A diet enriched with Mugil cephalus processed roes modulates the tissue lipid profile in healthy rats: a biochemical and chemometric assessment
A.
Rosa
*a,
A.
Atzeri
a,
D.
Putzu
a and
P.
Scano
b
aDepartment of Biomedical Sciences, University of Cagliari, Cittadella Universitaria, Km 4.5 SS 554, 09042 Monserrato, CA, Italy. E-mail: anrosa@unica.it; Fax: +39 070 6754032; Tel: +39 070 6754124
bDepartment of Chemical and Geological Sciences, University of Cagliari, Cittadella Universitaria, SS 554, Km 4.5, 09042 Monserrato, Cagliari, Italy
First published on 13th November 2015
Abstract
The effect of a diet enriched with mullet bottarga on the lipid profile (total lipids, total cholesterol, unsaturated fatty acids, α-tocopherol, and hydroperoxides) of plasma, liver, kidney, brain, and perirenal adipose tissues of healthy rats was investigated. Rats fed a 10% bottarga enriched-diet for 5 days showed body weights and tissue total lipid and cholesterol levels similar to those of animals fed control diet. Univariate and multivariate results showed that bottarga enriched-diet modified the fatty acid profile in all tissues, except brain. Significant increases of n-3 PUFA, particularly EPA, were observed together with a 20:4 n-6 decrease in plasma, liver, and kidney. Perirenal adipose tissue showed a fat accumulation that reflected the diet composition. The overall data suggest that mullet bottarga may be considered as a natural bioavailable source of n-3 PUFA and qualify it as a traditional food product with functional properties and a potential functional ingredient for preparation of n-3 PUFA enriched foods.
Introduction
A number of epidemiological, clinical, and experimental studies indicate the pleiotropic effects of long chain omega-3 (or n-3) polyunsaturated fatty acids (n-3 PUFA), in particular, eicosapentaenoic acid (EPA, 20:5 n-3) and docosahexaenoic acid (DHA, 22:6 n-3), in cardiovascular disease prevention, tumor growth and metastasis decrease, reduction of the risk of insulin resistance, and prevention of age-related cognitive decline.1–4 Humans, like most animals, have a very limited ability to synthesize these fatty acids from the essential precursor α-linoleic acid (18:3 n-3), thus the dietary intake of these functional constituents is a key aspect of human nutrition.1,3,5,6 The current recommended intake is 250 mg per day EPA + DHA for the general population, with an additional 100–200 mg DHA per day for pregnant women (European Food Safety Authority (EFSA)).7 The main dietary sources of these n-3 PUFA are fish and fish oils.5,6,8 Marine oils are obtained from the flesh of fatty fish (sardines, mackerel, herring, tuna, and salmon), liver of white lean fish (cod and halibut) and blubber of marine mammals (seals and whales).8,9 Individuals who do not eat fish or fish oils (e.g. vegans and non-fish-eating vegetarians, and meat-eaters) could be at risk of a low or inadequate n-3 PUFA status.5 Therefore, the use of nutraceutical and functional foods containing n-3 PUFA has become a topic of great interest for the health of the world population.9,10 Nowadays, there has been an increased production in Europe and USA of functional food products enriched with n-3 PUFA (such as milk and its derivatives, eggs, juices, bread and bakery products etc.) and, in most cases, fish oil is the main natural source of these beneficial health components.8 In view of all these factors and since the supply of wild fish is under threat and supplies are compromised, there is an obvious need for an alternative, sustainable source of high quality EPA and DHA.6Eggs of aquatic organisms, usually referred to as “roes”, are highly valued gourmet products, consumed raw or as salted, smoked, boiled or canned products.11 Fish roe products contain a significant amount of lipids having high levels of long chain n-3 PUFA (30–50% of total fatty acids).12,13 Salted–dried fish roes are one of the most popular forms of roe products in many countries. Among these, the salted and semi-dried mullet (Mugil genus) ovary product is a food delicacy produced in several countries, with the name of bottarga in Italy,14,15 avgotaracho in Greece,16 and karasumi in Japan.12 In recent studies we have determined the lipid composition,14,17,18 oxidative stability,18,19 browning processes,20 water-soluble low molecular weight metabolite profile,21,22 and biological activity19,21 of bottarga (as whole and grated products) manufactured in Sardinia (Italy), that has a long tradition in making high quality bottarga. The total lipid content of bottarga samples is estimated to be in the range 220–325 mg per g of edible portion and its lipid fraction is characterized by a high portion of wax esters (ca. 50–65% of lipid classes), triacylglycerols, phospholipids, and cholesterol.14,18 Mullet bottarga represents an important natural and stable source of health beneficial EPA and DHA, which amount to 10–13 mg per g and 20–33.5 mg per g of edible portion, respectively (13–25% of the total fatty acids).14,18,19 Furthermore, the salted and dried mullet roe is a source of high quality proteins (35–45% of fresh weight), and the moisture and ash contents of this product are 25 and 3% of fresh weight, respectively.16 Preliminary results have also shown the ability of bottarga lipids to reduce viability in colon adenocarcinoma cells and to induce a significant modification of the fatty acid composition in normal and cancer colon cells with a selective increase in n-3 PUFA levels, indicating the cellular bioavailability of these bioactive food components.19,21 The bioavailability of n-3 PUFA may be influenced by the lipid structures in which they are incorporated (free fatty acids bound in ethyl esters, triacylglycerols, or phospholipids), and by the food matrix.23,24 Several studies have reported the in vivo bioavailability (in humans or animal models) of n-3 PUFA from fish oils, algal oils, enriched foods, and fish roe oils.23–27 Only a few studies have been conducted to assess the bioavailability of n-3 PUFA in the form of synthetic wax esters or from wax ester-rich marine oil.28,29
Broadly, the present research aims to investigate the effect of a diet enriched with mullet bottarga (10%), a food product commonly consumed in the Mediterranean region, on the lipid profile of plasma, liver, kidney, brain, and perirenal adipose tissue of healthy rats, in order to evidence the short-term (5 days) in vivo bioavailability of n-3 PUFA when given in the form of wax ester-rich marine food and to assess the extent to which DHA and EPA are absorbed into the studied tissues. Results of this study will rely not only on statistical significance based on univariate tests but also on a multivariate approach aimed to show the modification of the overall fatty acid profile in the studied tissues.
