polyethylene by SI-PIMP | 100–150 | 0.05–0.13b | 0.98 N (29 MPa) | v s = 50 mm s−1 | ||
| PSPMA | Grafted from silicon by SI-ATRP | 45 | 0.005–0.24a | 0.5 N (0.23 MPa) | v s = 2 mm s−1 | Tribo-pair: Si/PDMS; friction increases in the following order: K+ < TBAB < DTAB < CTAB (gradually); minor change upon exchange with Cu2+ and Fe3+ | 18 |
| 50 | 0.01–0.8a | 0.5 N (0.32 MPa) | v s = 2 mm s−1 | Tribo-pair: Si/PDMS; high μ value in CTAB solution | 20 | ||
| — | 0.015a | 0.49 N (139 MPa) | v s = 150 mm s−1 | Tribo-pair: Si/glass (both surfaces were grafted) | 71 | ||
| Grafted from PNIPAAm microgels, and PDMS surface by SI-ATRP | 142 (microgels) | 0.005–0.015c | 1–10 N | v s = 1.67 mm s−1 | Tribo-pair: Si/PDMS; measured with solution containing hydrogels; μ < 0.003 when PDMS counterface was additionally grafted | 21 | |
| P(SPMA-co-METAC) | Grafted from silicon by SI-ATRP | — | 0.015a | 0.49 N (139 MPa) | v s = 150 mm s−1 | Tribo-pair: Si/glass (both surfaces were grafted) | 71 |
| PMMA-b-PSGMA | Grafted onto mica | 3 | <0.0006–0.005e | 10–50 μN (0.15–0.30 MPa) | v s = 220–550 nm s−1 | Tribo-pair: mica/mica; μ increases to 1 at around 0.3 MPa load | 41 |
| P2VP | Grafted onto silicon | 5 | 0.008–0.011d | 0–50 nN | v s=6–1000 μm s−1 | Tribo-pairs: Si/Si, Si/Si3N4, Si/SiO2; measured under dry conditions | 80 |
Polyzwitterionic brushes
Polyzwitterionic brushes are extremely efficient hydrophilic boundary lubricants, which withstand quite high pressures.40,55,71,73,74 In contrast to polyanionic and polycationic brushes they have no net charge and therefore are not associated with mobile counterions. This makes their friction properties relatively insensitive to environmental factors such as pH or the presence of counterions, as has been shown by Wei et al., who investigated poly(sulfobetaine methacrylate) (PSBMA)-based boundary lubricants.18,20 PSBMA and poly(2-methacryloylethyl phosphorylcholine) (PMPC)56,67,71,75 are two examples of zwitterionic polymers, which have been extensively studied in the literature. Table 3 presents an overview of the PSBMA- and PMPC-based boundary lubricants reported to date. Recently, Tairy et al.47 have grafted PMPC brushes from silica surfaces and found extremely low friction coefficients (μ = 10−5 – 7 × 10−4) at pressures as high as 14.9 MPa by pin-on-disk tribometry, which outperforms typical polyanionic and polycationic lubricating systems. Another interesting improvement has been reported by Kobayashi et al.,71 who increased the stability of PMPC brushes by crosslinking them with bis(2-iodoethoxy)ethane. These brushes showed relatively low friction coefficients (μ = 0.08–0.13) without any visible wear even after 200 friction cycles at pressures as high as 139 MPa.| Polymer | Substrate | d dry [nm] | μ | Pressure or normal load | Conditions | Remarks | Ref. |
|---|---|---|---|---|---|---|---|
|
The coefficient of friction was measured by:
a Pin-on-disk tribometer.
b Ball-on-plate tribometer.
c AFM.
d SFA.
e A blocks correspond to copolymers of quaternized PDMAEMA with MMA, while B blocks consist of an MMA backbone decorated with PMPC branches.
