 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Teaching old compounds new tricks: efficient N2 fixation by simple Fe(N2)(diphosphine)2 complexes†
Laurence R.
Doyle
a,
Peter J.
Hill
a,
Gregory G.
Wildgoose
b and
Andrew E.
Ashley
*a
aDepartment of Chemistry, Imperial College London, Exhibition Road, South Kensington, London SW7 2AZ, UK. E-mail: a.ashley@imperial.ac.uk; Tel: +44 (0) (20) 75945810
bSchool of Chemistry, University of East Anglia, Norwich Research Park, Norwich, NR4 7TJ, UK
First published on 5th April 2016
Abstract
The Fe(0) species Fe(N2)(dmpe)2 exists in equilibrium with the previously unreported dimer, [Fe(dmpe2)2(μ-N2)]. For the first time these complexes, alongside Fe(N2)(depe)2, are shown unambiguously to produce N2H4 and/or NH3 upon addition of triflic acid; for Fe(N2)(depe)2 this represents one of the highest electron conversion efficiencies for Fe complexes to date.
Homogeneous catalysts capable of fixing N2 to NH3 under mild conditions have been researched for over 50 years.1 Fe, which catalyses the industrial Haber–Bosch process (as Fe metal), is also considered to perform a crucial role in biological N2 fixation, performed at the Fe–Mo cofactor of the most abundant nitrogenase enzyme and mediated by successive proton-coupled electron transfers.2 Whilst the active site for N2 binding and reduction at the Fe–Mo cofactor is contested, less common nitrogenases with closely related Fe–V and Fe-only cofactors implicate the importance of Fe,3 and a mechanism for Fe-mediated N2 fixation has been proposed.4
The first major breakthrough in N2 fixation by a homogeneous Fe complex was reported in 1991 by Leigh et al., utilising chelating Me2PCH2CH2PMe2 (dmpe) as an ancillary ligand.5 In the eponymous reaction cycle, the Fe(II) complex [trans-Fe(H)(N2)(dmpe)2][BPh4] was reductively deprotonated to form the Fe(0) intermediate Fe(N2)(dmpe)2 (1) which, upon in situ acidification of the reaction mixture using various strong proton sources, was documented to produce NH3 (isolated as NH4+via a base distillation onto fresh acid and quantified using the spectrophotometric indophenol method);6 the highest yields were obtained using HCl.5,7,8 Since Fe was recovered as Fe(II), the yields of NH3 (up to 20%) were calculated based on each Fe providing a maximum of 2 electrons (out of a total of 6) to reduce N2; accordingly Fe(0) must be consumed as the sacrificial reductant. Analogous deprotonation/reprotonation experiments performed on related phosphine complexes – [trans-Fe(H)(N2)(depe)2]+ (depe = Et2PCH2CH2PEt2),8 [cis-Fe(H)(N2){E(CH2CH2PPh2)3}]+ (E = N, P),8,9 and [trans-Fe(H)(N2)(DMeOPrPE)2]+ (DMeOPrPE = [(MeOCH2CH2CH2)2PCH2]2)10 – have also been shown to generate similar yields of NH3 and/or N2H4. However, in all of these experiments the Fe(0) species were not isolated; in the case of the archetypal dmpe system, 1 was reported to be unstable with respect to dissociation of N2in vacuo, leading to its decomposition.5,8
In contrast with these findings, Komiya et al. successfully synthesised pure Fe(N2)(depe)2 (2)11 using an alternative route and discovered that only N2 and H2 were produced upon treatment with HCl; this result cast uncertainty on the candidacy of Fe(N2)L4 (L = 2 electron donor) complexes being the active NH3 producing species in Leigh-type experiments (Fig. 1). Furthermore, Field et al. recently showed that the positive detection of NH3 (as NH4+) using the indophenol method can arise from interference caused by free phosphine ligands, which may contaminate the analyte during the base distillation step;12 this was corroborated by the absence of resonances for NH4+ in the 1H and 14N{1H} NMR spectra of the analyte from the Leigh reaction of [trans-Fe(H)(N2)(dmpe)2]+. Clearly, the isolation of pure samples of such species, and their subsequent reaction with acids to assess their capability of producing reduced forms of N2, is crucial to clarifying this long-standing conundrum.
