Chiral and non-chiral assemblies from lipidated serine-based pseudopeptidic molecules†
M. B.
Bijesh
a,
N. U.
Dheepthi
a,
Appa Rao
Sapala
a,
Ashutosh
Shandilya
a,
Kedar
Khare
b and
V.
Haridas
*a
aDepartment of Chemistry, Indian Institute of Technology Delhi, Hauz Khas, New Delhi, 110016, India. E-mail: h_haridas@hotmail.com; haridasv@iitd.ac.in
bDepartment of Physics, Indian Institute of Technology Delhi, India
First published on 12th February 2016
Abstract
A series of lipidated molecules L-A1–L-A5 containing two serine units linked by a variety of aromatic spacers were designed and synthesized. Compound L-A1 containing a m-xylylene unit self-assembled into intertwined twisted-ribbons, while compound L-A2 containing a p-xylylene unit formed nano-sized twisted-ribbons. A racemic mixture of L-A1 and D-A1 showed tapes, while a racemate from L-A2 and D-A2 showed fibres. In contrast, compounds L-A3 and L-A4 containing a benzene-1,3-dicarbonyl spacer showed a fibrillar morphology. Control over self-assembly was achieved by the choice of aromatic spacers and N-terminal substituents. The need for molecular curvature for the formation of twisted ribbons was demonstrated by the inability of control compound L-A5 to form twisted-ribbons.
Introduction
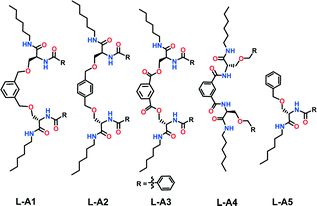
Self-assembly bridges the top–down and bottom–up approaches, hence allows the design of systems that mimic a biological system's complexities and hierarchical order.1 Besides self-assembly, chiralities at the molecular and macroscopic levels are hallmarks of biology.2 Building a defined supramolecular assembly using a designed molecule is a challenging endeavor. Controlling the assembly to a specific order and chirality requires an in depth understanding of non-covalent forces.3 Studies on dynamic non-covalent assemblies provide deep insight into the biology and open up potential avenues to fabricate materials with unique architecture and properties.4 Despite the large number of reports on supramolecular helical structures, a basic design strategy is still lacking.5 Further, the design of supramolecular helices with defined parameters such as helical width and pitch is even more challenging.6Herein we report a design strategy along with the synthesis of a series of serine-based molecules which self-assemble into chiral and non-chiral supramolecular structures. We envisaged that the suitably protected chiral amino acid serine (Ser) linked through the side chain to an aromatic spacer may facilitate self-assembly.7 We chose the amino acid serine due to diverse possibilities for functionalization of its side chain –OH, apart from the N and C-termini. The designed molecules have two serine amino acid units, linked by different aromatic spacers through the side chain –OH of serine. The C-terminus carries an alkyl chain with six carbons, whereas the N-terminus is functionalized with various moieties (Fig. 1 and Fig. S1, ESI†).
Compound L-A1 has two serine amino acids linked by an m-xylylene spacer through the Ser side chain and carries a benzoyl group in its N-terminus. L-A2 is similar to L-A1, except that it contains p-xylylene as a spacer. Replacement of the m-xylylene spacer with p-xylylene is expected to provide a relatively less bent architecture. Compound L-A3 contains a benzene-1,3-dicarbonyl unit as the spacer. The position of amide and ether linkage in L-A1 has been interchanged in compound L-A4. Being an isomer of L-A1, L-A4 acts as an excellent control compound to study the specific involvement of the amide and bis-ether functionalities in self-assembly. Compound L-A5, representing the half of L-A1/L-A2 and having a single serine unit with N-benzoyl and O-benzyl groups, was synthesized to study the role of the bent molecular structure in the assembly. Compounds L-A1–L-A5 were synthesized from the hexylamide derivative of N-Boc-L-serine (Fig. S1, ESI†).