All measurements were done with a polymer-free solution as a lubricant. |
|||||||
| PSBMA | Grafted from silicon by SI-ATRP | 15 | 0.006a | 0.5 N (0.23 MPa) | v s = 2 mm s−1 | Tribo-pair: Si/PDMS | 18 |
| 20 | 0.015a | 0.5 N (0.32 MPa) | v s = 2 mm s−1 | Tribo-pair: Si/PDMS | 20 | ||
| PMPC | Grafted from silicon by SI-ATRP | 4–10 | 0.03–0.09c | Up to 100 nN | v s =8 μm s−1 | Tribo-pair: Si/Si3N4; friction increased with increasing brush thickness; σ = 0.17 chains per nm2 | 56 |
| — | 0.09a | 0.49 N (139 MPa) | v s = 150 mm s−1 | Tribo-pair: Si/glass; wear occurred during the test | 71 | ||
| Grafted from mica by SI-ATRP | 7.5–19 | 0.00001–0.0007d | 0–60 mN (up to 14.9 MPa) | v s = 260–300 nm s−1 | Tribo-pair: mica/mica (both surfaces were grafted); σ = 0.18 chains per nm2 | 47 | |
| 4–12.2 | <0.001d | Up to 1 mN (2–2.5 MPa) | v s = 20–3000 nm s−1 | Tribo-pair: mica/mica | 75 | ||
| 0.0004d | 1–15 μN (up to 7.5 MPa) | ||||||
| Grafted from crosslinked polyethylene by SI-PIMP | 100–150 | 0.015b | 0.98 N (29 MPa) | v s = 50 mm s−1 | Tribo-pair: crosslinked PE/Co–Cr–Mo alloy; lubricants: pure water, acellular simulated body fluid, 25 vol% bovine serum | 67 | |
| Unmodified, crosslinked or quaternized P(MPC-co-DMAEMA) | Grafted from silicon by SI-ATRP | 85 | 0.1a | 0.49 N; 139 MPa | v s = 150 mm s−1 | Tribo-pair: Si/glass; stable friction for cross-linked poly(MPC) | 71 |
| ABA bottle-brush polymere | Grafted onto mica | 5 | 0.0025–0.0115d | Up to 11 mN (2.1 MPa) | v s = 2.5 μm s−1 | Tribo-pair: mica/mica | 55 |
Hydrophobic polymer brushes
Hydrophobic polymer brushes are very attractive boundary lubricants that can combine the lubrication properties of polymer brushes with the rheology and high temperature advantages of oil. An overview of hydrophobic polymer brush-based boundary lubricants is given in Table 4. The first studies involved polystyrene (PS) chains functionalized with zwitterionic groups such as N+(CH3)2(CH2)3SO3− that are able to readily adsorb onto mica surfaces.34 Related studies on hydrophobic polymer brushes generated by grafted-from approaches have been reported. Besides polystyrene, there is a number of reports on poly(methyl methacrylate) (PMMA), poly(ethyl methacrylate) (PEMA), poly(butyl acrylate) (PBA), poly(hexyl methacrylate) (P6MA), poly(dodecyl methacrylate) (P12MA) and poly(octadecyl methacrylate) (P18MA) brushes.37,38,52,54,56,76–81 A thick layer (250 nm) of P12MA has been found to withstand up to 460 MPa in hexadecane and maintain relatively low friction coefficients, i.e. 0.02–0.12.54| Polymer | Substrate | d dry [nm] | σ [chains per nm2] | μ | Pressure or normal load | Conditions | Remarks | Ref. |
|---|---|---|---|---|---|---|---|---|
| The coefficient of friction was measured by: a Pin-on-disk tribometer. b Ball-on-plate tribometer. c AFM. d Colloidal probe AFM. e SFA. | ||||||||
| PS-X | Grafted onto mica | — | — | <0.0015e | 0.1 mN; 1 MPa | v s = 100 nm s−1; toluene | Tribo-pair: mica/mica;X = −N+(CH3)2(CH2)3SO3− | 34 |
| PS | Grafted onto silicon | 6–12.1 | 0.071–0143 | 0.024–0.046d | 600–2400 nN; | v s = 10 μm s−1; dry | Tribo-pair: Si/polyethylene; measured under dry conditions | 52 |
| 5 | 0.063 | 0.004–0.010c | 0–50 nN | v s = 6–1000 μm s−1 | Tribo-pairs: Si/Si, Si/Si3N4, Si/SiO2; measured under dry conditions | 80 | ||
| — | — | 0.2–0.3b | 200 μN; 34.3 MPa | v s = 330 μm s−1 | Tribo-pair: Si/glass; measured under dry conditions | 77 | ||
| Grafted from silicon by SI-ATRP | 25–29 | 0.44–0.49 | 0.0001–0.3d | 0–30 nN | v s from 4 to 1000 μm s−1 | Tribo-pair: Si/SiO2; measured with toluene–2-propanol mixtures | 37 | |
| Grafted from silicon by SIP | 30 | — | ca. 0.0016d (in toluene);0.16d | 0–45 nN (95.6 MPa at 20 nN) | v s = 1400 nm s−1 | Tribo-pair: Si/SiO2; measured with toluene, 2-propanol and n-butanol | 81 | |
