The isoflavone content of two new alfalfa-derived products for instant beverage preparation
M. Guadalupe
Soto-Zarazúa
ab,
Francisca
Rodrigues
a,
Filipa B.
Pimentel
a,
M. M.
Bah
b and
M. Beatriz P. P.
Oliveira
*a
aREQUIMTE, Department of Chemical Sciences, Faculty of Pharmacy, University of Porto, Rua de Jorge Viterbo Ferreira, 228, 4050-313 Porto, Portugal. E-mail: beatoliv@ff.up.pt; Fax: +351 226 093 390; Tel: +351 220 428 640
bFacultad de Química, Universidad Autónoma de Querétaro, Centro Universitario, Cerro de las Campanas, C.P. 76010, Querétaro, Qro., México
First published on 26th October 2015
Abstract
The frequent use of plant-based products to promote health leads to the search for scientific information related to efficacy and safety of those products for human consumption. Two alfalfa-derived products (ADP), freeze-dried juice (FDJ) and dehydrated powder (DP), from alfalfa harvested in Mexico, are being developed as new possible nutraceuticals. To the best of our knowledge, any study reports the real composition of such products used to prepare instant beverages in what concerns isoflavone contents. Seven isoflavones (glycitein, formononetin, biochanin A, daidzein, genistein, daidzin and genistin) were assessed by HPLC-DAD analysis as well as its variation in five different batches of these products. Different solvents were tested in order to choose the best one to extract isoflavones. The results showed the presence of daidzein, genistein, genistin and daidzin in most samples while glycitein, formononetin and biochanin A were not detected. Significant differences between isoflavone contents were found with different solvent systems. Water was the best option to extract daidzein (0.40–1.08 mg per unit and 1.30–4.90 mg per unit for DP and FDJ, respectively) whereas the water–methanol–formic acid mixture was efficient to extract genistein (0.19–0.43 mg per unit and 0.15–0.72 mg per unit for DP and FDJ, respectively). In all cases, the total isoflavone content was higher in freeze-dried juices than in dehydrated powders. Genistein and daidzein were the more abundant isoflavones quantified. Further physiological and nutritional studies are needed to complete the validation of effectiveness and safety of these products.
1. Introduction
Isoflavones are among the most common and well known types of phytoestrogens.1 Since these compounds are structurally similar to estradiol, they have the ability to bind to estrogen receptors and mimic the endogenous estrogens’ actions.2 Consequently, several effects related to alleviation of menopause symptoms have been associated with phytoestrogens’ intake.3 During menopause, the decrease of physiological estrogen production can promote the development of different symptoms related to cardiovascular diseases or osteoporosis. These dysfunctions are frequently prevented with the use of Hormone Replacement Therapy (HRT). Nevertheless, some studies have correlated HRT with an increased risk of breast cancer development. Another approach of different researchers suggests its replacement by natural alternatives.4–6 Based on this fact, the use of products containing isoflavones is growing, mainly by menopausal women who prefer natural alternatives to avoid the referred symptoms. Positive and negative effects of phytoestrogens’ consumption have been reported, depending on the amounts consumed.7Within the main natural sources of phytoestrogens, soybeans and soy products are the most common, being extensively studied.8,9 Other sources, such as red clover (Trifolium pratenses L.) or alfalfa (Medicago sativa L.), containing phytoestrogens, mainly isoflavone aglycones (formononetin, biochanin A, genistein and daidzein) as well as glycosides (daidzin and genistin) have been described.10,11 Particularly, alfalfa has been used as feed and recently extended to human consumption. Besides its nutritional value, alfalfa contains a large number of phenolic compounds,12 being a promising raw material for food supplements or ingredients.
Currently, Mexican local companies are developing, using natural resources, new functional foods aiming to prevent and combat some of the most prevalent diseases in the country. However, scientific studies are needed to ensure the effectiveness and safety of these products and to guarantee beneficial properties to the consumer.
Two alfalfa-derived products (ADP), freeze-dried juice (FDJ) and dehydrated powder (DP), are being developed with alfalfa locally harvested. These products are trying to be launched as a new shelf raw material for instant beverage preparation. Both products are recommended to be reconstituted in water or juice, or to be a base for smoothies or other daily beverages. Despite their intended use as nutraceuticals, the evaluation of their nutritional composition and possible pharmacological effects has not been done. As alfalfa is considered an important source of phytoestrogens, the quantification of isoflavones is of utmost importance to consider these ADP as a good source of phytoestrogens.
The alfalfa phytoestrogen content may vary with different factors. Some studies revealed changes in its content according to harvesting time and origin.13–15 Among the most used methods for isoflavone analysis, HPLC-DAD has been the first choice due to its characteristics, easy access and reproducibility.16 Nevertheless, the choice of the best extractor solvent is a critical step as isoflavone aglycones (free form) are moderately polar but glycosides increased these properties.17 Also, co-solubility and the abundance of compounds may influence the optimal extraction. Based on this, organic solvents, such as methanol or ethanol, are frequently used to extract phenolic compounds.18,19 Water could be added in different proportions in order to generate a system which is able to extract both isoflavone forms, aglycones and glycosides. Some additional treatments like ultra-sound irradiation showed significant difference in the isoflavone amount, particularly glycoside extraction yields, due to cavitation, that increases the plant tissues permeability.20,21 The aim of this study was to quantify the isoflavone amounts present in two Mexican ADP obtained with alfalfa harvested at five different periods. The influence of different solvents on isoflavone extraction was also studied.