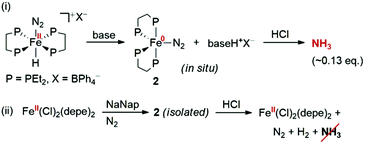 | ||
| Fig. 1 Synthesis and acidification of 2 performed by (i) Leigh et al. and (ii) Komiya et al.; NaNap = sodium naphthalenide, highlighting the disparate results for NH3 production. | ||
Recently we reported convenient multi-gram syntheses of dmpe and depe,13 and sought to reinvestigate the historically curious N2-fixation chemistry mediated by their Fe(N2) complexes. Herein we report the synthesis and characterisation of the Fe(0) species, [Fe(dmpe)2]2(μ-N2) (3), which reacts with N2 cleanly to produce 1. Alongside 2,‡ these isolated compounds react with TfOH (CF3SO3H) to produce N2H4 and/or NH3, thus unambiguously confirming that these complexes are active for the fixation of N2, for the first time.
KC8 reduction of trans-Fe(Cl)2(dmpe)2 under a 15N2 atmosphere in hexane (Scheme 1), as previously described by Field et al.,14 generates solutions of 1-15N2in situ [31P{1H} NMR: δ (ppm) = 63.3 ppm (s, fwhm = 6 Hz); 15N{1H} NMR: δ (ppm) = –48.8 (d), −47.0 (d), 1JNα–Nβ = 5.9 Hz] along with a trace amount of the known decomposition product [Fe(dmpe)2]2(μ-dmpe) (4) [31P{1H} NMR: δ (ppm) = 61.4 ppm (d), 8.2 ppm (m)]; see Fig. 2. However, another broader singlet was also observed downfield in the 31P{1H} NMR spectrum [δ (ppm) = 66.0 ppm, fwhm = 14 Hz], in addition to an upfield singlet (−54.9 ppm) in the 15N{1H} NMR spectrum of this solution. To assess the reported instability of 1 in the absence of N2, the solvent was removed in vacuo and the remaining oily solid dried for several hours at ca. 10−3 mbar pressure. Unexpectedly, subsequent dissolution of this solid in hexane under Ar revealed 1 to still be present by 31P{1H} NMR spectroscopy, albeit in a lower ratio relative to the unassigned resonances. Curiously, the amount of 4 remained almost unchanged. Gratifyingly, slow evaporation of the solvent (Ar atmosphere) yielded large, deep red crystals whose solution-phase 31P{1H} and 15N{1H} NMR spectra corresponded to the aforementioned unidentified resonances, and which were solved by single crystal X-ray diffraction as the new compound [Fe(dmpe)2]2(μ-N2) (3, Fig. 3).
 | ||
| Fig. 2 (i) 31P{1H} and (ii) 15N{1H} NMR spectra of the reduction of trans-Fe(Cl)2(dmpe)2 under 15N2 with KC8 (4 eq.) in hexane. | ||
The solid-state structure shows the independent [Fe(dmpe)2N] fragments in 3 both adopt near ideal trigonal bipyramidal coordination, with the two equatorial Fe(1)P(4)P(14) and Fe(2)P(24)P(34) best planes bisecting one another almost perpendicularly [82.08(4)°]. The bridging N2 ligand is approximately linear, exhibiting typical bond lengths for both the single Fe–N and triple N–N bonds; the latter is comparable with the previously reported structure of 2 [1.139(13) Å]15 and indicates weak activation of the N2 unit in both complexes. The bond lengths and angles seen in 3 are in close agreement with the geometry optimised structure reported by Tyler et al. in their theoretical study of N2 fixation mediated by various Fe(dmpe)2 intermediates, in which dimerisation of 1 (with concomitant loss of N2) to form 3 was calculated to be unfavourable by 20 kcal mol−1.16 Furthermore, a low energy barrier of only 6 kcal mol−1 was calculated for the dissociation of 3 to 1 and [Fe(dmpe)2]. Despite this, it has been possible to prepare 3 on a multi-gram scale (using 14N2; see ESI† for further details): after generating a crude solution of 1, the hexane solvent was mostly removed in vacuo until a slurry of solid (mixture of 1 and 3) in a small volume of solvent remained, after which this suspension was stirred for several days under Ar. Using this protocol, less soluble 3 selectively crystallises as N2 is slowly depleted upon condensation of 1, and residual 1 and 4 are subsequently removed by rinsing with additional cold hexane. The resulting sample was then rapidly recrystallised (redissolved in hexane, filtered and cooled to −35 °C) yielding a microcrystalline solid of ≥98% purity (31P NMR spectroscopy) that provided satisfactory elemental (CHN) analysis. Crystalline 3 is thermally unstable and is best stored under Ar at ≤−30 °C; under these conditions decomposition (to a mixture of 1, 4, and Fe metal) appears to be minimal after several months.