| PMMA | Grafted from silicon by SI-ATRP | 5 | 0.19 | 0.04–0.08c | Up to 100 nN | v s = 8 μm s−1 | Tribo-pair: Si/Si3N4; measured in toluene | 56 |
| 5–30 | 0.56 | 0.18–0.25b | 0.20–0.98 N | v s = 90 mm min−1 | Tribo-pair: Si/steel; measured under dry conditions | 79 | ||
| 0.12–0.15b | 0.20–0.98 N | v s = 90 mm min−1 | Tribo-pair: Si/steel; measured with hexane or cyclohexane | |||||
| 0.055–0.13b | 0.20–0.98 N | v s = 90 mm min−1 | Tribo-pair: Si/steel; measured with acetone or toluene | |||||
| PEMA | Grafted from silicon by SI-ATRP | 75 | 0.53–0.68 | 0.005d | 10–95 nN | v s = 5 μm s−1 | Tribo-pair: Si/SiO2; μ = 0.013 under dry conditions | 76 |
| 0.013d | 10–95 nN | v s = 5 μm s−1 | Tribo-pair: Si/SiO2; measured with hexadecane, FC-40 | |||||
| 20–206 | 0.16–0.82 | 0.0122–0.0456d | 10–95 nN | v s = 5 μm s−1 | Tribo-pair: Si/SiO2; measured under dry conditions; friction decreased with increasing ddry and σ | 78 | ||
| PBA | Grafted onto silicon | Up to 0.94 | 0.05–0.78 | 0.38–0.44b | 200 μN; 34.3 MPa | v s = 330 μm s−1 | Tribo-pair: Si/glass; measured under dry conditions | 77 |
| PS/PBA | 1.95–3.61 | 0.26–0.33 | 0.2–0.3b | |||||
| P6MA | Grafted from silicon by SI-ATRP | 40–110 | — | 0.151d | 0–130 nN | — | Tribo-pair: Si/SiO2 | 54 |
| 0.463d | ||||||||
| Grafted from silicon and borosilicate glass by SI-ATRP | 90 | — | 0.09–0.16b | 20 mN | v s = 0.1 cm s−1 | Tribo-pair: Si/glass; measured under dry conditions, with ethanol, hexadecane or PF350cSt oil | 38 | |
| 0.01b | 20 mN | v s = 0.1 cm s−1 | Tribo-pair: Si/glass; measured with toluene | |||||
| P12MA | Grafted from silicon by SI-ATRP | 170–330 | — | 0.019d | 0–130 nN | — | Tribo-pair: Si/SiO2 | 54 |
| 0.464d | ||||||||
| <0.04a,b | 3 N (460 MPa) | v s = 0.002–1.5 cm s−1 | Tribo-pair: Si/Si | |||||
| Grafted from silicon and borosilicate glass by SI-ATRP | 250 | — | 0.08–0.15b | 20 mN | v s = 0.1 cm s−1 | Tribo-pair: Si/glass; measured in dry conditions or with ethanol | 38 | |
| 0.01–0.02b | 20 mN | v s = 0.1 cm s−1 | Tribo-pair: Si/glass; measured with toluene, hexadecane or PF350cSt oil | |||||
| 0.06–0.16b | 20 mN | v s = 0.1–5 cm s−1 | Tribo-pair: Si/glass; measured with a set of oils (more details in Fig. 3) | |||||
| Grafted from Fe(III) oxide and steel by SI-ATRP | 250 | — | <0.02b | 20 mN | v s = 0.1 cm s−1 | Tribo-pair: Fe2O3/steel; measured with hexadecane or EO500 oil | ||
| P18MA | Grafted from silicon by SI-ATRP | 80–200 | — | 0.013d | 0–130 nN | — | Tribo-pair: Si/SiO2 | 54 |
| 0.253d | ||||||||
| Grafted from silicon and borosilicate glass by SI-ATRP | 230 | — | 0.12–0.14b | 20 mN | v s = 0.1 cm s−1 | Tribo-pair: Si/glass; measured under dry conditions or with ethanol | 38 | |
| 0.01–0.05b | 20 mN | v s = 0.1 cm s−1 | Tribo-pair: Si/glass; measured with toluene, hexadecane or PF350cSt oil | |||||
| PS/P2VP mixed binary brushes | Grafted onto silicon | 6–7 | 0.092 | 0.006–0.11c | 0–50 nN | v s = 6–1000 μm s−1 | Tribo-pairs: Si/Si, Si/Si3N4, Si/SiO2; measured under dry conditions; friction was tuned by pretreatment with toluene, ethanol or acidic water | 80 |
| PDMS | Grafted onto silicon | 0.8–13.8 | 0.05–0.79 | 0.0063–0.0405d | 500–3500 nN | v s = 10 μm s−1; dry | Tribo-pair: Si/polyethylene; measured under dry conditions | 52 |
Fluorinated polymer brushes
Fluorinated materials have a set of attractive properties, such as water repellency, chemical and thermal resistance, non-adhesion and low friction.76 These outstanding properties are attributed to the unique properties of the C–F bond, which is characterized by a high binding energy and low polarizability. Low-friction fluoropolymer coatings are usually prepared via chemical vapor deposition, spin coating or spray coatings. Some work has also been done to explore SI-ATRP for the preparation of fluorinated polymer brush-based lubricant layers (Table 5). Bhairamadgi et al. have examined the lubrication properties of poly(2,2,2-trifluoroethylmethacrylate)) (SPF3), 2,2,3,3,4,4,4-heptafluorobutyl methacrylate (SPF7) and poly(2-perfluorooctylethyl methacrylate) (SPF17).76,78 Their coefficient of friction was measured to be 0.004–0.006 in hexadecane and fluorocarbon-based fluids, such as Fluoroinert FC-40.| Polymer | Substrate | d dry [nm] | σ [chains per nm2] | μ | Pressure or normal load | Conditions | Remarks | Ref. |