2. Materials and methods
2.1. Materials
Standards of fatty acids and fatty acid methyl esters, cholesterol, triolein, trilinolein, Desferal (deferoxamine mesylate salt), and all solvents used, of the highest available purity, were purchased from Sigma-Aldrich (Milan, Italy). Methanolic HCl (3 N) was purchased from Supelco (Bellefonte, PA). cis,trans-13-Hydroperoxyoctadecadienoic acid (c,t-13-HPODE) and cis,trans-9-hydroperoxyoctadecadienoic acid (c,t-9-HPODE) were obtained from Cascade (Cascade Biochem Ltd, London, UK). All of the other chemicals used in this study were of analytical grade. A grated bottarga sample of mullet was kindly supplied by the company “Stefano Rocca s.r.l.” located in Sardinia (Quartucciu, Italy); ingredients reported on the label were mullet roe and salt.2.2. Animals and diets
All animal procedures were performed in accordance with the European Communities Council Directive of 22 September 2010 (2010/63/EU) for animal care, including adequate measures to minimize pain or discomfort. Adult male Wistar rats were purchased from Charles River Italy (Calco, Italy). The rats (initial body weight, 110 to 130 g) were housed in solid bottom polycarbonate cages with wire tops in a room maintained at 22 ± 2 °C and had free access to tap water and food, with a 12 h dark/12 h light photoperiod regime. Rats were divided into two experimental groups with comparable mean body weights. One group (n = 10) received a control rat chow diet GLP-4RF25 (rat food pellets; standard diet, Mucedola, Milan, Italy; containing 3.5% fat, w/w) (Ctrl diet) for 11 days, another group (n = 8) received (from day 6 to 11) a diet supplemented with 10% mullet bottarga (Diet Bott 10%) for 5 days. This amount of bottarga has been chosen in order to increase the level of n-3 fatty acids (EPA + DHA + 22:5 n-3) 6 times in the experimental diet (0.5%, w/w) with respect to Ctrl diet (0.084%). To prepare the experimental diet, GLP-4RF25 pellets were ground into a homogeneous powder (900 g), which was then mixed with the commercial grated mullet bottarga (100 g) and distilled water (50 mL). Addition of bottarga was compensated for by the removal of 10 g GLP-4RF25 per 100 g diet. This mixture was thoroughly blended and then formed into approximately 8 × 16 mm flat rectangles and air dried for 6 h at room temperature to make the experimental diet into pellets. Experimental diet was mixed weekly, vacuum-sealed, and stored at 4 °C. The body weight of the rats was monitored during the study period.2.3. Tissues and blood samples
Animals were deeply anaesthetized and heparinized blood, liver, kidney, brain, and perirenal adipose (PA) tissue were collected. The blood was immediately centrifuged at 4 °C for 10 min at 500g to obtain plasma. Tissues were snap-frozen in liquid nitrogen, and stored at −80 °C until further analysis. The total time from tissue harvest to freezing was less than 5 min.2.4. Lipid extraction and saponification
Total lipids (TL) were extracted from portions of grated mulled bottarga (60 mg), Ctrl diet (100 mg), Diet Bott 10% (100 mg), liver (500 mg), kidney (500 mg), brain (500 mg), PA tissue (30 mg) and from an aliquot (0.5 ml) of plasma by the Folch procedure,30 using 12 mL of the mixture, CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH 2
MeOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v). After addition of 4 mL H2O and centrifugation at 900g for 1 h, the CHCl3 fraction was separated from the MeOH/H2O mixture. Total lipids in the CHCl3 fraction were quantified by the method of Chiang.31 The dried CHCl3 fractions, containing the lipids, from each sample were dried down and dissolved in EtOH. Separation of lipid components (total cholesterol, α-tocopherol, and fatty acids) was carried out by mild saponification as previously reported.21 The unsaponifiable (total cholesterol and α-tocopherol) and saponifiable (fatty acids and conjugated diene fatty acid hydroperoxides (HP)) fractions were collected, the solvent was evaporated, and a portion of the dried residues, dissolved in MeOH and CH3CN with 0.14% CH3COOH (v/v), respectively, was injected into a high-performance liquid chromatograph (HPLC).21 The recovery of fatty acids and cholesterol during the saponification was calculated using an external standard mixture prepared by dissolving 1 mg of triolein, trilinolein, and cholesterol in EtOH and processed as samples. All solvent evaporation was performed under vacuum. An aliquot of dried fatty acids from saponification was methylated21,32 with 1 mL of methanolic HCl (3 N) for 30 min at room temperature. After the addition of n-hexane and H2O, samples were centrifuged for 20 min at 900g. The hexane phase with fatty acid methyl esters was collected and aliquots of the samples were injected into the GC system.
1 (v/v). After addition of 4 mL H2O and centrifugation at 900g for 1 h, the CHCl3 fraction was separated from the MeOH/H2O mixture. Total lipids in the CHCl3 fraction were quantified by the method of Chiang.31 The dried CHCl3 fractions, containing the lipids, from each sample were dried down and dissolved in EtOH. Separation of lipid components (total cholesterol, α-tocopherol, and fatty acids) was carried out by mild saponification as previously reported.21 The unsaponifiable (total cholesterol and α-tocopherol) and saponifiable (fatty acids and conjugated diene fatty acid hydroperoxides (HP)) fractions were collected, the solvent was evaporated, and a portion of the dried residues, dissolved in MeOH and CH3CN with 0.14% CH3COOH (v/v), respectively, was injected into a high-performance liquid chromatograph (HPLC).21 The recovery of fatty acids and cholesterol during the saponification was calculated using an external standard mixture prepared by dissolving 1 mg of triolein, trilinolein, and cholesterol in EtOH and processed as samples. All solvent evaporation was performed under vacuum. An aliquot of dried fatty acids from saponification was methylated21,32 with 1 mL of methanolic HCl (3 N) for 30 min at room temperature. After the addition of n-hexane and H2O, samples were centrifuged for 20 min at 900g. The hexane phase with fatty acid methyl esters was collected and aliquots of the samples were injected into the GC system.
