Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Magneto-optical investigations of molecular nanomagnet monolayers†
J.
Rozbořil
ab,
Y.
Rechkemmer
c,
D.
Bloos
c,
F.
Münz
ab,
C. N.
Wang
ab,
P.
Neugebauer
c,
J.
Čechal
de,
J.
Novák
*ab and
J.
van Slageren
*c
aCEITEC MU, Masaryk University, Kamenice 5, 62500 Brno, Czech Republic
bDepartment of Condensed Matter Physics, Faculty of Science, Masaryk University, Kotlářská 2, 61137 Brno, Czech Republic. E-mail: novak@physics.muni.cz
cInstitut für Physikalische Chemie, Universität Stuttgart, Pfaffenwaldring 55, 70569 Stuttgart, Germany. E-mail: slageren@ipc.uni-stuttgart.de
dCEITEC BUT, Brno University of Technology, Purkyňova 123, 61200 Brno, Czech Republic
eInstitute of Physical Engineering, Brno University of Technology, Technická 2896/2, 61669, Brno, Czech Republic
First published on 11th April 2016
Abstract
We report field-dependent magnetization measurements on monolayers of [Dy(Pc)2] on quartz, prepared by the Langmuir–Blodgett technique. The films are thoroughly characterized by means of X-ray reflectivity and atomic force microscopy. The magnetisation of the sample is measured through the magnetic circular dichroism of a ligand-based electronic transition.
Lanthanide single-molecule magnets possess much higher effective energy barriers towards relaxation of the magnetization than transition metal based ones.1 In addition, the blocking temperature of the magnetization has strongly increased during the last decade and has now reached 20 K.2 Here the blocking temperature of the magnetization is defined as the highest temperature at which coercivity in the magnetic hysteresis curve can be observed at conventional field sweep rates of ca. 1 T min−1. As a result of these recent successes, application in magnetic data storage devices, the main original aim of research in molecular nanomagnetism, has now come again into focus. It has become clear that successful improvement of lanthanide single-molecule magnets hinges on careful engineering of the crystal field (CF) splitting of the ground Russell–Saunders multiplet of the lanthanide ion. This can only be achieved through improved understanding of the factors influencing the CF splitting. Such understanding is now rapidly improving due to the synergetic combination of ab initio theory and advanced spectroscopy and magnetometry.3–5 It is clear that no device will be based on powder or single-crystal materials, but rather thin films or even monolayers. This was already realized in the molecular nanomagnetism community over a decade ago and this realization led to many investigations of molecular nanomagnets on surfaces.6 The majority of the earlier studies were largely topological, looking at the structure rather than the function of the thin layers. Later, synchrotron-based X-ray techniques such as X-ray absorption spectroscopy and X-ray magnetic circular dichroism started to be adopted. The early usage of the methods was to investigate the chemical stability of molecular nanomagnets on different surfaces. Later on, the physical properties of these were studied, culminating in the observation of quantum tunnelling of the magnetization in a monolayer of single-molecule magnets.7 In a different line of research, individual single-molecule magnets were investigated, by means of their insertion in break junctions and their attachment to carbon nanotubes.8 These studies are all necessarily performed at large scale facilities and/or extremely low temperatures. Such methods are eminently suitable for fundamental physical investigations, but less convenient for rapid material screening. Consequently, more easily accessible methods that have monolayer sensitivity would be of great benefit. A magnetometric method that may possess monolayer sensitivity is cantilever torque magnetometry. However, in magnetometric techniques the sum of signals due to all species in the sample is measured in contrast to the case of spectroscopic methods.
