Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Well-defined coinage metal transfer agents for the synthesis of NHC-based nickel, rhodium and palladium macrocycles†
Rhiann E.
Andrew
,
Caroline M.
Storey
and
Adrian B.
Chaplin
 *
*
Department of Chemistry, University of Warwick, Gibbet Hill Road, Coventry CV4 7AL, UK. E-mail: a.b.chaplin@warwick.ac.uk
First published on 9th May 2016
Abstract
With a view to use as carbene transfer agents, well-defined silver(I) and copper(I) complexes of a macrocyclic NHC-based pincer ligand, bearing a central lutidine donor and a dodecamethylene spacer [CNC–(CH2)12, 1], have been prepared. Although the silver adduct is characterised by X-ray diffraction as a dinuclear species anti-[Ag(μ-1)]22+, variable temperature measurements indicate dynamic structural interchange in solution involving fragmentation into mononuclear [Ag(1)]+ on the NMR time scale. In contrast, a mononuclear structure is evident in both solution and the solid-state for the analogous copper adduct partnered with the weakly coordinating [BArF4]− counter anion. A related copper derivative, bearing instead the more coordinating cuprous bromide dianion [Cu2Br4]2−, is notable for the adoption of an interesting tetranuclear assembly in the solid-state, featuring two cuprophilic interactions and two bridging NHC donors, but is not retained on dissolution. Coinage metal precursors [M(1)]n[BArF4]n (M = Ag, n = 2; M = Cu, n = 1) both act as carbene transfer agents to afford palladium, rhodium and nickel complexes of 1 and the effectiveness of these precursors has been evaluated under equivalent reaction conditions.
Introduction
The chemistry of N-heterocyclic carbene (NHC) complexes of the transition elements is rich, varied and at the forefront of contemporary organometallic chemistry and catalysis.1 While the formation of these adducts via direct coordination of the singlet carbene, isolated or generated in situ through deprotonation of the corresponding azolium salt, is conceptually the simplest method, the high chemical reactivity of these free carbene intermediates or incompatibility of the reaction conditions with acidic functionalities have necessitated the development of alternative approaches involving isolable ‘protected’ carbenes and transmetallation procedures. In this context, Ag(I)–NHC complexes have proved to be effective carbene transfer agents for a wide variety of late-transition elements (Scheme 1).2–4 These reagents are conveniently formed through reaction of the respective pro-ligand NHC·HX with Ag2O, resulting in mono- or bis-ligated complexes [Ag(NHC)X] or [Ag(NHC)2]X depending on the nature of the anion (X).2,3 Although comparatively less common, Cu(I)–NHC complexes have also been employed as carbene transfer agents (Scheme 1).2,5 Such copper reagents can be prepared from reactions between Cu2O and NHC·HX, but are generally prepared via free carbene intermediates.2 Interestingly, reflecting the relative M(I)–NHC bond strengths of the coinage metals (Au > Cu > Ag),6 transmetallation of both silver and copper complexes can be used to prepare gold derivatives as a result of the more robust nature of Au(I)–NHC bonds.2–5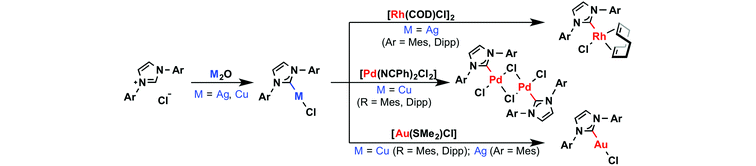 | ||
| Scheme 1 Selected synthesis and reactions of Cu(I) and Ag(I)–NHC complexes.4,5 | ||
The application of carbene transfer methodology is not limited to monodentate examples and the use of silver-based transmetallation protocols is also prevalent in the coordination chemistry of mer-tridentate “pincer” ligands bearing terminal NHC donors.7,8 While the corresponding silver transfer agents are often generated in situ, well defined and characteristically bimetallic Ag(I)–CEC (E = C, N) complexes such as A–C have been isolated and crystallographically characterised (Scheme 2).9–15 Copper adducts of CEC (E = C, N) ligands have also been prepared (e.g.D and E),10,16–18 however, their application as transfer agents has yet to be realised to our knowledge.
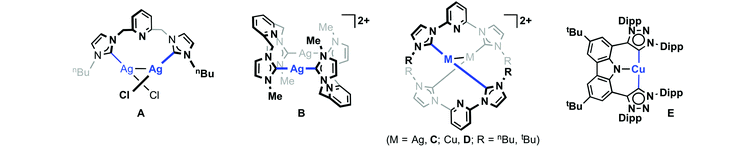 | ||
| Scheme 2 Examples of isolated Ag(I) and Cu(I) complexes of CNC pincer ligands.12–17 | ||
As part of our work involving the organometallic chemistry of macrocyclic CNC pincers,19 we now report the synthesis and characterisation of well-defined Ag(I) and Cu(I) adducts of a lutidine-based pincer ligand bearing a dodecamethylene spacer [CNC–(CH2)12, 1]. The use of these coinage metal species as transfer agents is then detailed for the synthesis of rhodium, palladium, and nickel complexes of 1.