Solutions of 3 prepared under an Ar atmosphere decompose to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 and Fe metal;7 this occurs relatively slowly in non-polar alkane solvents (pentane, t1/2 = 13 d) yet more readily in donor solvents (THF, t1/2 ≈ 1.5 d; εr = 7.52). Dissolution of 3 in N2-saturated solvents quantitatively generates 1, which proceeds more slowly in aliphatics than ethereal donor solvents (THF, 0.25 M, 2 d), whilst in the highly polar non-donor organic solvent 1,2-difluorobenzene (εr = 13.8),18 conversion to 1 is almost instantaneous. Thus, it would appear that a large solvent polarity facilitates dissociation, rather than the donor ability of the solvent.
17 and Fe metal;7 this occurs relatively slowly in non-polar alkane solvents (pentane, t1/2 = 13 d) yet more readily in donor solvents (THF, t1/2 ≈ 1.5 d; εr = 7.52). Dissolution of 3 in N2-saturated solvents quantitatively generates 1, which proceeds more slowly in aliphatics than ethereal donor solvents (THF, 0.25 M, 2 d), whilst in the highly polar non-donor organic solvent 1,2-difluorobenzene (εr = 13.8),18 conversion to 1 is almost instantaneous. Thus, it would appear that a large solvent polarity facilitates dissociation, rather than the donor ability of the solvent.
The Raman active ν(N–N) stretch of solid 3 (1933 cm−1) indicates a significant increase in the activation of the N2 ligand compared to the monomeric complex 1 [IR(KBr): ν(N–N) = 1975 cm−1)].5 In fact, neutral 3 has one of the lowest ν(N–N) stretches recorded for a low-spin Fe system, which is comparable with those found in the anionic complexes [(P3E)Fe(N2)][Na(12-crown-4)2] (P = 2-PiPr2C6H4; E = B, Si; IR(THF): ν(N–N) = 1918, 1920 cm−1)19 reported by Peters et al. [(P3B)Fe(N2)][Na(12-crown-4)2] is notable for being the first synthetic Fe complex able to catalyse the fixation of N2 to NH3 from proton and electron equivalents, demonstrating the feasibility of a single Fe site to perform this fundamental transformation;20 here, a very strong reductant (KC8) and a powerful acid [H(OEt2)2(BArF24); BArF24 = B(3,5-(CF3)2C6H3)4] were used in excess. In contrast, for Leigh-type chemistry, electron equivalents for the N2 reduction are ultimately supplied by Fe(0) species, generated via reductive deprotonation of a Fe–H bond in the Fe(II) precursor. Thus, to assess the reducing power of such Fe(0)N2-phosphine complexes, cyclic voltammetry measurements were performed on 1 (generated from 3/N2), 2, and 3 (2 mM in Et2O; [nBu4N][BArF24] electrolyte; Cp2Fe+/0 reference). For both compounds 2 and 3 a reversible one-electron oxidation was observed at various scan rates which can be assigned to the [Fe(0)/Fe(I)] redox couple (centred at −2.03 V and −2.23 V, for 2 ↔ [2]+ and 3 ↔ [3]+ respectively; see ESI†). Conversely, the cyclic voltammogram of 1 revealed a single irreversible oxidation at ca. −2.0 V [Fe(0) → Fe(I)], and three smaller unassigned reduction processes between ca. −2.0 and −2.4 V. Accordingly it appears that [1]+ is unstable under these conditions, and the additional reduction processes may involve highly reactive [Fe(dmpe)2]+ (via N2 dissociation from [1]+), or an Et2O adduct, or solvent-activation product(s) thereof. Nonetheless, the neutral Fe(0) compounds 1–3 are notably powerful reducing agents, and considerably stronger than the commonly employed CoCp2 and CoCp*2 (−1.33 and −1.84 V vs. Cp2Fe+/0 in 1,2-dimethoxyethane),21 which have been used as external reductants in catalytic N2 fixation by Mo complexes.22,23