Results and discussion
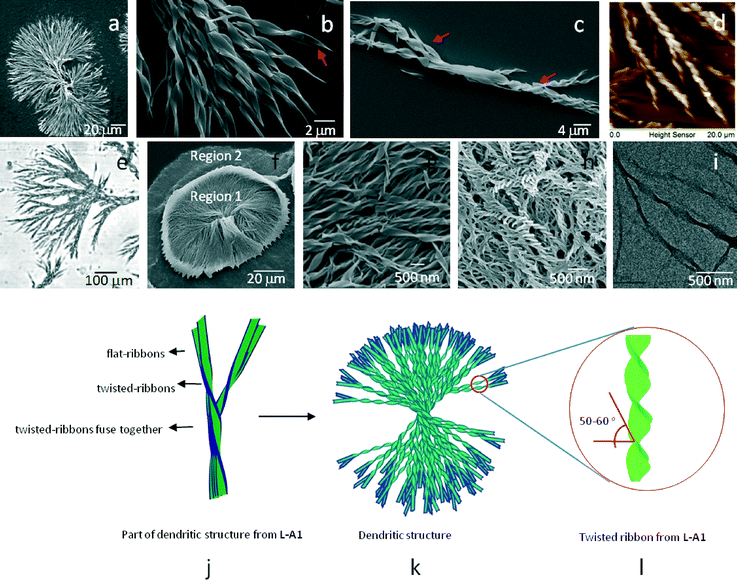
Scanning electron microscopy (SEM) images of the dried samples of L-A1 (1 mM, CH3OH) displayed well-defined discrete dendritic structures (Fig. 2a).8 Closer inspection of the dendritic structures revealed that each of the structures was formed by the intertwining of several left-handed twisted-ribbons (Fig. 2b). Analysis of several dendritic structures showed that twisted-ribbons intertwine, fuse, and then part from each other to form the characteristic dumbbell-like shape. The twisted-ribbons displayed a thickness of ∼0.8–1.5 μm with a pitch measuring ∼4–6 μm and a helix angle of 50–60° (Fig. S2, ESI†). Analysis of several SEM micrographs proved that L-A1 could only form discrete dendritic structures as no individual twisted-ribbons were seen throughout the surface. Apart from the fully grown dendritic structures, we observed a few partially grown dendrites, which also displayed similar organization of twisted-ribbons in specific order (Fig. 2c, Fig. S3, ESI†).Atomic force microscopy (AFM) images of a dendritic structure from L-A1 showed left-handed twisted-ribbons (Fig. 2d and Fig. S4a and b, ESI†). The twisted-ribbon morphology was proved by the zig-zag pattern obtained in the AFM cross sectional analysis (Fig. S4c, ESI†). As expected, D-A1, an enantiomer of L-A1, showed twisted-ribbons with right-handed helical sense (Fig. S5, ESI†), implying that the supramolecular chirality is controlled by the chirality of the amino acid. The optical microscopy analysis of L-A1 or D-A1 revealed the presence of dendritic structures in the peripheral regions of the dried samples (Fig. 2e and Fig. S6a–c, ESI†). Partially grown dendritic structures were also observed as a minor component, indicating that these structures may be the intermediates formed during the formation of the fully-grown dendrites. The twisting in the structure was seen in more detail using a digital holographic microscope (DHM). The phase map shows that the distance between two phase bumps is ∼2 μm (Fig. S6d–f, ESI†).
SEM images of L-A2 (1 mM, CH3OH) showed two distinct regions (Fig. 2f and Fig. S7a, ESI†). Region 1 (Fig. 2g and Fig. S7b, ESI†) is composed of left-handed twisted-ribbons of 100–200 nm thickness (helix angle = 50–60°), and several micrometers in length. Twisted-ribbons were found to be overlapped and arranged to form circular bundles in region 1. Region 2 showed abundance of nano-sized twisted-ribbons of ∼50–100 nm thickness (Fig. 2h and Fig. S7c, ESI†). Important features of these ribbons are their low pitch (∼100–200 nm) and twisting angle (40–45°). Right handed twisted-ribbons were obtained from D-A2, an enantiomer of L-A2 (Fig. S7d and e, ESI†). High resolution transmission electron microscopy (HR-TEM) of L-A2 showed that the twisted-ribbons have a diameter of ∼80–120 nm (Fig. 2i). Interestingly, L-A2 could not form intertwined twisted-ribbons as observed in the case of L-A1. Detailed analysis of the SEM images showed the presence of randomly overlapped twisted-ribbons with different sizes. Twisted-ribbons with a thickness of 50–100 nm were found to be the major self-assembled structure in L-A2.