Results and discussion
Our preceding work exploring the coordination chemistry of 1 employed in situ generation of silver transfer agents from the reaction between 1·2HBr and Ag2O in CH2Cl2 solution.19 As a convenient means of incorporating a weakly coordinating [BArF4]− anion20 and conferring solution solubility, these reactions were carried out in the presence of Na[BArF4] (ArF = 3,5-C6H3(CF3)2) as halogen ion abstractor. Using a slightly adapted protocol to facilitate isolation, well-defined silver derivative 2 was prepared via reaction in diethyl ether (Scheme 3). Filtration to remove insoluble silver and sodium bromide salts, and subsequent recrystallization from CHCl3/pentane, afforded analytically pure 2 as a white powder in reproducibly high isolated yields of ca. 80%.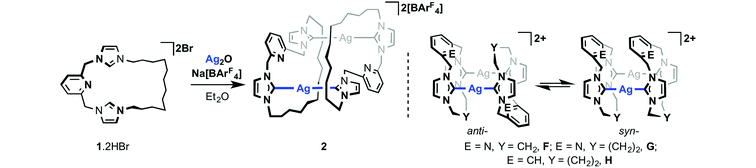 | ||
| Scheme 3 Preparation of 2 and related literature precedents.11 | ||
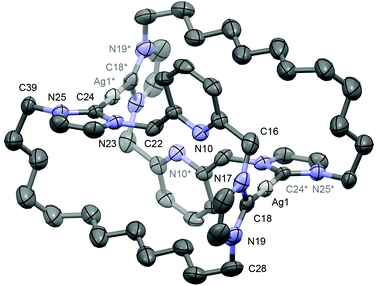
Single crystals grown from diethyl ether/pentane and analysed by X-ray crystallography enabled structural elucidation of 2 in the solid-state as a dinuclear complex anti-[Ag(μ-1)]2[BArF4]2 (Fig. 1) – as for closely related precedents B, F and G.11,13 Interestingly, while the principle geometric metrics about silver in 2 are directly comparable to the aforementioned precedents (e.g. Ag–NHC = 2.088(4), 2.093(4) Å, B; 2.077(3), 2.080(3) Å, 2; NHC–Ag–NHC = 176.78(9)°, B; 178.99(13)°, 2), the ligand topology is significantly altered. At the heart of the structural difference is the adoption of near orthogonal NHC–Ag–NHC geometries in 2 [N25–C24–C18*–N19* = 100.2(4)°], in contrast to coplanar NHC–Ag–NHC arrangements observed in B, F and G. This change in geometry is presumably necessary to accommodate the long aliphatic chain and as a consequence results in a very large Ag⋯Ag* separation [7.2732(6) cf. 3.7171(5), B; 3.538(2), F; 4.6636(7) Å, G], precluding the adoption of any argentophilic interaction as seen in A and C.12,14,15.
1H and 13C NMR data recorded in CD2Cl2 confirm the expected 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand to anion ratio and reveal time averaged C2v symmetry for 2 at 298 K (500 MHz). Such high symmetry is inconsistent with retention of the solid-state structure and instead implies highly fluxional behaviour in solution, involving fragmentation into [Ag(1)]+ (cf. structure of 4vide infra). Such an assertion is supported by ESI-MS, where only a singly charged species (i.e. integer mass spacing) was evident in the mass spectrum ([Ag(1)]+, 512.1927; calc. 512.1938 m/z). Not surprisingly the carbene signals of 2 were not observed by 13C{1H} NMR spectroscopy at 298 K, although a chemical shift of ca. δ 181 ppm can be inferred from 2D heteronuclear correlation experiments in line with expected values for Ag(I)–NHC complexes.2,3b,21 Progressive cooling to 250 K lead to decoalescence of the (broadened) 1H signals of 2 observed in CD2Cl2 at 298 K and establishment of an equilibrium mixture comprised of three major compounds, one of C2v symmetry and two of apparent Cs symmetry (1
1 ligand to anion ratio and reveal time averaged C2v symmetry for 2 at 298 K (500 MHz). Such high symmetry is inconsistent with retention of the solid-state structure and instead implies highly fluxional behaviour in solution, involving fragmentation into [Ag(1)]+ (cf. structure of 4vide infra). Such an assertion is supported by ESI-MS, where only a singly charged species (i.e. integer mass spacing) was evident in the mass spectrum ([Ag(1)]+, 512.1927; calc. 512.1938 m/z). Not surprisingly the carbene signals of 2 were not observed by 13C{1H} NMR spectroscopy at 298 K, although a chemical shift of ca. δ 181 ppm can be inferred from 2D heteronuclear correlation experiments in line with expected values for Ag(I)–NHC complexes.2,3b,21 Progressive cooling to 250 K lead to decoalescence of the (broadened) 1H signals of 2 observed in CD2Cl2 at 298 K and establishment of an equilibrium mixture comprised of three major compounds, one of C2v symmetry and two of apparent Cs symmetry (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). These species are tentatively assigned as [Ag(1)]+, syn-[Ag(μ-1)]22+ and anti-[Ag(μ-1)]22+, respectively, on the basis of related behaviour observed for F and G involving rapid equilibration between syn- and anti-isomers in solution (Scheme 3); dynamics that necessitate Ag–NHC bond cleavage and invoke coordination of the central lutidine donor through comparison to the less fluxional m-xylylene bridged analogue H.11 In the case of 2, the NMR data suggests that incorporation of the long dodecamethylene spacer destabilises the dinuclear structures relative to entropically favored fragmentation into [Ag(1)]+ at ambient temperature.22 Further cooling to 200 K resulted in a shift in the equilibrium toward the species assigned to anti-[Ag(μ-1)]22+ (ca. 60%) consistent with the pseudo Ci symmetric structure observed in the solid-state being enthalpically favoured in solution. In the context of 2 being used as a carbene transfer agent, these NMR data ultimately demonstrate facile Ag–NHC bond cleavage under conditions relevant to synthesis of other transition metal adducts of 1via transmetallation.
1). These species are tentatively assigned as [Ag(1)]+, syn-[Ag(μ-1)]22+ and anti-[Ag(μ-1)]22+, respectively, on the basis of related behaviour observed for F and G involving rapid equilibration between syn- and anti-isomers in solution (Scheme 3); dynamics that necessitate Ag–NHC bond cleavage and invoke coordination of the central lutidine donor through comparison to the less fluxional m-xylylene bridged analogue H.11 In the case of 2, the NMR data suggests that incorporation of the long dodecamethylene spacer destabilises the dinuclear structures relative to entropically favored fragmentation into [Ag(1)]+ at ambient temperature.22 Further cooling to 200 K resulted in a shift in the equilibrium toward the species assigned to anti-[Ag(μ-1)]22+ (ca. 60%) consistent with the pseudo Ci symmetric structure observed in the solid-state being enthalpically favoured in solution. In the context of 2 being used as a carbene transfer agent, these NMR data ultimately demonstrate facile Ag–NHC bond cleavage under conditions relevant to synthesis of other transition metal adducts of 1via transmetallation.