|---|---|---|---|---|---|---|---|---|
| The coefficient of friction was measured by: a Colloidal probe AFM. | ||||||||
| SPF3 (PTFEMA), SPF7 (PHFMA), SPF17 (PFOEMA) | Grafted from silicon by SI-ATRP | 78–80 | 0.1–0.69 | 0.004–0.006a | 10–95 nN | v s = 5 μm s−1 | Tribo-pair: Si/SiO2; measured under dry conditions | 76 |
| 0.006a | 10–95 nN | v s = 5 μm s−1 | Tribo-pair: Si/SiO2; measured with hexadecane or FC-40 | |||||
| SPF3 (PTFEMA) | Grafted from silicon by SI-ATRP | 10–140 | 0.09–073 | 0.0031–0.0085a | 10–95 nN | v s = 5 μm s−1, dry conditions | Tribo-pair: Si/SiO2; measured under dry conditions; μ decreased with increasing ddry | 78 |
Potential applications
Polymer brush-based boundary lubricants are attractive for prosthetic applications, including replacement of human synovial joints, such as hips or knees.15,23,44,57,67,74 These major joints normally operate under very harsh conditions and are prone to damage when insufficient lubrication is provided.21 Wear of cartilage often leads to serious health problems, such as osteoarthritis, which are difficult to treat. Artificial joint replacement is often the last choice, as it is very costly, but brings great relief from pain.20,23 An alternative treatment can involve injection of biocompatible synthetic lubricants, typically macromolecules or liposomes.21,82–84 One interesting, even more advanced system combines soft PNIPAAm microgels with the PSPMA polymer brush as a boundary lubricant.21 These microgels not only support load by viscoelastic deformations, but can be also used as drug reservoirs. Another potential application of polymer brush lubricants is in contact lenses, where low friction is also a concern.15 Lubricity is also important for biomaterials used for blood pump bearings, endoscope surfaces, and catheters.56Water-based lubrication is also desired in industry, although oils have been extensively used due to their high performance. Nevertheless, moving tribology to aqueous systems brings environmental advantages, i.e. reducing the exposure of organic compounds. Water is also a nice alternative because of its low cost and high heat capacity.59 This is an essential property for cooling and lubricating the tools in metal cutting.60 Other industrial areas with aqueous systems are reservoir pumps and hydraulic liquids in the mining industry, where water is used to exclude flammable materials from underground areas. Also, textile, food and pharmaceutical sectors prefer water-based systems, as they reduce oil contamination in products.60 Some specific applications inherently prevent the use of oil-based lubricants, such as microelectromechanical (MEM) devices developed for some aerospace applications and advanced steam engines.61 It is also important to note that especially for microfluidic MEMs and self-administered syringes the lubrication system must be designed to function on the length scale of these devices.52