A closer look at the HR-TEM and AFM images of the periphery of the dendritic structures formed from L-A1 showed the presence of flat-ribbons coiling to form a twisted-ribbon (Fig. S8a and b, ESI†). AFM cross sectional analysis showed that the flat-ribbons have a width of ∼540 nm and a height ∼100 nm, which are lesser than the height of the twisted-ribbons (∼600 nm), implying that these flat-ribbons might be the initially formed structures (height of the ribbon increases while twisting) (Fig. S8c, ESI†). From the above observations, it appears that in the case of L-A1, the flat molecular sheets assemble that induces twist and hence the formation of a twisted-ribbon which finally gives rise to the dendritic structures (Fig. 2j–l). No noticeable changes were observed in the dendritic structures obtained from aged solutions (8 months), indicating the stability of the supramolecular structure (Fig. S9, ESI†).
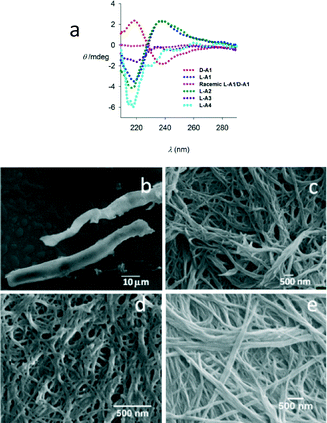
The CD spectrum of L-A1 (Fig. 3a) displayed a bisignate signal with a negative first Cotton effect having a maximum at 238 nm and a minimum at 218 nm with a zero crossing at ∼228 nm, which matches with the absorption maxima (Fig. S10, ESI†) of L-A1 indicating the right handed helicity of the structures. As expected, D-A1 showed opposite CD signals (Fig. 3a). A similar spectrum was also observed for L-A2, indicating a helical structure. In the case of L-A1/L-A2, while the twisted-ribbons observed in the electron microscopy images were left-handed, the CD spectrum indicated a right handed helical conformation. Contradiction of the helical sense obtained from the microscopy data and CD data were also reported by other researchers.9 In many cases, the initially formed helical aggregates of molecules adopt an oppositely biased helical arrangement during the growth of assembly. The temperature dependent CD data (in the range of 5–55 °C) of L-A1 showed no significant changes, indicating the stable nature of the assembly (Fig. S10, ESI†).
The self-assembly pattern of L-A1 is highly solvent dependent (Fig. S11a–h, ESI†). L-A1 in acetonitrile and in ethylacetate showed an urchin-like morphology,10 while fibres were observed in THF. In isopropanol, no specific morphology was observed, emphasizing that a specific polar protic solvent is needed for the formation of twisted-ribbons. The CD spectra of L-A1 in THF, ethylacetate and isopropanol showed non-bisignate CD spectra, which further corroborated the microscopy results (Fig. S12, ESI†). L-A1 forms a dendritic assembly on an aluminium sheet (Fig. S13, ESI†) and a copper grid, indicating that the formation of dendritic structures is independent of the surface used. The powder X-ray diffraction (PXRD) patterns of L-A1 showed the crystalline order as indicated by the well-defined peaks (Fig. S14a, ESI†). The periodic lamellar structure was evident from the occurrence of peaks at d = 2.03 and 0.98 which arises from the consecutive layers of the structure (Fig. S14b, ESI†).11 Concentration-dependent 1H NMR of L-A1 in CDCl3 showed the presence of weak intermolecular hydrogen bonding as indicated by the downfield shift (Δδ = 0.12) of amide NHs upon increasing the concentration from 6.5 mM to 76 mM (Fig. S14c, ESI†). The solution IR spectra in chloroform also proved the presence of hydrogen bonded and non-hydrogen bonded NHs (Fig. S14d, ESI†). Thus NMR spectroscopy, IR spectroscopy, and PXRD prove that the helical structures contain molecules arranged in a sheet-like fashion by virtue of hydrogen bonding and van der Waals interactions between the alkyl chains.