The synthesis of copper adducts of 1 was targeted by low temperature deprotonation of 1·2HBr in THF in the presence of excess copper bromide. In this manner [Cu(1)]2[Cu2Br4] 3 was formed and subsequently isolated in 76% yield (Scheme 4). Further treatment of 3 with Na[BArF4] in toluene resulted in incorporation of the weakly coordinating [BArF4]− anion in place of [Cu2Br4]2− to afford 4 in 59% isolated yield.20,23 In CD2Cl2 solution, the 1H and 13C NMR characteristics of both 3 and 4 point towards simple mononuclear complexes of 1, with sharp resonances and apparent C2v symmetry in the respective spectra at 298 K (400 MHz). Moreover, only minor differences in chemical shift are found for the equivalent 1H (<0.15 ppm) and 13C (<2 ppm) signals of 3/4, and presumably attributed to greater ion pairing in 3. Of most relevance to the coordination of 1, the carbenic centres were readily identified from 13C{1H} NMR spectra by their characteristically high frequency chemical shifts (δ 178.7, 3; δ 180.3, 4).2 Strong parent cation signals are observed by ESI-MS with correct isotope patterns and integer mass spacing (468.2185, 3; 468.2186, 4; calc. 468.2183 m/z), further supporting the presence of discrete [Cu(1)]+ in solution, irrespective of the counter anion.
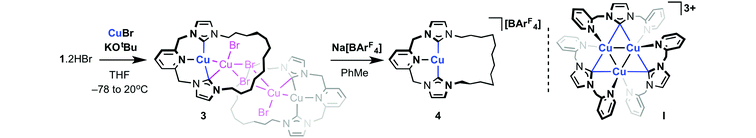 | ||
| Scheme 4 Preparation of 3, 4 and a related literature precedent.25 | ||
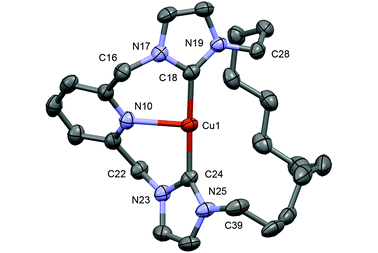
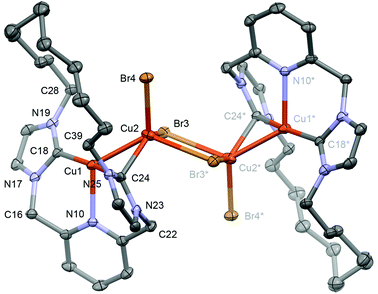
Despite similar solution characteristics, in the solid-state the nature of the counter anion impacts significantly on the coordination geometries of 3 and 4 (Fig. 2 and 3). Two independent, but well-separated cation/anion pairs are observed in the solid-state structure of 4. The cationic fragments are structurally similar, displaying near ideal T-shaped coordination geometries with the flexible dodecamethylene spacer notably skewed to one side. Focusing on the metrics associated with the independent cation shown in Fig. 2,24 the complex displays an approximately linear NHC–Cu–NHC angle (176.37(13)°), equivalent Cu–NHC bond lengths within error (1.905(3)/1.906(3) Å), and a Cu–N bond length of 2.233(3) Å. These parameters are in good agreement with those reported for E, although the anionic nature of the component CNC pincer ligand leads to a shorter Cu–N bond length than that in 4 (2.017(2) Å). In the case of 3, an anion bridged dimer/tetranuclear formulation is instead observed, viz [Cu(1)]2{μ-[Cu2Br4]}, featuring two formally bridging NHC donors (Cu1–C24, 1.980(3); Cu2–C24, 2.070(3) Å) and two cuprophilic interactions (Cu–Cu, 2.5209(5) Å). The bonding interaction with the anion results in a significant distortion of the coordination geometry observed in 4, with the NHC–Cu–NHC angle deviating from linearity (167.92(11)°) alongside elongation of the non-bridged Cu–NHC (1.925(3) cf. 1.905(3)/1.906(3) Å) and Cu–N (Cu–N = 2.246(2) cf. 2.233(3)) bonds. The bridging μ2-NHC coordination mode is an unusual feature of 3, but has striking precedence in trinuclear copper clusters, such as I, that contain three ligands in this coordination mode (Cu–NHC ca. 2.026(5)–2.044(5) Å for I).25,26 In these trinuclear clusters, the bridging coordination mode is retained in solution and characterised by δ13C 167–169 for imidazolyline based variants; values to significantly lower frequency than found for 3 (δ13C 178.7).25,26
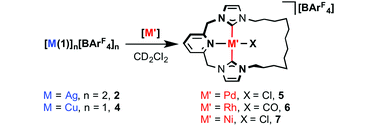
With well-defined coinage metal complexes 2 and 4 in hand, we turned to evaluation of their capacity to act as transfer agents of macrocyclic 1 in CH2Cl2. As convenient benchmarks we targeted preparation of known and soluble palladium and rhodium adducts of 1, [Pd(1)Cl][BArF4] 5 and [Rh(1)(CO)][BArF4] 6, through reactions with [Pd(NCMe)2Cl2] and [Rh(CO)2Cl]2, respectively (Table 1).19b,c Following these reactions in situ by 1H NMR spectroscopy in CD2Cl2, using the [BArF4]− resonances as a convenient internal standard, revealed rapid and high yielding transmetallation reactions of 2 in both cases (>70% yield). Consistent with these high yields, 5 and 6 have previously been isolated in 58% and 52% yield, respectively, through in situ generation of the silver transfer agent.19b,c In the case of copper, although complete consumption of 4 was apparent within 30 min in both cases, a large difference in selectivity is apparent: rhodium-based 6 was formed with an excellent yield of 98% (and subsequently isolated in 82% yield), while palladium-based 5 was formed with a significantly inferior yield of 23%.
