Ionic liquids as tailored media for the synthesis and processing of energy conversion materials
Gebrekidan Gebresilassie
Eshetu
ab,
Michel
Armand
c,
Hiroyuki
Ohno
d,
Bruno
Scrosati
*abd and
Stefano
Passerini
 *ab
*ab
aHelmholtz Institute Ulm (HIU), Helmholtzstrasse 11, 89081 Ulm, Germany. E-mail: stefano.passerini@kit.edu; bruno.scrosati@gmail.com
bKarlsruhe Institute of Technology (KIT), P.O. Box 3640, 76021 Karlsruhe, Germany
cCIC Energigune, Parque Tecnologico de Alava, Albert Einstein 48, ED. CIC, 01510, Minano, Spain
dDepartment of Biotechnology, Tokyo University of Agriculture and Technology, 2-24-16 Nakacho, Koganei, Tokyo 184-8588, Japan
First published on 22nd December 2015
Abstract
Though in its infancy stage, ionic liquid (IL)-assisted synthesis and processing of energy conversion materials is triggering a wide interest to eco-friendly production of already existing and novel materials. ILs possess the potential of overcoming the limitations of conventional synthesis approaches. Due to their unique characteristics such as high chemical and thermal stabilities, nearly negligible vapour pressure, wide electrochemical window, broad liquidus range, tunable polarity, hydrophobicity/hydrophilicity, ionic liquids are opening new frontiers in the ionothermal synthesis of tailored materials, enabling products that are difficult or impossible to achieve by using other, more conventional preparation routes. Trying to offer an exhaustive review on the burgeoning role of ionic liquids for the synthesis and processing of energy conversion materials, this perspective article focuses on IL-assisted production of electrode materials and catalysts for fuel cells, photo-induced water splitting and dye-sensitized solar cells. A brief excursion on the use of ionic liquids for the processing of natural fuels for biofuel cells is also made. The state-of-the-art research endeavours are firstly evaluated, followed by the exploration of future research directions with some thoughts on emerging challenges and opportunities.
Broader contextThe advent of ionic liquids (ILs) as eco-friendly and designer reaction media has opened new avenues in the field of energy storage and conversion devices. ILs offer many distinct advantages, such as negligible vapour pressure, good thermal stability, wide liquidus range, broad electrochemical window, and high synthetic flexibility. These peculiar properties endow ILs as designer reaction media in production and processing of highly engineered and tailored products via acting as advanced solvents, precursors, charge-compensating groups, and structure-directing/inducing agents. IL-assisted synthesis help to obtain task-oriented specific materials with non-typical features, suitable for a variety of specific applications. The synthesis and processing of energy conversion materials is one of the most rapidly growing applications of ILs. Herein, a critical assessment of the current status quo of the application of ILs in the fabrication and processing of materials for use in fuel cells, photoelectrochemical water splitting, dye sensitized solar cells (DSSC), and biofuels is made. This review also critically highlights crucial issues that should be addressed and provides perspectives for the future research directions in the field. |
1. Introduction
Global environmental issues associated with climatic changes induced by greenhouse gases are dramatic concerns of today's energy-conscious society.1–4 The ever-increasing world population and its increasing affluence with associated higher energy consumption put huge pressure for expanding our current energy infrastructure, which has been, and still is, heavily relying on limited and environmentally unfriendly sources, such as coal, oil and gas. The increasing atmospheric pollution, global warming, depletion of fossil-fuels and lack of energy security, represent the most important imperatives to be urgently addressed for a sustainable future. These challenges can only be met through the development of new materials, systems and technologies, enabling the transformation of environmentally friendly and renewable energy sources, such as sunlight, wind and biomass. However, all these sources rely on the availability of novel materials and systems capable of exploiting their full possibilities to the grand energy challenge. For instance, electrical energy is converted from chemical energy in fuel cells and from light in photovoltaics. Chemical energy, on the other hand, can be generated from water splitting by using appropriate catalysts. Key to the effective performance of all the aforementioned conversion systems are the materials that rule the primary process, such as catalysts and electrode materials in fuels cells, semiconductors in photovoltaics, processing strategies in biofuels, and so on. In this respect, the procedure for the materials fabrication plays a key role in tuning their properties and performance. Amid the synthetic routes reported in literature, the ionothermal method is expected to be economically competitive due to the low processing temperature coupled with its eco-friendliness. Indeed, this method has gained great attention in the materials science community since it allows obtaining and processing a wide range of attractive high performance energy conversion materials, otherwise not accessible via conventional synthesis approaches. As a result, ionothermal synthesis routes, utilizing ionic liquids (ILs) as tailored and green reaction media, have opened new frontiers in the field of energy storage and conversion devices. In a recent review,5 we have shown the impact of IL-based synthesis and processing on electrochemical energy storage devices, such as batteries and supercapacitors. Here we complete the information by critically discussing the role of ionic liquids on the preparation of materials for the development of other classes of timely important technologies, including fuel cells (comprising PEMFCs, DMFCs and SOFCs), dye sensitized solar cells (DSSC), water splitting and biofuel systems and processing.ILs can be broadly defined as molten salts formed by the combination of weakly coordinating cations and anions, with a melting point well below 100 °C.6–10 The small lattice enthalpies and large entropy changes, resulting from the large size and conformational flexibility of the weakly coordinating ions, favor the occurrence of the liquid state.9,11 Accordingly, some ILs possess unique physical properties compared to conventional electrolyte media, e.g., organic solvents, including, among others, negligible vapor pressure (thus, low flammability), high thermal stability, broad liquidus range, tunable electrochemical window, and ability to dissolve and process a wide range of materials (e.g., synthesis precursors, enzymes) as well as to stabilize metastable compounds.10–13 Such a wide range of unique properties endows ILs as suitable alternatives to molecular solvents for the synthesis of engineered materials, by acting as advanced solvents, reactants, dopants, as well as capping, templating agents and catalysts. For instance, owing to a series of beneficial aspects, electrochemical deposition is usually rated as the most convenient method to fabricate semiconducting materials for energy conversion (e.g., water splitting). However, electrodeposition is strongly temperature-dependent since throwing power and film quality are enhanced by operating at high temperatures in order to prevent yields of discrete materials being affected by poor oxygen evolution efficiency, as instead is the case for low temperature operations. Conventional aqueous, and even, non-aqueous electrolytes (e.g., used in the SIGAL process14), do not allow high deposition temperatures, and, consequently, the search for thermally stable media has been one of the top priorities in the field. In this regard, IL-based electrolytes have been identified as perfect candidates due to their high thermal stability, as well as to their ability to allow atom diffusion on the surface during growth, a factor which positively influences the quality of the final film.15–19 Another bonus of ILs is that theoretically they can be formed by unlimited cation/anion combinations. While the number of conventional solvents used in industry is of the order of about 6 × 102, that of ILs that can be simply prepared in laboratory exceed 106, this resulting in more than 1012 binary and about 1018 ternary combinations. This in turn provides the opportunity to generate the required system of ILs of improved reactivity, viscosity, solubility, purification and recyclability, and thereby open their way towards industrial applications. However, the life-cycle cost, sensitivity to contaminants, more efficient and scalable commercial synthesis, precise recyclability data, green and optimized distillation and recyclable strategies are still crucial in evaluating ILs viability in commercial applications. In other words, there is a long way to go before large-scale implementation of ILs for the fabrication of energy conversion materials and their eco-friendly processing. However, despite the excitement behind the use of ILs-mediated syntheses, the methodology is yet at its early stage being still affected by a series of issues, such as high cost and lack of well documented toxicity data. Nevertheless, these apparent drawbacks appear to be compensated by recyclability and re-usability, actions that can be performed without affecting the quality of the resulting products, as in fact demonstrated in practice by the well-known BASIL process, see also later discussion in Section 3.