Compounds with different N-terminal substituents ranging from aliphatic to aromatic such as acetyl (L-A7), biphenyl acetyl (L-A8), p-bromo benzoyl (L-A9) and naphthoyl (L-A6) were synthesized (Fig. S1, ESI†) and their morphologies were investigated by SEM to understand the role of the N-terminal protecting group (Fig. S15a–d, ESI†). SEM analysis of these compounds showed that L-A6–L-A9 form nanofibres. The inability of these compounds to form twisted-ribbons indicates that the benzoyl group at the N-terminus is essential for the formation of twisted-ribbons. Kimura et al. have shown that in twisted-ribbons, packing of molecules is weak compared to that in helical ribbons and tubes,12 hence it is reasonable to assume that the N-benzoyl substituent confers an adequate molecular packing of L-A1 necessary for the formation of the twisted-ribbons.
To understand the role of chirality in the assembly, we studied self-assembly of racemic mixtures of L-A1/D-A1 and L-A2/D-A2. SEM of an equimolar mixture of L-A1 and D-A1 showed micrometer sized plates (Fig. 3b). Similarly an equimolar mixture of L-A2 and D-A2 showed a fibrous morphology (Fig. 3c). These morphological differences between enantiopure and racemic compounds indicate a more dense packing between D-A1 and L-A1. The CD spectrum of a racemic mixture of L-A1/D-A1 also supported the non-chiral arrangement (Fig. 3a). The absence of a self-sorted structure in the assembly of a racemic mixture indicates stronger heterochiral association between the molecules.
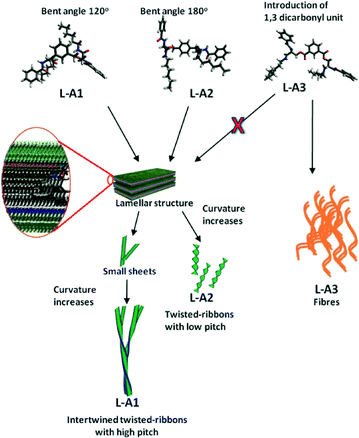
Control compound L-A3, in which the flexible units (–CH2) were replaced by rigid units (carbonyl), showed nanofibres with diameters ranging from 25 to 100 nm (Fig. 3d), hence justifying the crucial role of the flexible ether linkages in the formation of the twisted-ribbons. The introduction of the ester linkage in L-A3 is assumed to provide a relatively flat structure with respect to the spacer unit, hence preferring a fibrillar assembly. This notion was supported by the molecular dynamic (MD) studies on L-A1–L-A3 (Fig. 4). The MD simulated structures of L-A1–L-A3 in methanol was generated using the Amber 12 package. The MD simulated structures showed that L-A3 has a relatively planar structure compared to L-A1 and L-A2. The planar structure of L-A3 may be the reason for the formation of the fibres. The CD spectrum of L-A3 showed only a minimum at ∼217 nm, confirming the non-helical conformation in solution (Fig. 3a). The SEM image of compound L-A4 revealed fibres with a diameter of 25–100 nm (Fig. 3e). These observations underscore the importance of the bis-ether linkage in the formation of the twisted-ribbons. Interestingly the PXRD pattern of fibre forming L-A3 and L-A4 did not show any lamellar structure, indicating the requirement of specific molecular packing for the formation of chiral assembly (Fig. S16, ESI†).