| [M′] | M | t /h | T/°C | Product | Yieldc |
|---|---|---|---|---|---|
| a Reactions carried out in J. Young's NMR tubes, which were periodically placed in a ultrasound bath during the course of the reaction. b Complete conversion unless otherwise noted. c Determined by integration of 1H NMR data. d Incomplete reaction. | |||||
| [Pd(NCMe)2Cl2] | Ag | 0.5 | 20 | 5 | 73% |
| [Pd(NCMe)2Cl2] | Cu | 0.5 | 20 | 5 | 23% |
| [Rh(CO)2Cl]2 | Ag | 0.5 | 20 | 6 | 72% |
| [Rh(CO)2Cl]2 | Cu | 0.5 | 20 | 6 | 98% |
| [NiCl2(glyme)] | Ag | 20d | 20 | 7 | 22% |
| [NiCl2(glyme)] | Ag | 20 | 40 | 7 | 76% |
| [NiCl2(glyme)] | Cu | 20d | 20 | 7 | 86% |
| [NiCl2(glyme)] | Cu | 5 | 40 | 7 | 90% |
Seeking to expand the scope of this transmetallation methodology, we targeted the preparation of nickel derivative 7 – the lighter and Earth abundant group 10 congener of 5. Using the aforementioned methodology in combination with [NiCl2(glyme)], resulted in slow dissolution of the largely insoluble nickel(II) precursor, and gratifying (albeit gradual) formation of 7 at room temperature over 20 hours. The reaction with copper-based 4 notably proceeded ca. 4 times faster, suggesting the explanation for this behaviour is more complex than low solubility of [NiCl2(glyme)] alone. Repeating under more forcing conditions (40 °C) resulted in complete consumption of 2 (20 h) and 4 (5 h) and formation of 7 in 76% and 90% yield, respectively. The new air and moisture stable complex 7 was subsequently isolated from these reactions in ca. 30% yield following purification on alumina and fully characterised. Alternatively, 7 can also be prepared in similar yield using in situ generation of 2 from 1·2HBr and Ag2O (29% isolated yield).
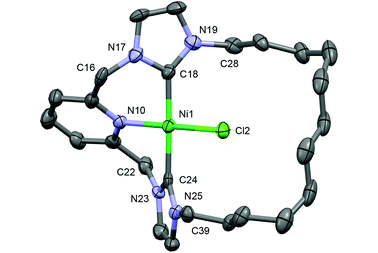
In the solid-state, 7 shows the expected contraction of metal–ligand bond lengths in comparison to 5 (Ni–Cl, 2.145(2); Ni–N, 1.928(5); Ni–C, 1.898(6), 1.916(6) Å; Pd–Cl, 2.287(4); Pd–N, 2.077(10); Pd–C, 2.036(12), 2.056(13) Å), but is otherwise isostructural with the palladium-based analogue (Fig. 4).19c Most notably, the chloride ancillary ligand is easily accommodated within the macrocyclic ring, which is orientated to maintain pseudo C2 symmetry (cf. twisting observed in 4), with an essentially linear N–Ni–Cl bond angle (179.06(15) cf. 176.2(3)° for the N–Pd–Cl angle in 5). In solution the solid-state structure is fully retained as indicated by the observation of diastereotopic methylene bridge (pyC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) and N-methylene (N-C
2) and N-methylene (N-C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH2) resonances at δ 5.14/6.30 (2JHH = 15.0 Hz) and δ 3.73/4.73, respectively. A single carbenic carbon signal is observed at δ 162.0 (cf. δ 164.5, 5).
2CH2) resonances at δ 5.14/6.30 (2JHH = 15.0 Hz) and δ 3.73/4.73, respectively. A single carbenic carbon signal is observed at δ 162.0 (cf. δ 164.5, 5).
Summary
Well-defined silver(I) and copper(I) complexes of a macrocyclic NHC-based pincer ligand, bearing a central lutidine donor and a dodecamethylene spacer [CNC–(CH2)12, 1], have been prepared. In the solid-state silver adduct 2 is characterised by X-ray diffraction as a dinuclear species anti-[Ag(μ-1)]22+, with the two metal centres held distant from each other (Ag⋯Ag, >7 Å) as a consequence of the conformation of the bridging macrocyclic ligand. However, variable temperature 1H NMR spectroscopy indicates dynamic structural interchange in solution involving fragmentation into mononuclear [Ag(1)]+. In contrast, a mononuclear structure is evident in both solution and the solid-state for the analogous copper adduct 4 partnered with the weakly coordinating [BArF4]− counter anion. A related copper derivative, bearing instead the more coordinating cuprous bromide dianion [Cu2Br4]2−, is notable for the adoption of an interesting tetranuclear assembly in the solid-state, featuring two cuprophilic interactions (Cu–Cu, 2.5209(5) Å) and two bridging NHC donors, but is not retained in solution. Coinage metal complexes [M(1)]n[BArF4]n (M = Ag, n = 2, 2; M = Cu, n = 1, 4) both act as carbene transfer agents to afford palladium (5), rhodium (6) and nickel complexes (7) of 1. Although not extensive, these reactions suggest that while silver-based transfer agents are more reliable, copper-based alternatives can result in significantly faster and higher yielding transmetallation reactions.Experimental
General experimental methods
Manipulations were performed under an inert atmosphere, using Schlenk and glove box techniques unless otherwise stated. Glassware was oven dried and flamed under vacuum prior to use. Anhydrous solvents (<0.005% H2O) were purchased from ACROS or Aldrich and used as supplied: Et2O, CHCl3, pentane, CH2Cl2, THF and toluene. CD2Cl2 was dried over molecular sieves (4 Å) and stored under an atmosphere of argon. Na[BArF4],27 [Rh(CO)2Cl]2,28 and 1·2HBr19c were synthesised using literature procedures. All other reagents are commercial products and were used as received. NMR spectra were recorded on Bruker HD-300, DPX-400, AV-400, DRX-500 and AVIII-500 HD spectrometers at 298 K unless otherwise stated. Chemical shirts are quoted in ppm and coupling constants in Hz. ESI-MS were recorded on a Bruker MaXis mass spectrometer. Microanalyses were performed at the London Metropolitan University by Stephen Boyer.Synthesis of 2
A mixture of 1·2HBr (100 mg, 0.176 mmol), Ag2O (42 mg, 0.181 mmol) and Na[BArF4] (170 mg, 0.192 mmol) was suspended in Et2O (5 mL) and stirred under argon in the absence of light for 48 h. The resulting grey suspension was allowed to settle and the solution filtered through a celite plug (pipette, 3 cm) under a flow of nitrogen. The colourless filtrate was concentrated to afford the crude product as white foam, which was subsequently dissolved in hot chloroform and filtered through a second celite plug (pipette, 3 cm). The analytically pure product was obtained on addition of excess pentane, washed with pentane and dried. Yield: 200 mg (82%, white powder). Crystals suitable for X-ray diffraction were grown from ether/pentane at 20 °C.