Here we will critically evaluate these aspects by discussing the use of ionic liquids for the synthesis of various timely and relevant materials outlining the technological importance, as well as the issues which may possibly affect their application for large scale production and at the same time, providing suggestions on how to successfully address them.
For the sake of convenience for readers, the acronyms/abbreviations along with the descriptions of ionic liquids utilized in this review manuscript are provided in Table 1.
| Acronyms/abbreviation | Ionic liquid |
|---|---|
| [EMI][TFSI] | 1-Ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide |
| [BMI][TFSI] | 1-Butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide |
| [MTBD][BETI] | 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene-bis(pentafluoroethanesulfonyl)imide |
| [BMP][DCA] | N-Butyl-3-methylpyrrolidinium cyanamide |
| [C4mim][Br] | 1-Butyl-3-methylimidazolium bromide |
| [3-MBP][DCA] | 1-Butyl-3-methylpyridinium dicyanamide |
| [C16mim][Br] | 1-Hexadecyl-3-methylimidazolium bromide |
| [BMP][TF2N] | 1-Butylpyrrolidinium bis(trifluoromethanesufonyl)imide |
| [C4mim][PF6] | 1-Butyl-3-methylimidazolium hexafluorophosphate |
| [C4mim][CH3CO2] | 1-Butyl-3-methylimidazolium acetate |
| [C2mim][Cl] | 1-Ethyl-3-methylimidazolium chloride |
| [C4mim][BF4] | 1-Butyl-3-methylimidazolium tetrafluoroborate |
| [Cmim][HSO4] | 3-Carboxymethyl-1-methylimidazolium bisulfate |
| [C4C1Py][TF2N] | 1-Butyl-3-methylpyridinium bis(trifluoromethanesulfonyl)imide |
| [C8mim][PF6] | 1-Octyl-3-methylimidazolium hexafluorophosphate |
| [VEI][NO3] | 1-Vinyl-3-ethylimidazolium nitrate |
| [C2mim][DCA] | 1-Ethyl-3-methylimidazolium dicyanide |
| [Ch Sac] | Cholinium saccharinate |
| [C4mpyr][DCA] | N-Butyl-N-methylpyrrolidinium dicyanide |
| [Ch][dhp] | Cholinium dihydrogen phosphate |
| [C4mim][dhp] | 1-Butyl-3-methylimidazolium dihydrogen phosphate |
| [Ch][dbp] | Cholinium dibutyl phosphate |
| [C4mim][CH3CO2] | 1-Butyl-3-methylimidazolium acetate |
| [C4mim][MeSO4] | 1-Butyl-3-methyl imidazolium methanesulfonate |
| [Ch][Lac] | 1-Butyl-3-methylimidazolium lactate |
| (Hy[Ch][dhp]) | Hydrated choline dihydrogen phosphate |
| [Dimim][MeO][H]PO2 | 1,3-Dimethylimidazolium methylphosphonate |
2. Synthesis of energy conversion materials
2.1. Catalysts for fuel cell technologies (FCs)
From the synthesis methodology perspective, ILs have emerged as tailorable media for the fabrication of highly active, molecularly engineered electrocatalysts, since they benefit by a series of relevant specific advantages, including: (i) the production of materials with very low particle size, due to low surface tension, resulting in a slow coarsening during the growth phase; (ii) electro-deposition at high temperature, due to the high thermal stability; (iii) flexibility in the solubility of precursors, due to the tunable polarity, and (iv) rapid crystal growth in microwave (MW) synthesis with a high susceptibility. In the following paragraphs, the role of IL-based synthesis is discussed for the various types of fuel cells.
Carbon nanomaterials (e.g., graphene) doped with heteroatoms, due to a series of specific features, which include excellent electrocatalytic activity, high conductivity and large surface area,25–27 are among the strongest contenders for use as metal-free catalysts in PEMFCs. However, the available synthetic methods such as chemical vapor deposition (CVD), arc discharge, segregation growth, Hummer's oxidation followed by hydrazine (N2H4) reduction or N-doping by nitrogen plasma treatment, require rigorous reaction conditions such as high temperature and vacuum, as well as the use of highly toxic and explosive reagents, and possible metal contaminants. Thus, the fabrication of trace-metal-free N-doped-graphene via low energy and benign synthesis conditions is of paramount importance. In this regard, Lu et al.28 showed a clever way of fabricating N-doped graphene via electrochemical exfoliation, followed by in situ nitrogen doping of graphite, using nitrate (ethyl ammonium nitrate – EAN)-based ILs, serving both as exfoliating and doping agent. The electrochemical exfoliation of graphite in the presence of EAN, containing 10% (v/v) water, resulted in a metal-free, N-doped graphene having an excellent catalytic activity towards ORR. The presence of water facilitates the degree of exfoliation via oxidation, generating oxygen species. While the nitrate ions of the ionic liquid are responsible for the fast and low stage intercalation, the ethylammonium cations serve for the in situ N-doping of the electrogenerated intermediate material.
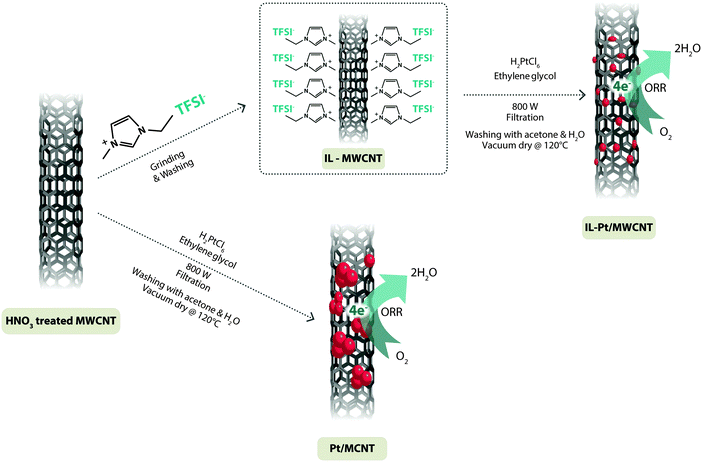
The sluggish ORR kinetics can be further improved by engineering the coating of the nanoparticle catalysts via ionic liquids, as demonstrated in the case of IL-impregnated nanoporous Ni–Pt, where ILs have been efficaciously applied to engineer metal–Pt alloy coatings.29 It is important in this respect to point out that the incorporation of the hydrophobic, but protic [MTBD][BETI] into the pores of the high surface-area Ni–Pt alloy nanoparticles leads to consistent improvements in the kinetics of ORR.29,30 The high O2 solubility in [MTBD][BETI], combined with the confined environment (due to encapsulation in Ni/Pt nanoparticles), boosts the attempt frequency, namely the parameter that characterizes the rate of surface diffusion through a jumping process of adatoms residing at adsorption sites to nearer-neighbour ones, thus finally enhancing the ORR rate. Indeed, the IL-impregnated Ni–Pt carbon-supported nanoporous nanoparticle catalyst (IL–Ni–Pt/C) shows a catalytic activity one order of magnitude higher than that of commercial Pt/C. Another evidence of the key role of ILs for the synthesis of PEMFC catalysts is provided by the work of Hasché et al.,31 who evaluated mesoporous N-doped, carbon-supported Pt catalysts, synthesized in n-butyl-3-methylpyridinium dicyanamide, [BMP][DCA], which acts as N and C precursor. Compared to the benchmark commercial Pt ones, the new catalyst fabricated via the IL-assisted method presented a comparable activity, however, with a consistent enhancement in the electrochemical active surface area (ECSA) and long-term stability, thus, finally confirming its suitability for promoting ORR. Finally, improvements in the ORR kinetics in PEMFCs, have been also reported for other electrocatalysts such as Pt-loaded carbon aerogels,32 and IL-functionalized MWCNTs decorated with Au nanoparticles.33
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio) at room temperature. When tested as catalyst in a DMFC, these particles showed a much superior peak current density than that obtained by using a single element, i.e., 9.56 mA cm−2versus 0.17 mA cm−2 (Au only) and 1.93 mA cm−2 (Pt only). The corresponding mass activity of the Au–Pt catalyst is 3.2 times higher than that reported for the Pt/C, i.e., 133.5 mA mg−1versus 41.1 mA mg−1. The key role of the IL in promoting the synthesis of the Au–Pt bimetallic particles was finally demonstrated by comparing their catalytic activity when prepared by a similar, but [C4mim][Br]-free procedure.