2. Materials and methods
2.1 Chemicals and reagents
Daidzin (≥95%), genistin (≥95%), glycitein (≥97%), daidzein (≥98%), genistein (≥98%), biochanin A (≥97%), formononetin (≥99%) and the internal standard (IS) 2-methoxyflavone were purchased from Sigma-Aldrich (St. Louis, MO, USA).Methanol, formic acid and acetonitrile (all of HPLC grade) were from Merck (Darmstadt, Germany). Purified water was obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA). Water was purified with a “Seradest LFM 20” system (Seral, Ransbach-Baumbach, Germany).
2.2 Standards
Isoflavone standard solutions (1 g L−1) were prepared in water/methanol (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) and then serially diluted to obtain the concentrations for the calibration curves. An internal standard (IS) solution (5 mg mL−1) was also prepared in the same solvent. All the solutions were stored in amber glass vials at −20 °C.
90) and then serially diluted to obtain the concentrations for the calibration curves. An internal standard (IS) solution (5 mg mL−1) was also prepared in the same solvent. All the solutions were stored in amber glass vials at −20 °C.
2.3 Samples
Samples of the two ADP obtained from five different batches (MS-1, MS-2, MS-3, MS-4 and MS-5) were kindly provided by Productora Sativa SPR de RL de CV (Querétaro, México). These batches are constituted by the aerial parts of alfalfa harvested in five months (May, July, August, September and October, respectively) during 2014 in a farm located in Queretaro, Mexico.The plant material was cleaned and washed before being extruded to obtain the juice and the residual material. The juice was freeze-dried to give the FDJ and the residual material was dried at room temperature under ventilation and milled, and protected from light, to give the DP.
2.4 Sample extraction
The extraction procedure for isoflavone determination was carried out using five different solvent systems: water (100%); methanol (100%); methanol–water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v); ethanol (100%) and methanol–water–formic acid (80
50 v/v); ethanol (100%) and methanol–water–formic acid (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v). A sample of 50 mg of FDJ or DP was mixed with 75 μL of IS at 0.1 mg mL−1 and adjusted to 1 mL with the different solvents. The suspension formed was shaken in a Vortex for one minute and subjected to ultra-sound irradiation for 10 minutes at room temperature. Then, samples were centrifuged at 5000 rpm for 10 minutes and the supernatant was recovered, filtered and stored at −20 °C in amber glass vials until analysis by HPLC-DAD.
2 v/v). A sample of 50 mg of FDJ or DP was mixed with 75 μL of IS at 0.1 mg mL−1 and adjusted to 1 mL with the different solvents. The suspension formed was shaken in a Vortex for one minute and subjected to ultra-sound irradiation for 10 minutes at room temperature. Then, samples were centrifuged at 5000 rpm for 10 minutes and the supernatant was recovered, filtered and stored at −20 °C in amber glass vials until analysis by HPLC-DAD.
2.5 HPLC equipment
The chromatographic analysis was performed using an HPLC integrated system (Jasco, Tokyo, Japan), consisting of two Jasco PU-980 Plus HPLC pumps, an AS-2957 automated injector (20 μL loop), and a MD-2010 Plus multiwavelength diode-array detector (DAD). The chromatographic separation of the compounds was achieved with a C18 RP Luna column (4.6 × 250 mm, 5 μm) from Phenomenex (Torrance, CA, USA), operating at a controlled temperature of 40 °C (Jasco CO-2060 Plus, Jasco, Japan).The mobile phase consisted of 0.1% formic acid (A) and acetonitrile (B). The gradient program was previously developed by Almeida et al. (2015), with some modifications: 0 min 10% B, 15 min 32% B, 18 min 45% B, 23 min 50% B, 25 min 50% B, 26 min 10% B, maintaining these conditions for 3 min and returning to the initial conditions.22 The flow rate of the mobile phase was 1 mL min−1 and the injection volume was 20 μL. Peak purity measurements of all compounds were based on spectral comparison at three different peak heights through DAD information. Analytes were monitored at 254 nm and quantified on the basis of the internal standard method. Chromatographic data were processed with ChromNAV Software (Jasco, Tokyo, Japan).
2.6 Statistical analysis
Data were reported as mean ± standard deviation (Mean ± S.D.) of at least triplicate experiments. Statistical analysis of the results was performed with Minitab 15 (Pennsylvania, RI, USA). One-way ANOVA was used to investigate the differences between different methods of extraction for both alfalfa product analysis. Post-hoc comparisons of the means were performed according to Tukey's HSD test. In all cases, P < 0.05 was accepted as denoting significance.3. Results and discussion
Isoflavones are phenolic compounds biosynthesized by several vegetal species, mainly from the Fabaceae family, as secondary metabolites with a specific purpose for plant survival. The production of these natural products is based on plant requirements. Several phenolic compounds, including phytoestrogens, are produced by Medicago species with different biological functions such as beneficial plant interaction,23 defense against insects,24 allelopathy25 or even as developmental regulators.26 Indeed, the phenolic content clearly depends on plant growth conditions.27 In addition, these compounds are capable of promoting biological effects in humans. Several physiological actions are attributed to phenolic compounds’ intake. Isoflavones are associated with antioxidant activity but estrogenic actions are mainly attributed to their chemical structure similar to estradiol. Fig. 1 presents the chemical structures of the seven isoflavones studied in this work.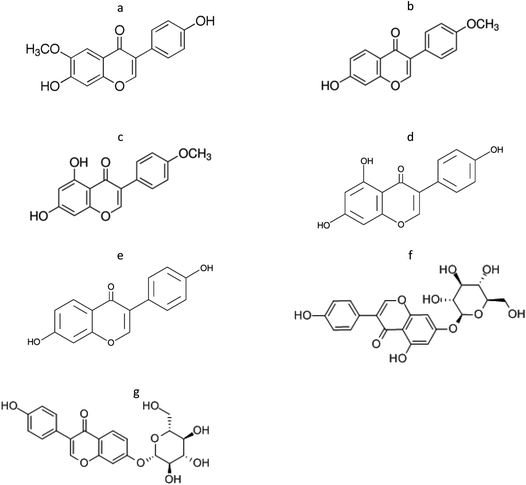 | ||
| Fig. 1 Chemical structures of the seven isoflavones investigated. (a) Glycitein; (b) formononetin; (c) biochanin A; (d) genistein; (e) daidzein; (f) genistin; (g) daidzin. | ||
Genistein and daidzein are the most studied isoflavones due to their high potency as phytoestrogens.28 However, these isoflavones can also be found in their natural source as glycosides (genistin and daidzin, respectively) or with methoxyl substituents as biochanin A, formononetin and glycitein.27 For this reason, the study of all the chemical forms of these compounds should be conducted.
In order to evaluate the range of isoflavones in the FDJ from alfalfa cultivated and harvested at five different months, HPLC-DAD analysis was performed. The seven isoflavones mentioned are the most reported in Medicago sativa, which were used to perform this study.
The retention time, linear regression data, limit of detection (LOD) and limit of quantification (LOQ) values are summarized in Table 1.
| Isoflavones | t R (minutes) | Regression equationa | Linear range (mg mL−1) | r | LOD (μg mL−1) | LOQ (μg mL−1) |
|---|---|---|---|---|---|---|
| a y, standard peak area/internal standard peak area; x, concentration (mg mL−1 of injected solution). | ||||||
| Daidzin | 9.25 | Y = 192.53X − 0.977 | 0.025–0.125 | 0.998 | 0.43 | 1.29 |
| Genistin | 12.20 | Y = 181.81X − 0.1468 | 0.006–0.075 | 0.993 | 0.11 | 0.34 |
| Daidzein | 16.6 | Y = 300.91X − 0.2414 | 0.025–0.125 | 0.999 | 0.03 | 0.10 |
| Glycitein | 16.80 | Y = 236.2X − 0.1226 | 0.003–0.025 | 0.999 | 0.04 | 0.13 |
| Genistein | 20.40 | Y = 333.1X − 0.4223 | 0.003–0.025 | 0.996 | 0.03 | 0.09 |
| Formononetin | 22.26 | Y = 17.74X − 0.0081 | 0.02–0.10 | 0.999 | 3.72 | 11.27 |
| Biochanin A | 25.5 | Y = 467.65X − 2.675 | 0.005–0.05 | 0.987 | 0.07 | 0.21 |
All standard curves were obtained by plotting standard solutions, at five concentrations (mg mL−1) as a function of the ratio between the peak areas of each standard and the IS.
The LOD and LOQ for each isoflavone were calculated as 3.3 and 10 times the standard deviation of the background divided by the slope of the calibration curve, respectively. The values obtained were in the range of 0.03–3.72 μg mL−1 and 0.10–11.27 μg mL−1 for LOD and LOQ, respectively.
Results are expressed as mg of isoflavones per unit of ADP (mg per unit), considering that one unit represents 3 grams of the product, since it is the recommended daily dose by the manufacturer.
3.1 Influence of extractor solvent
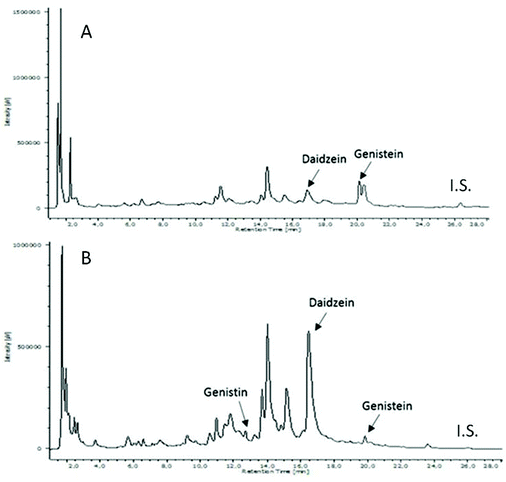
Although isoflavones come from the same biosynthetic route, they may have different chemical features caused by the hydroxyl substituents in their two aromatic rings and due to the presence of sugar molecules or methoxyl groups in their structure. These facts influence the polarity and solubility of the compounds. Consequently, the solvents selected to extract isoflavones are a critical aspect in the chemical analysis.Chromatograms of the FDJ sample extracted with water (A) and water–methanol–formic acid (B) are represented in Fig. 2.
As can be observed, the chromatographic profiles of samples extracted with water (Fig. 2A) are less complex than the ones extracted with water–methanol–formic acid (Fig. 2B). Even though FDJ is totally soluble in water, the chromatogram obtained from the sample treated with water–methanol–formic acid demonstrates that other polar compounds are extracted from FDJ using less polar solvents. This fact is totally justified due to the complexity of the matrix, and the mixture of solvents generates an optimal polarity to improve the extraction of both, moderately polar and polar compounds. This complex mixture of compounds is eluted generating a complex chromatogram in the base of their interaction with the stationary phase.
In this study, five different solvent systems were used to compare the efficacy of isoflavone extraction in the two ADP samples. Results are summarized in Tables 2 and 3.
| Solvent extractor | Isoflavone content in dehydrated powder (mean ± DS) | ||||||
|---|---|---|---|---|---|---|---|
| Harvesting month | Sample | Daidzin | Genistin | Daidzein | Genistein | Total | |
| n.d. – not determined. | |||||||
| Water | May | MS-1 | n.d. | n.d. | 1.08 ± 0.14 | n.d. | 1.08a |
| July | MS-2 | n.d. | n.d. | 0.85 ± 0.00 | n.d. | 0.85a | |
| August | MS-3 | n.d. | n.d. | 0.94 ± 0.00 | n.d. | 0.94a | |
| September | MS-4 | n.d. | n.d. | 0.40 ± 0.07 | n.d. | 0.40b | |
| October | MS-5 | n.d. | n.d. | 0.43 ± 0.00 | n.d. | 0.43b | |
| Methanol | May | MS-1 | n.d. | n.d. | n.d. | 0.14 ± 0.01 | 0.14a |
| July | MS-2 | n.d. | n.d. | n.d. | 0.22 ± 0.00 | 0.22b | |
| August | MS-3 | n.d. | n.d. | n.d. | 0.14 ± 0.00 | 0.14a | |
| September | MS-4 | n.d. | n.d. | n.d. | 0.22 ± 0.01 | 0.22b | |
| October | MS-5 | n.d. | n.d. | n.d. | 0.18 ± 0.02 | 0.18a,b | |
Water–methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
May | MS-1 | n.d. | n.d. | 0.58 ± 0.09 | 0.17 ± 0.00 | 0.75a,c |
| July | MS-2 | n.d. | n.d. | 0.83 ± 0.02 | 0.37 ± 0.00 | 1.20b | |
| August | MS-3 | n.d. | n.d. | 0.60 ± 0.04 | 0.15 ± 0.00 | 0.75a,c | |
| September | MS-4 | n.d. | n.d. | 0.65 ± 0.07 | 0.34 ± 0.01 | 0.94a,b | |
| October | MS-5 | n.d. | n.d. | 0.40 ± 0.01 | 0.20 ± 0.00 | 0.60c | |
| Ethanol | May | MS-1 | n.d. | n.d. | n.d. | 0.10 ± 0.00 | 0.10a |
| July | MS-2 | n.d. | n.d. | n.d. | 0.17 ± 0.20 | 0.17b | |
| August | MS-3 | n.d. | n.d. | n.d. | 0.12 ± 0.00 | 0.12a,b | |
| September | MS-4 | n.d. | n.d. | n.d. | 0.17 ± 0.00 | 0.17b | |
| October | MS-5 | n.d. | n.d. | n.d. | 0.14 ± 0.00 | 0.14a,b | |
Methanol–water–formic acid (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 v/v) 8 v/v) |
May | MS-1 | n.d. | n.d. | 0.50 ± 0.04 | 0.19 ± 0.01 | 0.69a |
| July | MS-2 | n.d. | n.d. | 0.60 ± 0.00 | 0.43 ± 0.01 | 1.03b | |
| August | MS-3 | n.d. | n.d. | 0.46 ± 0.02 | 0.20 ± 0.01 | 0.66a | |
| September | MS-4 | n.d. | n.d. | 0.45 ± 0.01 | 0.42 ± 0.03 | 0.87a,b | |
| October | MS-5 | n.d. | 0.12 ± 0.01 | 0.26 ± 0.03 | 0.28 ± 0.03 | 0.66a | |
| Solvent extractor | Isoflavone content in freeze-dried juice (mean ± DS) | ||||||
|---|---|---|---|---|---|---|---|
| Harvesting month | Sample | Daidzin | Genistin | Daidzein | Genistein | Total | |
| n.d. – not determined. | |||||||
| Water | May | MS-1 | n.d. | n.d. | 1.30 ± 0.03 | 0.14 ± 0.00 | 1.44a |
| July | MS-2 | 0.82 ± 0.01 | n.d. | 3.52 ± 0.14 | n.d. | 4.43c | |
| August | MS-3 | n.d. | n.d. | 2.70 ± 0.02 | n.d. | 2.70b | |
| September | MS-4 | n.d. | 0.84 ± 0.33 | 4.90 ± 1.77 | 0.23 ± 0.08 | 5.97d | |
| October | MS-5 | n.d. | n.d. | 1. 81 ± 0.24 | 0.17 ± 0.00 | 1.98a | |
| Methanol | May | MS-1 | n.d. | 0.71 ± 0.10 | n.d. | 0.38 ± 0.05 | 1.09a |
| July | MS-2 | n.d. | n.d. | n.d. | 0.10 ± 0.00 | 0.10b | |
| August | MS-3 | n.d. | n.d. | n.d. | 0.13 ± 0.00 | 0.13b | |
| September | MS-4 | n.d. | n.d. | n.d. | 0.10 ± 0.00 | 0.10b | |
| October | MS-5 | n.d. | 0.33 ± 0.00 | n.d. | 0.19 ± 0.00 | 0.52c | |
Water–methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
May | MS-1 | n.d. | n.d. | 1.17 ± 0.02 | 0.47 ± 0.00 | 1.64a |
| July | MS-2 | 0.70 ± 0.07 | n.d. | 1.50 ± 0.11 | n.d. | 2.20a,b | |
| August | MS-3 | n.d. | n.d. | 1.80 ± 0.12 | 0.15 ± 0.00 | 1.95a | |
| September | MS-4 | 1.14 ± 0.04 | n.d. | 1.50 ± 0.09 | 0.11 ± 0.01 | 2.75b,c | |
| October | MS-5 | n.d. | 0.63 ± 0.02 | 2.28 ± 0.07 | 0.27 ± 0.00 | 3.18c | |
| Ethanol | May | MS-1 | n.d. | n.d. | n.d. | 0.23 ± 0.02 | 0.23a |
| July | MS-2 | n.d. | n.d. | n.d. | 0.09 ± 0.00 | 0.09b | |
| August | MS-3 | n.d. | n.d. | n.d. | 0.09 ± 0.01 | 0.09b | |
| September | MS-4 | n.d. | n.d. | n.d. | 0.09 ± 0.02 | 0.09b | |
| October | MS-5 | n.d. | n.d. | n.d. | 0.13 ± 0.00 | 0.13b | |
Methanol–water–formic acid (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 v/v) 8 v/v) |
May | MS-1 | n.d. | n.d. | 1.19 ± 0.13 | 0.72 ± 0.00 | 1.91a,c |
| July | MS-2 | n.d. | n.d. | 0.91 ± 0.05 | 0.15 ± 0.00 | 1.06a | |
| August | MS-3 | n.d. | n.d. | 1.29 ± 0.11 | 0.21 ± 0.03 | 1.50a,c | |
| September | MS-4 | n.d. | n.d. | 0.94 ± 0.01 | 0.15 ± 0.02 | 1.09a | |
| October | MS-5 | n.d. | 0.88 ± 0.32 | 1.88 ± 0.39 | 0.39 ± 0.03 | 3.15c | |
As can be observed, glycitein, formononetin and biochanin A were not found in any ADP sample. Although formononetin and glycitein have been described in alcoholic extracts of Medicago sativa cultivated in Europe, the amount of isoflavones in those samples was smaller (2.40 ± 0.06 mg per kg db and 0.45 ± 0.01 mg per kg db, respectively) when compared to other Medicago species.29 These differences could be due to environmental or harvesting conditions found in Mexico. As is well known, biotic and abiotic factors are the most important conditions that lead to differences in the isoflavone biosynthesis.30 Also, the processing mechanism used to produce the two ADP can influence the isoflavone content.
Meanwhile, daidzein was detected in the ADP extracted with water, methanol–water and water–methanol–formic acid. In the DP samples treated with these solvents, the content of daidzein did not show significant differences. Samples extracted with water presented a higher daidzein content (0.40–1.08 mg per unit). Regarding FDJ, significant differences in the daidzein content were found between samples treated with water (1.30–4.90 mg per unit) and water–methanol–formic acid (0.91–1.88 mg per unit). These results suggest that daidzein is the highest polar isoflavone evaluated among the aglycone groups, which means that the extractor solvents should be clearly polar. In this way, the use of water as the solvent extractor could be considered as the best option to extract this isoflavone.
Genistein, an isoflavone with a similar chemical structure to daidzein (Fig. 1d and e), was detected in all samples of DP extracted with methanol (0.14–0.22 mg per unit), methanol–water (0.15–0.37 mg per unit), ethanol (0.10–0.17 mg per unit) and water–methanol–formic acid (0.19–0.43 mg per unit) with significant differences among the samples treated with ethanol and water–methanol–formic acid. The highest content of genistein was found in DP treated with water–methanol–formic acid. However, when the percentage of methanol is modified, genistein content decreases. Based on this, the mixture of water–methanol–formic acid seems to be the best system to extract genistein.
Also, in almost FDJ samples genistein was detected (Table 3), but the content of daidzein was greater than genistein.
Moreover, the glycoside daidzin was only detected in some FDJ treated with water (MS-2) and methanol–water (MS-2 and MS-4). In these cases, daidzin was better extracted from FDJ MS-2 sample in water (0.82 ± 0.01 mg per unit) than methanol–water (0.70 ± 0.07 mg per unit). Regarding MS-4, a higher amount of daidzin was extracted with methanol–water (1.14 ± 0.04 mg per unit). This could be justified based on the chemical structure of daidzin. As represented in Fig. 1 this compound has a glucose molecule at position 7, which provides a higher polarity and solubility in polar solvents such as water. Also, genistin was detected in some FDJ but not in DP, excep for MS-5 and methanol–water–formic acid.
The difference found in the total isoflavone content in the two ADP depends on several factors but the behavior of natural compounds against a solvent entirely depends on the matrix. For example, the glycosides genistin and daidzin that are very polar compounds are well extracted from soy extracts in 50% methanol or 50% ethanol.18 For this, several solvent mixtures are used to extract phenolic compounds, due to the variation in the matrix composition. Rodrigues et al. (2014) showed that the highest content of isoflavones from the aerial parts of M. sativa was obtained with ethanolic extracts.29
The optimal extraction and quantification of bioactive compounds from natural sources is totally dependent on the solvents used. One of the biggest challenges is the co-solubility factor. Plants biosynthesize thousands of compounds that remain in the original matrix. These compounds have chemical characteristics that allow them to stay together in solution. However, when the plant matrix is subjected to extraction with a polar solvent, for example, not only polar compounds are extracted inasmuch as the co-solubility factor also improves the solubility and extraction of non-polar compounds.18
The present results demonstrate that isoflavones with similar structures, such as genistein or daidzein, have different behaviors in the same solvents. The first is better extracted with ethanol, methanol or mixtures, while daidzein extraction is optimized with more polar solvents such as water. These aspects should be taken into account in the analysis of isoflavones from other ADP.
3.2 Influence of harvest month on isoflavone content
One of the biggest challenges in the standardization of plant-based products is the composition variations of plants grown in different latitudes and harvested along the year. These conditions affect the concentration of compounds and plant active ingredients, the evaluation of these fluctuations being crucial.In this study, the concentration of isoflavones in the ADP with alfalfa harvested in five different months, in 2014, was analyzed. The total isoflavone content from each ADP obtained from the five extractions to each harvest (from May, July, August, September and October) were compared. The results are presented in Tables 2 and 3.
Samples treated with water, methanol–water and water–methanol–formic acid presented the highest values, with variations in the harvest dates. FDJ from alfalfa cultivated in May (MS-1) and extracted with water had a total isoflavone content of 1.44 mg per unit while the samples harvested in September (MS-4) had 5.97 mg per unit, four times more.
As mentioned in the previous section, daidzein was better extracted with water. Comparing the concentration of those extracts through batches it is possible to observe that DP from MS-1 had the major content of daidzein (1.08 ± 0.14 mg per unit) while their respective FDJ showed the smallest value (1.30 ± 0.00 mg per unit). In contrast, the DP harvested in September (batch MS-4), had the lowest value (0.40 ± 0.07 mg per unit) and its FDJ had the highest (4.90 ± 1.77 mg per unit). The same behavior is observed in genistein (Tables 2 and 3) where DP from the sample MS-1 had the lowest value while the FDJ showed the highest (0.19 ± 0.01 mg per unit and 0.72 ± 0.00 mg per unit, respectively). In the meantime, DP and FDJ from MS-2 showed the opposite trend (0.43 ± 0.01 mg per unit and 0.15 ± 0.00 mg per unit, respectively).
It is well known that natural products production is affected by environmental factors. Studies using controlled environmental chamber showed that variations in temperature along with high carbon dioxide and water contents could modify, almost 65%, the chemical composition of plants during seed development.14 In Mexico, alfalfa is cultivated under wild conditions, where the environment changes according to the months of the year. May and July are the sunniest months while September and October are rainy months. These factors not only affect the isoflavone content but also the extraction rate of the compounds between the two alfalfa products. In this case, the degree of hydration of the plant could modify the amount of isoflavones extracted in the juice and in the residual material. Indeed, it is possible that the high water content observed in alfalfa cultivated in September could help the extraction of daidzein in the juice and not in the residual material.
According to the results, the total isoflavone content was higher in FDJ than DP, but the variation in the isoflavone content through different harvesting periods does not show a clear tendency. This could be due to the fact that samples are obtained from the same feedstock. The aerial part of alfalfa is squeezed to obtain the juice which is then freeze-dried, and the residues are dehydrated and milled. Therefore, the FDJ obtained contain the major number of water soluble compounds such as aglycones and their glycosides.
In order to use alfalfa as a new source of nutraceuticals, it is essential to ensure the content of bioactive compounds and their effectiveness and safety in the final products. However, several harvest conditions such as temperature, light quality, water, availability and pest pressure significantly modify the quality of plants.31 Other factor to consider in the variation of the chemical composition is the maturity stage of alfalfa, which significantly affects the concentration of phytoestrogens as these compounds are in lower amounts in the early plant vegetative stages.30 The fresh alfalfa used to manufacture these ADP is harvested in different periods and isoflavone content could be different in the later stages of maturation. Further studies are needed to determine the ideal maturity phase of plants to obtain the optimal concentration of isoflavones.