L-A5 was synthesized to study the effect of molecular curvature in self-assembly. SEM images of L-A5 in methanol showed sheets of ∼1 μm width (Fig. S17, ESI†). Surprisingly, L-A5 displayed a highly crystalline structure as indicated by the PXRD pattern (Fig. S16, ESI†). The inability of L-A5 to form a twisted-ribbon morphology is attributed to the lack of molecular curvature, hence favoring the sheets. As the width of the twisted-ribbons is greater than the molecular length of L-A1/L-A2 (∼3 nm obtained from the MD simulated structure), it can be assumed that each twisted-ribbons is composed of multi molecular layers, which are also evident from the observation of the lamellar arrangement of the molecules in the PXRD pattern. The schematic representation (Fig. 4) shows that the twisted-ribbons are composed of a lamellae-like arrangement of molecules of L-A1/L-A2 which induces twist as the assembly grows due to the curvature of the molecules. L-A1 forms more curved lamellar sheets, due to its bent structure (120° with respect to m-xylylene). L-A1 forms bigger twisted-ribbons with high pitch to minimize the curvature energy of the lamellar sheets. The flat lamellar sheets of L-A2 assemble into a twisted-ribbon like morphology without intertwining. Branching and clustering of the twisted helices in L-A1 happens due to the lateral interaction between the twisted-ribbons,4b which finally yields dendritic architectures. The p-xylylene unit in L-A2 provides less curvature to the molecule, hence it is more amenable to stacking. The better stacking ability of L-A2 leads to twisted-ribbons with lower pitch. The rapid gel formation in chloroform further supports the high π–π stacking ability of L-A2. The introduction of carbonyl groups in L-A3 restricts the conformational flexibility of the molecule. Thus, even though L-A3 has a bent structure, the rigidity and the inability to form a lamellar structure induce the molecule to adopt a fibrillar assembly. Similarly L-A4 also assembled into fibres. The formation of fibrous assembly was also observed in our earlier studies of similar compounds.7b
Conclusions
In summary, we demonstrated a simple strategy for the design and synthesis of molecules with unique self-assembly properties. Aromatic spacers play an important role in governing the size and dimensions of twisted-ribbons. The p-xylylene spacer-based compound L-A2 formed well-defined discrete twisted-ribbons, while m-xylylene-based L-A1 self-assembled into dendritic structures composed of intertwined twisted-ribbons. Introduction of a benzene-1,3-dicarbonyl spacer in L-A3–LA4 resulted in non-chiral assembly. The studies presented here show that the chirality of the amino acid was the principal factor governing the supramolecular chirality, but the width and the pitch of the self-assembled structures are majorly determined by the spacer units. Apart from these findings, our studies highlight the influence of solvents and the N-terminal substituent on self-assembly.Acknowledgements
We acknowledge the Department of Science and Technology (DST) of India for funding. We thank Prof. A. Ramanan for the discussion regarding the PXRD data. We thank the Nano Research Facility (NRF), IITD for AFM and AIIMS, New Delhi for HR-TEM measurements. We acknowledge the Department of Textile Technology, IITD for SEM measurements and the Kusuma School of Biological Sciences, IITD for CD measurements. BMB thanks the University Grants Commission, India for JRF.Notes and references
- (a) T. Kunitake, Angew. Chem., Int. Ed. Engl., 1992, 31, 709 CrossRef; (b) D. Philp and J. F. Stoddart, Angew. Chem., Int. Ed. Engl., 1996, 35, 1154 CrossRef; (c) A. Petitjean, H. Nierengarten, A. van Dorsselaer and J.-M. Lehn, Angew. Chem., Int. Ed., 2004, 43, 3695 CrossRef CAS PubMed; (d) A. M. Kushner and Z. Guan, Angew. Chem., Int. Ed., 2011, 50, 9026 CrossRef CAS PubMed; (e) M. Liu, L. Zhang and T. Wang, Chem. Rev., 2015, 115, 7304 CrossRef CAS PubMed; (f) I. Alfonso, Chem. Commun., 2016, 52, 239 RSC.