Here we present the results of our investigations of monolayers of the lanthanide double-decker [Dy(Pc)2] (1) on quartz. The monolayers are prepared by Langmuir–Blodgett (LB) techniques and characterized by atomic force microscopy (AFM) and X-ray reflectivity (XRR) techniques. Magnetic circular dichroism (MCD) spectroscopy is used to investigate the electronic structure and magnetic properties of the adsorbed species.‡
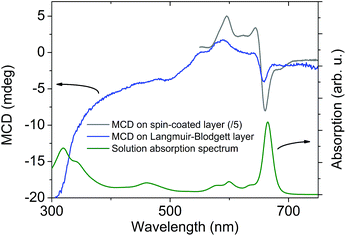
MCD spectroscopy measures the absorption difference of left- and right-hand circularly polarized light in the presence of a magnetic field. It allows study of the electronic spectrum of complexes based on transition metals,9 or lanthanides,5 with greater precision than absorption spectroscopy because the MCD signal is a signed quantity. In addition, the MCD intensity is related to the magnetization of the sample, and can thus be used to study the magnetic properties of the sample, e.g., by recording MCD-detected magnetic hysteresis curves.10–20 In order to assess the sensitivity of MCD for thin layer samples, we prepared a thin film on quartz substrate by means of the spin coating technique from a 1 mM solution of (NBu4)[Dy(Pc)2] (2) in chloroform solvent. The film morphology was characterized using profilometry. The average film thickness is (150 ± 30) nm. Interestingly, the MCD spectrum of the spin-coated layer reflects the features of the electronic spectrum of the neutral species 1 (Fig. 1 and S1†), rather than those of the anionic species 2, indicating that the starting compound has oxidized during the deposition process. Oxidation of the anions of the lanthanide double-deckers in chlorinated solvents is not uncommon (Fig. S1†).21 We cannot exclude that (part of) the oxidation occurs after deposition. In any case, the oxidized species 1 is a better single-molecule magnet than 2.22 The electronic transitions that are excited in the 600–700 nm range have ππ* character and are localized on the phthalocyanine ligands.23 The favourable signal-to-noise ratio of these measurements prompted us to prepare and study monolayers of 1.
We have chosen the LB technique to deposit monolayers, because this technique is generally applicable. In contrast, UHV techniques such as organic molecular beam deposition require volatility and thermal stability of the compounds.11 The LB technique has been used previously to prepare monolayers of single-molecule magnets.24 We have started from a 1 mM solution of 2 in chloroform, which was spread on the surface of a de-ionized water sub-phase in the Langmuir trough at a density of 30 μL per 100 cm2. The molecular monolayer formed on top of the sub-phase was then compressed to the target surface pressure of 15 mN m−1. Finally, the ultra-thin film of 1 on quartz was obtained by pulling out the substrate from the sub-phase at a speed of 0.5 mm min−1 while keeping the surface pressure constant. X-ray photoelectron measurements on the film show the clear presence of all the elements in 1 (Fig. S2†). Furthermore, the binding energies for the N 1s peaks have typical values for phthalocyanines.25 The resulting film was studied by means of AFM in the semi-contact mode (Fig. 2a). The AFM measurements show predominantly a molecular monolayer with some cracks and sparsely distributed thicker islands, due to locally deposited bi-layers. Fig. 2b displays the height histogram of the film, with data-points collected over the morphology of the entire AFM image displayed in Fig. 2a. The bimodal distribution is interpreted as follows: the first peak corresponds to the bare surface, while the second corresponds to the top of the monolayer. The distance difference between the two peaks (1.7 nm) corresponds very well to the edge-on configuration of the phthalocyanine molecules (molecular diameter 1.49 nm), which is common for weakly interacting substrates.26 The finite width of the peaks corresponds to surface roughness of the substrate and the molecular layer, respectively.
In order to obtain mesoscopically averaged characteristics of the film relevant for the MCD, we measured X-ray reflectivity (Fig. 3). The data show a well pronounced interference fringe. The data are very well simulated considering a one box model that includes a single layer of (2.12 ± 0.05) nm thickness. This thickness is slightly more than the usual height of the molecular steps and accounts for the regions with molecular bilayer observed by AFM. The average molecular surface density obtained from the data fit amounts to (0.89 ± 0.07) nm−2.
 | ||
| Fig. 3 X-ray reflectivity curve recorded on a Langmuir–Blodgett monolayer of 1 on quartz. The solid line is the fit using a model consisting of substrate and one layer. | ||
We then investigated the LB film by MCD. The spectrum (Fig. 1) shows several peaks that correspond well to those found for the spin-coated layer. The peak position (656 nm) of the lowest-energy band corresponds very well to that found in the spin-coated layer (660 nm), proving intactness of the compound. Finally, we were able to measure a magnetization curve by monitoring the MCD intensity at 656 nm as a function of magnetic field (Fig. 4). We observe a clear magnetization signal, which changes on increasing the temperature, thus indicating its magnetic origin. We observe no coercivity which suggests that the magnetic field is not applied along the easy axis of the molecule. Unfortunately, 1 crystallizes in a monoclinic space group with two differently oriented molecules in the unit cell,27 which precludes clarifying the orientation of the easy axis by single crystal magnetometry. Recently, it was reported that 200 nm thick films of the terbium derivative of 1 on quartz displayed both hysteresis and orientation-dependence.11 We found no orientation dependence of the magnetization curve.
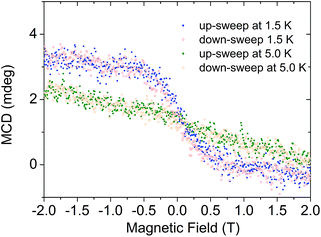 | ||
| Fig. 4 Magnetization curve recorded by MCD on the sample at λ = 658 nm and different temperatures and sweep directions as indicated. | ||
Our results demonstrate monolayer sensitivity of MCD for the investigation of the magnetic properties of monolayers of molecular nanomagnets on surfaces. This establishes MCD as a viable alternative to XMCD for the investigation of monolayers.28,29 In contrast to the latter, the former is relatively inexpensive and can be performed in a normal laboratory. XMCD is restricted to synchrotron sources and hence to large scale facilities.
In conclusion, we have measured the spectroscopic and magnetic properties of ultra-thin films of [Dy(Pc)2] at a molecular surface density of 0.89 nm−2 by means of magnetic circular dichroism (MCD) spectroscopy, demonstrating monolayer sensitivity of the MCD technique.
Acknowledgements
We acknowledge financial support from the projects CEITEC 2020 (grant no. LQ1601 financed by the MEYS of the Czech Republic), CEITEC – open access (grant LM2011020 from MEYS of Czech Republic), DAAD/AWTR/MEYS MOBILITY grant 57154055/7AMB15DE005, DFG INST 41/864-1, SL104/5-1.Notes and references
- D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110–5148 CrossRef CAS PubMed.
- Y.-C. Chen, J.-L. Liu, L. Ungur, J. Liu, Q.-W. Li, L.-F. Wang, Z.-P. Ni, L. F. Chibotaru, X.-M. Chen and M.-L. Tong, J. Am. Chem. Soc., 2016, 138, 2829–2837 CrossRef CAS PubMed.
- L. Ungur and L. F. Chibotaru, in Lanthanides and Actinides in Molecular Magnetism, Wiley-VCH Verlag GmbH & Co. KGaA, 2015, pp. 153–184 Search PubMed.
- K. S. Pedersen, D. N. Woodruff, J. Bendix and R. Clérac, in Lanthanides and Actinides in Molecular Magnetism, ed. R. A. Layfield and M. Murugesu, Wiley-VCH, Weinheim, 2015 Search PubMed.
- Y. Rechkemmer, J. E. Fischer, R. Marx, M. Dörfel, P. Neugebauer, S. Horvath, M. Gysler, T. Brock-Nannestad, W. Frey, M. F. Reid and J. van Slageren, J. Am. Chem. Soc., 2015, 137, 13114–13120 CrossRef CAS PubMed.
- A. Cornia and M. Mannini, in Molecular Nanomagnets and Related Phenomena, ed. S. Gao, Springer, Berlin, Heidelberg, 2015, pp. 293–330 Search PubMed.
- M. Mannini, F. Pineider, C. Danieli, F. Totti, L. Sorace, P. Sainctavit, M. A. Arrio, E. Otero, L. Joly, J. C. Cezar, A. Cornia and R. Sessoli, Nature, 2010, 468, 417–421 CrossRef CAS PubMed.
- R. Vincent, S. Klyatskaya, M. Ruben, W. Wernsdorfer and F. Balestro, Nature, 2012, 488, 357–360 CrossRef CAS PubMed.
- J. van Slageren, S. Piligkos and F. Neese, Dalton Trans., 2010, 39, 4999–5004 RSC.
- M. Gonidec, E. S. Davies, J. McMaster, D. B. Amabilino and J. Veciana, J. Am. Chem. Soc., 2010, 132, 1756 CrossRef CAS PubMed.
- L. Malavolti, M. Mannini, P.-E. Car, G. Campo, F. Pineider and R. Sessoli, J. Mater. Chem. C, 2013, 1, 2935–2942 RSC.
- M. R. Cheesman, V. S. Oganesyan, R. Sessoli, D. Gatteschi and A. J. Thomson, Chem. Commun., 1997, 1677–1678 RSC.
- L. Bogani, L. Cavigli, M. Gurioli, R. L. Novak, M. Mannini, A. Caneschi, F. Pineider, R. Sessoli, M. Clemente-Leon, E. Coronado, A. Cornia and D. Gatteschi, Adv. Mater., 2007, 19, 3906–3911 CrossRef CAS.
- P. Gerbier, N. Domingo, J. Gómez-Segura, D. Ruiz-Molina, D. B. Amabilino, J. Tejada, B. E. Williamson and J. Veciana, J. Mater. Chem., 2004, 14, 2455 RSC.
- R. Moroni, R. Buzio, A. Chincarini, U. Valbusa, F. B. de Mongeot, L. Bogani, A. Caneschi, R. Sessoli, L. Cavigli and M. Gurioli, J. Mater. Chem., 2008, 18, 109 RSC.
- J. M. Bradley, A. J. Thomson, R. Inglis, C. J. Milios, E. K. Brechin and S. Piligkos, Dalton Trans., 2010, 9904–9911 RSC.
- M. Waters, F. Moro, I. Krivokapic, J. McMaster and J. van Slageren, Dalton Trans., 2012, 41, 1128–1130 RSC.
- M. Gonidec, I. Krivokapic, J. Vidal-Gancedo, E. S. Davies, J. McMaster, S. M. Gorun and J. Veciana, Inorg. Chem., 2013, 52, 4464–4471 CrossRef CAS PubMed.
- N. Domingo, B. E. Williamson, J. Gómez-Segura, P. Gerbier, D. Ruiz-Molina, D. B. Amabilino, J. Veciana and J. Tejada, Phys. Rev. B: Condens. Matter, 2004, 69, 052405 CrossRef.
- E. J. L. McInnes, E. Pidcock, V. S. Oganesyan, M. R. Cheesman, A. K. Powell and A. J. Thomson, J. Am. Chem. Soc., 2002, 124, 9219–9228 CrossRef CAS PubMed.
- I. S. Kirin, P. N. Moskalev and Y. A. Makashev, Russ. J. Inorg. Chem., 1965, 10, 1065–1066 Search PubMed.
- N. Ishikawa, M. Sugita, N. Tanaka, T. Ishikawa, S. Y. Koshihara and Y. Kaizu, Inorg. Chem., 2004, 43, 5498–5500 CrossRef CAS PubMed.
- M. S. Haghighi and H. Homborg, Z. Anorg. Allg. Chem., 1994, 620, 1278–1284 CrossRef CAS.
- M. Clemente-Leon, H. Soyer, E. Coronado, C. Mingotaud, C. J. Gomez-Garcia and P. Delhaes, Angew. Chem., Int. Ed., 1998, 37, 2842–2845 CrossRef CAS.
- S. Maldonado and K. J. Stevenson, J. Phys. Chem. B, 2004, 108, 11375–11383 CrossRef CAS.
- S. M. Bayliss, S. Heutz, G. Rumbles and T. S. Jones, Phys. Chem. Chem. Phys., 1999, 1, 3673–3676 RSC.
- P. N. Moskalev, G. N. Shapkin and A. N. Darovskikh, Russ. J. Inorg. Chem., 1979, 24, 340–346 CAS.
- S. Stepanow, J. Honolka, P. Gambardella, L. Vitali, N. Abdurakhmanova, T.-C. Tseng, S. Rauschenbach, S. L. Tait, V. Sessi, S. Klyatskaya, M. Ruben and K. Kern, J. Am. Chem. Soc., 2010, 132, 11900–11901 CrossRef CAS PubMed.
- M. Gonidec, R. Biagi, V. Corradini, F. Moro, V. De Renzi, U. del Pennino, D. Summa, L. Muccioli, C. Zannoni, D. B. Amabilino and J. Veciana, J. Am. Chem. Soc., 2011, 133, 6603–6612 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: UV/Vis and X-ray photoelectron spectra. See DOI: 10.1039/c6dt00839a |
| ‡ All thin layers were made starting from (NBu4)[Dy(Pc)2]. The results show that the compound oxidizes during the deposition process giving [Dy(Pc)2], where one of the rings has been oxidized. Spin coating was performed by means of an APT spin coater Spin150 at 1500 rpm for 2 min in ambient atmosphere. Langmuir–Blodgett films were prepared using a KSV Minimicro 2 trough with 100 cm2 surface area equipped with motorized compression barriers and a substrate dipper. AFM images were recorded using NT-MDT NTEGRA spectra. MCD measurements were performed on a home-built MCD spectrometer consisting of an Aviv 42 CD spectrometer equipped with an Oxford Instruments Spectromag 10T optical cryomagnet. X-ray reflectivity measurements were performed on Rigaku SmartLab diffractometer using the Cu Kα radiation collimated by parallel beam optics. Profilometry was measured using Veeco Dektak 150 instrument. XPS measurements were carried out using Phoibos 150 spectrometer and non-monochromatic XR50 X-ray source (Al Kα) in normal emission geometry. |
| This journal is © The Royal Society of Chemistry 2016 |