In the knowledge that 1–3 are potent reductants, we sought to establish conclusively whether they are able to convert N2 to the reduced forms N2H4 and NH3 in the presence of protons, and furthermore in the absence of any potential contaminants (synthetic by-products/decomposites) from Leigh-type deprotonation reactions. Our protocol (see ESI†) for the quantitative assay of NH3 used the relative integration of the NH4+ resonance in the 1H spectrum§ against a calibrated insert. Quantitative analysis of N2H4 employed a spectrophotometric method which relies on reaction with acidic para-dimethylaminobenzaldehyde indicator solution;24 by performing thorough control experiments we found, crucially, that neither NH3, nor dmpe, nor depe interfered with the results.¶ Using HCl to acidify pristine solutions of 1, we detected only trace amounts (<0.5% per Fe) of NH3 and no N2H4, including when 1 was prepared in situ from [trans-Fe(H)(N2)(PP)2]+ (PP = dmpe, depe) using the original method of Leigh et al.; the latter results corroborate those of Field et al.12 Identical results were also obtained for pure 2 and 3 which confirms that, under this acidification protocol (whether formed under Leigh-type conditions or using isolated pure samples), neither of these dmpe/depe complexes can produce the yields of NH3 previously reported.
Tyler et al. reported a marked difference in the yields of NH3 upon acidification of their Leigh-type prepared Fe(N2)(DMeOPrPE)2 complex with the following acids: HCl (4%), HBF4 (7%), and TfOH (up to 15%); in the latter case they showed, using a phenanthroline spectrophotometric test, that after acidification all Fe species are present as Fe(II), thus verifying the hypothesis that each Fe(0) can only supply a maximum of two electrons for the reduction of N2 (or H+ to H2). These yields were suggested to reflect increasing favourability of NH3 formation with decreasing coordination/ion-pairing of the anion of the acid. It should be noted that whilst NH3 was quantified either by NMR spectroscopy10 or the indophenol test,25 the DMeOPrPE ligand is expected to be far less volatile than dmpe/depe and thus unlikely to interfere with the latter method. To our delight, when using TfOH to acidify 1–3, we were able to detect significant amounts of N2H4 and/or NH3, which showed a marked dependence on solvent and/or temperature; these data are reported in Table 1, alongside other reported Fe(N2)L4 Leigh-type experiments for comparison. Historically, yields of NH3 from Leigh-type experiments are quoted per Fe centre, however since we have mixtures of N2H4/NH3 products we have also included two other measures in order to resolve the efficiency of the ability of Fe(N2)L4 species to produce these azanes: (1) a combined fixed-N electron yield was calculated on the basis that reduction of N2 to N2H4/NH3 requires four/three electrons (per mol of product), which takes into account that each Fe provides a maximum of two electrons;5,25 (2) a fixed-N atom yield, calculated by the fraction of N atoms from the starting material that end up as N2H4 or NH3. Clearly the yields for these reactions may be interpreted in several ways, and all may be worth considering in the absence of greater mechanistic understanding of these rapid, and complex, transformations.