- (a) R. E. Franklin and A. Klug, Acta Crystallogr., 1955, 8, 777 CrossRef CAS; (b) R. E. Franklin, Nature, 1955, 175, 379 CrossRef CAS PubMed; (c) D. Eyre, Science, 1980, 207, 1315 CrossRef CAS PubMed; (d) N. Rubin, E. Perugia, M. Goldschmidt, M. Fridkin and L. Addadi, J. Am. Chem. Soc., 2008, 130, 4602 CrossRef CAS PubMed; (e) J. Adamcik, J.-M. Jung, J. Flakowski, P. De Los Rios, G. Dietler and R. Mezzenga, Nat. Nanotechnol., 2010, 5, 423 CrossRef CAS PubMed.
- (a) J. H. K. K. Hirschberg, L. Brunsveld, A. Ramzi, J. A. J. M. Vekemans, R. P. Sijbesma and E. W. Meijer, Nature, 2000, 407, 167 CrossRef CAS PubMed; (b) A. Aggeli, I. A. Nyrkova, M. Bell, R. Harding, L. Carrick, T. C. B. McLeish, A. N. Semenov and N. Boden, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 11857 CrossRef CAS PubMed; (c) H. Nakade, B. J. Jordan, H. Xu, G. Han, S. Srivastava, R. R. Arvizo, G. Cooke and V. M. Rotello, J. Am. Chem. Soc., 2006, 128, 14924 CrossRef CAS PubMed; (d) P. A. Korevaar, C. Schaefer, T. F. A. de Greef and E. W. Meijer, J. Am. Chem. Soc., 2012, 134, 13482 CrossRef CAS PubMed; (e) Q. Li, C. Han, S. R. Horton, M. Fuentes-Cabrera, B. G. Sumpter, W. Lu, J. Bernholc, P. Maksymovych and M. Pan, ACS Nano, 2012, 6, 566 CrossRef CAS PubMed; (f) J. T. Simmons, Z. Yuan, K. L. Daykin, B. T. Nguyen, R. J. Clark, M. Shatruk and L. Zhu, Supramol. Chem., 2014, 26, 214 CrossRef CAS; (g) Y.-N. Li, L.-H. Huo, X. Zou, Z.-P. Deng, Z.-B. Zhu and S. Gao, Tetrahedron, 2015, 71, 8746 CrossRef CAS; (h) Y.-Z. Liu, K. Yuan, L.-L. Lv, Y.-C. Zhu and Z. Yuan, J. Phys. Chem. A, 2015, 119, 5842 CrossRef CAS PubMed.
- (a) N. Nakashima, S. Asakuma and T. Kunitake, J. Am. Chem. Soc., 1985, 107, 509 CrossRef CAS; (b) H. Engelkamp, S. Middelbeek and R. J. M. Nolte, Science, 1999, 284, 785 CrossRef CAS PubMed; (c) J. H. Jung, G. John, K. Yoshida and T. Shimizu, J. Am. Chem. Soc., 2002, 124, 10674 CrossRef CAS PubMed; (d) R. C. Claussen, B. M. Rabatic and S. I. Stupp, J. Am. Chem. Soc., 2003, 125, 12680 CrossRef CAS PubMed; (e) S. R. Diegelmann, J. M. Gorham and J. D. Tovar, J. Am. Chem. Soc., 2008, 130, 13840 CrossRef CAS PubMed; (f) J.-K. Kim, E. Lee, M.-C. Kim, E. Sim and M. Lee, J. Am. Chem. Soc., 2009, 131, 17768 CrossRef CAS PubMed; (g) X. Zhu, P. Duan, L. Zhang and M. Liu, Chem. – Eur. J., 2011, 17, 3429 CrossRef CAS PubMed; (h) L. Ziserman, H.-Y. Lee, S. R. Raghavan, A. Mor and D. Danino, J. Am. Chem. Soc., 2011, 133, 2511 CrossRef CAS PubMed; (i) C. Tomasini and N. Castellucci, Chem. Soc. Rev., 2013, 42, 156 RSC; (j) Y. Wang, W. Qi, R. Huang, X. Yang, M. Wang, R. Su and Z. He, J. Am. Chem. Soc., 2015, 137, 7869 CrossRef CAS PubMed; (k) M. Yamauchi, T. Ohba, T. Karatsu and S. Yagai, Nat. Commun., 2015, 6, 8936 CrossRef CAS PubMed.