1H NMR (400 MHz, CD2Cl2) δ 7.82 (br, 1H, py), 7.70–7.75 (m, 8 H, ArF), 7.56 (br, 4H, ArF), 7.42 (br, 2H, py), 7.17 (br, 2H, imid), 7.05 (s, 2H, imid), 5.25 (br, 4H, pyC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 4.06 (app. t, J = 7, 4H, NCH2), 1.79 (app. pent., J = 7, 4H, CH2) 1.10–1.40 (m, 16 H, CH2). 13C{1H} NMR (101 MHz, CD2Cl2) δ 162.3 (q, 1JCB = 50, ArF), 155.1 (s, py), 140.5 (br, py), 135.4 (s, ArF), 129.4 (qq, 2JFC = 32, 3JCB = 3, ArF), 125.3 (q, 1JFC = 273, ArF), 125.0 (br, py), 124.1 (br, imid), 120.2 (br, imid), 118.0 (pent., 3JFC = 4, ArF), 57.1 (s, py
2), 4.06 (app. t, J = 7, 4H, NCH2), 1.79 (app. pent., J = 7, 4H, CH2) 1.10–1.40 (m, 16 H, CH2). 13C{1H} NMR (101 MHz, CD2Cl2) δ 162.3 (q, 1JCB = 50, ArF), 155.1 (s, py), 140.5 (br, py), 135.4 (s, ArF), 129.4 (qq, 2JFC = 32, 3JCB = 3, ArF), 125.3 (q, 1JFC = 273, ArF), 125.0 (br, py), 124.1 (br, imid), 120.2 (br, imid), 118.0 (pent., 3JFC = 4, ArF), 57.1 (s, py![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2), 54 (obscured, NCH2), 31.4 (s, CH2), 27.5 (br, CH2), 25.8 (s, CH2). The carbene resonance was not unambiguously identified in the 13C{1H} NMR spectrum, but can be located at ca. δ 181 from an HMBC experiment. ESI-MS (CH3CN, 180 °C, 3 kV) positive ion: 512.1927 m/z, [M]+ (calc. 512.1938). Anal. Calcd for C57H47AgBF24N5·CHCl3 (1496.05 g mol−1): C, 46.28; H, 3.22; N, 4.64. Found: C, 46.08; H, 3.23; N, 4.58.
H2), 54 (obscured, NCH2), 31.4 (s, CH2), 27.5 (br, CH2), 25.8 (s, CH2). The carbene resonance was not unambiguously identified in the 13C{1H} NMR spectrum, but can be located at ca. δ 181 from an HMBC experiment. ESI-MS (CH3CN, 180 °C, 3 kV) positive ion: 512.1927 m/z, [M]+ (calc. 512.1938). Anal. Calcd for C57H47AgBF24N5·CHCl3 (1496.05 g mol−1): C, 46.28; H, 3.22; N, 4.64. Found: C, 46.08; H, 3.23; N, 4.58.
Synthesis of 3
A suspension of 1·2HBr (200 mg, 0.353 mmol) and CuBr (152 mg, 1.057 mmol) in THF (7 mL) under argon was cooled to −78 °C before addition of a solution of KOtBu (100 mg, 0.881 mmol) in THF (2 mL) via cannula. The resulting orange suspension was warmed to room temperature and sonicated to produce a yellow suspension, which was stirred for a further 30 h. The reaction mixture was filtered and the product precipitated by addition of excess pentane, isolated by filtration, washed with pentane and dried. Yield: 147 mg (76%, yellow powder). Crystals suitable for X-ray diffraction were grown from CD2Cl2/pentane at 20 °C.
1
H NMR (400 MHz, CD2Cl2) δ 7.78 (t, 3JHH = 7.7, 1H, py), 7.39 (d, 3JHH = 7.7, 2H, py), 7.22 (d, 3JHH = 1.7, 2H, imid), 6.98 (d, 3JHH = 1.7, 2H, imid), 5.37 (s, 4H, pyC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 4.17 (t, 3JHH = 6.8, 4H, NCH2), 1.83 (app. pent., J = 7, 4H, CH2), 1.14–1.34 (m, 16H, CH2). 13C{1H} NMR (101 MHz, CD2Cl2) δ 178.7 (s, NCN), 155.5 (s, py), 139.4 (s, py), 123.2 (s, py), 122.5 (s, imid), 120.8 (s, imid), 56.3 (s, py
2), 4.17 (t, 3JHH = 6.8, 4H, NCH2), 1.83 (app. pent., J = 7, 4H, CH2), 1.14–1.34 (m, 16H, CH2). 13C{1H} NMR (101 MHz, CD2Cl2) δ 178.7 (s, NCN), 155.5 (s, py), 139.4 (s, py), 123.2 (s, py), 122.5 (s, imid), 120.8 (s, imid), 56.3 (s, py![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2), 52.1 (s, NCH2), 31.2 (s, CH2), 28.1 (s, CH2), 27.8 (s, CH2), 25.8 (s, CH2). ESI-MS (CH3CN, 180 °C, 3 kV) positive ion: 468.2185 m/z, [M]+ (Calc. 468.2183). Anal. Calcd for C50H70Br4Cu4N10 (1384.96 g mol−1): C, 43.36; H, 5.09; N, 10.11. Found: C, 43.28; H, 4.97; N, 9.99.
H2), 52.1 (s, NCH2), 31.2 (s, CH2), 28.1 (s, CH2), 27.8 (s, CH2), 25.8 (s, CH2). ESI-MS (CH3CN, 180 °C, 3 kV) positive ion: 468.2185 m/z, [M]+ (Calc. 468.2183). Anal. Calcd for C50H70Br4Cu4N10 (1384.96 g mol−1): C, 43.36; H, 5.09; N, 10.11. Found: C, 43.28; H, 4.97; N, 9.99.
Synthesis of 4
A suspension of 3 (53 mg, 0.0383 mmol) and Na[BArF4] (90 mg, 0.1016 mmol) was sonicated in toluene (3 mL) and then stirred at room temperature for 2 days before filtration. The filtrate was concentrated to dryness and the crude product crystallised from THF/pentane. Yield: 62 mg (61%, pale yellow powder) as 4·0.5THF. Crystals suitable for X-ray diffraction were grown from ether/pentane at 20 °C.
1
H NMR (400 MHz, CD2Cl2) δ 7.83 (t, 3JHH = 7.8, 1H, py), 7.73 (bs, 8H, ArF), 7.56 (br, 4H, ArF), 7.42 (d, 3JHH = 7.8, 2H, py), 7.10 (d, 3JHH = 1.2, 2H, imid), 7.02 (d, 3JHH = 1.2, 2H, imid), 5.19 (s, 4H, pyC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 4.20 (t, 3JHH = 7.6, 4H, NCH2), 1.87 (app. pent., J = 7, 4H, CH2), 1.25–1.48 (m, 16 H, CH2).13C{1H} NMR (101 MHz, CD2Cl2) δ 180.3 (s, NCN), 162.3 (q, 1JCB = 50, ArF), 153.8 (s, py), 140.2 (s, py), 135.4 (s, ArF), 129.5 (qq, 2JFC = 32, 3JCB = 3, ArF), 125.2 (q, 1JFC = 271, ArF), 124.1 (s, py), 123.2 (s, imid), 119.6 (s, imid), 118.1 (pent., 3JFC = 4, ArF), 54.9 (s, py
2), 4.20 (t, 3JHH = 7.6, 4H, NCH2), 1.87 (app. pent., J = 7, 4H, CH2), 1.25–1.48 (m, 16 H, CH2).13C{1H} NMR (101 MHz, CD2Cl2) δ 180.3 (s, NCN), 162.3 (q, 1JCB = 50, ArF), 153.8 (s, py), 140.2 (s, py), 135.4 (s, ArF), 129.5 (qq, 2JFC = 32, 3JCB = 3, ArF), 125.2 (q, 1JFC = 271, ArF), 124.1 (s, py), 123.2 (s, imid), 119.6 (s, imid), 118.1 (pent., 3JFC = 4, ArF), 54.9 (s, py![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2), 52.9 (s, NCH2), 31.4 (s, CH2), 27.2 (s, CH2), 27.1 (s, CH2), 25.8 (s, CH2), 25.6 (s, CH2). ESI-MS (CH3CN, 180 °C, 3 kV) positive ion: 468.2185 m/z, [M]+ (Calc. 468.2183). Anal. Calcd for C57H47BCuF24N5 (1332.33 g mol−1): C, 51.38; H, 3.56; N, 5.26. Found: C, 51.48; H, 3.47; N, 5.34.
H2), 52.9 (s, NCH2), 31.4 (s, CH2), 27.2 (s, CH2), 27.1 (s, CH2), 25.8 (s, CH2), 25.6 (s, CH2). ESI-MS (CH3CN, 180 °C, 3 kV) positive ion: 468.2185 m/z, [M]+ (Calc. 468.2183). Anal. Calcd for C57H47BCuF24N5 (1332.33 g mol−1): C, 51.38; H, 3.56; N, 5.26. Found: C, 51.48; H, 3.47; N, 5.34.
In situ formation of 5 and 6
A J. Young's NMR tube was charged with 0.004/0.008 mmol of 2/4 and 1.1 equivalent (metal per metal) of [Pd(NCMe)2Cl2]/[Rh(CO)2Cl]2. CD2Cl2 (0.5 mL) was added and the reaction monitored by 1H NMR until complete consumption of 2/4. The samples were periodically placed in an ultrasound bath during the course of the reaction. In the case of the reaction between 4 and [Rh(CO)2Cl]2, the crude reaction mixture was subsequently passed through a short silica plug (pipette, 3 cm) with additional CH2Cl2 to afford 6 in 82% yield following removal of the solvent in vacuo.Synthesis of 7
1
H NMR (500 MHz, CD2Cl2): δ 7.82 (t, 3JHH = 7.7, 1H, py), 7.68–7.76 (m, 8H, ArF), 7.55 (br, 4H, ArF), 7.45 (d, 3JHH = 7.7, 2H, py), 7.11 (d, 3JHH = 1.7, 2H, imid), 6.87 (d, 3JHH = 1.7, 2H, imid), 6.30 (d, 2JHH = 15.0, 2H, pyC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 5.14 (d, 2JHH = 15.0, 2H, pyC
2), 5.14 (d, 2JHH = 15.0, 2H, pyC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 4.73 (app. t, J = 12, 2H, NCH2), 3.68–3.78 (m, 2H, NCH2), 1.94 (br, 2H, CH2), 1.66 (br, 2H, CH2), 1.18–1.50 (m, 14H, CH2), 1.09 (br, 2H, CH2). 13C{1H} NMR (126 MHz, CD2Cl2): δ 162.3 (q, 1JCB = 50, ArF), 162.0 (s, NCN), 156.5 (s, py), 140.9 (s, py), 135.3 (s, ArF), 129.4 (qq, 2JFC = 32, 3JCB = 3, ArF), 125.2 (q, 1JFC = 271, ArF), 125.1 (s, py), 123.0 (s, imid), 121.4 (s, imid), 118.0 (pent., 3JFC = 4, ArF), 55.0 (s, py
2), 4.73 (app. t, J = 12, 2H, NCH2), 3.68–3.78 (m, 2H, NCH2), 1.94 (br, 2H, CH2), 1.66 (br, 2H, CH2), 1.18–1.50 (m, 14H, CH2), 1.09 (br, 2H, CH2). 13C{1H} NMR (126 MHz, CD2Cl2): δ 162.3 (q, 1JCB = 50, ArF), 162.0 (s, NCN), 156.5 (s, py), 140.9 (s, py), 135.3 (s, ArF), 129.4 (qq, 2JFC = 32, 3JCB = 3, ArF), 125.2 (q, 1JFC = 271, ArF), 125.1 (s, py), 123.0 (s, imid), 121.4 (s, imid), 118.0 (pent., 3JFC = 4, ArF), 55.0 (s, py![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2), 51.3 (s, NCH2), 30.8 (s, CH2), 28.7 (s, CH2), 27.5 (s, CH2), 23.7 (s, CH2). ESI-MS (CH3CN, 180 °C, 4 kV) positive ion: 498.1929 m/z, [M]+ (calc. 498.1929). Anal. Calcd for C57H47BClF24N5Ni (1362.95 g mol−1): C, 50.23; H, 3.48; N, 5.14. Found: C, 50.62; H, 3.74; N, 5.05.
H2), 51.3 (s, NCH2), 30.8 (s, CH2), 28.7 (s, CH2), 27.5 (s, CH2), 23.7 (s, CH2). ESI-MS (CH3CN, 180 °C, 4 kV) positive ion: 498.1929 m/z, [M]+ (calc. 498.1929). Anal. Calcd for C57H47BClF24N5Ni (1362.95 g mol−1): C, 50.23; H, 3.48; N, 5.14. Found: C, 50.62; H, 3.74; N, 5.05.
Crystallography
Full details about the collection, solution and refinement are documented in the CIF, which have been deposited with the Cambridge Crystallographic Data Centre under CCDC 1470494–1470497.Acknowledgements
We thank the University of Warwick (R. E. A.), European Research Council (ERC, grant agreement 637313; C. M. S, A. B. C) and Royal Society (A. B. C.) for financial support. Crystallographic and high-resolution mass-spectrometry data were collected using instruments purchased through support from Advantage West Midlands and the European Regional Development Fund. Crystallographic data for 7 were collected using an instrument that received funding from the ERC under the European Union's Horizon 2020 research and innovation programme (grant agreement No 637313).References
-
(a) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef CAS PubMed
; (b) M. Soleilhavoup and G. Bertrand, Acc. Chem. Res., 2015, 48, 256–266 CrossRef CAS PubMed
; (c) S. Díez-González, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612–3676 CrossRef PubMed
; (d) P. de Frémont, N. Marion and S. P. Nolan, Coord. Chem. Rev., 2009, 253, 862–892 CrossRef
; (e) F. E. Hahn and M. C. Jahnke, Angew. Chem., Int. Ed., 2008, 47, 3122–3172 CrossRef CAS PubMed
.
- J. C. Y. Lin, R. T. W. Huang, C. S. Lee, A. Bhattacharyya, W. S. Hwang and I. J. B. Lin, Chem. Rev., 2009, 109, 3561–3598 CrossRef CAS PubMed
.
-
(a) I. J. B. Lin and C. S. Vasam, Coord. Chem. Rev., 2007, 251, 642–670 CrossRef CAS
; (b) J. C. Garrison and W. J. Youngs, Chem. Rev., 2005, 105, 3978–4008 CrossRef CAS PubMed
.
-
(a) X.-Y. Yu, B. O. Patrick and B. R. James, Organometallics, 2006, 25, 2359–2363 CrossRef CAS
; (b) P. de Frémont, N. M. Scott, E. D. Stevens and S. P. Nolan, Organometallics, 2005, 24, 2411–2418 CrossRef
.
- M. R. L. Furst and C. S. J. Cazin, Chem. Commun., 2010, 46, 6924–6922 RSC
.
- C. Boehme and G. Frenking, Organometallics, 1998, 17, 5801–5809 CrossRef CAS
.
-
(a) R. E. Andrew, L. González-Sebastián and A. B. Chaplin, Dalton Trans., 2016, 45, 1299–1305 RSC
; (b) M. Poyatos, J. A. Mata and E. Peris, Chem. Rev., 2009, 109, 3677–3707 CrossRef CAS PubMed
; (c) D. Pugh and A. A. Danopoulos, Coord. Chem. Rev., 2007, 251, 610–641 CrossRef CAS
; (d) E. Peris and R. H. Crabtree, Coord. Chem. Rev., 2004, 248, 2239–2246 CrossRef CAS
.
- Representative examples of transmetallation reactions involving other polydentate NHC ligands:
(a) D. T. Weiss, P. J. Altmann, S. Haslinger, C. Jandl, A. Pöthig, M. Cokoja and F. E. Kühn, Dalton Trans., 2015, 44, 18329–18339 RSC
; (b) A. Rit, T. Pape and F. E. Hahn, J. Am. Chem. Soc., 2010, 132, 4572–4573 CrossRef CAS PubMed
; (c) F. E. Hahn, C. Radloff, T. Pape and A. Hepp, Chem. – Eur. J., 2008, 14, 10900–10904 CrossRef CAS PubMed
; (d) X. Hu, I. Castro-Rodriguez, K. Olsen and K. Meyer, Organometallics, 2004, 23, 755–764 CrossRef CAS
; (e) Y. A. Wanniarachchi, M. A. Khan and L. M. Slaughter, Organometallics, 2004, 23, 5881–5884 CrossRef CAS
.
-
(a) J. Vaughan, D. J. Carter, A. L. Rohl, M. I. Ogden, B. W. Skelton, P. V. Simpson and D. H. Brown, Dalton Trans., 2016, 45, 1484–1495 RSC
; (b) M. Hernández-Juárez, J. López-Serrano, P. Lara, J. P. Morales-Cerón, M. Vaquero, E. Álvarez, V. Salazar and A. Suárez, Chem. – Eur. J., 2015, 21, 7540–7555 CrossRef PubMed
; (c) W. D. Clark, G. E. Tyson, T. K. Hollis, H. U. Valle, E. J. Valente, A. G. Oliver and M. P. Dukes, Dalton Trans., 2013, 42, 7338–7344 RSC
; (d) S. Wei, X. Li, Z. Yang, J. Lan, G. Gao, Y. Xue and J. You, Chem. Sci., 2012, 3, 359–363 RSC
; (e) K. Inamoto, J.-I. Kuroda, E. Kwon, K. Hiroya and T. Doi, J. Organomet. Chem., 2009, 694, 389–396 CrossRef CAS
; (f) D. H. Brown, G. L. Nealon, P. V. Simpson, B. W. Skelton and Z. Wang, Organometallics, 2009, 28, 1965–1968 CrossRef CAS
; (g) D. Pugh, A. Boyle and A. A. Danopoulos, Dalton Trans., 2008, 1087–1094 RSC
; (h) W. Chen, B. Wu and K. Matsumoto, J. Organomet. Chem., 2002, 654, 233–236 CrossRef CAS
; (i) A. Caballero, E. Díez-Barra, F. A. Jalón, S. Merino, A. M. Rodríguez and J. Tejeda, J. Organomet. Chem., 2001, 627, 263–264 CrossRef CAS
.
-
(a) V. Charra, P. de Frémont, P.-A. R. Breuil, H. Olivier-Bourbigou and P. Braunstein, J. Organomet. Chem., 2015, 795, 25–33 CrossRef CAS
; X. Liu, R. Pattacini, P. Deglmann and P. Braunstein, Organometallics, 2011, 30, 3302–3310 Search PubMed
.
-
(a) A. Biffis, M. Cipani, C. Tubaro, M. Basato, M. Costante, E. Bressan, A. Venzo and C. Graiff, New J. Chem., 2013, 37, 4176–4184 RSC
; (b) C. Radloff, H.-Y. Gong, C. Schulte to Brinke, T. Pape, V. M. Lynch, J. L. Sessler and F. E. Hahn, Chem. – Eur. J., 2010, 16, 13077–13081 CrossRef CAS PubMed
.
- (Scheme 2, A): R. S. Simons, P. Custer, C. A. Tessier and W. J. Youngs, Organometallics, 2003, 22, 1979–1982 CrossRef CAS
.
- (Scheme 2, B): D. J. Nielsen, K. J. Cavell, B. W. Skelton and A. H. White, Inorg. Chim. Acta, 2002, 327, 116–125 CrossRef CAS
.
- (Scheme 2, C and D): T. Nakamura, S. Ogushi, Y. Arikawa and K. Umakoshi, J. Organomet. Chem., 2016, 803, 67–72 CrossRef CAS
.
- (Scheme 2, C): A. Herbst, C. Bronner, P. Dechambenoit and O. S. Wenger, Organometallics, 2013, 32, 1807–1814 CrossRef CAS
.
- (Scheme 2, D): B. R. M. Lake and C. E. Willans, Chem. – Eur. J., 2013, 19, 16780–16790 CrossRef CAS PubMed
; B. Liu, X. Ma, F. Wu and W. Chen, Dalton Trans., 2015, 44, 1836–1844 RSC
.
- (Scheme 2, E): D. I. Bezuidenhout, G. Kleinhans, G. Guisado-Barrios, D. C. Liles, G. Ung and G. Bertrand, Chem. Commun., 2014, 50, 2431–2433 RSC
.
- A. V. Knishevitsky, N. I. Korotkikh, A. H. Cowley, J. A. Moore, T. M. Pekhtereva, O. P. Shvaika and G. Reeske, J. Organomet. Chem., 2008, 693, 1405–1411 CrossRef CAS
.
-
(a) R. E. Andrew, D. W. Ferdani, C. A. Ohlin and A. B. Chaplin, Organometallics, 2015, 34, 913–917 CrossRef CAS
; (b) R. E. Andrew and A. B. Chaplin, Inorg. Chem., 2015, 54, 312–322 CrossRef CAS PubMed
; (c) R. E. Andrew and A. B. Chaplin, Dalton Trans., 2014, 43, 1413–1423 RSC
.
- I. Krossing and I. Raabe, Angew. Chem., Int. Ed., 2004, 43, 2066–2090 CrossRef CAS PubMed
.
- D. Tapu, D. A. Dixon and C. Roe, Chem. Rev., 2009, 109, 3385–3407 CrossRef CAS PubMed
.
- Related observations have been made for Ag(I) and Au(I) complexes of di-NHC ligands: J. Gil-Rubio, V. Cámara, D. Bautista and J. Vicente, Inorg. Chem., 2013, 52, 4071–4083 CrossRef CAS PubMed
.
- Other instances of the [Cu2Br4]2− dianion see:
(a) B. R. M. Lake, A. Ariafard and C. E. Willans, Chem. – Eur. J., 2014, 20, 12729–12733 CrossRef CAS PubMed
; (b) E. Boess, D. Sureshkumar, A. Sud, C. Wirtz, C. Farès and M. Klussmann, J. Am. Chem. Soc., 2011, 133, 8106–8109 CrossRef CAS PubMed
; (c) P. Drożdżewski and M. Kubiak, Polyhedron, 2009, 28, 1518–1524 CrossRef
; (d) A. J. Canty, L. M. Engelhardt, P. C. Healy, J. D. Kildea, N. J. Minchin and A. H. White, Aust. J. Chem., 1987, 40, 1881–1891 CrossRef CAS
.
- The other independent cation features a significant degree of disorder about the copper centre.
- V. J. Catalano, L. B. Munro, C. E. Strasser and A. F. Samin, Inorg. Chem., 2011, 50, 8465–8476 CrossRef CAS PubMed
.
-
(a) C. Chen, H. Qiu and W. Chen, J. Organomet. Chem., 2012, 696, 4166–4172 CrossRef CAS
; (b) X. Liu and W. Chen, Organometallics, 2012, 31, 6614–6622 CrossRef CAS
.
- W. E. Buschmann, J. S. Miller, K. Bowman-James and C. N. Miller, Inorg. Synth., 2002, 33, 83–91 CAS
.
- J. A. McCleverty, G. Wilkinson, L. G. Lipson, M. L. Maddox and H. D. Kaesz, Inorg. Synth., 1990, 28, 84–86 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C{1H} NMR, and ESI-MS spectra of new complexes. Selected 1H NMR data for transmetallation reactions of 2 and 4. CCDC 1470494–1470497. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt01263a |
| This journal is © The Royal Society of Chemistry 2016 |