3 ratio) at room temperature. When tested as catalyst in a DMFC, these particles showed a much superior peak current density than that obtained by using a single element, i.e., 9.56 mA cm−2versus 0.17 mA cm−2 (Au only) and 1.93 mA cm−2 (Pt only). The corresponding mass activity of the Au–Pt catalyst is 3.2 times higher than that reported for the Pt/C, i.e., 133.5 mA mg−1versus 41.1 mA mg−1. The key role of the IL in promoting the synthesis of the Au–Pt bimetallic particles was finally demonstrated by comparing their catalytic activity when prepared by a similar, but [C4mim][Br]-free procedure.
As part of the research efforts towards the development of metal-free catalysts, it worth to stress that heteroatom (N, S or B)-doped carbon-based catalysts have shown promising features, such as high immunity towards methanol crossover (i.e., they do not catalyze the oxidation of CH3OH directly by oxygen), long-term stability, low cost and high electrocatalytic activity for ORR. Han et al.35 successfully demonstrated the synthesis of N-doped hollow carbon spheres using ILs with a nitrile group anion/cation as carbon precursor and silica as the templating agent. The obtained material, having high nitrogen content (∼10.9%) and degree of graphitization, combined with other features, demonstrated an excellent electrocatalytic activity with ∼4e− transfer, super-immunity towards methanol crossover, and a stability superior to commercial Pt/C. In addition, high surface area S and N-co-doped hierarchical porous carbon, obtained using [BMP][TF2N] as N, S and C precursors, showed improved catalytic activity, high methanol crossover tolerance, and good thermal stability.36 The ORR potential peaks were found to be directly related to the heteroatom loading, demonstrating the need to have a N/S optimal ratio for obtaining the best catalytic activity when both dopants were used.
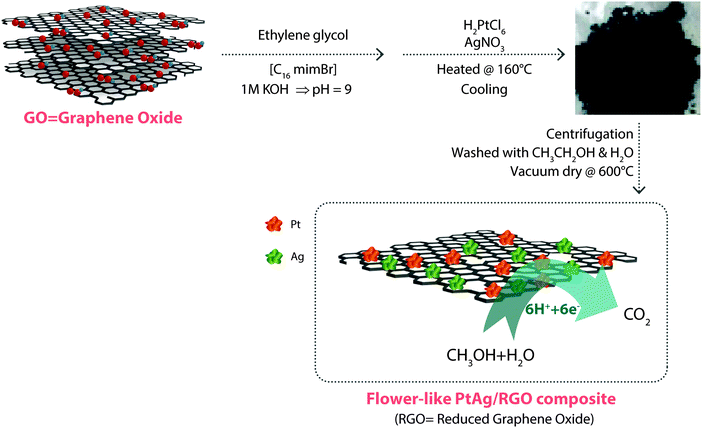
It has been reported that graphene, due to its high surface area, can be exploited as an active support to load noble metals, such as Pt, Au, Ag or bimetallic alloys (e.g., PtAg), for use as catalysts in DMFCs. For example, PtAg nanostructures decorated on reduced graphene oxide (PtAg/RGO) have been synthesized again using an IL, [C16mim][Br], which serves both as surface-capping and structure-directing agent.37 The IL-prepared PtAg/RGO nanocomposite has a large electrochemically active surface area, namely, 92% and 16%, higher than that of Pt/C and Pt-black, respectively, as well as higher catalytic activity, methanol tolerance and long-term durability for ORR in alkaline solution. Fig. 2 illustrates the IL-based synthesis of PtAg-decorated graphene.
Another promising approach towards the fabrication of highly efficient hybrid nanocatalysts for DMFC sees the direct mixing of IL-functionalized carbon nanotubes (CNTs) with metal (e.g., Pt) precursors.38 The imidazolium group, attached through N,N′-dicyclohexyl carbodiimide (DCC) coupling with the counter anion (PtCl42−), plays a crucial role in the formation of IL–CNTs/Pt hybrid materials. More recently, Do et al.39 reported the synthesis of GnP-supported Pt catalysts (Pt/GnP) by using a microwave process based on [C4mim][PF6] and [C4mim][CH3CO2] and using H2PtCl6·6H2O as Pt precursor and ethylene glycol (EG) as reducing agent. The IL addition to EG–GnP after that of the Pt precursor assured a uniform distribution of Pt on GnP, as promoted by the direct interaction of Pt ions with the π sites on the GnP basal plane and residual oxygen groups at the GnP edge surface. The size of Pt nanoparticles was found to be controlled by the polarity of the ILs, as well as by the IL-/GnP molar ratio. For instance, deposition in [C4mim][CH3CO2] produced small Pt nanoparticles, due to the higher basicity of the CH3CO2− anion with respect to PF6−, this being associated with its unsymmetrical arrangement of oxygen atoms. Electrochemical comparisons of IL-assisted Pt/GnP and commercial Pt/XC-72R catalysts towards methanol oxidation revealed that the former exhibited much higher (an 80% increase) catalytic activity, in turn ascribed to the higher dispersion of Pt particles and more favorable Pt-support interaction with GnP. Finally, it is here worth noting that a large number of electrocatalysts for use in DMFCs have been obtained via the ionothermal synthesis route. The most important of them are PtRu,32 Pt/C,40 C–PtAu-nanoparticle hybrids,41 flower-like Pd nanoparticles, Pt–WC/TiO2,42 polymeric ionic liquids (PIL)/Pt nanoparticle hybrids,43 CuPt–/IL–graphene,44 Pt/XC-72 coated N-doped carbon (Pt/XC-72@C–N),45 mixed ILs/graphene-supported Pt nanoparticles,46 Pt–Ru/C,47 and Pd/C.48
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio, followed by controlled oxidation, a highly performing nanostructured Au–Dy2O3 composite electrocatalyst could be easily obtained.51 In general, though fuel cells are considered as relatively exotic technologies, they are not yet a major commercial success. This is mainly attributed to their high cost, durability and related issues. IL-mediated synthesis has demonstrated to enable the synthesis of designable catalysts, one of the critical issues that needs to be solved with FCs. Moreover, the question of how well the synthesis methodology can be scaled up from the laboratory to large scale needs to be critically addressed.
2 molar ratio, followed by controlled oxidation, a highly performing nanostructured Au–Dy2O3 composite electrocatalyst could be easily obtained.51 In general, though fuel cells are considered as relatively exotic technologies, they are not yet a major commercial success. This is mainly attributed to their high cost, durability and related issues. IL-mediated synthesis has demonstrated to enable the synthesis of designable catalysts, one of the critical issues that needs to be solved with FCs. Moreover, the question of how well the synthesis methodology can be scaled up from the laboratory to large scale needs to be critically addressed.
| 2H2O → O2 + 4H+ + 4e− | (1) |
| 4H+ + 4e− → 2H2 | (2) |
| 2H2O → 2H2 + O2 | (3) |
Photoelectrochemical water splitting can be regarded as a cost-effective, convenient and simple technique, which involves the simultaneous oxidation/reduction of oxygen (eqn (1)) and hydrogen (eqn (2)) with the production of the two gases (eqn (3)). Compared to that associated with hydrogen production, the oxidation reaction, also denoted the oxygen evolution reaction (OER), is the most sluggish and rate-determinant step, indeed requiring an overpotential exceeding 0.45 V to drive the reaction at useful rates, which results in a substantial energy loss, usually as heat. This clearly accounts for the tremendous efforts recently devoted to the search of effective and tailor-made electrocatalysts based on earth-abundant elements (e.g., Mn, Co), aimed to allow the replacement of the precious metals (e.g., RuO2, IrO2,…)-based catalysts currently used in commercial electrolysers.
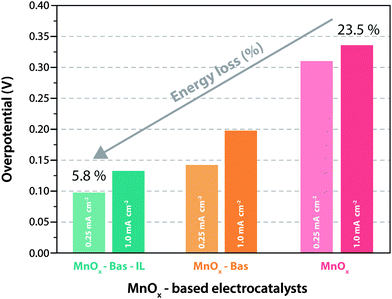
In this scenario, particular attention has been addressed to manganese oxides, MnOx (x = 1.5–2), which have been synthesized using a number of approaches, including precipitation, hydrothermally, sol–gel route, classical aqueous electrode deposition, etc. Once again, ILs have shown fascinating outcomes as solvents/electrolytes for the preparation of MnOx and related catalysts. A relevant example is provided by Zhou et al.53 who recently reported the use of ethylammonium nitrate as electrolyte for the electrosynthesis of MnOx layers at moderate temperatures (100–150 °C). This process was made possible only by exploiting an ionic liquid (ethylammonium nitrate, EAN), which in fact, allows the use of water (reactant) at high temperatures without need of a high-pressure vessel. As it can be seen from Fig. 3, the IL-synthesized catalyst presented a very good electrochemical performance, allowing significantly high currents at low overpotentials with an energy loss limited to 5.8%, i.e. a value much lower than the 23.5% associated with the production of MnOx-based catalyst via conventional synthesis routes. Unfortunately, the practical application of this catalyst is still hindered by its degradation over a relatively short operation time due to the fact that MnOx, as like the majority of the transition metal-based oxides, is affected by the oxo-anion solubility under prolonged exposure at high (oxidizing) potentials in aqueous solutions. This issue has been addressed attempting the stabilization of the metal oxide-based catalyst by developing water-insoluble multi-layer structures, again with the assistance of ionic liquids, as shown by Izgorodin et al.54 who demonstrated the incorporation of phosphates into the MnOx surface at high concentration of phosphate ions, enabling a suitable stabilization of the oxide surface, hence assuring its prolonged catalytic activity.
Still with the support of ethylammonium nitrate, EAN, Zhou et al.55 succeeded in electrodepositing a variety of MnOx phases having different catalytic performance, depending on the acidity and/or alkalinity of the IL used as the electrolyte. In this respect, while the film deposited from a highly basic medium mainly resulted in the hausmannite, spinel-structured (Mn3O4), those from slightly alkaline, acidic and neutral electrolytes, yielded Mn2O3 and birnessite-like MnOx films, having the highest catalytic activity for water oxidation among all those deposited from a neutral electrolyte. Recently, a rational design of highly performing boron (B)- and fluorine (F)-doped 2D-graphitic carbon nitrides (g-CN) via self-polymerization of urea in [C4mim][BF4] IL has also been reported.56 In this synthesis, the urea is used as delamination agent, while the IL serves as a multifunctional modifier to control the texture and surface chemistry, as well as to tune the semiconductor properties of the g-CN imparted by B and F doping. Finally, IL-assisted of efficient electrocatalysts, such as self-organized TiO2 nanotubes,57 TiO2 nanotube arrays,58 Ag/Pd nanoalloys,59etc. have also been reported to be effective novel catalysts in photoinduced water splitting.
The standard Gibbs free energy change (ΔG) for water splitting into H2 and O2 is +237.2 kJ mol−1, which corresponds to 1.23 eV per electron transferred, indicating that to run the overall process, a semiconductor photoelectrode with a band gap wider than 1.23 eV, along with suitable band edge positions, is needed. Owing to its chemical and photostability, environmental-friendly nature and low cost, titanium oxide, TiO2, is in principle a very appealing candidate. However, its wide band gap (over 3 eV) and the rapid recombination rate of photogenerated electron–hole pairs, is reflected in a very low efficiency of the overall H2 production. The recombination issue has been addressed by developing carbon–TiO2 composites, which provide enhanced interfacial contact with adsorbates. A recent example is provided by a TiO2/reduced graphene oxide (RGO) nanocomposite, prepared via the hydrothermal method using [C4mim][PF6] IL as a solvent. While the graphene sheets act as electron transfer channels, reducing the recombination of the photogenerated electron–hole pairs, ILs serve as tailorable solvents for yielding a material with engineered morphology and boosted properties. The obtained TiO2/RGO showed in fact, a much higher efficiency of hydrogen production than that achievable by composites synthesized making use of traditional solvents.60
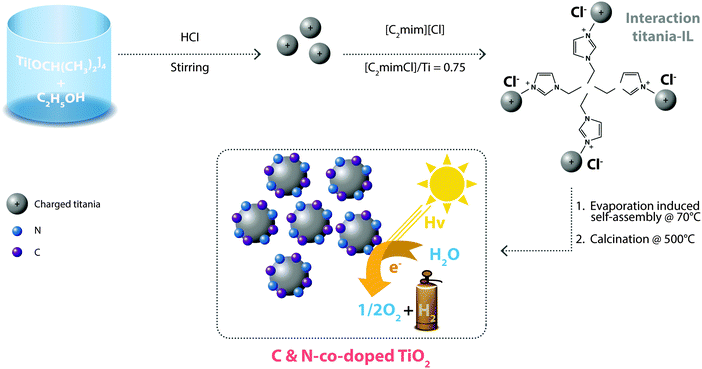
Surface modification of TiO2 by doping with heteroatoms is another proposed way to improve its photocatalytic activity. This strategy involves the preparation of nitrogen (N) and carbon (C) co-doped mesoporous TiO2via an evaporation-induced self-assembly method using [C2mim][Cl] ionic liquid, as schematically shown in Fig. 4. C and N co-doping are expected to tune the band gap, making it decrease to a value of 2.92 eV, compared to 3.2 eV for commercial TiO2, reflected by a significant increase in the absorbance edge and intensity of visible light.61 All these material improvements lead to a photocatalytic hydrogen generation of 81.8 μmol g−1 h−1, which is ∼37 times higher than that achievable by commercial TiO2. Other important and highly performing electrocatalysts that have been obtained by IL-assisted synthesis include: mesoporous TiO2 (using [C4mim][PF6]62,63 and [C4mim][BF4]64), mesoporous CeO2–TiO2 ([C16mim][Br]65) and TiO2/RGO ([C4mim][PF6]66).
2.2. Dye sensitized solar cells (DSSCs)
The dye-sensitized solar cell (DSSC), also occasionally referred to as the “2nd generation PV” or “Grätzel” cell,67 has the prospective to be technically and economically more viable than traditional semiconductor-based photovoltaics (PVs). A typical DSSC device consists of three components: the dye-sensitized, nanoparticle TiO2 photoanode, the redox electrolyte (most commonly iodide/triiodide), and the conducting, electrocatalytic layer, acting as counter electrode. Even if DSSCs use a number of the above described advanced dye and electrode materials, they are still less costly compared to the silicon-based solar cells, especially in terms of manufacturing steps.Due to these expected favorable properties, DSSCs have attracted considerable attention both in academic and industrial laboratories. However, despite the large efforts spent in developing the related technology, DSSCs are only commercialized on a small scale, since the required improvements in efficiency (presently limited to 13%68) and the stability requirement (<10% drop in performance after 1000 hours aging at 60 °C) are still key targets to be met. One promising strategy to address these issues is to optimize the porous-structured oxide film photoanode with the aim of enhancing its light absorption. In this scenario, a number of binary (e.g., TiO2, ZnO, SnO2) and ternary (BiOX, X = Cl, Br, I) oxide photoanodes have been developed, using IL-assisted fabrication, either alone or in synergy with microwave, ultrasound and other similar synthesis techniques.69
Optimized anatase TiO2, i.e., the photoanode of choice for DSSCs, may be prepared by sol–gel using [C4mim][BF4] IL as templating agent, so as to obtain a material characterized by high surface area, stable crystal structure, controlled porosity, tailored-designed pore size distribution, and good thermal stability.70 Due to these unique structural and morphological features, this modified TiO2 photoanode could benefit by larger absorption of dye molecules and longer electron lifetime, compared to commercial, non-porous TiO2, this finally allowing its operation with higher photocurrent, photovoltage and efficiency. In addition, the photocatalytic activity of this IL-prepared nanocrystalline TiO2 was found be at 100 °C comparable with that of standard TiO2, however obtained at the much higher temperature of 400 °C.
The best DSSC performance, in terms of short-circuit photocurrent (13.15 mA cm−2) and power conversion efficiency, was in fact achieved using TiO2 films having a nanoflower morphology, as that obtained with a [Cxmim][HSO4] IL-assisted synthesis.71 Another electrode choice for application in DSSCs is zinc oxide, ZnO, a material that also greatly benefits by IL-based synthesis. In this respect, it was found that the morphology of the IL-assisted prepared ZnO material varies from rod-like to star-like and flower-like depending on the nature of the ILs utilized.72 The control of the surface, which is assumed to play an important role in favouring the charge transfer mechanism at the ZnO-based heterojunction, is another aspect recently receiving attention. Amongst all the suggested strategies to enhance the surface properties, the fabrication of ZnO–organic hybrid materials has been reported to be a very promising approach because the complementary benefits of ZnO and the organic moieties can judiciously be combined to tune the formed material.73 In this regard, ILs can again be the electrolytes of choice, not only because of their self-assembling and templating properties, but also as convenient sources of ions which may interact electrostatically with the inorganic solid oxide surface, so as to promote its tuneable functional properties. An impressive proof of concept on the use of ILs as all-in-one (i.e., reaction media, morphology templating agent, and surface modifier) media was recently reported by Azaceta et al.,73 who, by exploiting this route, succeeded in preparing a nanostructured ZnO/[C4C1py][TF2N] having very promising properties as DSSC anode in terms of power conversion efficiency, electrochemical performance, extended absorption edge, and open circuit voltage. For instance, the power conversion efficiency recorded for the ZnO–IL hybrid film increased by a factor of about 2, compared to the IL-free ZnO one.
2.3. Ionic liquids for biofuel processing
Biofuel cells, see scheme of Fig. 5, are attracting a continuously growing interest. There are a few different strategies to prepare these cells whose function and performance depend on the materials used as catalysts, among which, enzymes are the most efficient. There are many studies on the fabrication and performance analysis of enzyme-based biofuel cells, but all of them are carried out in aqueous (buffer) solutions. In spite of being the best medium for biosystems, water is not a suitable solvent from the viewpoint of the required performance. Aqueous solutions, in fact, easily evaporate and require pasteurization to retain cleanliness, especially when used under open air. Accordingly, the challenge in the field is to replace these solutions with more efficient solvents. Ionic liquids, due to their properties of non-volatility and high ionic conductivity, are potentially excellent candidates. However, there is a critical requirement for the use of IL-based biofuel cells, namely whether they can allow the operation of enzymes. At present, there is no ionic liquid that completely satisfies this tough condition; however, several studies on enzyme-based biofuel cells are under way to address this issue. There are several challenges to dissolve proteins in ionic liquids. For instance, while lipase, a rather strong enzyme, can be stably dispersed in some polar ionic liquids still keeping its enzymatic activity, there are only few reports on the dissolution of active proteins, such as cytochrome C, in pure ionic liquids. Though a few chloride-based ILs have a low melting point, chloride generally forms strong H-bonds with proteins amide linkages which favour dissolution. Along this line, 1-allyl-3-methylimidazolium cation was coupled with a chloride anion to prepare a low melting point polar ionic liquid in which cytochrome C was reported to be soluble at 80 °C with a redox activity detected up to 140 °C.74 It was also reported that both the π* and β values, i.e., the Kamlet–Taft parameters representing polarizability and hydrogen bond basicity, respectively, of ionic liquids are in fact sufficiently high to dissolve cytochrome C. On the other hand, the overall stability of proteins is not sufficiently long in pure ionic liquids.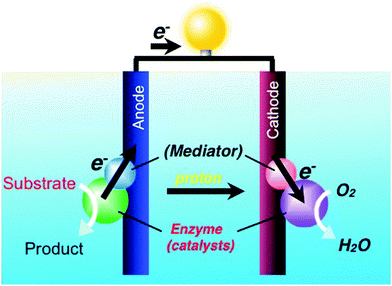 | ||
| Fig. 5 Schematic representation of the operating mechanism of a biofuel cell. The biofuel requires preliminary processing which can be proficiently performed in IL media. | ||
Therefore, various strategies have been attempted to address this issue. Fujita et al. have reported that cholinium dihydrogen phosphate mixed with small amount of water (Hy-IL) is an excellent solvent for proteins.75 Such a Hy-IL contains 3–5 water molecules per ion pair, all of them strongly hydrated to ions. Consequently, Hy-IL has no unbound water and thus, it can be regarded as an ionic liquid derivatives. It has been reported that the electron transfer activity of cytochrome C dissolved in cholinium dihydrogen phosphate, containing a small amount of water, maintained for more than one year. This exceptional long shelf life is suitable for application in enzyme-based biofuel cells. Indeed, Fujita et al. succeeded, for the first time, to obtain the enzymatic oxidation of cellobiose with cellobiose dehydrogenase (CDH) in the Hy-IL, with two electrons taken from cellobiose to the CDH, and further transferred to cytochrome C, to finally promote its oxidation, as spectroscopically demonstrated.76 It was also shown that some metalloproteins, such as horseradish peroxidase, ascorbate oxidase, azurin II and fructose dehydrogenase, all dissolve in the Hy-IL with enzymatic activity.77 Although some property changes depending on their species cannot be excluded, most of these enzymes in the Hy-IL showed an improvement in thermal stability with respect to that obtained in buffered aqueous solutions. Harvesting electrons from saccharides by corresponding dehydrogenases in the Hy-IL, was also reported to be a successful strategy to obtain prototypes of enzyme-based biofuel cells.78 Ohno et al. reported that ionic liquids may dissolve cellulose,79 as well as promoting its extraction not only from biomass,80,81 but also even from wood,82 finally demonstrating that exploiting ionic liquids is a powerful method to make biofuel cells become a practical reality. On this line, it is expected that in the near future grass, wood chips, used papers and even cotton T-shirts may be thrown into ionic liquids to generate electrons with the aid of enzyme-based biofuel cells.
3. Challenges, prospects and opportunities
In this review, we have shown that tailorable ionic liquids show some potential for assisting the production and processing of energy conversion materials. Table 2 summarises all materials presented in this review.| Material obtained | IL(s) used | Role of the IL |
|---|---|---|
| IL–Pt/CNT | [EMI][TFSI], [BMI][TFSI] | Reaction media |
| N-Doped graphene | EAN-based IL + 10% (v/v) H2O | Exfoliating agent and in situ dopant |
| (IL–Ni–Pt)/C | [MTBD][BETI] | Advanced solvent, surface modifier |
| Pt-loaded carbon aerogels | [C4mim][BF4] | Stabilizer |
| IL-functionalized MWCNTS–Au | 1-(3-Aminopropyl)-3-methylimidazolium bromide | Surface modifier |
| N-doped Pt/C | [BMP][DCA] | N- and/or C-precursor |
| Core–shell Au–Pt bimetallic | [C4mim][Br] | Solvent and morphology directing |
| N-doped hollow C spheres | [3-MBP][DCA] | C precursor |
| S- and N-co-doped porous C | [BMP][TF2N] | N, S and C precursor |
| PtAg/RGO | [C16mim][Br] | Surface capping, structure-directing |
| IL–CNTS/Pt | 1-(3-Aminopropyl)-3-methylimidazolium bromide (NH2-IL) | Surface functionalizing |
| Pt/GnP | [C4mim][PF6], [C4mim][CH3CO2] | Structure-directing |
| PtRu | [C4mim][BF4] | Stabilizer |
| Pt/C | [C4mim][BF4] | Advanced solvent |
| Pd nanoparticles | [C4mim][Br], [C4mim][Cl] | Morphology/structure directing |
| Pt–WC/TiO2 | [C4mim][BF4] | Structure and morphology directing |
| CuPt–/IL–graphene | [C8mim][PF6] | Increase conductivity |
| PIL/Pt hybrids | [BviM][Br] | Monomer |
| Pt/XC-72@C–N | [VEIM][NO3], [C2mim][DCA] | Dopant |
| ILs/graphene supported Pt | [C4mim][BF4], [C4mim][PF6] | Surface functionalizing |
| Pt–Ru/C | [C4mim][BF4], [C4mim][PF6] | Advanced solvent |
| Pd/C | [C4mim][BF4] | Advanced solvent |
| Dy2O3 | Eutectic choline chlorine/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, molar ratio) 2, molar ratio) |
Electrolyte for electrodeposition |
| MnOx (x = 1.5–2) | EAN (ethyl ammonium nitrate)-based IL | Electrolyte for electrodeposition |
| Phosphate surface-modified MnOx | Phosphate buffer ionic liquid | Surface modification |
| B- and F-doped graphitic carbon carbides | [C4mim][BF4] | Texture and chemistry modifier |
| TiO2/RGO | [C4mim][PF6] | Advanced solvent |
| N- and C-co-doped TiO2 | [C2mim][Cl] | N- and C-precursor |
| Mesoporous TiO2 | [C4mim][PF6], [C4mim][BF4] | Templating |
| CeO2–TiO2 | [C16mim][Br] | As template |
| TiO2/RGO | [C4mim][PF6] | Reducing agent |
| Anatase TiO2 | [C4mim][BF4] | Templating agent |
| TiO2 films | [Cmim][HSO4] | Structure-directing agent |
| ZnO | [C4C1Py][TF2N] | Reaction media, functional surface modifier |
| Cytochrome C protein | [C4mim][MeSO4], [C4mim][CH3CO2], [Ch][dbp], [C4mim][dhp], [Ch][dhp], [C4mpyr][DCA], [Ch][Sac], [Ch][Lac] | Advanced solvent and extracting agent |
| Oxidized cellobiose | [Ch][dbp] | Advanced solvent and extracting agent |
| Metalloprotein | (Hy[Ch][dhp]) | Advanced solvent and extracting agent |
| Fructose dehydrogenase mobilized on Au or C | (Hy[Ch][dhp]) | Advanced solvent and extracting agent |
| Cellobiose dehydrogenase mobilized on Au or C | (Hy[Ch][dhp]) | Advanced solvent and extracting agent |
| Dissolved cellulose | [C2mim][MeO][R][PO2], R = H, Me, MeO | Advanced solvent and extracting agent |
| Extracted cellulose and xylan from wheat bran | [C1mim][MeO][PO2] | Advanced solvent and extracting agent |
Indeed, ILs can play multiple roles, such as functional solvents, electrolytes for electrodeposition, structure-directing agents, stabilizers and precursors, opening a low-temperature and eco-friendly synthesis approach, and new avenues towards the synthesis of highly effective materials for fuel cells, photocatalytic water splitting and dye sensitized solar cells. In addition, ILs have capability to process biofuels, a newly emerging area of potential practical importance. However, the research accounts that have been so far observed represent the tip of an iceberg and intensified research work is still needed to fully understand the effect of the IL structure in promoting advancements of the above listed materials. For instance, the interactions between ILs and precursors/intermediate products, as well as the detailed mechanism of enzyme dissolution in ILs are matters to be methodically investigated. Understanding of the governing mechanisms of the ILs towards tuning the reaction conditions via theoretical modeling is also another desirable, but not yet achieved goal. As announced by BASF in the large-scale and multi-ton BASIL (biphasic acid scavenging using ILs) process, ILs are starting to leave academic labs and find their way into industrial-scale applications.83 However, for the not yet fully commercialized technologies (e.g., FCs, DSSCs, water splitting) a suitable approach of scaling up of the IL-assisted ionothermal synthesis and processing need to be well thought and developed. As the concepts and paradigms of IL-assisted synthesis in the aforesaid applications is new, a very close cooperation between academia and industry is necessary towards their deployment in large-scale applications. Considering the research work in this field is still at its early stage, there is hope that these aspects will be successfully clarified in the near future.
Acknowledgements
Dr G. G. Eshetu and Prof. Dr S. Passerini would like to acknowledge the financial support of the Helmholtz Institute of Ulm (HIU). Prof. Dr B. Scrosati is grateful to HIU and to the Tokyo University of Agriculture and Technology (TUAT) for the visiting professor positions.References
- D. R. MacFarlane, N. Tachikawa, M. Forsyth, J. M. Pringle, P. C. Howlett, G. D. Elliott, J. H. Davis, M. Watanabe, P. Simon and C. A. Angell, Energy Environ. Sci., 2014, 7, 232 CAS.
- Z. Gong and Y. Yang, Energy Environ. Sci., 2011, 4, 3223 CAS.
- N.-S. Choi, Z. Chen, S. A. Freunberger, X. Ji, Y.-K. Sun, K. Amine, G. Yushin, L. F. Nazar, J. Cho and P. G. Bruce, Angew. Chem., Int. Ed., 2012, 51, 9994–10024 CrossRef CAS PubMed.
- G. G. Eshetu, J.-P. Bertrand, A. Lecocq, S. Grugeon, S. Laruelle, M. Armand and G. Marlair, J. Power Sources, 2014, 269, 804–811 CrossRef CAS.
- G. G. Eshetu, M. Armand, B. Scrosati and S. Passerini, Angew. Chem., Int. Ed., 2014, 53, 13342–13359 CrossRef PubMed.
- J. Kalhoff, G. G. Eshetu, D. Bresser and S. Passerini, ChemSusChem, 2015, 8, 2154–2175 CrossRef CAS PubMed.
- I. Katsounaros, S. Cherevko, A. R. Zeradjanin and K. J. J. Mayrhofer, Angew. Chem., Int. Ed., 2014, 53, 102–121 CrossRef CAS PubMed.
- I. Krossing, J. M. Slattery, C. Daguenet, P. J. Dyson, A. Oleinikova and H. Weingärtner, J. Am. Chem. Soc., 2006, 128, 13427–13434 CrossRef CAS PubMed.
- H. Weingärtner, Angew. Chem., Int. Ed., 2008, 47, 654–670 CrossRef PubMed.
- M. Armand, F. Endres, D. R. MacFarlane, H. Ohno and B. Scrosati, Nat. Mater., 2009, 8, 621–629 CrossRef CAS PubMed.
- J. Reiter, S. Jeremias, E. Paillard, M. Winter and S. Passerini, Phys. Chem. Chem. Phys., 2013, 15, 2565–2571 RSC.
- M. Kunze, M. Montanino, G. B. Appetecchi, S. Jeong, M. Winter and S. Passerini, J.Phys.Chem. A, 2010, 114, 1776–1782 CrossRef CAS PubMed.
- X. Duan, J. Ma, J. Lian and W. Zheng, CrystEngComm, 2014, 16, 2550–2559 RSC.
- W. Kautek and H. Lehmkuhl, Electrochim. Acta, 1989, 34, 1213–1218 CrossRef.
- M. Tulodziecki, J.-M. Tarascon, P. L. Taberna and C. Guery, J. Electrochem. Soc., 2009, 156, D503 CrossRef.
- M. Armand, F. Endres, D. R. MacFarlane, H. Ohno and B. Scrosati, Nat. Mater., 2009, 8, 621–629 CrossRef CAS PubMed.
- A. Matic and B. Scrosati, MRS Bull., 2013, 38, 533–537 CrossRef CAS.
- R. E. Morris, Chem. Commun., 2009, 2990–2998 RSC.
- F. Endres, ChemPhysChem, 2002, 3, 144–154 CrossRef CAS.
- E. Antolini, Energy Environ. Sci., 2009, 2, 915 CAS.
- Y. Bing, H. Liu, L. Zhang, D. Ghosh and J. Zhang, Chem. Soc. Rev., 2010, 39, 2184 RSC.
- B. C. H. Steele and A. Heinzel, Nature, 2001, 414, 345–352 CrossRef CAS PubMed.
- S. Guo, S. Zhang and S. Sun, Angew. Chem., Int. Ed., 2013, 52, 8526–8544 CrossRef CAS PubMed.
- Z. W. Zhao, Z. P. Guo, J. Ding, D. Wexler, Z. F. Ma, D. Y. Zhang and H. K. Liu, Electrochem. Commun., 2006, 8, 245–250 CrossRef CAS.
- W. Y. Wong, W. R. W. Daud, A. B. Mohamad, A. A. H. Kadhum, K. S. Loh and E. H. Majlan, Int. J. Hydrogen Energy, 2013, 38, 9370–9386 CrossRef CAS.
- Z. Yang, H. Nie, X. Chen, X. Chen and S. Huang, J. Power Sources, 2013, 236, 238–249 CrossRef CAS.
- L. Feng, L. Yang, Z. Huang, J. Luo, M. Li, D. Wang and Y. Chen, Sci. Rep., 2013, 3, 3306 Search PubMed.
- X. Lu and C. Zhao, Phys. Chem. Chem. Phys., 2013, 15, 20005–20009 RSC.
- J. Snyder, T. Fujita, M. W. Chen and J. Erlebacher, Nat. Mater., 2010, 9, 904–907 CrossRef CAS PubMed.
- J. Snyder, K. Livi and J. Erlebacher, Adv. Funct. Mater., 2013, 23, 5494–5501 CrossRef CAS.
- F. Hasché, T.-P. Fellinger, M. Oezaslan, J. P. Paraknowitsch, M. Antonietti and P. Strasser, ChemCatChem, 2012, 4, 479–483 CrossRef.
- Y. Iflah, I. Zilbermann, E. Korin and A. Bettelheim, ECS Electrochem. Lett., 2013, 2, F55–F59 CrossRef CAS.
- Z. Wang, Q. Zhang, D. Kuehner, X. Xu, A. Ivaska and L. Niu, Carbon, 2008, 46, 1687–1692 CrossRef CAS.
- Z. Li and Q. Niu, ACS Sustainable Chem. Eng., 2014, 2, 533–536 CrossRef CAS.
- C. Han, J. Wang, Y. Gong, X. Xu, H. Li and Y. Wang, J. Mater. Chem. A, 2014, 2, 605 CAS.
- Z. Cui, S. Wang, Y. Zhang and M. Cao, J. Power Sources, 2014, 259, 138–144 CrossRef CAS.
- J.-J. Lv, J.-X. Feng, S.-S. Li, Y.-Y. Wang, A.-J. Wang, Q.-L. Zhang, J.-R. Chen and J.-J. Feng, Electrochim. Acta, 2014, 133, 407–413 CrossRef CAS.
- S. Guo, S. Dong and E. Wang, Adv. Mater., 2010, 22, 1269–1272 CrossRef CAS PubMed.
- I. Do and L. T. Drzal, ACS Appl. Mater. Interfaces, 2014, 6, 12126–12136 CAS.
- X. Xue, C. Liu, T. Lu and W. Xing, Fuel Cells, 2006, 6, 347–355 CrossRef CAS.
- Z. Li, H. Gong, T. Mu and Y. Luan, CrystEngComm, 2014, 16, 4038 RSC.
- X. Lang, M. Shi, Y. Chu, W. Liu, Z. Chen and C. Ma, J. Solid State Electrochem., 2013, 17, 2401–2408 CrossRef CAS.
- J. Yang, L. Qiu, B. Liu, Y. Peng, F. Yan and S. Shang, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 4531–4538 CrossRef CAS.
- Y. Liu, Y. Huang, Y. Xie, Z. Yang, H. Huang and Q. Zhou, Chem. Eng. J., 2012, 197, 80–87 CrossRef CAS.
- J. Cao, Y. Chu and X. Tan, Mater. Chem. Phys., 2014, 144, 17–24 CrossRef CAS.
- G. Shi, Z. Wang, J. Xia, S. Bi, Y. Li, F. Zhang, L. Xia, Y. Li, Y. Xia and L. Xia, Electrochim. Acta, 2014, 142, 167–172 CrossRef CAS.
- X. Xue, T. Lu, C. Liu, W. Xu, Y. Su, Y. Lv and W. Xing, Electrochim. Acta, 2005, 50, 3470–3478 CrossRef CAS.
- F.-B. Zhang and H.-L. Li, Carbon, 2006, 44, 3195–3198 CrossRef CAS.
- A. B. Stambouli and E. Traversa, Renewable Sustainable Energy Rev., 2002, 6, 433–455 CrossRef CAS.
- T. A. Adams, J. Nease, D. Tucker and P. I. Barton, Ind. Eng. Chem. Res., 2013, 52, 3089–3111 CrossRef CAS.
- C. Mele and B. Bozzini, Energies, 2012, 5, 5363–5371 CrossRef CAS.
- G. Xie, K. Zhang, B. Guo, Q. Liu, L. Fang and J. R. Gong, Adv. Mater., 2013, 25, 3820–3839 CrossRef CAS PubMed.
- F. Zhou, A. Izgorodin, R. K. Hocking, L. Spiccia and D. R. MacFarlane, Adv. Energy Mater., 2012, 2, 1013–1021 CrossRef CAS.
- A. Izgorodin, R. Hocking, O. Winther-Jensen, M. Hilder, B. Winther-Jensen and D. R. MacFarlane, Catal. Today, 2013, 200, 36–40 CrossRef CAS.
- F. Zhou, A. Izgorodin, R. K. Hocking, V. Armel, L. Spiccia and D. R. Macfarlane, ChemSusChem, 2013, 6, 643–651 CrossRef CAS PubMed.
- Z. Lin and X. Wang, ChemSusChem, 2014, 7, 1547–1550 CrossRef CAS PubMed.
- H. Wender, A. F. Feil, L. B. Diaz, C. S. Ribeiro, G. J. Machado, P. Migowski, D. E. Weibel, J. Dupont and R. Teixeira, ACS Appl. Mater. Interfaces, 2011, 3, 1359–1365 CAS.
- H. Li, J. Qu, Q. Cui, H. Xu, H. Luo, M. Chi, R. A. Meisner, W. Wang and S. Dai, J. Mater. Chem., 2011, 21, 9487 RSC.
- A. Safavi, S. H. Kazemi and H. Kazemi, Fuel, 2014, 118, 156–162 CrossRef CAS.
- J. Shen, M. Shi, B. Yan, H. Ma, N. Li and M. Ye, Nano Res., 2011, 4, 795–806 CrossRef CAS.
- S.-H. Liu and H.-R. Syu, Int. J. Hydrogen Energy, 2013, 38, 13856–13865 CrossRef CAS.
- K. S. Yoo, T. G. Lee and J. Kim, Microporous Mesoporous Mater., 2005, 84, 211–217 CrossRef CAS.
- H. Choi, Y. J. Kim, R. S. Varma and D. D. Dionysiou, Chem. Mater., 2006, 18, 5377–5384 CrossRef CAS.
- Y. Zhou and M. Antonietti, J. Am. Chem. Soc., 2003, 125, 14960–14961 CrossRef CAS PubMed.
- H. Liu, M. Wang, Y. Wang, Y. Liang, W. Cao and Y. Su, J. Photochem. Photobiol., A, 2011, 223, 157–164 CrossRef CAS.
- G. Nagaraju, G. Ebeling, R. V. Gonçalves, S. R. Teixeira, D. E. Weibel and J. Dupont, J. Mol. Catal. A: Chem., 2013, 378, 213–220 CrossRef CAS.
- B. O’Regan and M. Gratzel, Nature, 1991, 353, 737–740 CrossRef.
- S. Mathew, A. Yella, P. Gao, R. Humphry-baker, B. F. E. Curchod, N. Ashari-astani, I. Tavernelli, U. Rothlisberger, K. Nazeeruddin and M. Gratzel, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- J. Xia, L. Xu, J. Zhang, S. Yin, H. Li, H. Xu and J. Di, CrystEngComm, 2013, 15, 10132 RSC.
- C. C. Han, Y. P. Lin, S. Y. Ho, Y. C. Lai, S. Y. Chen, J. Huang and Y. W. Chen-Yang, J. Phys. D: Appl. Phys., 2010, 43, 035102 CrossRef.
- H. Process, C. A. Betty, P. N. Bhosale, P. S. Patil and C. K. Hong, Sci. Rep., 2014, 4, 2–9, DOI:10.1038/srep05451.
- I. Yavari, A. R. Mahjoub, E. Kowsari and M. Movahedi, J. Nanopart. Res., 2008, 11, 861–868 CrossRef.
- E. Azaceta, J. Idigoras, J. Echeberria, A. Zukal, L. Kavan, O. Miguel, H.-J. Grande, J. A. Anta and R. Tena-Zaera, J. Mater. Chem. A, 2013, 1, 10173 CAS.
- K. Tamura, N. Nakamura and H. Ohno, Biotechnol. Bioeng., 2012, 109, 729–735 CrossRef CAS PubMed.
- K. Fujita, D. R. Macfarlane, M. Forsyth, M. Yoshizawa-Fujita, K. Murata, N. Nakamura and H. Ohno, Biomacromolecules, 2007, 8, 2080–2086 CrossRef CAS PubMed.
- K. Fujita, N. Nakamura, K. Igarashi, M. Samejima and H. Ohno, Green Chem., 2009, 11, 351 RSC.
- K. Fujita and H. Ohno, Biopolymers, 2010, 93, 1093–1099 CrossRef CAS PubMed.
- K. Fujita, N. Nakamura, K. Murata, K. Igarashi, M. Samejima and H. Ohno, Electrochim. Acta, 2011, 56, 7224–7227 CrossRef CAS.
- Y. Fukaya, K. Hayashi, M. Wada and H. Ohno, Green Chem., 2008, 10, 44 RSC.
- M. Abe, Y. Fukaya and H. Ohno, Green Chem., 2010, 12, 1274 RSC.
- K. Kuroda, H. Kunimura, Y. Fukaya, N. Nakamura and H. Ohno, ACS Sustainable Chem. Eng., 2014, 2, 2204–2210 CrossRef CAS.
- M. Abe, T. Yamada and H. Ohno, RSC Adv., 2014, 4, 17136–17140 RSC.
- http://https://www.basf.com/en/404.html .
| This journal is © The Royal Society of Chemistry 2016 |