- (a) A. E. Rowan and R. J. M. Nolte, Angew. Chem., Int. Ed., 1998, 37, 63 CrossRef CAS; (b) C. Schmuck, Angew. Chem., Int. Ed., 2003, 42, 2448 CrossRef CAS PubMed; (c) M. V. Escarcega-Bobadilla and A. W. Kleij, Chem. Sci., 2012, 3, 2421 RSC.
- (a) R. Oda, I. Huc, M. Schmutz, S. J. Candau and F. C. MacKintosh, Nature, 1999, 399, 566 CrossRef CAS PubMed; (b) C. C. Lee, C. Grenier, E. W. Meijer and A. P. H. J. Schenning, Chem. Soc. Rev., 2009, 38, 671 RSC; (c) Y. Wang, J. Xu, Y. Wang and H. Chen, Chem. Soc. Rev., 2013, 42, 2930 RSC; (d) C. Liu, Q. Jin, K. Lv, L. Zhang and M. Liu, Chem. Commun., 2014, 50, 3702 RSC.
- (a) D. Ranganathan, V. Haridas, R. Gilardi and I. L. Karle, J. Am. Chem. Soc., 1998, 120, 10793 CrossRef CAS; (b) V. Haridas, M. B. Bijesh, A. Chandra and S. Sharma, Chem. Commun., 2014, 50, 13797 RSC.
- (a) D. Chen, J. E. Maclennan, R. Shao, D. K. Yoon, H. Wang, E. Korblova, D. M. Walba, M. A. Glaser and N. A. Clark, J. Am. Chem. Soc., 2011, 133, 12656 CrossRef CAS PubMed; (b) H. Cao, Q. Yuan, X. Zhu, Y.-P. Zhao and M. Liu, Langmuir, 2012, 28, 15410 CrossRef CAS PubMed; (c) G. Qing, X. Shan, W. Chen, Z. Lv, P. Xiong and T. Sun, Angew. Chem., Int. Ed., 2014, 53, 2124 CrossRef CAS PubMed; (d) A. Fernández-Mato, M. Sánchez-Andújar, B. Pato-Doldán, M. A. Señarís-Rodríguez, C. Platas-Iglesias, D. Tordera, H. J. Bolink, J. M. Quintela, C. Peinador and M. D. García, Cryst. Growth Des., 2014, 14, 3849 CrossRef.
- (a) J. Bae, J.-H. Choi, Y.-S. Yoo, N.-K. Oh, B.-S. Kim and M. Lee, J. Am. Chem. Soc., 2005, 127, 9668 CrossRef CAS PubMed; (b) A. Lohr, M. Lysetska and F. Würthner, Angew. Chem., Int. Ed., 2005, 44, 5071 CrossRef CAS PubMed; (c) A. Ajayaghosh, C. Vijayakumar, R. Varghese and S. J. George, Angew. Chem., Int. Ed., 2006, 45, 456 CrossRef CAS PubMed.
- S. Naik and V. Haridas, RSC Adv., 2013, 3, 21356 RSC.
- (a) J. Cui, A. Liu, Y. Guan, J. Zheng, Z. Shen and X. Wan, Langmuir, 2010, 26, 3615 CrossRef CAS PubMed; (b) D. Xu, Y. Ma, Z. Jing, L. Han, B. Singh, J. Feng, X. Shen, F. Cao, P. Oleynikov, H. Sun, O. Terasaki and S. Che, Nat. Commun., 2014, 5, 4262 CAS.
- A. Uesaka, M. Ueda, A. Makino, T. Imai, J. Sugiyama and S. Kimura, Langmuir, 2014, 30, 1022 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, NMR, mass spectra, and microscopy images. See DOI: 10.1039/c6me00009f |
| This journal is © The Royal Society of Chemistry 2016 |