2.5. HPLC analyses
Analyses of α-tocopherol, total cholesterol, unsaturated fatty acids, and oxidative products were carried out with an Agilent Technologies 1100 liquid chromatograph (Agilent Technologies, Palo Alto, CA) equipped with a diode array detector (DAD) (Agilent Technologies). Cholesterol and α-tocopherol, detected at 203 nm and 292 nm, respectively, were measured with the use of an Inertsil ODS-2 column, 150 × 4.6 mm, 5 μm particle size (Superchrom, Milan, Italy), and MeOH as the mobile phase, at a flow rate of 0.7 mL min−1. Analyses of unsaturated fatty acids (detected at 200 nm) and HP (detected at 234 nm), were carried out with an XDB-C18 Eclipse (150 mm × 4.6 mm, 3.5 μm particle size) (Agilent Technologies) equipped with a Zorbax XDB-C18 Eclipse (12.5 mm × 4.6 mm, 5 μm particle size) guard column (Agilent Technologies), with a mobile phase of CH3CN/H2O/CH3COOH (75/25/0.12, v/v/v), at a flow rate of 2.3 mL min−1.21 The temperature of the column was maintained at 37 °C. The identification of fatty acids and HP was made using standard compounds and the second derivative and conventional UV spectra, generated with the Agilent OpenLAB Chromatography Data System. Calibration curves of all of the compounds were constructed using standards and were found to be linear, with correlation coefficients >0.995.2.6. GC analyses
Fatty acid methyl esters were analysed on a gas chromatograph Hewlett-Packard HP-6890 (Hewlett-Packard, Palo Alto, CA) with a flame ionization detector and equipped with a cyanopropyl methylpolysiloxane HP-23 FAME column (30 m × 0.32 mm × 0.25 μm) (Hewlett-Packard). Nitrogen was used as a carrier gas at a flow rate of 2 mL min−1; the oven temperature was set at 175 °C; the injector temperature was set at 250 °C; the detector temperature was set at 300 °C. The fatty acid methyl esters were identified by comparing the retention times to those of standard compounds. The relative composition of individual fatty acids was calculated as a percentage of the total amount of fatty acids (g%), using Hewlett-Packard A.05.02 software.2.7. Statistical analyses
Graph Pad INSTAT software (GraphPad software, San Diego, CA) was used to calculate the means and standard deviations. Evaluation of the statistical significance of differences between the two groups was performed using Student's unpaired t-test with Welch's correction.2.8. Chemometrics
The HPLC fatty acid profiles were subjected to multivariate statistical data analysis (MVA). For each analyzed tissue, a matrix was built, where rows are the samples and columns are the fatty acid concentrations expressed as a percentage of the total amount of fatty acids (g%) for each sample. When MVA was carried out for all tissues at once, due to the different total fatty acid concentrations, to make samples comparable the HPLC data were normalized to 100 row-wise. Data were mean centered and unit scaled column-wise. The MVA tools employed here were: (a) PCA for sample distribution overview; (b) pair wise PLS-DA and its OPLS-DA variant to stress differences among the Ctrl diet and Diet Bott 10% sample groups. Internal cross-validation was used. PLS-DA's R2Y and Q2Y parameters indicate the classification power of models, and of models in cross-validation, respectively. The used visualization tools were: (a) the PLS-DA and OPLS-DA score plots; in the latter, the interclass variability is expressed in the first predictive component reported in the x-axis, while intra-class variability is expressed in the component orthogonal to the first (y-axis); (b) the PLS-DA loading plot and the OPLS-DA column loading plot. In this latter tool, the vertical axis reports the loading values in the predictive component, and error bars indicate the error, for each variable, in cross-validation.333. Results
3.1. Lipid composition of mullet bottarga and diets
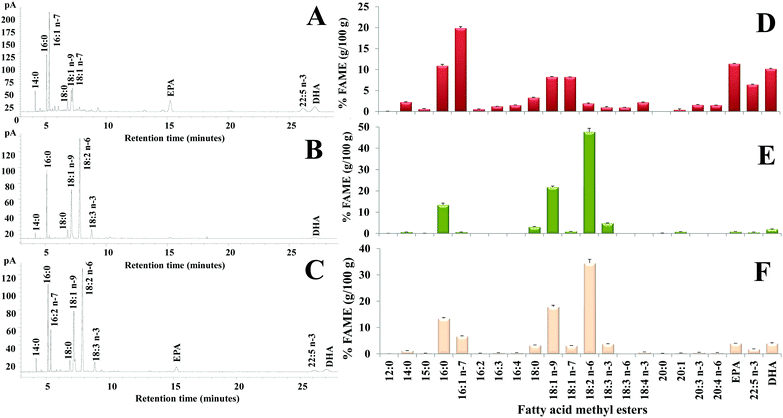
The lipid fraction was extracted from the grated mullet bottarga sample used for diet supplementation (total lipids, TL, 380.0 ± 2.7 mg per g of the edible portion) and fatty acids, hydroperoxides (HP), total cholesterol, and α-tocopherol concentrations were measured. Fig. 1 shows the chromatographic profile (Fig. 1A) and the fatty acid composition (expressed as % of the total fatty acids) (Fig. 1D) of the bottarga sample by GC. Bottarga showed a concentration of approximately 17% of saturated fatty acids (SFA) (mainly palmitic acid 16:0 and stearic acid 18:0), 37% of monounsaturated (MUFA) (mainly palmitoleic acid 16:1 n-7, oleic acid 18:1 n-9, and cis-vaccenic acid 18:1 n-7), and 40% of polyunsaturated (PUFA) (mainly EPA and DHA). By HPLC, the levels of total cholesterol, α-tocopherol, EPA, DHA, 22:5 n-3, and HP in the bottarga sample were detected as follows: 7.39 ± 0.69 mg g−1, 67.13 ± 3.50 μg g−1, 20.03 ± 0.35 mg g−1, 20.74 ± 0.17 mg g−1, 11.62 ± 0.48 mg g−1, and 0.64 ± 0.10 μmoles per g of the edible portion.Then, the lipid fraction was analyzed in Ctrl diet and diet supplemented with 10% bottarga. The diets were characterized by comparable values of TL (40.46 and 32.34 mg g−1 in Ctrl diet and Diet Bott 10%, respectively), α-tocopherol (approximately 38 μg g−1), and oxidative products (approximately 2 μmoles per g of HP), while experimental diet contained a higher amount of total cholesterol (0.83 mg g−1) than Ctrl diet (0.13 mg g−1), as reported in Table 1. Quali–quantitative information on the individual fatty acids that compose the diet lipids was obtained by GC and HPLC-DAD analyses. Fig. 1 shows the fatty acid chromatographic profile and composition (expressed as % of the total fatty acids) of Ctrl diet (Fig. 1B and E) and Diet Bott 10% (Fig. 1C and F) by GC. The Ctrl diet lipids showed a relative concentration of approximately 18% of SFA (mainly 16:0 and 18:0, 13 and 3%, respectively), 24% of MUFA (mainly 18:1 n-9, 22%), and 56% of PUFA, mainly constituted by linoleic acid 18:2 n-6 and linolenic acid 18:3 n-3, 48 and 5%, respectively, with low amounts of the EPA and DHA (0.9 and 2%, respectively). The bottarga supplementation increased the MUFA amount (28%, especially 16:1 n-7) and reduced the total PUFA level (51%) in the diet, even though the levels of EPA (4%) and DHA (4%) increased. The absolute levels of the main unsaturated fatty acids determined by HPLC for lipids in both diets are reported in Table 2. The amounts of EPA and DHA in Ctrl diet were detected as 0.25 ± 0.12 mg g−1 and 0.59 ± 0.03 mg g−1, respectively, while values of 1.85 ± 0.10 mg g−1 and 2.15 ± 0.11 mg g−1 were measured in Diet Bott 10%.