The lubricant layer is easiest to maintain between conformal low-modulus elastomeric (LME) contacts, as opposed to non-conformal contacts of hard materials, such as gears or ball bearings.59,60 Low coefficients of friction ca. 0.01 can be readily obtained for LME contacts with water as a lubricant.85–87 For systems with contacts made from hard materials, the water-based lubrication presents more challenges, as a good lubrication performance is usually limited to high-speed conditions. For instance, Hartung et al. observed an increase of friction from ca. 0.05 to 0.2 with a decrease of sliding speed from 100 to 1 mm s−1 for self-mating Si3N4 and SiC in the presence of PLL-g-PEG solution.64 Another interesting aspect to explore is the “self-healing” mechanism that has been used for some of the grafted-onto polymer brushes. This strategy helps to maintain the same performance with wear of the polymer-grafted lubricant.64 Interestingly, polyelectrolyte brush-based boundary lubricants can be used for designing smart surfaces, whose friction can respond to a range of stimuli including pH, salt concentration, counterions or ionic surfactants.18,53
Some approaches focus on polymer brush films intended for oil-based lubrication.54 On the other hand, there have also been some attempts to provide more universal lubrication, working also under dry conditions.52,88 Fluorinated polymer brushes can serve as solid lubricants in micro/nanoelectromechanical systems (MEMs/NEMs), where liquids cannot be introduced.76 These structures may have superior lubrication and stability properties, as compared to widely used polytetrafluoroethylene and their dry performance in MEMs/NEMs has not yet been extensively studied.
Conclusions and outlook
Surface-grafted polymers are an attractive class of boundary lubricants that can operate under both aqueous and non-aqueous conditions. Triggered by the pioneering discoveries by Klein et al., the examples presented in this article powerfully underline the importance of polymer chain conformation on materials properties. More recent work impressively demonstrates how fine-tuning the chemistry of these surface-grafted polymer films can be used to generate robust boundary lubricant films with outstanding friction coefficients. The importance and impact of the collective work discussed here is not only of fundamental but also of great technological relevance. On the one hand, these studies help to shed light on the fundamental aspects that provide the basis for the extraordinary lubrication properties of biological brush-like systems, such as those found in e.g. synovial joints. On the other hand, the work summarized here has also paved the way towards very efficient and highly promising lubrication systems that are of great interest e.g. for prosthetic applications.Acknowledgements
The authors gratefully acknowledge financial support from the Swiss National Science Foundation (SNSF). We thank Prof. Nicholas D. Spencer for providing us with Fig. 3.References
-
E. R. Booser, Handbook of lubrication, Theory and practice of tribology, CRC Press, Boca Raton, FL, 1983, p. 33431 Search PubMed
.
-
Y. Nakanishi, Hydrated Materials: Applications in Biomedicine and the Environment, CRC Press, Boca Raton, FL, 2015, pp. 33487–2742 Search PubMed
.
-
T. Mang and W. Dresel, Lubricants and lubrication, Wiley-VCH, Weinheim, Germany, 2007 Search PubMed
.
- S. Lee, M. Müller, M. Ratoi-Salagean, J. Vörös, S. Pasche, S. M. De Paul, H. A. Spikes, M. Textor and N. D. Spencer, Tribol. Lett., 2003, 15, 231–239 CrossRef CAS
.
- M. Müller, S. Lee, H. A. Spikes and N. D. Spencer, Tribol. Lett., 2003, 15, 395–405 CrossRef
.
- M. T. Müller, X. Yan, S. Lee, S. S. Perry and N. D. Spencer, Macromolecules, 2005, 83, 3861–3866 CrossRef
.
- M. T. Müller, X. Yan, S. Lee, S. S. Perry and N. D. Spencer, Macromolecules, 2005, 38, 5706–5713 CrossRef
.
- S. Lee, M. Müller, R. Heeb, S. Zürcher, S. Tosatti, M. Heinrich, F. Amstad, S. Pechmann and N. D. Spencer, Tribol. Lett., 2006, 24, 217–223 CrossRef CAS
.
- S. Lee and N. D. Spencer, Tribol. Lett., 2005, 38, 922–930 CrossRef CAS
.
- T. Drobek and N. D. Spencer, Langmuir, 2008, 24, 1484–1488 CrossRef CAS PubMed
.
- I. Tzanakis, M. Hadfield, B. Thomas, S. M. Noya, I. Henshaw and S. Austen, Renewable Sustainable Energy Rev., 2012, 16, 4126–4140 CrossRef CAS
.
- IEA Key World Energy Statistics 2013, OECD/IEA International Energy Agency, Paris, 2013 Search PubMed.
- K. Holmberg and A. Erdemir, presented in part at the Serbiatrib '15, 14th International Conference on Tribology, Belgrade, Serbia, 2015 Search PubMed.
- J. Klein, Osteoarthr. Cartil., 2015, 23, A17 CrossRef
.
- J. Seror, L. Zhu, R. Goldberg, A. J. Day and J. Klein, Nat. Commun., 2015, 6, 6497–6503 CrossRef CAS PubMed
.
- G. W. Greene, L. L. Martin, R. F. Tabor, A. Michalczyk, L. M. Ackland and R. Horn, Biomaterials, 2015, 53, 127–136 CrossRef CAS
.
- C. Klein, C. R. Iacovella, C. McCabe and P. T. Cummings, Soft Matter, 2015, 11, 3340–3346 RSC
.
- Q. Wei, M. Cai, F. Zhou and W. Liu, Macromolecules, 2013, 46, 9368–9379 CrossRef CAS
.
- J. Yang, H. Chen, S. Xiao, M. Shen, F. Chen, P. Fan, M. Zhong and J. Zheng, Langmuir, 2015, 31, 9125–9133 CrossRef CAS PubMed
.
- R. Zhang, S. Ma, Q. Wei, Q. Ye, B. Yu, J. van der Gucht and F. Zhou, Macromolecules, 2015, 48, 6186–6196 CrossRef CAS
.
- G. Liu, Z. Liu, N. Li, X. Wang, F. Zhou and W. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 20452–20463 CAS
.
- A. Dėdinaitė, Soft Matter, 2012, 8, 273–284 RSC
.
- J. Klein, Science, 2009, 323, 47–48 CrossRef CAS PubMed
.
- C. P. Neu, K. Komvopoulos and A. H. Reddi, Tissue Eng., Part B, 2008, 14, 235–247 CrossRef CAS PubMed
.
- B. Zhao and W. J. Brittain, Prog. Polym. Sci., 2000, 25, 677–710 CrossRef CAS
.
- J. Pyun, T. Kowalewski and K. Matyjaszewski, Macromol. Rapid Commun., 2003, 24, 1043–1059 CrossRef CAS
.
- S. Edmondson, V. L. Osborne and W. T. Huck, Chem. Soc. Rev., 2004, 33, 14–22 RSC
.
- W. Senaratne, L. Andruzzi and C. K. Ober, Biomacromolecules, 2005, 6, 2427–2448 CrossRef CAS PubMed
.
- P. Jain, G. L. Baker and M. L. Bruening, Annu. Rev. Anal. Chem., 2009, 2, 387–408 CrossRef CAS PubMed
.
- R. Barbey, L. Lavanant, D. Paripovic, N. Schüwer, C. Sugnaux, S. Tugulu and H.-A. Klok, Chem. Rev., 2009, 109, 5437–5527 CrossRef CAS PubMed
.
- O. Azzaroni, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3225–3258 CrossRef CAS
.
- J. Klein, Y. Kamiyama, H. Yoshizawa, J. N. Israelachvili, G. H. Fredrickson, P. Pincus and L. J. Fetters, Macromolecules, 1993, 26, 5552–5560 CrossRef CAS
.
- J. Klein, E. Kumacheva, D. Mahalu, D. Perahla and L. J. Fetters, Nature, 1994, 370, 634–636 CrossRef CAS
.
- J. Klein, Colloids Surf., A, 1994, 86, 63–76 CrossRef
.
- M. Hamel, A. Addali and D. Mba, Lubricants, 2013, 1, 61–74 CrossRef
.
- M. Kobayashi, H. Tanaka, M. Minn, J. Sugimura and A. Takahara, ACS Appl. Mater. Interfaces, 2014, 6, 20365–20371 CAS
.
- A. Nomura, K. Okayasu, K. Ohno, T. Fukuda and Y. Tsujii, Macromolecules, 2011, 44, 5013–5019 CrossRef CAS
.
- R. M. Bielecki, M. Crobu and N. D. Spencer, Tribol. Lett., 2013, 49, 263–272 CrossRef CAS
.
- R. Tadmor, J. Janik, J. Klein and L. J. Fetters, Phys. Rev. Lett., 2003, 91, 115503 CrossRef PubMed
.
- M. Chen, W. H. Briscoe, S. P. Armes and J. Klein, Science, 2009, 323, 1698–1701 CrossRef CAS PubMed
.
- U. Raviv, S. Giasson, N. Kampf, J.-F. Gohy, R. Jérôme and J. Klein, Langmuir, 2008, 24, 8678–8687 CrossRef CAS PubMed
.
- P. Pincus, Macromolecules, 1991, 24, 2912–2919 CrossRef CAS
.
- E. B. Zhulina, O. V. Borisov and T. M. Birshtein, Macromolecules, 1999, 32, 8189–8196 CrossRef CAS
.
- T. Pettersson, A. Naderi, R. Makuška and P. M. Claesson, Langmuir, 2008, 24, 3336–3347 CrossRef CAS PubMed
.
- N. Kampf, U. Raviv and J. Klein, Macromolecules, 2004, 37, 1134–1142 CrossRef CAS
.
- U. Raviv, S. Giasson, N. Kampf, J.-F. Gohy, R. Jérôme and J. Klein, Nature, 2003, 425, 163–165 CrossRef CAS
.
- O. Tairy, N. Kampf, M. J. Driver, S. P. Armes and J. Klein, Macromolecules, 2015, 48, 140–151 CrossRef CAS
.
- L. Ma, A. Gaisinskaya-Kipnis, N. Kampf and J. Klein, Nat. Commun., 2015, 6, 6060–6065 CrossRef CAS
.
- E. Broitman, Friction, 2014, 2, 40–46 CrossRef
.
- S. Bistac and A. Galliano, Tribol. Lett., 2005, 18, 21–25 CrossRef CAS
.
- E. Liu, B. Blanpain, J. P. Celis and J. R. Roos, J. Appl. Phys., 1998, 84, 4859–4865 CrossRef CAS
.
- L. J. Landherr, C. Cohen, P. Agarwal and L. A. Archer, Langmuir, 2011, 27, 9387–9395 CrossRef CAS PubMed
.
- G. Dunér, E. Thormann, O. Ramström and A. Dėdinaitė, J. Dispersion Sci. Technol., 2010, 31, 1285–1287 CrossRef
.
- R. M. Bielecki, E. M. Benetti, D. Kumar and N. D. Spencer, Tribol. Lett., 2012, 45, 477–487 CrossRef CAS
.
- X. Banquy, J. Burdyńska, D. W. Lee, K. Matyjaszewski and J. Israelachvili, J. Am. Chem. Soc., 2014, 136, 6199–6202 CrossRef CAS
.
- K. Kitano, Y. Inoue, R. Matsuno, M. Takai and K. Ishihara, Colloids Surf., B, 2009, 74, 350–357 CrossRef CAS
.
- S. Jahn and J. Klein, Macromolecules, 2015, 48, 5059–5075 CrossRef CAS
.
- W. Hartung, T. Drobek, S. Lee, S. Zürcher and N. D. Spencer, Tribol. Lett., 2008, 31, 119–128 CrossRef CAS
.
- S. Lee, R. Iten, M. Müller and N. D. Spencer, Macromolecules, 2008, 37, 8349–8356 CrossRef
.
- S. Lee and N. D. Spencer, Science, 2008, 319, 575–576 CrossRef CAS PubMed
.
- X. Yan, S. S. Perry, N. D. Spencer, S. Pasche, S. M. De Paul, M. Textor and M. S. Lim, Langmuir, 2004, 20, 423–428 CrossRef CAS PubMed
.
- U. Raviv, J. Frey, R. Sak, P. Laurat, R. Tadmor and J. Klein, Langmuir, 2002, 18, 7482–7495 CrossRef CAS
.
- J. Klein and P. F. Luckham, Macromolecules, 1983, 17, 1041–1048 CrossRef
.
- W. Hartung, A. Rossi, S. Lee and N. D. Spencer, Tribol. Lett., 2009, 34, 201–210 CrossRef CAS
.
- B. Lin, A. K. Tieu, H. Zhu, B. Kosasih, O. Novareza and G. Triani, Wear, 2013, 302, 1010–1016 CrossRef CAS
.
- M. A. Plunkett, A. Feiler and M. W. Rutland, Langmuir, 2003, 19, 4180–4187 CrossRef CAS
.
- M. Kyomoto, T. Moro, K. Saiga, M. Hashimoto, H. Ito, H. Kawaguchi, Y. Takatori and K. Ishihara, Biomaterials, 2012, 33, 4451–4459 CrossRef CAS
.
- W. M. de Vos, P. M. Biesheuvel, A. de Keizer, J. M. Kleijn and M. A. Cohen Stuart, Langmuir, 2009, 25, 9252–9261 CrossRef CAS
.
- R. Heeb, R. M. Bielecki, S. Lee and N. D. Spencer, Macromolecules, 2009, 42, 9124–9132 CrossRef CAS
.
- E. P. K. Currie, A. B. Sieval, M. Avena, H. Zuilhof, E. J. R. Sudhölter and M. A. C. Stuart, Langmuir, 1999, 15, 7116–7118 CrossRef CAS
.
- M. Kobayashi, M. Terada and A. Takahara, Faraday Discuss., 2012, 156, 403–412 RSC
.
- A. Dedinaite, T. Pettersson, B. Mohanty and P. M. Claesson, Soft Matter, 2010, 6, 1520–1526 RSC
.
- M. Kobayashi, Y. Terayama, N. Hosaka, M. Kaido, A. Suzuki, N. Yamada, N. Torikai, K. Ishihara and A. Takahara, Soft Matter, 2007, 3, 740–746 RSC
.
- T. Moro, Y. Takatori, K. Ishihara, T. Konno, Y. Takigawa, T. Matsushita, U.-I. Chung, K. Nakamura and H. Kawaguchi, Nat. Mater., 2004, 3, 829–836 CrossRef CAS PubMed
.
- M. Chen, W. H. Briscoe, S. P. Armes, H. Cohen and J. Klein, Eur. Polym. J., 2011, 47, 511–523 CrossRef CAS
.
- N. S. Bhairamadgi, S. P. Pujari, C. J. van Rijn and H. Zuilhof, Langmuir, 2014, 30, 12532–12540 CrossRef CAS PubMed
.
- M. C. LeMieux, D. Julthongpiput, K. N. Bergman, P. D. Cuong, H.-S. Ahn, Y.-H. Lin and V. V. Tsukruk, Langmuir, 2004, 20, 10046–10054 CrossRef CAS PubMed
.
- N. S. Bhairamadgi, S. P. Pujari, F. A. Leermakers, C. J. van Rijn and H. Zuilhof, Langmuir, 2014, 30, 2068–2076 CrossRef CAS PubMed
.
- H. Sakata, M. Kobayashi, H. Otsuka and A. Takahara, Polym. J., 2005, 37, 767–775 CrossRef CAS
.
- M. Kumar Vyas, K. Schneider, B. Nandan and M. Stamm, Soft Matter, 2008, 4, 1024–1032 RSC
.
- F. T. Limpoco, R. C. Advincula and S. S. Perry, Langmuir, 2007, 23, 12196–12201 CrossRef CAS PubMed
.
- S. Sivan, A. Schroeder, G. Verberne, Y. Merkher, D. Diminsky, A. Priev, A. Maroudas, G. Halperin, D. Nitzan, I. Etsion and Y. Barenholz, Langmuir, 2010, 26, 1107–1116 CrossRef CAS PubMed
.
- G. Verberne, A. Schroeder, G. Halperin, Y. Barenholz and I. Etsion, Wear, 2010, 268, 1037–1042 CrossRef CAS
.
- M. Wathier, B. A. Lakin, P. N. Bansal, S. S. Stoddart, B. D. Snyder and M. W. Grinstaff, J. Am. Chem. Soc., 2013, 135, 4930–4933 CrossRef CAS
.
- D. F. Moore, Wear, 1975, 35, 159–170 CrossRef CAS
.
- J. B. Medley, A. B. Strong, R. M. Pilliar and E. W. Wong, Wear, 1980, 63, 25–40 CrossRef
.
- S. C. Richards and A. D. Roberts, J. Phys. D: Appl. Phys., 1992, 25, A76–A80 CrossRef CAS
.
- Z. Rymuza, Microsyst. Technol., 1999, 5, 173–180 CrossRef
.
- N. Xu, W. Li, M. Zhang, G. Zhao and X. Wang, RSC Adv., 2015, 5, 2427–2448 Search PubMed
.
- S. Chen, L. Li, C. Zhao and J. Zheng, Polymer, 2010, 51, 5283–5293 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |