In vitro starch digestibility and in vivo glycemic response of foxtail millet and its products
Xin
Ren
a,
Jing
Chen
a,
Mohammad Mainuddin
Molla
a,
Chao
Wang
a,
Xianmin
Diao
b and
Qun
Shen
*a
aNational Engineering and Technology Research Center for Fruits and Vegetables, College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China. E-mail: shenqun@cau.edu.cn; Fax: +86-10-62737524; Tel: +86-10-62737524
bNational Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Science, Chinese Academy of Agricultural Sciences, Beijing 100081, China
First published on 12th October 2015
Abstract
Foxtail millet, as a leading variety in arid and semi-arid areas of Asia and Africa, can provide broad potential benefits to human health. However, its digestion properties have not been reported. So in this study, the in vitro starch digestibilities and in vivo glycemic indices (GI) of foxtail millet and pure millet products were investigated. The results showed that starch digestibility of the foxtail millet flour is obviously lower than that of wheat flour. However, deproteinization and heating significantly increased its rapidly digestible starch and decreased its slowly digestible starch and resistant starch. The GIs of pure millet products were in the following order: millet porridge (93.6 ± 11.3) > millet steamed bread (89.6 ± 8.8) > No. 1 millet pancake (75.0% millet flour and 25.0% extrusion flour, 83.0 ± 9.6) > No. 2 millet pancake (without extrusion flour, 76.2 ± 10.7) > cooked millet (64.4 ± 8.5). They were significantly positively correlated with the rapidly digestible starch (r = 0.959), degree of gelatinization (r = 0.967) and estimated glycemic index (r = 0.988). Both in vitro and in vivo tests suggested that boiling, steaming and extrusion enhanced the formation of digestible starch and subsequently increased the GI values. Additionally, the No. 1 millet pancake and cooked millet had a relatively gentle stimulation on β-cell. Therefore, foxtail millet, especially the cooked millet, may serve as a potential source of nutraceutical and functional food that could delay the development of type 2 diabetes.
Introduction
Type 2 diabetes has become a major health-threatening problem in many countries of the world. Already, it has reached epidemic proportions, specifically, in China, with up to 92.4 million people (9.7% of the general adult population) experiencing type 2 diabetes, and up to 148.2 million people (15.5%) experiencing prediabetes.1 As is known, the quantity and quality of dietary carbohydrates play a critical role in the control of postprandial blood glucose.2 Several studies have shown that slowly digested and absorbed carbohydrates were independently associated with the decreased risk of developing type 2 diabetes,3–5 and several official dietary guidelines have recommended using the glycemic index (GI) for food choices.6 Many factors may decrease the rate and extent of starch digestion and subsequently GI values, including the enzyme resistance of amylose–lipid complexes,7 the encapsulation of protein matrix,8,9 and the processing method with low temperature, short time and insufficient water.3,10 Nowadays, it is possible to produce low-GI foods, such as millet, pasta and foods containing modified starch, by controlling the ingredients and processing conditions.4,11Millet is a generic term that includes a range of small seeded cereals, such as pearl millet (Pennisetum glaucum), foxtail millet (Setaria italica), proso millet (Panicum miliaceum), finger millet (Eleusine coracana), and common millet (Panicum miliaceum). It has been used to produce porridge, wine, nutrition powder and several national products like kunu, fura, upma and Laddu.12 Foxtail millet is the leading variety in China and it was first domesticated and selected as grain food in the Yellow River basin as early as 8700 years ago.13,14 It is one of the most important drought-resistant crops and plays a critical role in food security in arid and semi-arid areas of Asia and Africa.12 It has been reported that foxtail millet can lower the risk of type 2 diabetes15 and cardiovascular disease.16 It has a high phytochemical content with antioxidative and antiproliferative activities.17 Feeding foxtail millet decreased the C-reactive protein and triacylglycerol levels in hyperlipidemic rats18 and improved insulin sensitivity and cholesterol metabolism in genetically type 2 diabetic mice.16 Additionally, both haematological and histological changes confirmed that foxtail millet bran oil was capable of attenuating ethanol-induced hepatic injury.19 There has been growing interest in its nutritive value and potential health benefits in recent years, however, it has remained not fully studied and utilized.20 Starch, as a major component of foxtail millet, may determine the nutritional qualities and physiological properties of millet products. However, there are still no such reports regarding the starch digestion characteristics and glycemic responses of foxtail millet.
Therefore, the objectives of this study were (a) to evaluate the effects of lipid and protein on the contents of different starch fractions of foxtail millet under raw and cooked conditions with wheat flour as a positive control; (b) to determine the effects of different processing methods on the in vitro starch digestion characteristics, the degree of gelatinization (DG) and the estimated glycemic index (eGI); and (c) to monitor the blood glucose and insulin responses in ten healthy adults after the ingestion of five pure foxtail millet products.
Materials and methods
Foxtail millet and enzymes
Foxtail millet (Setaria italica) was purchased from Jinguzi Company (Tianjin, China). It was milled by a WF-20B pulverizer (Keyi Machinery, Nanjing, China) and ground through 0.2 mm sieves, and then stored at 4 °C. Amyloglucosidase (catalogue no. 10113), invertase (catalogue no. I4504), pancreatin (catalogue no. P7545) and pepsin (catalogue no. P7000) were purchased from Sigma-Aldrich (St Louis, MO, U.S.).Samples preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio of 1
water ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and millet porridge with a millet
1.5 and millet porridge with a millet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio of 1
water ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9. The freshly prepared products were wet-ground for 3 seconds by a JYL-C012 machine (Joyoung, Hangzhou, China) and subjected to the in vitro test in a form that resembles the food “as eaten”.2
9. The freshly prepared products were wet-ground for 3 seconds by a JYL-C012 machine (Joyoung, Hangzhou, China) and subjected to the in vitro test in a form that resembles the food “as eaten”.2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 w/v) thereto, stirring and mixing well for defatting, sealing the flasks with tin foil paper and placing them in a water bath at 45 °C under continuous stirring at 160 rpm for 120 min, vacuum-filtering by a vacuum suction pump to collect residues, and drying the obtained residues via air stream to obtain the defatted millet flour. In addition, the foxtail millet flour and defatted millet flour were placed into several flasks, respectively, followed by adding freshly-prepared pepsin solution (5.0 g L−1 pepsin in 0.05 mol L−1 HCl), stirring and mixing well for deproteining, placing them in a water bath at 37 °C under continuous stirring at 160 rpm for 30 min, then centrifuging at 1500g for 10 min to collect residues, and freeze-drying the obtained residues to obtain the deproteined millet flour and millet starch. The contents of protein and lipid of each sample are listed in Table 1. Finally, the foxtail millet flour and the flour with lipid removed, protein removed, or both lipid and protein removed were dispersed in 5.0 mL of water and heated in a boiling water bath for 20 min to obtain the cooked samples. The wheat flour (Jinshahe Flour Manufacturing, Hebei, China) was used as a positive control.
5 w/v) thereto, stirring and mixing well for defatting, sealing the flasks with tin foil paper and placing them in a water bath at 45 °C under continuous stirring at 160 rpm for 120 min, vacuum-filtering by a vacuum suction pump to collect residues, and drying the obtained residues via air stream to obtain the defatted millet flour. In addition, the foxtail millet flour and defatted millet flour were placed into several flasks, respectively, followed by adding freshly-prepared pepsin solution (5.0 g L−1 pepsin in 0.05 mol L−1 HCl), stirring and mixing well for deproteining, placing them in a water bath at 37 °C under continuous stirring at 160 rpm for 30 min, then centrifuging at 1500g for 10 min to collect residues, and freeze-drying the obtained residues to obtain the deproteined millet flour and millet starch. The contents of protein and lipid of each sample are listed in Table 1. Finally, the foxtail millet flour and the flour with lipid removed, protein removed, or both lipid and protein removed were dispersed in 5.0 mL of water and heated in a boiling water bath for 20 min to obtain the cooked samples. The wheat flour (Jinshahe Flour Manufacturing, Hebei, China) was used as a positive control.
| Sample | Protein (g per 100 g) | Lipid (g per 100 g) | Remove ratio of protein (%) | Remove ratio of lipid (%) |
|---|---|---|---|---|
| Values (mean ± SD) followed by a different letter in each column were significantly different (P < 0.05). | ||||
| Millet flour | 9.76 ± 0.03 d | 1.85 ± 0.012 d | — | — |
| Defatted millet flour | 9.41 ± 0.03 c | 0.29 ± 0.009 b | 3.61 ± 0.28 a | 84.40 ± 0.49 b |
| Deproteined millet flour | 2.54 ± 0.003 a | 0.89 ± 0.004 c | 73.99 ± 0.03 b | 51.98 ± 0.24 a |
| Millet starch | 2.60 ± 0.001 b | 0.22 ± 0.005 a | 73.33 ± 0.01 b | 88.10 ± 0.25 c |
In vitro starch digestibility
The samples were analyzed for in vitro starch digestion based on Englyst et al.2,23 with some modifications. The samples (containing about 0.5 g of starch) were dispersed in 25.0 mL of acetate buffer (0.1 M, pH 5.2) in 50 mL centrifuge tubes with 2 glass balls. After being vortex-mixed vigorously, the tubes were placed into a boiling water bath for 30 min and cooled to 37 °C, and then invertase (3000 U mL−1, 0.3 mL) was added, vortex-mixed and incubated at 37 °C for 30 min. Finally 0.2 mL of each sample was added into 4 mL of absolute ethanol and mixed well to obtain the free glucose (FG) portion.As above, the other samples were dispersed in 10.0 mL of freshly-prepared pepsin solution (5.0 g L−1 pepsin and 5.0 g L−1 guar gum in 0.05 mol L−1 HCl, 5 glass balls), placed in a water bath at 37 °C for 30 min, and 10 mL of acetate buffer (0.1 M, pH 5.5, 37 °C) was added. A 5.0 mL of the enzyme mixture was added to initiate starch digestion, wherein the enzyme mixture was prepared by dispersing 3.0 g of pancreatin in 20.0 mL of water via a magnetic stirrer for 10 min, then centrifuging at 1500g for 10 min to obtain pancreatin supernatant (15.0 mL), and adding 0.75 mL of amyloglucosidase (1200 U mL−1) and 1 mL of invertase (3000 U mL−1) thereto. The samples were digested at 37 °C for 2 h under horizontal shaking at 160 rpm. After exactly 20 and 120 min of digestion, 0.2 mL of each sample was added into 4.0 mL of absolute ethanol and mixed well to obtain the glucose portion for 20 min (G20) and 120 min (G120).
After 0.2 ml of G120 samples had been collected, the tubes were vortex-mixed vigorously. After boiling-water incubation for 30 min, the contents were cooled to 0 °C and mixed with 10.0 mL of 7.0 mol L−1 potassium hydroxide. After ice-water incubation for 30 min, 0.2 mL of each sample was added to 1.0 mL of 1.0 mol L−1 acetic acid containing 40.0 μL of amyloglucosidase (100.0 U mL−1), followed by placing in 70 °C water bath for 30 min and boiling-water bath for 10 min, then cooling to room temperature and adding 20.0 mL of water to obtain the total glucose portion (TG).
All the above collected samples (FG, G20, G120 and TG) were centrifuged at 1500g for 5 min. The glucose content in the supernatant was measured using the glucose oxidase-peroxidase method by using a GOD-POD diagnostic kit (Applygen Technologies, Beijing, China). The OD values (x-axis) were measured by Thermo Scientific Multiskan GO (Thermo Fisher Scientific, MA, USA). Standard glucose solutions with concentrations of 125.0, 250.0, 500.0, 1000.0 and 2000.0 μM L−1 each were subjected to the same tests, respectively, at the same time to thereby obtain a standard curve (y = 4526x − 21.7, R2 = 0.9998).
Degree of gelatinization (DG)
An enzyme method24 for detecting DG was applied in this study. In short, 50.0 mg of freshly wet-ground sample was accurately weighed into a 10.0 mL centrifuge tube, together with 1.0 mL of amyloglucosidase (50.0 U mL−1) and 4.0 mL of acetate buffer (0.1 M, pH 4.75). The contents were vortex-mixed and the tubes were placed in a 37 °C water bath for 30 min under horizontal shaking at 160 rpm. Then 0.2 mL of each sample was added into 4.0 mL of absolute ethanol, mixed well and centrifuged at 1500g for 5 min. The glucose content in the supernatant was measured as described above. The other samples were autoclaved at 121 °C for 30 min for full gelatinization and thereafter subjected to the same procedures. The DG was defined as the glucose content of per gram of original sample, expressed as a percentage of that for per gram of fully gelatinized sample.Estimated glycemic index (eGI)
The kinetics of the in vitro starch digestibility and the eGI were calculated on the basis of glucose measurements at different times (20, 40, 60, 80, 100, 120 and 180 min) during starch hydrolysis. A first order equation [C = C∞(1 − e−kt)] was applied,25 where C, C∞ and k represented the percentage of starch hydrolyzed at time t (min), the maximum hydrolysis extent and the kinetic constant, respectively. The hydrolysis index (HI) was obtained based on the relationship between area under hydrolysis curve (AUC) for the millet product and the AUC for a reference food (fresh white bread). The eGI (bread = 100) was calculated using the equation eGI = 39.71 + 0.549HI and it was multiplied by 0.7 to obtain the eGI value with glucose as the reference food (glucose = 100).6In vivo glycemic response
The in vivo glycemic response of five freshly-prepared pure foxtail millet products were determined in ten healthy subjects (three males and seven females, mean age = 26.0, mean BMI = 20.8 kg m−2).26,27 The consumption amount of test foods which can provide 50.0 g of available carbohydrate is listed in Table 2. Each subject consumed the test foods and standard glucose solution in a random order on separate mornings (3 days apart) after 10–12 h of overnight fasting. For collection of venous blood samples, an intravenous catheter (BD Insyte 20GA × 1.16IN, 1.1 × 30 mm; Becton Dickinson Infusion Therapy Systems) was applied in this study. After collection of 2 mL of fasting blood sample, the subjects ate a test meal at a comfortable speed within 15 min and then 2 mL of further blood samples were collected at 15, 30, 45, 60, 90 and 120 min, respectively. Test meals were provided together with 200.0 mL of water and standard glucose solution was measured twice. Blood samples were collected into tubes and immediately separated by centrifugation and stored at −80 °C for analysis. Plasma glucose was measured using the Roche P800 analyzer (Roche-Diagnostics, Basel, Switzerland) by enzymatic determination. Plasma insulin was measured with an immunoluminometric assay using the Siemens ADVIA Centaur XP analyser (Siemens Healthcare Diagnostics, Washington, USA). Ethical permission for this study was obtained from the Biomedicine Ethical Committee of Peking University, and the written informed consent was given to subjects.| Variety | Water content (%) | Available carbohydrate content (%) | Available carbohydrate amount (g) | Consumption amount (g) |
|---|---|---|---|---|
| a MSB, millet steamed bread; MP-1, No. 1 millet pancake (75.0% millet flour and 25.0% extrusion flour); MP-2, No. 2 millet pancake (without extrusion flour). | ||||
| MSB | 42.0 | 50.1 | 50.0 | 100.0 |
| MP-1 | 59.0 | 35.4 | 50.0 | 141.0 |
| MP-2 | 52.0 | 41.2 | 50.0 | 121.0 |
| Cooked millet | 65.4 | 29.7 | 50.0 | 169.0 |
| Millet porridge | 89.4 | 9.1 | 50.0 | 550.0 |
| Glucose solution | 80.0 | 20.0 | 50.0 | 250.0 |
Statistical analysis
According to Englyst et al.,2 from the data of in vitro starch digestion, the contents of different starch fractions: rapidly digestible starch (RDS), slowly digestible starch (SDS), and resistant starch (RS), as well as the contents of different available glucose fractions: rapidly available glucose (RAG) and slowly available glucose (SAG) on a dry basis were calculated as follows:| RAG = G20 | (1) |
| SAG = G120 − G20 | (2) |
| RDS = (G20 − FG) × 0.9 | (3) |
| SDS = (G120 − G20) × 0.9 | (4) |
| Total starch = (TG − FG) × 0.9 | (5) |
| RS = (TG − G120) × 0.9 | (6) |
The results in this study were expressed as a percentage of total starch or total available glucose.28 The AUCs of blood glucose and insulin were calculated according to the trapezoidal rule in geometry, ignoring any area beneath the fasting level. The GI and insulin index (II) for the test foods were calculated by the average of individual values.27
In all cases, at least three replicates were performed for each analysis. All analyses were performed using SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA). Data for in vitro digestibility was presented as the mean ± standard deviation (SD) and for in vivo digestibility (GI and II) was presented as the mean ± standard error of the mean (SEM). One-Way ANOVA was used followed by Tukey's test and a p value under 0.05 was considered to indicate significance.
Results and discussion
Effects of components and heating on the in vitro starch digestibility
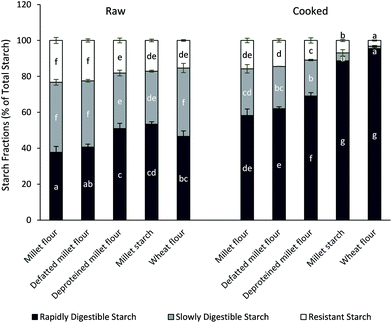
Starch, protein and lipid are three major components in cereal-based foods. Interactions among them play an important role in starch digestibility in the human small intestine and therefore influence further blood glucose response.11 The effects of lipid and protein on in vitro starch digestion characteristics of foxtail millet were investigated in this study. As shown in Fig. 1, the different starch fractions (RDS, SDS and RS) were determined in the millet flour, defatted millet flour, deproteined millet flour, and millet starch, respectively, with wheat flour as a positive control. The content of RDS in raw millet flour was the lowest (37.7 ± 3.2, mean ± SD). By comparison, it can be seen that the content of RDS increased slightly in defatted millet flour (40.1 ± 1.6) but significantly in deproteined millet flour and millet starch (50.9 ± 2.9 and 53.4 ± 1.3, respectively). Conversely, both the content of SDS and RS in raw millet flour was the highest, followed by that of defatted millet flour being slightly decreased, and that of deproteined millet flour and millet starch being drastically decreased respectively. These results were consistent with those obtained in previous studies, i.e. fats formed complexes with amylose and proteins blocked enzyme adsorption sites on the surface of the starch granule.7,9,29 The amylose–lipid complexes had enzymatic resistance which increased with increasing lipid chain length, amylose degree of polymerization and complexation temperature.7 Additionally, the hydrophobicity of lipids also significantly affect the starch digestibility. Proteins reduced starch granule surface accessibility and therefore influenced the enzyme susceptibility. More specifically, protein fractions, such as albumin, globulin and glutenin, were glued into a matrix surrounding starch granules acting as a barrier against starch digestion.11 This phenomenon has been confirmed by adding protease to corn flour or removing gluten from wheat flour, both of which resulted in a significant enhancement of in vitro starch digestibility.11 Many other studies also reported the presence of a protein barrier, such as sorghum kafirin29 and pasta.9In addition, it could be suggested that, under the present study conditions, the surface protein has a greater effect than surface lipid on in vitro starch digestibility of foxtail millet. This result, however, was inconsistent with those found by Annor et al.30 in kodo millet flour, possibly due to the different interactive model among different free fatty acids, proteins and starch.7
After cooking, the changes among different samples were almost the same (Fig. 1). In detail, the RDS of all samples increased significantly, accompanied by a considerable decrease of SDS and RS. This phenomenon was the most obvious in wheat flour, in which the RDS content increased from 46.6 ± 3.0 to 95.5 ± 0.7. In general, starch gelatinization was characterized by physical and chemical changes, such as swelling, rupturing and the disruption of crystalline structure. From the result of this study, it can be further suggested that in terms of biochemical change, the starch gelatinization was such a process that SDS and RS turned into RDS. Martine et al.31 had reported that the starch granule swelling behavior can be classified into three classes, and the data from cooked samples showed that foxtail millet starch should be classified as the second class: slow swelling, which can be converted to rapid swelling by extraction of surface proteins and lipids.
More interestingly, the starch digestibility of millet flour was significantly lower than that of wheat flour both under raw and cooked conditions. The RDS content of cooked millet flour was just 61.0% of that of cooked wheat flour (Fig. 1). That is, the millet flour had relatively low enzyme susceptibility and resisted enzymatic hydrolysis to some extent. This result was consistent with those observed in kodo millet, finger millet and barnyard millet, but not consistent with that observed in the proso millet.20 After both lipid and protein were removed from the millet flour, the digestibility was slightly higher than that of wheat flour in raw materials and almost the same as in the cooked ones, which indicated once more that the presence of the protein and lipid decreased the starch digestion rate of foxtail millet.
Effects of different processing methods on the in vitro starch digestibility and estimated glycemic index
There were many factors contributing to the in vitro starch digestibility, such as amylose content, type of cultivar, partial size, processing and storage conditions.11,27 Among all these, food processing was the major determinant of starch gelatinization, digestion and absorption, and eventually influenced the final postprandial glycemic response.3,32 There were pieces of evidence that the starch fractions of finger millet (ragi)10 and the glycemic index of potato3 varied significantly depending on the different cooking methods. In the present study, the effects of different processing methods (steaming, pan-baking, cooking and boiling) on starch digestion and gelatinization properties of foxtail millet were investigated (Table 3). The DG of millet porridge was the highest (93.5 ± 0.1, mean ± SD), followed by MSB (86.5 ± 0.2) and the two MP (70.2 ± 1.9 for MP-1 and 67.7 ± 1.1 for MP-2 respectively), and the DG of cooked millet was the lowest (55.5 ± 2.9). This trend was also observed in the contents of RAG and RDS, suggesting that the DG of specific food strongly affects its digestibility.24 From the point of available glucose, the RAG content ranged from 51.3 ± 5.8 to 65.1 ± 5.6 after various cooking methods, that is, the RAG always was the dominant component in five pure millet products. From the point of starch, millet porridge showed the highest RDS content, which may support the opinion of Englyst23 who reported that the RDS content of boiled millet was about 56.0%. Except for millet porridge, there was no significant difference between the contents of RDS and SDS. However, the RS content showed a wide variation from 8.8 ± 3.9 to 24.9 ± 3.6, and the highest RS content was observed in the cooked millet which was corresponding to the lowest DG. RS is considered as a source of dietary fiber and can provide a number of beneficial effects. The decrease in digestible starch and increase in RS content of the cooked millet would be expected to improve human health.| Sample | Starch fraction | Available glucose | DG % | |||
|---|---|---|---|---|---|---|
| RDS % | SDS % | RS % | RAG % | SAG % | ||
| a MSB, millet steamed bread; MP-1, No. 1 millet pancake (75.0% millet flour and 25.0% extrusion flour); MP-2, No. 2 millet pancake (without extrusion flour); RDS, rapidly digestible starch; SDS, slowly digestible starch; RS, resistant starch; RAG, rapidly available glucose; SAG, slowly available glucose; DG, degree of gelatinization. Values (mean ± SD) followed by a different letter in each column were significantly different (P < 0.05). | ||||||
| MSB | 46.3 ± 6.7 ab | 44.9 ± 4.6 a | 8.8 ± 3.9 a | 55.4 ± 6.6 a | 44.7 ± 6.6 a | 86.5 ± 0.2 c |
| MP-1 | 43.0 ± 1.3 abc | 46.3 ± 5.9 a | 10.7 ± 5.0 a | 53.0 ± 3.9 a | 47.0 ± 3.9 a | 70.2 ± 1.9 b |
| MP-2 | 39.1 ± 2.3 bc | 45.0 ± 6.3 a | 15.9 ± 4.3 ab | 51.3 ± 5.8 a | 48.7 ± 5.8 a | 67.7 ± 1.1 b |
| Cooked millet | 36.9 ± 1.4 c | 38.3 ± 2.2 ab | 24.9 ± 3.6 c | 52.9 ± 0.6 a | 47.1 ± 0.6 a | 55.5 ± 2.9 a |
| Millet porridge | 50.7 ± 4.2 a | 40.5 ± 3.2 ab | 8.8 ± 2.7 a | 65.1 ± 5.6 b | 34.9 ± 5.6 b | 93.5 ± 0.1 d |
The kinetics of in vitro starch digestibility and eGI of pure foxtail millet products are listed in Table 4. The maximum hydrolysis extent, or equilibrium concentration, C∞, ranged between 76.5 ± 1.6 and 92.1 ± 2.0. These results were obviously higher than those of legumes ranging from 33.1 to 43.1,33 but lower than those of gluten-free breads with an average of 96.5.34 The kinetic constant, k, which reflects the rate of hydrolysis in the early stage, ranged between 0.030 ± 0.002 and 0.040 ± 0.001. The k was the lowest in MP-2, which was almost the same as MP-1. More interestingly, the trend of C∞ and k were not fully consistent with each other. The k of cooked millet was higher than that of pancake but its C∞was much lower. That is, in terms of the cooked millet, although its hydrolysis rate was faster in the early stage, its equilibrium hydrolysis extent was smaller. The eGI, either in white bread or glucose used as the reference food, followed the order: millet porridge > MSB > MP-1 > MP-2 > cooked millet. This trend was consistent with the results of DG and RDS. According to the above discussion, it can be concluded that the eGI was a result of joint effect of C∞ and k, and reflected the starch digestibility more succinctly in this portion.
| Sample | C ∞ | k | eGI (bread = 100) | eGI (glucose = 100) | GI | II | II/GI |
|---|---|---|---|---|---|---|---|
| a MSB, millet steamed bread; MP-1, No. 1 millet pancake (75.0% millet flour and 25.0% extrusion flour); MP-2, No. 2 millet pancake (without extrusion flour); C∞, maximum hydrolysis extent; k, kinetic constant; HI, hydrolysis index; Egi, estimated glycemic index; GI, glycemic index; II, insulin index; II/GI, II to GI ratio. Values followed by a different letter in each column were significantly different (P < 0.05). | |||||||
| MSB | 90.8 ± 1.8 b | 0.036 ± 0.001 b | 86.3 ± 1.1 c | 60.4 ± 0.8 c | 89.6 ± 8.8 ab | 109.3 ± 11.5 c | 1.2 ± 0.2 b |
| MP-1 | 92.1 ± 2.0 b | 0.031 ± 0.001 a | 84.9 ± 0.7 c | 59.4 ± 0.5 c | 83.0 ± 9.6 ab | 65.0 ± 4.0 ab | 0.8 ± 0.1 a |
| MP-2 | 86.5 ± 3.8 b | 0.030 ± 0.002 a | 81.7 ± 1.3 b | 57.2 ± 0.9 b | 76.2 ± 10.7 ab | 84.5 ± 14.4 bc | 1.1 ± 0.1 ab |
| Cooked millet | 76.5 ± 1.6 a | 0.033 ± 0.001 ab | 77.6 ± 0.6 a | 54.3 ± 0.4 a | 64.4 ± 8.5 a | 49.8 ± 7.6 a | 0.8 ± 0.1 a |
| Millet porridge | 91.8 ± 1.7 b | 0.040 ± 0.001 c | 86.8 ± 0.6 c | 60.7 ± 0.5 c | 93.6 ± 11.3 b | 85.8 ± 9.8 bc | 0.9 ± 0.1 ab |
Roasting, autoclaving and pressure-cooking enhanced the formation of RDS in finger millet;10 while boiling, mashing and extrusion-cooking contributed to significant increase of digestible starch in potato.3 In the present study, although the raw materials of MSB and MP-1 were exactly the same, the RAG, RDS, DG and eGI of MSB were always higher than those of MP-1, which represented that steaming enhanced the formation of digestible starch. Although two millet pancakes were obtained by the same processing method, the MP-1 which had 25.0% extrusion flour, exhibited higher digestibility during the whole hydrolyzation. Nowadays, extrusion cooking has been widely used for the production of precooked flours, snack foods, and breakfast cereals. During the extrusion process, high temperatures, pressures and shear forces destroy the starch granular structure, thereby decreasing the crystallinity and lead to partial depolymerisation, and therefore increase its gelatinization extent and enzymes availability. Many researchers have reported that the extrusion cooking significantly increased the in vitro starch digestibility of potatoes, beans, corn and barley.3,11 Our results confirmed this phenomenon in foxtail millet. The additional amount of water during processing was also an important factor determining the DG and starch digestibility.4,11 When starch-based materials were heated in excess water, such as millet porridge, the water molecules linked to the exposed hydroxyl groups of amylose and amylopectin, which caused an increase in granule swelling and complete gelatinization.11 Thus the cooked millet, when compared with the millet porridge, had a smaller RDS, DG and eGI for the quite low water content. This phenomenon was also observed in biscuits32 and fried potatoes.3 In addition, milling can increase the surface area and subsequent enzyme susceptibility of the starch granule. Therefore, although the water contents of MSB and MP were lower than that of cooked millet, their DG and starch digestibility was still much higher.
Effects of different processing methods on the in vivo starch digestibility
The GI, which was first introduced by Jenkins et al.26 in 1981, has been widely accepted as a golden standard of carbohydrate classification and primary guidance of food choice.5,6 However, it is still controversial whether the in vitro digestion characteristics can reflect the glycemic responses accurately.6,35 And only a few foods have been subjected to both in vitro and in vivo testing for comparison.3 So in order to give a comprehensive evaluation of the effects of different processing methods on starch digestibility of foxtail millet, we further investigated the blood glucose and insulin responses after ingestion of pure millet products (Fig. 2). The peak time and concentration were two main factors of the blood glucose curve. From the data (Fig. 2A), it can be observed that the peak concentration of millet porridge was the highest (8.0 ± 0.4, mean ± SD), even higher than that of the standard glucose solution (7.4 ± 0.2), followed by MP-1 (7.3 ± 0.3), MSB (7.1 ± 0.6), MP-2 (6.6 ± 0.2) and cooked millet (6.4 ± 0.2). The peak time of millet porridge, MP-1 and MSB was 45 min, while that of others was 30 min. The blood glucose concentration of cooked millet, followed by MP-2, was always apparently lower than that of standard glucose solution within 2 h. In detail, the maximum increase of blood glucose in cooked millet was just 63.4% of that in standard glucose solution and 55.7% of that in the millet porridge. Moreover, only the blood glucose level of MP-2 at 120 min was lower than fasting level. From the blood insulin reaction curves (Fig. 2B), it can be observed that when compared with a standard glucose solution (555.0 ± 107.8), the peak concentration of millet porridge (608.1 ± 97.5) was slightly higher and that of MSB (551.5 ± 137.0) and MP-2 (522.0 ± 140.0) were slightly lower, and the peak concentration of MP-1 (383.0 ± 47.9) and cooked millet (288.8 ± 64.2) were the lowest. The peak time of cooked millet was 30 min, while that of others was 45 min. Similar to the blood glucose curve, the insulin concentration of cooked millet, followed by MP-1, was always apparently lower than that of standard glucose solution within 2 h. The maximum increase of blood insulin in cooked millet was just 49.1% of that in standard glucose solution and 43.3% of that in millet porridge.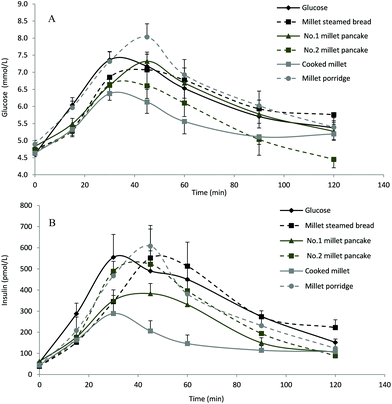 | ||
| Fig. 2 Mean (±SD) plasma glucose (A) and insulin (B) after ingestion of pure foxtail millet products or standard glucose solution. Error bars showed standard deviation among ten subjects. | ||
The GI, II and II to GI ratio (II/GI) were calculated (Table 4). The results showed that the GI of millet porridge was the highest, followed by MSB, MP-1, MP-2 and cooked millet. These findings were similar to the international tables which reported that the GI of millet flour porridge (Kenya) was 107,6 but apparently higher than the results provided by Yang et al. who found that the GI of millet porridge was just 61.5 ± 9.36 Many factors (such as food ingredients and processing methods) may result in the differences in starch digestibility and subsequently GI values for apparently similar foods.6,11,27 For instance, the published GI values of potatoes and potato-products varied from 23 to 144.3 The above difference can be attributed to the inherent botanical differences and methodological factors, especially the measurement of the available carbohydrate content. Among the five pure foxtail millet products, only cooked millet was classified as medium-GI food (from 55 to 70 on the glucose reference scale). MP-2, MP-1, MSB and millet porridge were all available in high-GI forms (70 or greater). Even so, the GI of foxtail millet was apparently lower than those of wheat and rice,6 and this result has been confirmed by the above investigation which showed the starch digestibility of foxtail millet flour was significantly lower than wheat flour no matter in raw materials or cooked ones.
Furthermore, the GI of pure millet produce was significantly positively correlated with DG (r = 0.967, p = 0.007), RDS (r = 0.959, p = 0.01) and eGI (r = 0.988, p = 0.002). But no significantly positive relationship between GI and RAG was observed. This may be due to different forms of raw materials: flour and grain. To verify this hypothesis, millet products were sorted for comparison based on their material forms and the correlation turned apparent. Our results suggested that to some extent the in vitro starch digestion was a reliable index of the in vivo postprandial glycemic responses for a certain kind of food. But for the complexity of the food matrix and gastrointestinal system, different kinds of food may be suitable for different predictions. Therefore, more wide and concrete work needs to be carried out before the in vitro results of a specific kind of food can be used in clinical applications or epidemiologic research.
Considering the fact that insulin resistance is a key feature of type 2 diabetes and metabolic syndrome, another objective of the present study was to evaluate the effect of specific food on blood insulin response. The II of five pure foxtail millet products followed the order: MSB > millet porridge > MP-2 > MP-1 > cooked millet. Just based on the fact that the insulin/glucose ratio may be used to evaluate β-cell response,37,38 the II/GI was defined to evaluate the insulin demand for a specific food (Table 4). The II/GI of MSB and the II/GI of MP-2 were larger than 1.0, which indicated that ingesting MSB and MP-2 may induce a strong stimulation to β-cell. That is, quite a large amount of insulin was needed after ingestion of MSB and MP-2. In contrast, the II/GI of MP-1 and the II/GI of cooked millet was smaller than 1.0, so after ingestion of such foods, there was no need for β-cells to secrete too much insulin, and the blood glucose can be maintained at a stable level. Coincidentally, the insulin AUC/glucose AUC, a similar concept to II/GI, has been used by Holt et al.,39 who have found that the AUC ratio of white pasta was more than twice of that of brown pasta and the protein-rich foods stimulated a large amount of insulin secretion relative to their glycemic responses. In conclusion, the cooked millet was the most suitable pure foxtail millet product for type 2 diabetics.
Conclusions
Blood glucose and insulin are essential for the health of both normal and diabetic subjects. Several prospective epidemiological studies have shown that diet, especially starch-based food, is crucial for maintaining homeostasis of blood glucose and insulin. Interestingly, results from this study confirmed that foxtail millet, as a kind of functional food, had a quite low GI value and a relatively gentle stimulation to β-cell. Moreover, different processing methods had a great influence on the digestibility and glycemic responses of foxtail millet, which suggested that in daily life, it is necessary for humans to select appropriate processing methods according to their own health conditions. Additionally, it is necessary to carry out further research studies about diet intervention with foxtail millet among pre-diabetics or diabetics. The investigation of the hypoglycemic effect of foxtail millet will be beneficial to promote the development of millet industry and to popularize the millet-based foods.Abbreviations
| MSB | Millet steamed bread |
| MP-1 | No. 1 millet pancake (75.0% millet flour and 25.0% extrusion flour) |
| MP-2 | No. 2 millet pancake (without extrusion flour) |
| RDS | Rapidly digestible starch |
| SDS | Slowly digestible starch |
| RS | Resistant starch |
| RAG | Rapidly available glucose |
| SAG | Slowly available glucose |
| DG | Degree of gelatinization |
| eGI | Estimated glycemic index |
| GI | Glycemic index |
| II | Insulin index |
Acknowledgements
The authors thank Chang Shu and Zenglong Chen for improving the language and providing valuable comments on this manuscript. This research was supported by the China Agriculture Research System (CARS-07-12.5-A17).References
- W. Yang, J. Lu, J. Weng, W. Jia, L. Ji, J. Xiao, Z. Shan, J. Liu, H. Tian and Q. Ji, N. Engl. J. Med., 2010, 362, 1090–1101 CrossRef CAS PubMed.
- K. N. Englyst, H. N. Englyst, G. J. Hudson, T. J. Cole and J. H. Cummings, Am. J. Clin. Nutr., 1999, 69, 448–454 CAS.
- B. Nayak, J. D. J. Berrios and J. Tang, Food Res. Int., 2014, 56, 35–46 CrossRef CAS PubMed.
- U. Lehmann and F. Robin, Trends Food Sci. Technol, 2007, 18, 346–355 CrossRef CAS PubMed.
- B. Jennie, S. Hayne, P. Petocz and C. Stephen, Diabetes Care, 2003, 26, 2261–2267 CrossRef.
- K. Foster-Powell, S. H. Holt and J. C. Brand-Miller, Am. J. Clin. Nutr., 2002, 76, 5–56 CAS.
- G. G. Gelders, J. P. Duyck, H. Goesaert and J. A. Delcour, Carbohydr. Polym., 2005, 60, 379–389 CrossRef CAS PubMed.
- L. I. Ezeogu, K. G. Duodu, M. N. Emmambux and J. R. Taylor, Cereal Chem., 2008, 85, 397–402 CrossRef CAS.
- E. H.-J. Kim, J. R. Petrie, L. Motoi, M. P. Morgenstern, K. H. Sutton, S. Mishra and L. D. Simmons, Food Biophys., 2008, 3, 229–234 CrossRef.
- S. Roopa and K. Premavalli, Food Chem., 2008, 106, 875–882 CrossRef CAS PubMed.
- J. Singh, A. Dartois and L. Kaur, Trends Food Sci. Technol, 2010, 21, 168–180 CrossRef CAS PubMed.
- A. S. Saleh, Q. Zhang, J. Chen and Q. Shen, Compr. Rev. Food Sci. Food Saf.., 2013, 12, 281–295 CrossRef CAS PubMed.
- H. Lu, J. Zhang, K.-b. Liu, N. Wu, Y. Li, K. Zhou, M. Ye, T. Zhang, H. Zhang and X. Yang, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 7367–7372 CrossRef CAS PubMed.
- C. Wang, G. Jia, H. Zhi, Z. Niu, Y. Chai, W. Li, Y. Wang, H. Li, P. Lu and B. Zhao, G3: Genes, Genom, Genet., 2012, 2, 769–777 CAS.
- S. Li and X. Luo, Compendium of Materia Medica:(Bencao Gangmu), Foreign Languages Press, 2003 Search PubMed.
- Y.-Y. Choi, K. Osada, Y. Ito, T. Nagasawa, M.-R. Choi and N. Nishizawa, Biosci., Biotechnol., Biochem., 2005, 69, 31–37 CrossRef CAS PubMed.
- L. Z. Zhang and R. H. Liu, Food Chem., 2015, 174, 495–501 CrossRef CAS PubMed.
- S. H. Lee, I.-M. Chung, Y.-S. Cha and Y. Park, Nutr. Res., 2010, 30, 290–296 CrossRef CAS PubMed.
- M. Pang, S. He, L. Wang, X. Cao, L. Cao and S. Jiang, Food Funct., 2014, 5, 1763–1770 CAS.
- F. Zhu, Food Res. Int., 2014, 64, 200–211 CrossRef CAS PubMed.
- C. AACC, Methods, 2000, 54, 21 Search PubMed.
- J. Frias, C. Vidal-Valverde, C. Sotomayor, C. Diaz-Pollan and G. Urbano, Eur. Food Res. Technol., 2000, 210, 340–345 CrossRef CAS.
- N. Englyst, S. Kingman and J. Cummings, Eur. J. Clin. Nutr., 1992, 46, S33–S50 Search PubMed.
- K. Liu and J. Han, J. Agric. Food Chem., 2012, 60, 4212–4221 CrossRef CAS PubMed.
- I. Goñi, A. Garcia-Alonso and F. Saura-Calixto, Nutr. Res., 1997, 17, 427–437 CrossRef.
- D. Jenkins, T. Wolever, R. H. Taylor, H. Barker, H. Fielden, J. M. Baldwin, A. C. Bowling, H. C. Newman, A. L. Jenkins and D. V. Goff, Am. J. Clin. Nutr., 1981, 34, 362–366 CAS.
- F. Brouns, I. Bjorck, K. Frayn, A. Gibbs, V. Lang, G. Slama and T. Wolever, Nutr. Res. Rev., 2005, 18, 145–171 CrossRef CAS PubMed.
- L. R. Brewer, L. Cai and Y.-C. Shi, J. Agric. Food Chem., 2012, 60, 4379–4387 CrossRef CAS PubMed.
- P. Belton, I. Delgadillo, N. Halford and P. Shewry, J. Cereal Sci., 2006, 44, 272–286 CrossRef CAS PubMed.
- G. A. Annor, M. Marcone, E. Bertoft and K. Seetharaman, Cereal Chem., 2013, 90, 211–217 CrossRef CAS.
- M. R. Debet and M. J. Gidley, Carbohydr. Polym., 2006, 64, 452–465 CrossRef CAS PubMed.
- K. N. Englyst, S. Vinoy, H. N. Englyst and V. Lang, Br. J. Nutr., 2003, 89, 329–339 CrossRef CAS PubMed.
- H.-J. Chung, Q. Liu, R. Hoover, T. D. Warkentin and B. Vandenberg, Food Chem., 2008, 111, 316–321 CrossRef CAS PubMed.
- E. de la Hera, C. M. Rosell and M. Gomez, Food Chem., 2014, 151, 526–531 CrossRef CAS PubMed.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carrière, R. Boutrou, M. Corredig and D. Dupont, Food Funct., 2014, 5, 1113–1124 CAS.
- Y.-X. Yang, H.-W. Wang, H.-M. Cui, Y. Wang, L.-D. Yu, S.-X. Xiang and S.-Y. Zhou, World J. Gastroenterol.: WJG, 2006, 12, 3430–3433 Search PubMed.
- R. A. DeFronzo, J. D. Tobin and R. Andres, Am. J. Physiol.-Gastr. L, 1979, 237, G214–G223 Search PubMed.
- R. L. Hanson, R. E. Pratley, C. Bogardus, K. V. Narayan, J. M. Roumain, G. Imperatore, A. Fagot-Campagna, D. J. Pettitt, P. H. Bennett and W. C. Knowler, Am. J. Epidemiol., 2000, 151, 190–198 CrossRef CAS.
- S. Holt, J. Miller and P. Petocz, Am. J. Clin. Nutr., 1997, 66, 1264–1276 CAS.
| This journal is © The Royal Society of Chemistry 2016 |