| Treatment | TL (mg g−1) | TC (mg g−1) | α-Toc (μg g−1) | HP (μmoles per g) |
|---|---|---|---|---|
| Ctrl diet | 40.36 ± 1.25 | 0.13 ± 0.01 | 38.10 ± 2.65 | 2.50 ± 0.78 |
| Diet Bott 10% | 32.34 ± 1.35 | 0.83 ± 0.06 | 38.12 ± 1.99 | 1.70 ± 0.08 |
| Fatty acid | Ctrl diet (mg g−1) | Diet Bott 10% (mg g−1) |
|---|---|---|
| 16:1 n-7 | 0.15 ± 0.02 | 2.43 ± 0.17 |
| 16:2 | — | 0.11 ± 0.01 |
| 16:3 | — | 0.08 ± 0.00 |
| 16:4 | — | 0.04 ± 0.01 |
| 18:1 n-7 + 18:1 n-9 | 7.30 ± 0.39 | 8.77 ± 0.23 |
| 18:2 n-6 | 15.22 ± 0.92 | 14.09 ± 0.13 |
| 18:3 n-3 | 1.76 ± 0.08 | 1.91 ± 0.07 |
| 18:3 n-6 | 0.01 ± 0.00 | 0.09 ± 0.01 |
| 18:4 n-3 | 0.03 ± 0.00 | 0.20 ± 0.01 |
| 20:3 n-3 + 20:3 n-6 | 0.04 ± 0.01 | 0.15 ± 0.03 |
| 20:4 n-6 | 0.05 ± 0.00 | 0.24 ± 0.02 |
| 20:5 n-3 | 0.25 ± 0.12 | 1.85 ± 0.10 |
| 22:5 n-3 | — | 1.10 ± 0.21 |
| 22:6 n-3 | 0.59 ± 0.03 | 2.15 ± 0.11 |
3.2. Animal weights
After 11 days, the animals increased their weight by approximately 1.5 times and there were no statistically significant differences in the growth performance between different treatments, the final average body weight of groups being 184 ± 10 g and 191 ± 12 g, for Ctrl diet and Diet Bott 10% groups, respectively.3.3. Total lipid, total cholesterol, and α-tocopherol composition of tissues
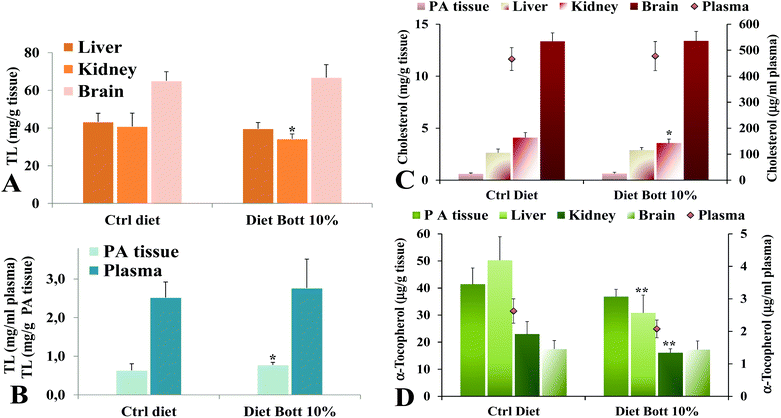
Lipid components were extracted from rat tissues and the levels of TL, total cholesterol, and the liposoluble antioxidant α-tocopherol were analyzed. Fig. 2 shows the values of TL measured in liver, kidney, brain (Fig. 2A), plasma, and PA tissue (Fig. 2B) of rats alimented with Ctrl diet and Diet Bott 10%. No statistically significant differences were observed in the TL amount in plasma, liver, and brain of the two groups, nevertheless the Diet Bott 10% group showed a lower and higher TL level (P < 0.05) in kidney and PA tissue, respectively, compared with the Ctrl group. Remarkably, feeding of the diet containing 10% bottarga for the period of 5 days did not result in an increase of total cholesterol levels in plasma, liver, brain, and PA tissue of the experimental rat group, while a lower cholesterol level was measured in the kidney of the Diet Bott 10% group (P < 0.05) compared to the Ctrl group (Fig. 2C). α-Tocopherol levels (Fig. 2D) were significantly lower in the liver and kidney of the group fed the Diet Bott 10% compared to the Ctrl group (P < 0.01). Decreased α-tocopherol levels were also observed in plasma and PA tissue of rats alimented with Diet Bott 10%, although this reduction was not statistically significant; no difference was observed between the groups for brain α-tocopherol levels.3.4. Fatty acid profile of tissues
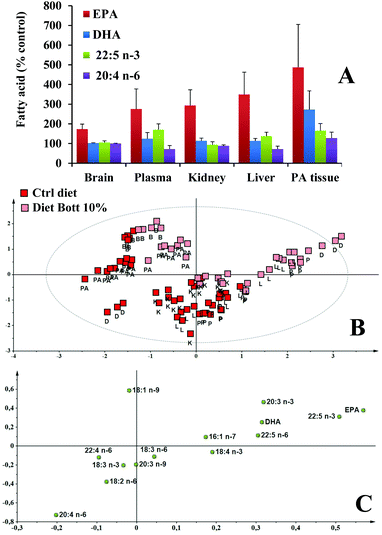
The fatty acid profiles and HP concentration were then analyzed in rat tissues. Dietary fatty acids altered the tissue fatty acid composition and the main differences between the two groups were observed for the unsaturated fatty acids. The absolute amount of unsaturated fatty acids measured by HPLC-DAD in tissues of rats alimented with Ctrl diet and Diet Bott 10% are reported in Fig. 3A–D for plasma, liver, kidney, and PA tissue, respectively. The main unsaturated fatty acids in plasma, liver, and kidney of control rats were 18:2 n-6, 20:4 n-6, 18:1 n-9, and DHA. The plasma fatty acid profile (Fig. 3A) of the Diet Bott 10% group showed significantly greater levels of the n-3 PUFA: EPA and 22:5 n-3 (P < 0.001), as compared to the Ctrl diet group; an evident, but not significant increase was also observed for DHA. No significant differences were observed in the plasma level of 16:1 n-7, 18:1 n-9, and 18:2 n-6 between groups, nevertheless, the Diet Bott 10% group showed a lower level of 20:4 n-6 (P < 0.01) than the Ctrl group. A similar modification in the unsaturated fatty acid profile was observed in the livers (Fig. 3B) of the Diet Bott 10% group, with a significant increase in the levels of EPA, 22:5 n-3 (P < 0.001), and DHA (P < 0.05), accompanied by a marked reduction (28%) of the 20:4 n-6 amount, with respect to the Ctrl diet group. An increased level of 16:1 n-7 was found in the liver of rats fed with the experimental diet, although no effect of the diet was observed for 18:1 n-9 and 18:2 n-6. EPA (P < 0.001) and DHA (P < 0.05) significantly increased in the kidney (Fig. 3C) of the Diet Bott 10% group, and 20:4 n-6 was also decreased but to a lesser extent than in the plasma and liver. An increasing significant level of all measured n-3 PUFA was shown in PA tissue (Fig. 3D) of the Diet Bott 10% group with respect to control animals. The levels of unsaturated fatty acids in the brain (data not shown) of rats fed the Diet Bott 10% were generally identical to those of the control group, except for EPA that significantly increased (from 3.58 ± 0.27 to 6.19 ± 0.93 μg per g brain, P < 0.01).Fig. 4A shows the values of EPA, DHA, 22:5 n-3, and 20:4 n-6 (expressed as % Ctrl group values) measured in the brain, plasma, kidney, liver, and PA tissue of rats alimented with Diet Bott 10%. Bottarga supplementation induced a marked increase of the EPA content in all the analyzed tissues, in the order: PA tissue > liver > kidney > plasma > brain. Also the level of 22:5 n-3 and DHA increased in different tissues, except in the brain, though to a lesser extent than EPA. Under our experimental conditions, no significant differences were observed in the levels of the oxidative products, HP, between Ctrl and Diet Bott 10% groups in all the studied tissues (data not shown).
3.5. Chemometrics
Following a holistic approach to highlight differences in the HPLC fatty acid profile between the 2 diets, multivariate statistical analyses were performed. At first, an overview of sample distribution was obtained by performing a PCA for each studied tissue, as a result, neither deviating features nor outliers were observed (data not shown). Consequently, for each tissue, a pair-wise OPLS-DA of the Ctrl diet group vs. the Diet Bott 10% group was performed. Sample distribution in the predictive and orthogonal components is depicted as a score scatter plot, in Fig. 5A–D for plasma, liver, kidney, and PA tissue, respectively. Here, we can see that Diet Bott 10% and Ctrl diet samples lie in different areas, along the predictive component (x-axis) of the plots. Scattering of samples along the orthogonal component (y-axis) indicated intra-class variability. The R2Y and Q2Y values of the pair-wise OPLS-DA models are reported in the caption of the corresponding figures. Higher values of both parameters indicate that a better separation between the 2 groups exists. These parameters confirmed that diet enriched with bottarga modified the overall fatty acid profile in all the studied tissues. The higher Q2Y values for the liver and kidney suggest that in these latter tissues modifications are more consistent. For each tissue, the most discriminant variables, i.e. those fatty acids that mostly changed between the Diet Bott 10% group and the Ctrl diet group, were reported as the loading column plot in Fig. 5A–D for plasma, liver, kidney, and PA tissue, respectively. Here we can see that generally EPA and 22:5 n-3 were the fatty acids that increased with bottarga diet, and 20:4 n-6 and 22:4 n-6 were those that decreased. In the kidney only EPA shows an increase (Fig. 5C). In the PA tissue model (Fig. 5D) it is clearly visible that all the fatty acids increased with bottarga enriched diet, with EPA and DHA being the most affected. In this regard, it is worth reporting the Pearson's correlation coefficients. They were r = −0.66, −0.87, −0.70, for EPA vs. 20:4 n-6, in plasma, liver, and kidney respectively; while, for all these latter tissues, no correlations between DHA and 20:4 n-6 were observed (r values between −0.16 and −0.35). In PA tissue, r = 0.84 for EPA vs. 20:4 n-6 and r = 0.82 for DHA vs. 20:4 n-6, were calculated.A snapshot of lipid profiles of all tissues and of diets, at once, is given in Fig. 4B and C. Here, the score plot (Fig. 4B) and loading (Fig. 4C) plots of the PLS-DA of HPLC fatty acid normalized data are reported. In the score we can see that along a diagonal that passes through the center, control diet and enriched diet samples are in the opposite part, and those samples of plasma and liver of rats fed enriched-diet are closer to the enriched-diet samples. In the corresponding multivariate space in the loading plot EPA and 20:4 n-6 are in the opposite corners. As to the brain and PA tissue, samples are pulled far apart from others by a relatively higher concentration of 18:1 n-9.
4. Discussion
In the Mediterranean countries, bottarga, the salted and semi-dried product of mullet roe, is considered a food delicacy, commonly eaten grated with spaghetti or cut into thin slices with extra virgin olive oil. It has been proposed as a rich source of health beneficial n-3 PUFA, containing high amounts of EPA and DHA.14,18,19 Moreover, bottarga may be regarded as a natural marine source of n-3 PUFA more stable than other sources like fish oils and their ethyl ester derivatives due to the fact that significant amounts of n-3 PUFA in mullet roe are wax ester components,14 and it was reported that wax esters enriched in n-3 fatty acids have a low degree of susceptibility to oxidation.18,20,28 Similar to other nutrients, the bioavailability of n-3 PUFA is highly variable and determined by numerous factors.23 In particular, the bioactivity of n-3 PUFA may be influenced by the lipid structures in which they are incorporated: phospholipids and free fatty acids have increased bioavailability compared to triacylglycerols and ethyl ester forms of n-3 PUFA.25,27 Previous studies have demonstrated the in vivo bioavailability of n-3 PUFA when given to animals in the form of synthetic wax esters28 or wax ester-rich oil extracted from the marine copepod, Calanus finmarchicus.29There is an increased demand for new bioavailable sources of high quality n-3 PUFA for human consumption, and mullet bottarga is a traditional promising product in this regard. We studied, for the first time, the effect of a diet enriched with 10% mullet processed roes on the lipid profile of healthy rat tissues. Short-term treatment (5 days) has been chosen in order to avoid potential adverse effects of this wax ester-rich food in rats, like gastrointestinal disturbances.34 Feces of animals fed bottarga enriched diet appeared normal by visual inspection during all days of treatment, as previously observed for mice fed with wax ester-rich oil from C. finmarchicus.29
The results of this study evidenced the modulating effects of bottarga lipids on the rat tissue lipid profile and the tissue bioavailability of mullet roe n-3 PUFA. The analysis of fatty acid composition of bottarga lipids demonstrated a high content of EPA (20 mg per g of edible portion), 22:5 n-3 (12 mg g−1), and DHA (21 mg g−1), resembling the fatty acid composition of grated bottarga published earlier.14,18,19 The diet supplemented with 10% bottarga was characterized by comparable values of TL, α-tocopherol, and HP, with a higher amount of total cholesterol, 16:1 n-7, EPA (2 mg g−1), 22:5 n-3 (1 mg g−1) and DHA (2 mg g−1) than Ctrl diet. Diet Bott 10% did not affect the body weight and similar TL levels were measured in plasma, liver, kidney, and brain of animals fed experimental diet, with a slight fat deposition in PA tissue. It is worth noting that bottarga diet, in spite of the fact that it contained a total cholesterol amount 6-fold greater than Ctrl diet, did not increase the cholesterol levels in all the studied rat tissues, probably due to the n-3 PUFA-induced down-regulation of transporter proteins that regulated intestinal cholesterol.35 A little increase in the total cholesterol level was observed in plasma lipids of mouse fed, for 1 week, with a diet rich in herring roe lipids (HR-L) (containing a high amount of phospholipids rich in EPA and DHA), although HR-L contained 9% cholesterol.13 The lower α-tocopherol levels measured in tissues of rats fed the Diet Bott 10% compared to Ctrl animals could be explained by the possible consumption of this liposoluble antioxidant for the protection of PUFA, which were more abundant in the experimental diet, from oxidative degradation. Moreover, the marked reduction in α-tocopherol levels observed in liver and kidney with respect to plasma and PA tissue could be due to a tissue-specific metabolism of this liposoluble compounds.
Diet Bott 10% significantly influenced the unsaturated fatty acid profile of the analysed tissues. Results of the multivariate approach highlighted that the fatty acid profile of tissue samples upon diet enriched with bottarga is significantly different from that of the Ctrl samples, with more robust discrimination for liver and kidney tissues when compared to plasma and PA tissue. By univariate tests, increased levels of EPA, 22:5 n-3, and DHA were detected in all tissues, except the brain, in which only EPA increased in a significant manner. The brain seems particularly resistant to changes in the fatty acid profile of dietary fat, with the duration of exposure and dietary concentration being key factors to detect significant alterations.36 The percentage of the EPA increase was much higher than that of DHA in all the examined tissues of rats fed Diet Bott 10%, despite a similar quantity in the experimental diet. This suggests that EPA was more efficiently absorbed or less excreted than DHA. Studies have shown that the DHA level in lipid pools has a less steep dose–response curve than EPA, which is easy to influence by supplementation.27 It is interesting to note that the amount of 20:4 n-6 significantly decreased in the plasma, liver, and kidney of the bottarga group. A recent study showed an n-6 PUFA decrease (8.2%) concomitant with the increase of EPA and DHA in the plasma lipids of young adults after two week supplementation with herring roe oil.27 A statistically significant reduction of 20:4 n-6 has also been observed in plasma phospholipids of rats fed for 4 weeks a diet enriched with synthetic wax esters.28 EPA and DHA are incorporated into the phospholipids of cell membranes in a dose-dependent way depending on the EPA and DHA intake, and their incorporation partly competes with the incorporation of 20:4 n-6 and EPA also competes with 20:4 n-6 regarding enzymes in the eicosanoid metabolism.37 The anti-inflammatory activity of long-chain n-3 PUFA has been attributed to the decrease of the production of inflammatory eicosanoids, cytokines, and the expression of adhesion molecules, and these beneficial health compounds act both directly (e.g., by replacing arachidonic acid as an eicosanoid substrate and inhibiting 20:4 n-6 metabolism) and indirectly (e.g., by altering the expression of inflammatory genes through effects on transcription factor activation) or give rise to a family of anti-inflammatory mediators termed resolvins.38 The hypothesis that can be stressed by these results, i.e. a diet rich in n-3 PUFA could move the enzymatic balance towards the n-3 route, is strengthened by the analysis of the correlation coefficients and of the OPLS-DA loadings that clearly indicated that the observed increase of n-3 PUFA (mainly EPA and 22:5 n-3) in samples with enriched diet is correlated to a decrease of n-6 PUFA. Exception to this observation is the PA tissue where all fatty acids increased EPA and 22:5 n-3 to a major extent, suggesting a mere passive storage of fatty acids in this tissue. An increase of 20:4 n-6 (not statistically significant), coupled to a significant increase of 16:1 n-7, EPA, 22:5 n-3, and DHA, was observed in PA tissue, due to their higher amounts in Diet Bott 10% than in Ctrl diet. The fatty acid composition of the PA tissue reflected to a large extent, the composition of the diet, as previously observed.29
This study clearly demonstrated that the enrichment of a typical rat chow diet with grated mullet bottarga can induce marked changes into the lipid profile of rat tissues and their enrichment with n-3 PUFA. A previous study evidenced that wax ester-derived n-3 PUFA are absorbed well in vivo in an animal model but less than fish oils.29 However, further studies are needed to compare the bioavailability/tissue incorporation of n-3 PUFA by dietary enrichment in the form of bottarga wax esters with that induced by a similar enrichment with fish oils.
5. Conclusions
The overall data suggest that mullet bottarga may be considered as a bioavailable source of n-3 PUFA. Bottarga was able to increase n-3 PUFA levels in rat plasma, liver, and kidney, significantly reducing the 20:4 n-6 tissue level, with potential positive health implication. The waxy nature of bottarga lipids confers oxidative stability to this Mediterranean product. Therefore, mullet bottarga qualifies as a traditional food product with functional properties and as a traditional ingredient for the preparation of n-3 PUFA enriched foods, like mozzarella, pasta and vegetables (artichokes, fennel) with grated bottarga.Acknowledgements
We thank the company, “Stefano Rocca s.r.l.”, for sample supplies.References
- B. M. Anderson and D. W. Ma, Are all n-3 polyunsaturated fatty acids created equal?, Lipids Health Dis., 2009, 8, 33 CrossRef PubMed.
- U. Gogus and C. Smith, n-3 Omega fatty acids: a review of current knowledge, Int. J. Food Sci. Technol., 2010, 45, 417–436 CrossRef CAS.
- G. Calviello, H. M. Su, K. H. Weylandt, E. Fasano, S. Serini and A. Cittadini, Experimental evidence of ω-3 polyunsaturated fatty acid modulation of inflammatory cytokines and bioactive lipid mediators: their potential role in inflammatory, neurodegenerative, and neoplastic diseases, Biomed. Res. Int., 2013, 2013, 743171 Search PubMed.
- D. Cutuli, P. De Bartolo, P. Caporali, D. Laricchiuta, F. Foti, M. Ronci, C. Rossi, C. Neri, G. Spalletta, C. Caltagirone, S. Farioli-Vecchioli and L. Petrosini, n-3 Polyunsaturated fatty acids supplementation enhances hippocampal functionality in aged mice, Front. Aging Neurosci., 2014, 6, 220 Search PubMed.
- A. A. Welch, S. Shakya-Shrestha, M. A. Lentjes, N. J. Wareham and K. T. Khaw, Dietary intake and status of n-3 polyunsaturated fatty acids in a population of fish-eating and non-fish-eating meat-eaters, vegetarians, and vegans and the product-precursor ratio [corrected] of α-linolenic acid to long-chain n-3 polyunsaturated fatty acids: results from the EPIC-Norfolk cohort, Am. J. Clin. Nutr., 2010, 92, 1040–1051 CrossRef CAS PubMed.
- M. Venegas-Calerón, O. Sayanova and J. A. Napier, An alternative to fish oils: metabolic engineering of oil-seed crops to produce omega-3 long chain polyunsaturated fatty acids, Prog. Lipid Res., 2010, 49, 108–119 CrossRef PubMed.
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA), Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol, EFSA J., 2010, 8, 1461 Search PubMed , http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/1461.pdf.
- N. Rubio-Rodríguez, S. Beltrán, I. Jaime, S. M. de Diego, M. T. Sanz and J. R. Carballido, Production of omega-3 polyunsaturated fatty acid concentrates: A review, Innovative Food Sci. Emerging Technol., 2010, 11, 1–12 CrossRef.
- F. Shahidi, Nutraceuticals, functional foods and dietary supplements in health and disease, J. Food Drug Anal., 2012, 20(S1), 226–230 CAS.
- E. Campioli, C. Rustichelli and R. Avallone, n-3 Dietary supplementation and lipid metabolism: difference between vegetable and fish-derived oils, J. Funct. Foods, 2012, 4, 207–212 CrossRef CAS.
- N. Kalogeropoulos, A. Mikellidi, T. Nomikos and A. Chiou, Screening of macro- and bioactive microconstituents of commercial finfish and sea urchin eggs, LWT – Food Sci. Technol., 2012, 46, 525–531 CrossRef CAS.
- G. E. Bledsoe, C. D. Bledsoe and B. Rasco, Caviars and fish roe products, Crit. Rev. Food Sci. Nutr., 2003, 43, 317–356 CrossRef CAS PubMed.
- H. Moriya, M. Hosokawa and K. Miyashita, Combination effect of herring roe lipids and proteins on plasma lipids and abdominal fat weight of mouse, J. Food Sci., 2007, 72, C231–C234 CrossRef CAS PubMed.
- P. Scano, A. Rosa, F. Cesare Marincola, E. Locci, M. P. Melis, M. A. Dessì and A. Lai, 13C NMR, GC and HPLC characterization of lipid components of the salted and dried mullet (Mugil cephalus) roe “bottarga”, Chem. Phys. Lipids, 2008, 151, 69–76 CrossRef CAS PubMed.
- R. Bernasconi, E. Bolzacchini, G. Galliani, F. Gugliersi, B. Rindone, M. Rindone, M. T. Tacconi and A. Terraneo, Determination of the content of wax esters in some sea food and their molecular composition. A comparison with ω-3 enriched wax esters, LWT – Food Sci. Technol., 2007, 40, 569–573 CrossRef CAS.
- N. Kalogeropoulos, T. Nomikos, A. Chiou, E. Fragopoulou and S. Antonopoulou, Chemical composition of greek avgotaracho prepared from mullet (Mugil cephalus): nutritional and health benefits, J. Agric. Food Chem., 2008, 56, 5916–5925 CrossRef CAS PubMed.
- P. Scano, A. Rosa, E. Locci, M. A. Dessì and A. Lai, NMR study of the lipid profile of mullet raw roe and bottarga, Eur. J. Lipid Sci. Technol., 2009, 111, 505–512 CrossRef CAS.
- A. Rosa, P. Scano, M. P. Melis, M. Deiana, A. Atzeri and M. A. Dessì, Oxidative stability of lipid components of mullet (Mugil cephalus) roe and its product “bottarga”, Food Chem., 2009, 115, 891–896 CrossRef CAS.
- A. Rosa, A. Atzeri, M. Deiana, M. P. Melis, D. Loru, A. Incani, B. Cabboi and M. A. Dessì, Effect of aqueous and lipophilic mullet (Mugil cephalus) bottarga extracts on the growth and lipid profile of intestinal Caco-2 cells, J. Agric. Food Chem., 2011, 59, 1658–1666 CrossRef CAS PubMed.
- A. Rosa, P. Scano, A. Atzeri, M. Deiana, S. Mereu and M. A. Dessì, Effect of storage conditions on lipid components and color of Mugil cephalus processed roes, J. Food Sci., 2012, 77(1), 107–114 CrossRef PubMed.
- A. Rosa, P. Scano, A. Atzeri, M. Deiana and A. M. Falchi, Potential anti-tumor effects of Mugil cephalus processed roe extracts on colon cancer cells, Food Chem. Toxicol., 2013, 60, 471–478 CrossRef CAS PubMed.
- P. Scano, A. Rosa, E. Locci, G. Manzo and M. A. Dessì, Modifications of the 1H NMR metabolite profile of processed mullet (Mugil cephalus) roes under different storage conditions, Magn. Reson. Chem., 2012, 50, 436–442 CrossRef CAS PubMed.
- J. P. Schuchardt and A. Hahn, Bioavailability of long-chain omega-3 fatty acids, Prostaglandins Leukotrienes. Essent. Fatty Acids, 2013, 89, 1–8 CrossRef CAS PubMed.
- L. B. Schram, C. J. Nielsen, T. Porsgaard, N. S. Nielsen, R. Holm and H. Mu, Food matrices affect the bioavailability of (n-3) polyunsaturated fatty acids in a single meal study in humans, Food Res. Int., 2007, 40, 1062–1068 CrossRef CAS.
- L. Burri, N. Hoem, S. Banni and K. Berge, Marine omega-3 phospholipids: metabolism and biological activities, Int. J. Mol. Sci., 2012, 13, 15401–15419 CrossRef CAS PubMed.
- K. Lane, E. Derbyshire, W. Li and C. Brennan, Bioavailability and potential uses of vegetarian sources of omega-3 fatty acids: a review of the literature, Crit. Rev. Food Sci. Nutr., 2014, 54, 572–579 CrossRef CAS PubMed.
- B. Bjørndal, E. Strand, J. Gjerde, P. Bohov, A. Svardal, B. W. Diehl, S. M. Innis, A. Berger and R. K. Berge, Phospholipids from herring roe improve plasma lipids and glucose tolerance in healthy, young adults, Lipids Health Dis., 2014, 13, 82 CrossRef PubMed.
- F. Gorreta, R. Bernasconi, G. Galliani, M. Salmona, M. T. Tacconi and R. Bianchi, Wax esters of n-3 polyunsaturated fatty acids: a new stable formulation as a potential food supplement. 1 − Digestion and absorption in rats, LWT – Food Sci. Technol., 2002, 35, 458–465 CrossRef CAS.
- A. M. Pedersen, W. Salma, A. C. Höper, T. S. Larsen and R. L. Olsen, Lipid profile of mice fed a high-fat diet supplemented with a wax ester-rich marine oil, Eur. J. Lipid Sci. Technol., 2014, 116, 1718–1726 CrossRef CAS.
- J. Folch, M. Lees and G. H. Sloane-Stanley, A simple method for the isolation and purification of total lipid from animals tissues, J. Biol. Chem., 1957, 226, 497–509 CAS.
- S. D. Chiang, C. F. Gessert and O. H. Lowry, Colorimetric determination of extracted lipids. An adaptation for microgram amounts of lipids obtained from cerumen, Curr. List Med. Lit. Res. Rep., 1957, 33, 56–113 Search PubMed.
- W. W. Christie, Preparation of ester derivatives of fatty acids for chromatographic analysis, in Advantage in Lipid Methodology – Two, ed. W. W. Christie, The Oily Press, Dundee, Scotland, 1993, pp. 69–111 Search PubMed.
- L. Eriksson, E. Johansson, N. Kettaneh-Wold, J. Trygg, C. Wikstrom and S. Wold, Multi-and megavariate data analysis, Umetrics Academy, Sweden, 3rd edn, 2013 Search PubMed.
- K. H. Ling, P. D. Nichols and P. P. But, Fish-induced keriorrhea, Adv. Food Nutr. Res., 2009, 57, 1–52 CAS.
- A. Alvaro, R. Rosales, L. Masana and J.-C. Vallvé, Polyunsaturated fatty acids down-regulate in vitro expression of the key intestinal cholesterol absorption protein NPC1L1: no effect of monounsaturated nor saturated fatty acids, J. Nutr. Biochem., 2010, 21, 518–525 CrossRef CAS PubMed.
- V. Di Marzo, M. Griinari, G. Carta, E. Murru, A. Ligresti, L. Cordeddu, E. Giordano, T. Bisogno, M. Collu, B. Batetta, S. Uda, K. Berge and S. Banni, Dietary krill oil increases docosahexaenoic acid and reduces 2-arachidonoylglycerol but not N-acylethanolamine levels in the brain of obese Zucker rats, Int. Dairy J., 2010, 20, 231–235 CrossRef CAS.
- T. Maaløe, E. B. Schmidt, M. Svensson, I. V. Aardestrup and J. H. Christensen, The effect of n-3 polyunsaturated fatty acids on leukotriene B4 and leukotriene B5 production from stimulated neutrophil granulocytes in patients with chronic kidney disease, Prostaglandins Leukotrienes. Essent. Fatty Acids, 2011, 85, 37–41 CrossRef PubMed.
- P. C. Calder, n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases, Am. J. Clin. Nutr., 2006, 83(Suppl. 6), 1505S–1519S CAS.
| This journal is © The Royal Society of Chemistry 2016 |