Comparing the total isoflavone content of the two ADP with some commercial soy-food supplements it is possible to observe that both ADP contain smaller amounts of isoflavones than soy-food supplements (4.5 mg per unit–110.9 mg per unit).32 Although both plants belong to the Fabaceae family, the differences in genus and species could be enough to produce these variations in the chemical composition.
Different studies report the positive effects of the standardized soy extract on climacteric symptoms, lipid profiles and bone markers. However, the daily doses consumed are high (35 mg–100 mg of isoflavones daily)33–35 and higher amounts of the two ADP should be consumed to achieve those concentrations. Also, other factors such as bioavailability and metabolism should be considered. Further assays are recommended to ensure the effects of these ingredients on menopausal symptoms.
The importance of this study lids on the fact that food supplements containing isoflavones are consumed worldwide to improve health. However, as previously reported by Almeida et al. 2015, the real isoflavone content in some products is different from the content mentioned on the label.32
The present study demonstrates differences in the isoflavone content between different harvesting periods which should be taken into account in the production of these products along with further pharmacological evaluation and possible side effects.
4. Conclusion
In Mexico, the use of local resources to generate functional products for human consumption represents an alternative to remain healthy but it is also a way to improve economic development in the country. Several new small companies are being created with these purposes. However, the lack of scientific evidence related to the effectiveness and safety of their products could represent danger for consumers. In this work, the total isoflavone content in two Mexican alfalfa-derived products were conducted. The isoflavone content through the different months of harvest were analyzed to determine variations in the amount of isoflavones per unit. Also, different solvents were used for isoflavone extraction to ensure optimal analysis.Results demonstrates that the two ADP do not contain formononetin, glycitein and biochanin A, but other isoflavones like daidzein, genistein, daidzin and genistin were detected and quantified. In all cases, the total isoflavone content was higher in FDJ than DP, daidzein and genistein being the most abundant compounds.
In terms of a solvent extractor, isoflavones with similar chemical structure are not extracted in the same way from the two samples of ADP, daidzein being better extracted with water while genistein showed best results with a mixture of water–methanol–formic acid.
The harvest month also influences the chemical composition of the two ADP showing differences in the total isoflavone content.
Further studies are needed to determine the optimal stage of maturity to harvest the raw material in order to obtain products with higher amounts of isoflavones. Also, correlations between isoflavone concentration and pharmacological effects should be conducted to find an effective daily dose intake.
Conflict of interest
The authors declare no conflict of interest.Acknowledgements
The authors are thankful to Productora Sativa S. P. R. de R. L. de C. V. for providing samples to carry out this work. This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT, México) (Project INNOVAPYME no. 213408). Guadalupe Soto is thankful to CONACyT for her grant during master degree studies. Francisca Rodrigues is thankful to the Foundation for Science and Technology (Portugal) for her PhD grant (SFRH/BDE/51385/2011) financed by POPH-QREN and subsidized by European Science Foundation. This work received financial support from the European Union (FEDER funds through COMPETE) and the National Funds (FCT, Foundation for Science and Technology) through project LAQV UID/QUI/50006/2013 and QREN through Project NORTE-07-0124-FEDER-000069. To all financing sources the authors are greatly indebted.References
- E. Poluzzi, C. Piccinni, E. Raschi, A. Rampa, M. Recanatini and F. D. Ponti, Curr. Med. Chem., 2014, 21, 417–436 CrossRef CAS.
- S. Bedell, M. Nachtigall and F. Naftolin, J. Steroid Biochem., 2014, 139, 225–236 CrossRef CAS PubMed.
- A. V. Sirotkin and A. H. Harrath, Eur. J. Pharmacol., 2014, 741, 230–236 CrossRef PubMed.
- V. S. Lagari and S. Levis, J. Steroid Biochem., 2014, 139, 294–301 CrossRef CAS PubMed.
- J. E. Rossouw, G. L. Anderson, R. L. Prentice, A. Z. LaCroix, C. Kooperberg, M. L. Stefanick, R. D. Jackson, S. A. Beresford, B. V. Howard, K. C. Johnson, J. M. Kotchen and J. Ockene, JAMA, J. Am. Med. Assoc., 2002, 288, 321–333 CrossRef CAS PubMed.
- A. Hajirahimkhan, B. M. Dietz and J. L. Bolton, Planta Med., 2013, 79, 538–553 CrossRef CAS PubMed.
- A. C. Moreira, A. M. Silva, M. S. Santos and V. A. Sardão, J. Steroid Biochem., 2014, 143, 61–71 CrossRef CAS PubMed.
- F. Borrelli and E. Ernst, Maturitas, 2010, 66, 333–343 CrossRef PubMed.
- C. Tsukamoto, S. Shimada, K. Igita, S. Kudou, M. Kokubun, K. Okubo and K. Kitamura, J. Agric. Food Chem., 1995, 43, 1184–1192 CrossRef CAS.