| Entry | Compound | Solvent | N2H4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (%) a (%) |
NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (%) a (%) |
N-atom yield (%) | e− yieldb (%) | Ref. |
|---|---|---|---|---|---|---|---|
| All reactions performed at 25 °C using TfOH, unless stated otherwise.a Yields per mol Fe.b Yield assuming each Fe supplies a max. of two electrons.c Performed at −78 °C.d This work.e Performed under Ar.f From deprotonation of [trans-Fe(H)(N2)(DMeOPrPE)2]+.g [H(OEt2)2][BArF24] used.h 2,6-Dimethylpyridinium (lutidinium) triflate used. NR = not reported. Yields are averaged over all runs (see ESI for more details). | |||||||
| 1 | 1 | THF | 0 | 0 | 0 | 0 | |
| 2 | 1 | Et2O | 9.1 | 0 | 9.1 | 18.2 | |
| 3 | 1 | Pentane | 9.1 | 0 | 9.1 | 18.2 | |
| 4 | 1 | Pentanec | 3.8 | 0 | 3.8 | 7.7 | |
| 5 | 2 | THF | 3.6 | 2.6 | 4.9 | 11.1 | |
| 6 | 2 | Et2O | 11.2 | 6.2 | 14.2 | 31.5 | |
| 7 | 2 | Et2Oc | 6.3 | 10.5 | 11.5 | 28.3 | |
| 8 | 2 | Pentane | 20.9 | 7.8 | 24.8 | 53.5 | |
| 9 | 2 | Pentanec | 24.0 | 4.5 | 26.3 | 54.8 | |
| 10 | 3 | Pentanee | 4.3 | 1.5 | 5.0 | 10.8 | |
| 11 | 3 | Pentanec,e | 2.0 | 0 | 2.0 | 4.1 | |
| 12 | FeN2(DMeOPrPE)2f | Et2O/THF | 2 | 15 | 9.5 | 26.5 | 10 |
| 13 | 1, prepared in situ | Hexane | NR | 0 | 0 | 0 | 12 |
| 14 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g g |
Et2O | 0.3 | 0.2 | 0.4 | 0.9 | |
| 15 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h h |
Et2O | 0 | 0 | 0 | 0 | |
Using TfOH, the highest electron yields were obtained for 2 (entries 5–9; ≤55%), followed by 1 (entries 1–4; ≤18%) and 3 (entries 10–11; ≤11%); these yields, in particular for 2, are amongst the highest reported for complexes of Fe, and highlight the delicate dependence on the acidification conditions, which is typical for N2 fixation chemistry.20,23,26 In these reactions initial protonation of the N2 ligand is a critical step, thereby triggering subsequent electron transfer; the efficacy of this process will presumably depend primarily on the strength of the H+ source employed. Previous calculations have shown that protonation at Fe is more thermodynamically favourable than at the terminal N atom in Fe(N2)(dmpe)2 by some 40 kcal mol−1, and it is expected that the latter process would result from kinetic factors, such as the use of a strong and sterically bulky acid source.16 The effect of solvent on the yields obtained for 2 is conspicuous, which generally decrease in the order: pentane > Et2O > THF. Whilst TfOH is insoluble in pentane and mass transfer effects may explain the high yields obtained from this medium, in both Et2O and THF [pKa(H2O) = −3.59 and −2.08, respectively]27 it is expected that acidity of TfOH [pKa(H2O) ≈ −12],28 will be levelled to the donor solvent, hence the protonating power of TfOH in the solvents used is expected to follow the same order, correlating with a greater efficiency of H+ attack on N2. Another factor may be the aggregation of TfOH due to strong intermolecular H-bonding,29 with a bulkier proton source favouring protonation at the exposed N2 ligand over the Fe centre.
Curiously, when H(OEt2)2(BArF24) in Et2O is employed as the acid source, only trace amounts of N2H4 and NH3 are observed; since TfOH and HCl [pKa(H2O) = −8] are expected to be levelled to protonated Et2O, taken together these experiments provide a situation where the solution pH can be viewed as approximately constant, and hence the effect of the anion on these reactions can be resolved. It is envisaged that strongly coordinating anions may bind/ion-pair more favourably to protonated intermediates along the N2-fixation pathway, which could sequester their reactivity and hence inhibit the formation of N2H4 or NH3; is it is therefore surprising that both HCl and H(OEt2)2(BArF24) are ineffective, since the coordinating ability of the counteranions follows the order Cl− ≫ TfO− > [BArF24]−.30 This trend has been previously observed in the catalytic reduction of N2 to NH3 by Mo PNP-pincer complexes, where proton sources incorporating TfO− as the counteranion were privileged in their activity in comparison with either Cl− or [BArF24]−.23 In our study, it is possible that the intermediate coordinating ability of TfO− strikes the best balance of lability properties to facilitate proton-coupled electron transfer events during N2 fixation mediated at the Fe centre. We have also probed the use of the weaker acid 2,6-dimethylpyridinium (lutidinium) triflate [pKa(H2O) = −6.77]31 with our most efficient compound 2; in this case no azanes were produced, and instead protonation at the metal centre resulted in clean conversion to the Fe(II) compound trans-[(H)Fe(N2)(depe)2]+,8 as ascertained by 31P{1H} NMR (δ = 81.4 ppm) and 1H NMR (hydride signal at δ = −18.20 ppm; 2JHP = 49 Hz) spectroscopy. Thus it appears that if too weak an acid source is used, formation of the thermodynamic Fe–H product is strongly favoured.
The increased yields of N2 fixation products for 2 relative to 1 may be attributed to the augmented steric bulk around the Fe centre conferred by the depe ligand, which also protects the metal centre from non-productive direct H+ attack. Despite a greater degree of N2 activation and a more negative reduction potential for 3, the conversion yields are lower than for 1. However, since the reduction of H+ to H2 competes with N2 fixation, the more potently reducing 3 may lead to poorer discrimination between the processes, translating to lower yields of N2H4 and NH3vs. H2 formation.
In conclusion, we have finally verified that simple Fe0(N2)(dmpe/depe)2 complexes, previously synthesised in situ from Leigh-type deprotonations, are capable of producing appreciable amounts of N2H4 and NH3 using TfOH as the acid source. In the case of the Fe0(N2)(depe)2 the reaction is particularly efficient based on the number of electrons available, and represents one of the highest conversions (55%) to date. The significant proportion of N2H4 produced in these reactions suggests that NH3 formation may proceed via N2H4 intermediates;32 further reduction may occur on Fe and/or via an outer sphere pathway. Mechanistic investigations into understanding this reactivity are currently underway.
We wish to thank the EPSRC for PhD studentship funding (LRD and PJH), the ERC (Starting Grant no. 307061, PiHOMER; GGW) and the Royal Society for University Research Fellowships (AA and GGW).
Notes and references
- N. Hazari, Chem. Soc. Rev., 2010, 39, 4044 RSC; K. C. MacLeod and P. L. Holland, Nat. Chem., 2013, 5, 559 CrossRef CAS PubMed; B. A. MacKay and M. D. Fryzuk, Chem. Rev., 2004, 104, 385 CrossRef PubMed.
- B. K. Burgess and D. J. Lowe, Chem. Rev., 1996, 96, 2983 CrossRef CAS PubMed; L. C. Seefeldt, B. M. Hoffman and D. R. Dean, Annu. Rev. Biochem., 2009, 78, 701 CrossRef PubMed; B. M. Hoffman, D. R. Dean and L. C. Seefeldt, Acc. Chem. Res., 2009, 42, 609 CrossRef PubMed.
- Y. Hu and M. W. Ribbe, J. Biol. Inorg. Chem., 2015, 20, 435 CrossRef CAS PubMed.
- B. M. Hoffman, D. Lukoyanov, Z.-Y. Yang, D. R. Dean and L. C. Seefeldt, Chem. Rev., 2014, 114, 4041 CrossRef CAS PubMed.
- J. G. Leigh and M. Jimenez-Tenorio, J. Am. Chem. Soc., 1991, 113, 5862 CrossRef.
- M. W. Weatherburn, Anal. Chem., 1967, 39, 971 CrossRef CAS.
- A. Hills, D. L. Hughes, M. Jimenez-Tenorio, G. J. Leigh and A. T. Rowley, J. Chem. Soc., Dalton Trans., 1993, 3041 RSC.
- D. A. Hall and G. J. Leigh, J. Chem. Soc., Dalton Trans., 1996, 3539 RSC.
- T. A. George, D. J. Rose, Y. D. Chang, Q. Chen and J. Zubieta, Inorg. Chem., 1995, 34, 1295 CrossRef CAS.
- J. D. Gilbertson, N. K. Szymczak and D. R. Tyler, J. Am. Chem. Soc., 2005, 127, 10184 CrossRef CAS PubMed.
- S. Komiya, M. Akita, A. Yoza, N. Kasuga, A. Fukuoka and Y. Kai, J. Chem. Soc., Chem. Commun., 1993, 787 RSC.
- L. D. Field, N. Hazari and H. L. Li, Inorg. Chem., 2015, 54, 4768 CrossRef CAS PubMed.
- L. R. Doyle, A. Heath, C. H. Low and A. E. Ashley, Adv. Synth. Catal., 2014, 356, 603 CrossRef CAS PubMed.
- L. D. Field, H. L. Li and A. M. Magill, Inorg. Chem., 2009, 48, 5 CrossRef CAS PubMed.
- C. Perthuisot and W. D. Jones, New J. Chem., 1994, 18, 621 CAS.
- R. B. Yelle, J. L. Crossland, N. K. Szymczak and D. R. Tyler, Inorg. Chem., 2009, 48, 861 CrossRef CAS PubMed.
- C. A. Tolman, S. D. Ittel, A. D. English and J. P. Jesson, J. Am. Chem. Soc., 1978, 100, 4080 CrossRef CAS.
- A. Mansingh and D. B. McLay, J. Chem. Phys., 1971, 54, 3322 CrossRef CAS.
- M.-E. Moret and J. C. Peters, Angew. Chem., Int. Ed., 2011, 50, 2063 CrossRef CAS PubMed; Y. Lee, N. P. Mankad and J. C. Peters, Nat. Chem., 2010, 2, 558 CrossRef PubMed.
- Examples of catalytic N2 to NH3 fixation mediated by Fe complexes: J. S. Anderson, J. Rittle and J. C. Peters, Nature, 2013, 501, 84 CrossRef CAS PubMed; S. E. Creutz and J. C. Peters, J. Am. Chem. Soc., 2014, 136, 1105 CrossRef PubMed.
- J. R. Aranzaes, M.-C. Daniel and D. Astruc, Can. J. Chem., 2006, 84, 288 CrossRef.
- D. V. Yandulov and R. R. Schrock, Science, 2003, 301, 76 CrossRef CAS PubMed.
- K. Arashiba, Y. Miyake and Y. Nishibayashi, Nat. Chem., 2011, 3, 120 CrossRef CAS PubMed.
- G. W. Watt and J. D. Chrisp, Anal. Chem., 1952, 24, 2006 CrossRef CAS.
- C. G. Balesdent, J. L. Crossland, D. T. Regan, C. T. López and D. R. Tyler, Inorg. Chem., 2013, 52, 14178 CrossRef CAS PubMed.
- H.-P. Jia and E. A. Quadrelli, Chem. Soc. Rev., 2014, 43, 547 RSC.
- E. M. Arnett and C. Y. Wu, J. Am. Chem. Soc., 1960, 82, 4999 CrossRef CAS.
- E. Raamat, K. Kaupmees, G. Ovsjannikov, A. Trummal, A. Kütt, J. Saame, I. Koppel, I. Kaljurand, L. Lipping, T. Rodima, V. Pihl, I. A. Koppel and I. Leito, J. Phys. Org. Chem., 2013, 26, 162 CrossRef CAS.
- C. M. Burba, N. M. Rocher and R. Frech, J. Phys. Chem. B, 2009, 113, 11453 CrossRef CAS PubMed.
- I. Krossing and I. Raabe, Angew. Chem., Int. Ed., 2004, 43, 2066 CrossRef CAS PubMed; S. H. Strauss, Chem. Rev., 1993, 93, 927 CrossRef.
- K. Clarke and K. Rothwell, J. Chem. Soc., 1960, 1885 RSC.
- NH3 formation from Fe(N2H4) complexes: J. L. Crossland, L. N. Zakharov and D. R. Tyler, Inorg. Chem., 2007, 46, 10476 CrossRef CAS PubMed and ref. 14.
- S. W. Gerritz and A. M. Sefler, J. Comb. Chem., 2000, 2, 39 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental procedures and characterization data. CCDC 1447532. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt00884d |
| ‡ We do not see any solution-phase spectroscopic evidence (31P, 15N, 1H NMR spectroscopy) for the formation of the depe analogue of 3, and we believe that the increased steric impact of replacing Me with Et in the ligand backbone is sufficient to preclude the formation of a dimeric species [Fe(depe)2]2(μ-N2). |
§ NH4+: 1H NMR (DMSO-d6: δ ≈ 7.3 ppm, t(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 1JNH = 51 Hz); 2,5-dimethylfuran33 insert (vinylic proton resonance): 1H NMR (DMSO-d6: δ = 5.83 ppm). 1), 1JNH = 51 Hz); 2,5-dimethylfuran33 insert (vinylic proton resonance): 1H NMR (DMSO-d6: δ = 5.83 ppm). |
| ¶ This methodology was validated by acidification experiments on authentic samples of NH4Cl or N2H4·2HCl, in the presence of trans-FeCl2(PP)2 (PP = dmpe, depe). |
| This journal is © The Royal Society of Chemistry 2016 |