- N. L. Booth, C. R. Overk, P. Yao, J. E. Burdette, D. Nikolic, S. N. Chen, J. L. Bolton, R. B. van Breemen, G. F. Pauli and N. R. Farnsworth, J. Altern. Complement. Med. (New York, N.Y.), 2006, 12, 133–139 CrossRef PubMed.
- T. Visnevschi-Necrasov, J. C. Barreira, S. C. Cunha, G. Pereira, E. Nunes and M. B. Oliveira, Phytochem. Anal., 2015, 26, 40–46 CrossRef CAS PubMed.
- E. Gawel, Acta Sci. Pol., Technol. Aliment., 2012, 11, 303–310 CAS.
- M. J. Morrison, E. R. Cober, M. F. Saleem, N. B. McLaughlin, J. Frégeau-Reid, B. L. Ma and L. Woodrow, Field Crop. Res., 2010, 117, 113–121 CrossRef PubMed.
- C. R. Caldwell, S. J. Britz and R. M. Mirecki, J. Agric. Food Chem., 2005, 53, 1125–1129 CrossRef CAS PubMed.
- P. Seguin and W. Zheng, J. Sci. Food Agric., 2006, 86, 765–771 CrossRef CAS PubMed.
- J. C. Gasparetto, F. S. F. Smolarek, T. M. G. de Francisco, L. C. Miranda, R. Pontarolo and P. F. Siqueira, J. Am. Oil Chem. Soc., 2012, 89, 1211–1222 CrossRef CAS.
- A. Kozlowska and D. Szostak-Wegierek, Rocz. Panstw. Zakl. Hig., 2014, 65, 79–85 Search PubMed.
- M. A. Rostagno, M. Palma and C. G. Barroso, Anal. Chim. Acta, 2007, 597, 265–272 CrossRef CAS PubMed.
- D. L. Luthria, R. Biswas and S. Natarajan, Food Chem., 2007, 105, 325–333 CrossRef CAS PubMed.
- R. Fahmi, F. Khodaiyan, R. Pourahmad and Z. Emam-Djomeh, J. Food Sci. Technol., 2014, 51, 2857–2861 CrossRef CAS PubMed.
- M. A. Rostagno, M. Palma and C. G. Barroso, J. Chromatogr., A, 2003, 1012, 119–128 CrossRef CAS.
- I. M. Almeida, F. Rodrigues, B. Sarmento, R. C. Alves and M. B. Oliveira, Food Funct., 2015, 6, 938–946 CAS.
- J. Zhang, S. Subramanian, Y. Zhang and O. Yu, Plant Physiol., 2007, 144, 741–751 CrossRef CAS PubMed.
- L. G. Kamphuis, A. H. Williams, H. Kuster, R. D. Trengove, K. B. Singh, R. P. Oliver and S. R. Ellwood, Mol. Plant Pathol., 2012, 13, 593–603 CrossRef CAS PubMed.
- G. Bonanomi, V. Antignani, E. Barile, V. Lanzotti and F. Scala, J. Plant Pathol., 2011, 93, 57–69 CAS.
- C. Laffont, S. Blanchet, C. Lapierre, L. Brocard, P. Ratet, M. Crespi, U. Mathesius and F. Frugier, Plant Physiol., 2010, 153, 1597–1607 CrossRef CAS PubMed.
- A. Gholami, N. De Geyter, J. Pollier, S. Goormachtig and A. Goossens, Nat. Prod. Rep., 2014, 31, 356–380 RSC.
- D. Vitale, C. Piazza, B. Melilli, F. Drago and S. Salomone, Eur. J. Drug Metab. Pharmacokinet., 2013, 38, 15–25 CrossRef CAS PubMed.
- F. Rodrigues, I. Almeida, B. Sarmento, M. H. Amaral and M. B. P. P. Oliveira, Ind. Crop. Prod., 2014, 57, 110–115 CrossRef CAS PubMed.
- P. Seguin, W. Zheng and A. Souleimanov, J. Agron. Crop Sci., 2004, 190, 211–217 CrossRef CAS PubMed.
- A. J. Parr and G. P. Bolwell, J. Sci. Food Agric., 2000, 80, 985–1012 CrossRef CAS.
- I. M. Almeida, F. Rodrigues, B. Sarmento, R. C. Alves and M. B. Oliveira, Food Funct., 2015, 6(3), 938–946 CAS.
- T.-S. Yang, S.-Y. Wang, Y.-C. Yang, C.-H. Su, F.-K. Lee, S.-C. Chen, C.-Y. Tseng, H.-J. Jou, J.-P. Huang and K.-E. Huang, Taiwan J. Obstet. Gynecol., 2012, 51, 229–235 CrossRef PubMed.
- A. Ferrari, J. Obstet. Gynaecol. Res., 2009, 35, 1083–1090 CrossRef CAS PubMed.
- E. A. Nahas, J. Nahas-Neto, F. L. Orsatti, E. P. Carvalho, M. L. Oliveira and R. Dias, Maturitas, 2007, 58, 249–258 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |