 Open Access Article
Open Access ArticleBlood–brain barrier shuttle peptides: an emerging paradigm for brain delivery
Benjamí
Oller-Salvia
 a,
Macarena
Sánchez-Navarro
a,
Ernest
Giralt
ab and
Meritxell
Teixidó
a
a,
Macarena
Sánchez-Navarro
a,
Ernest
Giralt
ab and
Meritxell
Teixidó
a
aInstitute for Research in Biomedicine (IRB Barcelona), The Barcelona Institute of Science and Technology (BIST), 08028 Barcelona, Spain. E-mail: meritxell.teixido@irbbarcelona.org; ernest.giralt@irbbarcelona.org
bDepartment of Organic Chemistry, University of Barcelona, 08028 Barcelona, Spain
First published on 18th May 2016
Abstract
Brain delivery is one of the major challenges in drug development because of the high number of patients suffering from neural diseases and the low efficiency of the treatments available. Although the blood–brain barrier (BBB) prevents most drugs from reaching their targets, molecular vectors – known as BBB shuttles – offer great promise to safely overcome this formidable obstacle. In recent years, peptide shuttles have received growing attention because of their lower cost, reduced immunogenicity, and higher chemical versatility than traditional Trojan horse antibodies and other proteins.
1. Introduction
Delivery to the brain is a major challenge in drug development because an ageing population and the growing prevalence of brain cancers are increasing the incidence of central nervous system (CNS) diseases.1 Moreover, the lack of efficient treatments generates high direct and indirect costs, which together correspond to 1/4th of the burden of all diseases in Europe and high-income countries.2 Therefore, improving CNS drugs would not only enhance the well-being of many people but also considerably reduce health costs. However, a formidable obstacle must be overcome to enable active compounds to reach their targets in therapeutically relevant amounts: the blood–brain barrier (BBB).3Although many strategies to circumvent the BBB have been proposed, to date none has shown a satisfactory efficiency–safety balance. At one end of the spectrum, direct drug administration into the brain has a high risk and is very local and, at the other end, the modification of molecules to enhance their diffusion through the barrier is applicable only for some small drugs. Among the non-invasive approaches, molecular vectors – also known as BBB shuttles – (Fig. 1) have proved their potential in preclinical research over the last two decades, and some of these compounds are in clinical trials. The BBB shuttle4 concept includes Trojan horse antibodies5 and any other molecule capable of transporting a cargo into the brain parenchyma without affecting the BBB integrity. Over the last five years, research into peptide shuttles has thrived because they overcome some of the weaknesses of classical protein shuttles, including complex derivatization and characterization, high immunogenicity, and costly production. Of note, here we will use the word peptide to refer to small proteins (with or without structure) containing up to 50 amino acid residues.
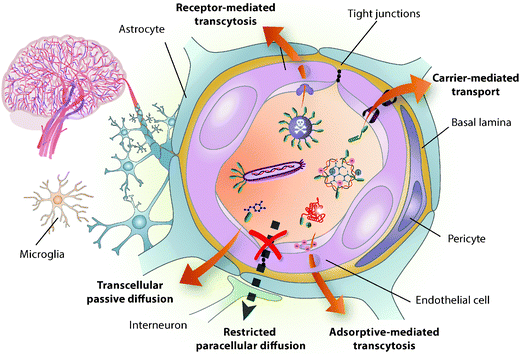 | ||
Fig. 1 BBB-shuttle mediated transport across the BBB. The BBB is formed mainly by the tight brain endothelium, which is surrounded by the basal lamina and regulated by the other cells in the neurovascular unit, including pericytes, glial cells and neurons.6 BBB shuttles mediate drug delivery across this barrier by taking advantage of endogenous transport pathways. Small molecules and peptides can be delivered using shuttles that undergo passive diffusion and carrier-mediated transport. However, passive diffusion is altered by the physicochemical characteristics of the cargo, including log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, pKa, molecular weight, topological polar surface area and hydrogen bonding.186 Carrier proteins usually undergo precise rearrangements to translocate constructs into the cystosol,9 thus they are also highly sensitive to the modification of substrate properties. Conversely, adsorptive- or receptor-mediated transcytosis allow the transport of a wider variety of cargoes, including proteins, viruses and nanocarriers. Despite the high efficiency of the former mechanism in mediating tissue uptake, the latter has focussed most attention on brain delivery due to its potential targeting capacity. P, pKa, molecular weight, topological polar surface area and hydrogen bonding.186 Carrier proteins usually undergo precise rearrangements to translocate constructs into the cystosol,9 thus they are also highly sensitive to the modification of substrate properties. Conversely, adsorptive- or receptor-mediated transcytosis allow the transport of a wider variety of cargoes, including proteins, viruses and nanocarriers. Despite the high efficiency of the former mechanism in mediating tissue uptake, the latter has focussed most attention on brain delivery due to its potential targeting capacity. | ||
In this review, crossing the BBB is presented as a tremendous challenge but also as an excellent opportunity for drug delivery into the brain. We first provide an overview of BBB shuttle peptides and subsequently present the most advanced ones, which are included in drug formulations that have reached clinical trials. Then, the focus is given to three representative case studies to illustrate some of the main achievements of shuttle transport of diverse cargoes. Additionally, some strategies that rescue unspecific shuttles are reported and the relevance of protease-resistance is highlighted. Finally, we point out the main trends in the field and the challenges to be addressed.
2. Toward minimized brain delivery vectors
2.1. The blood–brain barrier
The BBB is a physical, metabolic and transport barrier that tightly controls the transfer of substances from blood to neural tissues and vice versa, thereby contributing to brain homeostasis.6–8 Endothelial cells on brain capillary walls are the main constituents of this barrier and they form tight junctions that hinder paracellular passage. Additionally, these cells have many cytosolic and extracellular-membrane enzymes, down-regulated vesicular transport and efflux pumps. The permeability of the brain endothelium is influenced by the other cells belonging to the neurovascular unit (Fig. 1) and is affected by most CNS pathologies; however, BBB dysfunction is usually only significant in advanced stages of disease and in the most affected sites.Despite its efficient role as a barrier, the BBB is the main gateway to the brain as it grants access to necessary ions, nutrients and hormones – the maximal cell-capillary distance is 20 μm, which can be permeated by small molecules in half a second.9 Therefore, taking advantage of the endogenous transport mechanisms present on the BBB is potentially the most efficient way to deliver substances to any part of the brain.6 Many small hydrophobic compounds (<500 Da) diffuse across the endothelium membrane, while polar molecules such as glucose, amino acids, and several peptides have specific carriers; these highly selective transporters mediate transport into the endothelium cytosol and from there to the brain extracellular space. Indeed, even some macromolecules and also certain peptides cross the BBB through endocytic mechanisms involving receptor-mediated transcytosis (RMT) and/or adsorptive-mediated transcytosis (AMT). In the process of transcytosis, the vesicle formed circulates across the cell, bypassing the degradation pathway, and eventually releases its content into the parenchyma by exocytosis. AMT is considered non-specific and comprises all vesicular transport mechanisms that do not involve protein receptors; in AMT, endocytosis is generally promoted by the interaction of the often positively charged molecule with membrane phospholipids and the glycocalyx.
2.2. Brain delivery approaches
Nowadays most strategies for drug delivery into the brain that circumvents the BBB are invasive,10 involving the highest risk of brain damage or infection and requiring demanding set-ups.11,12 In addition, administration is often excessively localized and diffusion in the brain is very limited, especially for large molecules.13 Although alternative routes such as nasal delivery are under investigation, most attention is focused on achieving efficient distribution of the drug through the extensive brain vasculature.Temporal disruption of the tight junctions of brain endothelium by chemical or physical stimuli entails toxicity and the risk of neuronal dysfunction.14 Hence, much effort has been channelled into improving transport across endothelial cells. The most common strategies rely on enhancing lipophilicity and positive charge, in order to increase passive diffusion and mediate interaction with the anionic glycocalyx, respectively. However, these modifications lead to higher unspecific uptake in many tissues often resulting in off-target effects and, in addition, they enhance recognition by efflux pumps.12,15 A more selective way to boost the permeation of certain small molecules into the brain is to modify them by mimicking endogenous substrates of BBB carriers.16 However, all these approaches require a high degree of tailoring and are rarely applicable to large drugs such as biotherapeutics.
A more general approach for drug delivery to the CNS focuses on delivery vectors. Although biological vectors such as viruses17,18 and modified cells19 have been used to increase BBB transport, their safety, permeability across an intact barrier, and brain selectivity are still limited.18–20 Conversely, molecular vectors, dubbed BBB shuttles,4,21–33 aim to provide broadly applicable, selective and safer delivery systems.
2.3. BBB shuttle peptides
BBB shuttles allow the transport of a wide range of cargoes, comprising small molecules, proteins, nanoparticles and genetic material across the BBB. Substrates of natural carriers such as glucose and neutral amino acids have been applied to transport small molecules through their natural carriers on the BBB, while for nanoparticles and biomolecules the focus has been set on receptor ligand proteins since vesicular mechanisms tolerate a wide range of cargo sizes.3 Remarkably, peptides have bridged the gap between these two worlds.The BBB shuttle concept was conceived by William M. Pardridge in the mid-1980s,34 inspired by chimeric proteins targeting cell receptors. The first successful attempts relied first on cationized albumin,35 which lacked brain selectivity, and then on IgGs directed against insulin and transferrin receptors.36 However, the success of these initial antibodies was limited by their high affinity, which hampered an efficient release into the brain parenchyma.15,37 Therefore, a variety of protein shuttles have been investigated; most of them are ligands of receptors on the brain endothelium and include the following: apolipoproteins (Apo) A and E,38 receptor-associated protein (RAP),39 transferrin (Tf),40 lactotransferrin,41 melanotransferrin (p97),42 and leptin.43 However, these proteins compete with their endogenous counterparts. Although a few non-endogenous proteins, such as wheat germ agglutinin44 and a non-toxic mutant of diphtheria toxin (CRM197),45 have been used, they also have shown moderate efficacy and selectivity. In recent years, research on antibodies has been relaunched focussing on lower-affinity IgG derivatives.21,37,46,47
Despite the relative success of antibodies and other large proteins, their production is expensive and high immunogenicity is an issue. This is why research in this field in the last decade has focussed to a great extent on peptides. These molecules combine the low cost of small drugs with the high specificity of biologics.37,48 Peptides are easier to obtain and characterize than the latter and have very low immunogenicity, especially those without a rigid structure.49 Moreover, they display medium to low affinities, a property that has been critical to the development of anti-TfR shuttles.37 In addition, peptides are amenable to chemical synthesis. This feature opens up the possibility of applying a wide range of non-natural modifications and of introducing a plethora of functional groups for site-specific conjugation to proteins and nanocarriers. Furthermore, reduced functional alteration of the cargo and enhanced shelf-life are also important advantages over most proteins. Although peptides have often been undervalued in pharmaceutical chemistry because of their low resistance to proteolytic degradation, this limitation can now be overcome by means of various strategies that will be described in section 7.50
Although some peptides had long been shown to cross the BBB,51 the field of peptide shuttles was pioneered in 1999 by Stephen Dowdy and coworkers.52 In this seminal paper, the authors demonstrated the capacity of a fragment from the HIV TAT protein to deliver β-galactosidase into the brain and other organs. However, it was in 2007 with RVG2953 that a peptide was proven capable of transporting cargoes into the brain in a selective fashion. Soon after, the great potential of Angiopep-254 and glutathione (GSH)55,56 as BBB shuttles was unravelled – formulations including these peptides are currently in clinical trials. Remarkably, in the last 5 years over 30 BBB shuttle peptides with increasing efficiency and versatility have been reported (Table 1).
| Peptide | Typical sequence | Proposed transporter | Origin | Main cargoes | BBB passage evidence | Ref. |
|---|---|---|---|---|---|---|
| “BBB passage evidence” includes the main strategies used to assess the presence of the compound targeted by the BBB-shuttle in the brain parenchyma or the effects derived from it – these approaches do not provide information about brain selectivity and do not prove that the whole cargo-shuttle construct crosses the BBB. Abbreviations: Endog.: endogenous; Exog.: exogenous; nanopart.: nanoparticles; BBBCM: a cell-based BBB model; MPS: mucopolysaccharidosis; MLD: metachromatic leukodystrophy; AD: Alzheimer's disease. Nomenclature for cyclic peptides (&) is adapted to the 3-letter amino acid code from the one described by Spengler et al.188 [Dap] stands for diaminopropionic acid. Only selected references relevant to the study of these peptides as BBB-shuttles are cited here. | ||||||
| Angiopep-2 | TFFYGGSRGKRNNFKTEEY-OH | LRP1 | Neurotropic endog. Protein | Small drugs, proteins, nanopart., and DNA/RNA |
• BBBCM
• Capillary depletion • Fluorescence microscopy • TEM • Effect on glioma, epilepsy and Parkinson's mouse models |
54 and 113–135 |
| ApoB (3371–3409) | SSVIDALQYKLEGTTRLTRK-RGLKLATALSLSNKFVEGS |
LRP2
LDLR |
Neurotropic endog. Protein | Proteins |
• Capillary depletion
• Effect on MPS mouse model |
81 and 156 |
| ApoE (159–167)2 | (LRKLRKRLL)2 |
LRP1
LRP2 LDLR |
Neurotropic endog. protein | Proteins and nanopart. |
• BBBCM
• Capillary depletion • Fluorescence microscopy • Effect on the MLD mouse model |
82, 157 and 160 |
| Peptide-22 | Ac-C(&)MPRLRGC(&)-NH2 | LDLR | Phage display (receptor) | Nanopart. |
• BBBCM
• Live fluorescence microscopy |
27 |
| THR | THRPPMWSPVWP-NH2 | TfR1 | Phage display (cells) | RNA and nanopart. |
• BBBCM
• TEM |
88 and 183 |
| THR retro-enantio | pwvpswmpprht-NH2 | TfR1 | Phage display-derived | Small drugs and nanopart. |
• BBBCM
• Live fluorescence microscopy |
182 |
| CRT | C(&)RTIGPSVC(&) | TfR1 | Phage display (mice) | Virus | • Capillary depletion | 89 |
| Leptin30 | YQQILTSMPSRNVIQISND-LENLRDLLHVL | Leptin receptors | Neurotropic endog. protein | DNA | • Capillary depletion | 76 and 77 |
| RVG29 | YTIWMPENPRPGTPCDIFT-NSRGKRASNG-OH | nAchR | Neurotropic exog. protein | Nanopart. and RNA/DNA |
• Capillary depletion
• BBBCM • Effect in viral encephalitis and mouse models of Parkinson's disease |
53 and 146–154 |
| DCDX | GreirtGraerwsekf-OH | nAchR | Neurotoxin-derived | Nanopart. |
• BBBCM
• Effect on glioblastoma |
94 |
| Apamin | C(&1)NC(&2)KAPETALC(&1)-AR-RC(&2)QQH-NH2 | KCa channel? | Neurotoxin | Proteins and nanopart. | • BBBCM | 95 and 96 |
| MiniAp-4 | [Dap](&)KAPETALD(&) | KCa channel? | Neurotoxin-derived | Small drugs, proteins and nanopart. |
• Human BBBCM
• Fluorescence microscopy |
97 |
| GSH | γ-L-glutamyl-CG-OH | GSH transporter | Endog. peptide | Nanopart. |
• Intracerebral microdialysis
• Effect on glioma and MS mouse models |
55, 56 and 141–145 |
| G23 | HLNILSTLWKYRC | GM1 | Phage display (receptor) | Nanopart. |
• BBBCM
• Fluorescence microscopy |
107 |
| g7 | GFtGFLS(O-β-Glc)-NH2 | Unknown receptor | Endog. peptide-derived | Nanopart. |
• Fluorescence microscopy
• TEM |
100 and 164–166 |
| TGN | TGNYKALHPHNG | Unknown receptor | Phage display (in vivo) | Nanopart. |
• Fluorescence microscopy
• Effect on glioma and AD mouse models |
109–111 |
| TAT (47–57) | YGRKKRRQRRR-NH2 | AMT | Exog. protein | Proteins and nanopart. |
• BBBCM
• Capillary depletion • Fluorescence microscopy • TEM |
52 and 167–174 |
| SynB1 | RGGRLSYSRRRFSTSTGR | AMT | Toxin | Small drugs |
• Brain perfusion
• Capillary depletion |
62 and 63 |
| Diketopiperazines | &(N-MePhe)–(N-MePhe)Diketo-piperazines | Passive diffusion | Design (+serendipity) | Small drugs |
• PAMPA
• BBBCM |
32 and 180 |
| PhPro | (Phenylproline)4-NH2 | Passive diffusion | Design | Small drug | • PAMPA | 68 |
3. Aiming for selectivity
The discovery of TAT peptide as a brain delivery vector, directed initial research efforts into finding BBB shuttles with high permeability across cell membranes. Passive diffusion and AMT provide the highest transport since the first is considered unsaturable and saturation concentrations for AMT are 3 orders of magnitude higher than for RMT.57 Nevertheless, the need for safer therapeutics has pushed research towards targeted strategies in an attempt to achieve Paul Ehrlich's “magic bullet”.3.1. Unspecific uptake
BBB shuttle peptides capable of increasing the brain uptake of large cargoes in a non-selective way belong mainly to the cell-penetrating peptide (CPP) family. CPPs comprise short amphipathic and/or cationic sequences with a high capacity to cross cell membranes without the need of a receptor.58,59 Most peptides with this property have been derived from protein transduction domains (e.g. TAT60 and penetratin61), by hybridizing these domains with antibiotic peptides (e.g. SynB162,63 and transportan64), or through biomimetic design (e.g. oligoarginine65). Although CPPs can enter cells through different mechanisms, when linked to large cargoes they mostly undergo endocytosis. Therefore, it is generally assumed that they undergo AMT across the BBB.57 However, exocytosis of the entire BBB shuttle constructs from the endothelium is more controversial than in RMT and they may accumulate in the endothelium as has been reported for positively charged proteins such as lectins.44 By contrast, a recent study using CPPs of four different classes suggests that trapping in brain capillaries of peptides alone may be relatively low but indicates that brain parenchymal accumulation does not correlate with their cell internalization capacity.66For small drugs (<300 Da), peptide shuttles formed by 2–4 amino acid residues that cross the BBB through passive diffusion are more attractive since they may minimize the loss of activity upon conjugation. In peptides diffusing across the BBB, hydrogen bonding and water desolvation have a better correlation with permeability than log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P.67 Based on this criterion, three families of BBB shuttle peptides, namely diketopiperazines,32N-methylphenylalanines29–31 and phenylprolines,68 have been developed.
P.67 Based on this criterion, three families of BBB shuttle peptides, namely diketopiperazines,32N-methylphenylalanines29–31 and phenylprolines,68 have been developed.
3.2. Targeting transporters
Most BBB shuttle peptides that interact with transporters have been obtained from either neurotropic biomolecules or phage display biopanning (Fig. 2). Natural peptides or proteins targeting the brain can be endogenous, like hormones and apolipoproteins, or exogenous, such as certain viruses and neurotoxins.33,69 Regarding phage display, although it has been extensively applied for the last three decades,70 it has not been exploited to find BBB shuttles until the last few years. Filamentous phages, which are the most commonly used ones, measure 6.5 nm in diameter and 900 nm in length and are generally engineered to display only 5 copies of peptide per viral particle.71 These features, together with the high number of sequences that can be displayed in a phage library, explain why this screening technique has provided peptides capable of transporting large cargoes such as nanoparticles.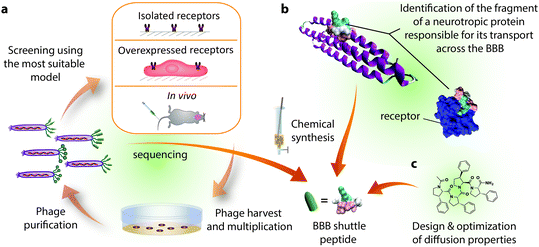 | ||
| Fig. 2 Sources of BBB shuttle peptides. (a) Phage display biopanning may be performed on isolated receptors, cells overexpressing a particular receptor or in vivo. The phages that bind the receptors or accumulate in the brain are recovered, amplified through bacterial infection, titrated and sequenced. The biopanning cycle is generally repeated 2–3 times and the most abundant sequences are chemically synthesized for further study. (b) For peptides coming from (neurotropic) proteins, the moiety responsible for transport is identified through one or more of the following techniques: sequence alignment, screening of synthesized fragments or structural studies involving X-ray and NMR. Here we highlight a sequence of ApoE (PDB: 1LPE) that has been shown to interact with LRP1 (PDB: 2KNY).187 (c) Passive diffusion shuttles have been designed taking into account the parameters involved in this permeation mechanism. The structure of phenylproline is presented here as the BBB peptide shuttle with the highest solubility and transport capacity. | ||
Ideally, BBB shuttles should target receptors with the following attributes: high expression in the luminal side of brain vasculature with respect to other tissues; capacity to mediate transcytosis; high turnover and broad substrate recognition.72 Moreover, the physiological role of the transporter should not be easily altered. Unfortunately, quantitative physiological information of the wide variety of brain endothelium receptors reported is very limited, thus screening together with trial and error rather than design has mainly driven the discovery of new shuttle peptides. Of note, although many sequences have been reported to target a particular receptor, the contribution of other mechanisms cannot be excluded.15 Determining whether the molecule has reached the brain parenchyma is already a challenge and studying the delivery route requires a multifocal and integrated approach (Table 2).
| Model | Advantages | Limitations | Observations & examples | Ref. |
|---|---|---|---|---|
| Despite the plethora of BBB models currently available,189–192 evaluating the capacity of BBB shuttles to deliver a cargo across the BBB into the brain parenchyma remains challenging. Thus, an integrated approach is required. Cell-based BBB models193 have often been applied to screen BBB shuttles mainly due to their balance between high-throughput and predictive values. Moreover, these models allow focusing on transcellular transport without any interference of other physiological factors, which is particularly useful in mechanistic studies provided paracellular diffusion is low enough; direct internalization assays can also deliver valuable information in this regard. In particular, experiments with human endothelial cells are essential to complement transport studies in animals. Notwithstanding, further refinement and validation of transporters in cell-based BBB models would boost their use. Regarding in vivo studies, the capacity of the best-validated shuttles has been evaluated in healthy mice and in animal models of disease. In situ brain perfusion in tandem with microdialysis of the brain interstitial fluid provides the most reliable quantification of the unbound drug capable of crossing the BBB. However, these techniques are seldom used due to their complexity, low throughput and limitations of probes for large cargoes. Conversely, a capillary depletion step is more common to minimize to a certain extent the quantification of the compound adsorbed onto or internalized in brain endothelium. This method, which tends to overestimate the parenchymal concentration,194 is often complemented with an image showing the construct outside the capillaries. Since it is difficult to unambiguously assign spots or a background increase in fluorescence, establishing co-localization with a localized target (e.g. amyloid plaques) is more reliable. Additionally, some studies take advantage of an intrinsic property of the cargo to show that it remains functional upon reaching the brain parenchyma and that a label or a metabolite is not quantified instead. The use of BBB integrity markers and controls including scrambled peptides gives further strength to these studies. Finally, an increase in the therapeutic effect of a drug in an animal model can also be an indirect proof that the drug-shuttle conjugate has reached the brain parenchyma providing the target is only in the CNS and the BBB is intact. | ||||
| Passive diffusion BBB models | • Suitable for high-throughput screening |
• Only useful for compounds that cross mainly through diffusion
• Cannot predict the effect of efflux pumps |
• E.g. diketopiperazine, N-methyl-Phe and phenylproline shuttles have been optimized using the parallel artificial membrane permeability assay (PAMPA) | 29–32, 68 and 180 |
| Cell uptake |
• High throughput
• Mechanistic understanding of the transport process using simple settings: active transport (temperature, sodium azide), endocytic mechanism (saturation, inhibition), and receptor type (competence with substrates) |
• Cannot be used to predict BBB permeability
• Expression of transporters may differ from physiological human BBB • Inhibitors may affect different pathways in diverse cell-lines • No evidence of whether the construct has been degraded |
• Immortalized cell-lines are preferred to primary endothelial cells since the lattter rapidly lose their natural phenotype; bEnd3 is the most used cell-line
• E.g. mechanisms of Angiopep-2 and TGN have been partly elucidated through cell uptake |
25, 109, 113 and 132 |
| Cell-based BBB models |
• Good correlation with in vivo permeability values of small molecules
• Compromise between costs, throughput and predictive value • Paracellular transport restricted by tight junction proteins • Functional expression of many transporters • Can be used to study transport mechanisms when paracellular contribution is low |
• Difficult to compare permeability values between different models
• Only certain trends and certain permeability values can be predicted for macromolecules & NPs • Trans-endothelial resistance is still low in robust models compared to in vivo (0.05–1 vs. >2–8 kΩ cm2) • To minimize paracellular contribution some authors perform pulse-chase assays • Expression of transporters may differ from physiologic human BBB • High cost of Transwell® and media |
• Various cell alternatives: non-cerebral cells or brain capillary endothelial cells (BCEC). BCEC can be from different species and primary or immortalized, generally in co-culture with glial cells or pericytes. hCMEC/D3 is the most used cell-line, although it is considered leaky
• E.g. Angiopep-2, THRre and MiniAp-4 were discovered in a bovine BBB cell-based model |
21, 54, 82, 94, 95, 97, 135, 160, 182 and 183 |
| In vivo intracerebral microdialysis |
• Measures unbound drug in the interstitial fluid site-specifically with a putatively intact BBB
• Fast sampling and possibility to measure during extended periods (days) • Current probes preclude sampling large constructs such as nanopart. |
• Very low throughput
• Requires highly skilled personnel • Tissue damage and glial activation produced by the probe • Interaction with receptors cannot always be extrapolated to humans (like all techniques applied to animals/tissues derived from them) |
• E.g. drugs carried by PEGylated liposomes targeted with GSH have been measured using microdialysis | 98 and 144 |
| In vivo non-invasive imaging |
• General: rough assessment of PK with a limited number of animals
• Fluorescence and luminescence are available without restrictions and are the fastest noninvasive methods (<1 min vs. rest >30 min); wide choice of fluorescent labels; luminescence is more sensitive • PET and SPECT: high sensitivity and penetration • MRI: high penetration and resolution (0.1–1 mm); no regulatory restrictions |
• General: lower resolution than ex vivo (generally >1 mm); no possibility to verify the integrity of the construct
• Fluorescence and luminescence: low-penetrating and semi-quantitative • PET and SPECT: regulatory restrictions for radioactivity; PET requires short-lived radiotracers and cyclotron • MRI: difficult to quantify |
• Fluorescence: most widely used
• Luminescence: luciferase gene delivery • PET: herpes simple virus thymidine kinase (e.g. CRT) • SPECT: 125I-labelled proteins (e.g. 9Lys-ApoE (151–170)) • MRI: iron oxide NPs and DTPA or DOTA–Ga3+ (e.g. Angiopep-2, cRGD) |
23, 89, 92, 93, 97, 126–134, 136, 154 and 161 |
| In vivo two-photon microscopy |
• High resolution (<10 μm)
• Only technique that provides live visual evidence of BBB permeation |
• Need for craniotomy
• Low throughput; requires skilled personnel |
• Quantum dots targeted with THR retro-enantio | 27 and 182 |
| Ex vivo quantification of entire organs |
• General: removal of blood and external tissues; minimal alteration of the sample
• Fluorescence: no special regulation, only the organs of same type can be compared (e.g. the amount of BBB-shuttle-cargo vs. cargo in brain) • High-energy radiotracers: accurate measure of the total amount of constructs; autoradiography of brain slices provides low-resolution brain distribution |
• General: requires the use of labels and does not allow the identification of the entities
• Fluorescence: semi-quantitative → only the same organ in different animals can be compared • High-energy radiotracers: specific regulations |
• Fluorescence is the most extensively used
• 125I and 131I have also been used to label BBB shuttle constructs, especially with Angiopep-2 |
23, 111, 126, 127, 132 and 134 |
| Ex vivo quantification in organ homogenates |
• Capillary depletion allows measurement of the amount of compound in brain parenchyma
• Identity of the compound can be verified • If extraction is quantitative, most techniques provide absolute amounts that can be compared between different organs • A wide variety of quantification techniques can be applied |
• Capillary depletion is not quantitative and results should be interpreted with great care
• Many steps before the final measure increase the chances of introducing artefacts |
• 3H and 14C have been used to label drugs encapsulated in GSH-coated PEGylated liposomes and Angiopep-2 constructs
• ICP-MS is the reference technique for metal NPs (e.g. THR, Angiopep-2) • Absorbance, fluorescence and luminescence have been used to quantify delivered molecules/enzymes/genes encoding them (e.g. RVG29, RDPs) • Flow cytometry (e.g. TAT, RVG29) |
36, 37, 39, 44, 53, 54, 82, 83, 91, 111, 127, 157, 162 and 176 |
| Ex vivo microscopy |
• Optical microscopy gives rough localization of the cargo (i.e. enzyme)
• TEM is the most reliable, sensitive and precise technique for metal nanoparticles • Fluorescence microscopy can reveal the real position of cargo (staining the capillaries or colocalization with certain cell types, such as neurons) |
• Fixation efficiency of the constructs may be different
• Processing of the sample may introduce artefacts • Punctuated or diffuse patterns without a clear localization may be difficult to distinguish from noise |
• Optical microscopy: β-galactosidase (e.g. TAT and RDPs)
• TEM (e.g. THR) • Fluorescence microscopy (e.g. ApoB and ApoE peptides, g7) |
21, 44, 45, 81, 82, 161 and 183 |
The receptors with high expression on the BBB for which transcytosis has been best characterized include: transferrin (TfR1),40 low-density lipoproteins (LDLRs),73 insulin74 and leptin.75 However, while the first two have been widely used for brain delivery, very few BBB shuttle peptides derive from the others.76,77
LDLRs have been extensively studied for their roles in transport and signalling78 and they are the most exploited receptors for delivery across the BBB using peptides. Moreover, some of them (particularly LRP1) are overexpressed in the brain79 and in tumours.80 Peptides targeting this family of receptors either are based on natural protein ligands, namely ApoB and ApoE fragments81,82 and Angiopep-2, or they are found by phage display biopanning against LDLR, like Peptide-22.27
TfR1 is also well characterized among BBB transport receptors. Furthermore, it has even higher expression than LRP1 on the brain endothelium83–85 and is widely present in tumours.86 Peptides interacting with TfR1 were discovered by applying phage display biopanning in various ways. B6 was identified in a nonamer library screened against the extracellular domain of human TfR,87 whereas THR and T7 were found through panning against a human receptor expressed in chicken fibroblasts (chicken TfR does not bind human Tf),88 and the CRT peptide was found to selectively target mouse brain parenchyma in vivo.89
In addition to the aforementioned receptors, many other pathways have been explored in an attempt to increase brain delivery efficiency and selectivity. The widespread occurrence of some ion channels in the CNS, as well as their intracellular traffic and recycling ability, have inspired several shuttles, comprising RVG29, RDPs,90,91 KC2S,92LCDX,93DCDX,94 apamin,95,96 and MiniAp-4.97 By contrast, the endogenous peptide GSH was identified as a BBB shuttle following from its reported capacity to reach the brain through a saturable and specific mechanism. Many transporters, some of which are preferentially expressed in the CNS,98 mediate the influx and efflux of GSH and its endogenous conjugates;99 however, further research is required to elucidate the putative transcytotic mechanism of GSH. Another shuttle derived from an endogenous peptide is g7,100 which is discussed later in this review.
Although integrin receptors do not display a particularly high expression in the brain microvasculature, they have been extensively used for targeting brain tumours and inflamed regions of the CNS.101 Cyclic RGD (cRGD)102 is a peptide derived from a sequence present in many proteins that recognise these receptors. Because integrin αvβ3 is overexpressed in the neovasculature, cRGD has been extensively used to target nanoparticles into gliomas.103 However, it can mediate transcytosis only indirectly through internalization into leukocytes and other immune cells, which are recruited into the brain in response to inflammation.19
Protein transporters are not the sole means to achieve a certain degree of selectivity in the transcytosis across brain endothelium. Gangliosides have a heterogeneous tissue distribution and can also mediate transport across polarized cells.104,105 This particular selective AMT mechanism has been exploited by G23 peptide, which was found by phage display biopanning against gangliosides GT1b106 and GM1,107 the latter of which is present in caveolae. G23 has been shown to promote the transport of nanoparticles across the BBB107 and provide a targeting effect.108
Finally, several peptide shuttles have been found through in vivo phage display biopanning without aiming for a particular receptor. The most prominent example is that of TGN.109 This sequence is actively transported across brain endothelial cells and its brain selectivity suggests that the mechanism is receptor-mediated. The brain delivery capacity of this shuttle is supported by enhanced therapeutic effect in glioblastoma and Alzheimer's mouse models.110,111
4. On the way to clinical application
The first generation of BBB shuttle peptides has reached clinical trials in the last few years. Here we will describe the two best-documented examples, Angiopep-2 and GSH. However, others are in advanced pre-clinical stages, and MTfp,112 a dodecapeptide derived from melanotransferrin, has been announced to be ready to enter clinical development.4.1. Angiopep-2: an example of versatility
Angiopep-2 was identified by sequence alignment of aprotinin with other human proteins having a Kunitz domain, which interacts with LRP1 (Fig. 3).54,113 This BBB shuttle was initially exploited to transport small molecules such as doxorubicin,114 etoposide,114 paclitaxel,115 and also peptides.116 Its conjugate with paclitaxel (ANG1005 or GRN1005)117,118 showed good tolerance in Phase I clinical studies119,120 and reached Phase II for the treatment of recurrent high-grade glioma in combination with bevacizumab (http://ClinicalTrials.gov identifier: NCT01480583). ANG1005 is currently also in Phase II clinical trials for breast cancer (http://ClinicalTrials.gov identifier: NCT02048059), and preliminary results show that this compound reduces tumours up to 60% in patients.121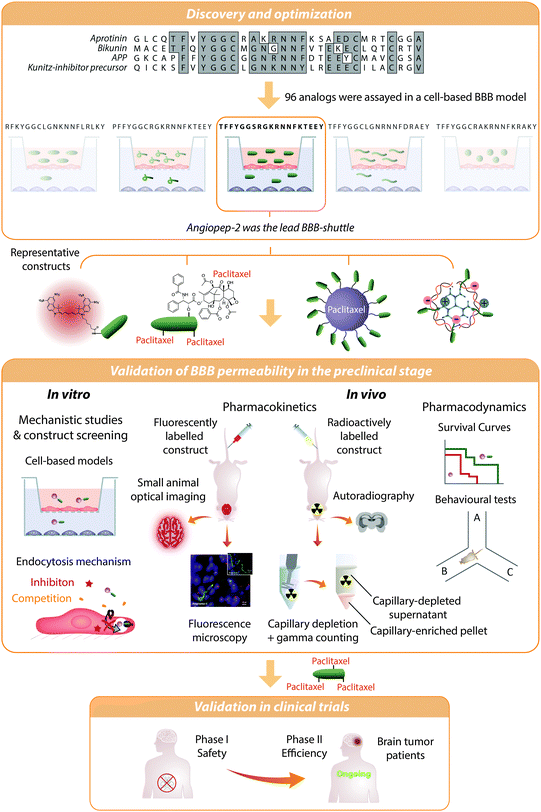 | ||
| Fig. 3 Angiopep-2 discovery and validation as a BBB shuttle. Angiopep-2 was discovered by alignment of Kunitz protein domains followed by a screening of 96 analogues in a bovine cell-based blood–brain barrier model. The transport of the selected peptide was inhibited by low temperature, RAP protein, and α2-macroglobulin, thereby indicating that the transcytosis was active and probably mediated by LRP-1.113 However, several uptake mechanisms may contribute to the transport of conjugates.25 This peptide has proven its efficiency as a BBB shuttle in vitro and in vivo. Whole-animal fluorescence imaging and autoradiography among other techniques have shown that Angiopep-2 constructs accumulate in the brain. Additionally, there is qualitative (e.g. microscopy. Image reproduced with permission from ref. 195. Copyright 2010 Elsevier) as well as quantitative (e.g. radioisotopic labelling and capillary depletion) evidence proving that this peptide reaches the brain parenchyma. Angiopep-2 conjugated to paclitaxel is currently in Phase II clinical trials. | ||
Angiopep-2 has been used to transport a wide variety of nanocarriers loaded with small molecules, proteins or genetic material into the CNS. These carriers include liposomes,122,123 nanotubes,124 dendrimers made of polyamidoamine125–128 and poly-L-lysine,129 and also nanoparticles made of PEG–polycaprolactam,130–133 PEG–poly(lactic-co-glycolate) (PEG–PLGA),134 thermoresponsive hydrogels,135 upconversion nanocrystals136 and gold.137 The diameter of these particles ranged from 7 to 200 nm thus further confirming the versatility of this shuttle. Moreover, the number of peptides required for efficient delivery is relatively low; four peptides are considered optimal for 7–8 nm dendrons127 and 53 peptides on the surface of a 90 nm nanoparticle provided efficient transport.25 The increase in brain delivery for most constructs is in the range of 1.5- to 3-fold in mice.
The vast majority of studies with Angiopep-2 describe about conjugates for the diagnosis125,136 or the treatment124,131,137 of brain tumours; in the second case a significant increase in survival with respect to the free drug or untargeted nanocarriers has often been reported. Although this peptide has been used mainly to transport small molecules and nanoparticles, conjugation of the shuttle to trastuzumab has recently been shown to enhance the therapeutic effect of this antibody in mice bearing HER2+ brain tumours.138 Angiopep-2 also increases the antifungal activity of amphotericin B in meningoencephalitis139,140 and the therapeutic index of phenytoin sodium against epilepsy in rats.135 Additionally, delivery of Angiopep-2-coated nanoparticles loaded with hGDNF boosts the neuroprotective effect of this protein in a Parkinsonian rat model, improving the locomotor activity and recovery of dopaminergic neurons.129 Although in some of these models, especially in those involving tumours, the BBB may be compromised, permeation has also been assessed in healthy mice and cell-based BBB models.135
4.2. GSH: a highly specialized shuttle
Together with Angiopep-2, GSH is the BBB shuttle peptide that has reached most advanced stages in the route towards clinical application. GSH has been mainly applied to target PEGylated nanoliposomes loaded with drugs, which are thereby protected from degradation and clearance. This formulation, known as G-Technology®, has been applied to a wide range of compounds, encompassing small molecules,141–143 peptides144 and, very recently, biologics.145G-Technology® for doxorubicin delivery (2B3-101) has reached Phase I/IIa clinical trials for brain cancer treatment (http://ClinicalTrials.gov identifier: NCT01386580). This nanoplatform has also been exploited for the delivery of methylprednisolone (2B3-201), enhancing its transport up to 6.5-fold.142 2B3-201 is capable of reducing neuroinflammation in rats with encephalomyelitis, and reached Phase I clinical trials for multiple sclerosis55 (http://ClinicalTrials.gov identifier: NCT02048358). Even more remarkable is the selective increase in brain delivery of single domain antibodies against amyloid plaques in APP/PS1 mice.145
5. One shuttle, one cargo
The large change in physicochemical properties induced by therapeutic cargoes and the distinct location of the targets of these drugs inside the brain has limited the universal aspiration of most BBB shuttles. Hence, in general, each peptide shuttle is prominent in the delivery of a particular family of cargoes. Although many peptides have been reported for each type of cargo, here we will focus on three well-documented case studies.5.1. Gene delivery with RVG29
RVG29 was found when studying the neurotropism of the rabies virus, which is mediated by its glycoprotein (RVG).53 Although a tail with 9 arginines was introduced to bind siRNA, the unmodified peptide was shown to reach the brain parenchyma. This observation suggested that the increase in brain delivery of the oligoarginine-RVG29 construct was due to the targeting peptide and not to the potential opening of tight junctions promoted by the policationic sequence. This construct was first used to transport oligonucleotides into healthy mouse brains to silence GFP in GFP-transgenic mice as well as endogenous SOD1 in the CNS. As a further demonstration of its value, this delivery strategy was successfully applied to protect mice with JEV-induced encephalitis. The authors reasoned that transport across the BBB could take place by RMT through interaction with the α7 subunit of the nicotinic acetylcholine receptor (nAchR), as shown by the selective binding of the peptide to neurons and its competition with α-bungarotoxin. In a subsequent study,146 RVG29 was intravenously injected into mice and was found inside cells that overexpress nAchR, unlike a scrambled version of the sequence. Moreover, RVG29 was not detected when administered to knockout animals devoid of this receptor.The high potential of this sequence for gene delivery has been confirmed using either the oligoarginine tail,147,148 a polylysine dendrigraft,149 polyethylenimine,150,151 polyasparthydrazide151 or polyamidoamide dendrimers152 to bind the oligonucleotide chains. RVG29 linked to exosomes is particularly efficient as it mediates higher protein knockdown than when linked to oligoarginine with five-fold less RNA.153 As an example of therapeutic effect, the polylysine dendrigraft targeted delivery of caspase-3 RNAi reduced caspase-3 levels, improving locomotor activity and rescuing dopaminergic neuronal loss in a rat model of Parkinson's disease.154
5.2. Apolipoprotein-derived peptides for enzyme delivery
Apolipoproteins have been applied for brain delivery taking advantage of their roles in lipid transport. Although the whole proteins have been used to transport nanoparticles,155 the peptides derived from them have mainly been applied to enzymes, probably because of the lower effect of the targeting moiety on the enzymatic activity.Apolipoprotein B100 (ApoB) is the primary component of low-density lipoprotein and it interacts with LDLR and LRP2.79 Spencer and Verma81 achieved hepatic expression of proteins in the liver fused to the binding domain of ApoB (3371–3409, P04114 UniProt), which resulted in sustained brain delivery. The cDNA was introduced into the liver and spleen using a lentivirus vector. Through this strategy, GFP and glucocerebrosidase were delivered into the brain. Fluorescence microscopy revealed the co-localization of these molecules in neurons and astrocytes in the regions of the brain with a high expression of LDLR. Also using ApoB (3371–3409) to target iduronate-2-sulphatase, the overall brain pathology was improved in a mouse model of mucopolysaccharidosis type IIIA.156
Despite the successful cases of ApoB BBB shuttles, peptides derived from ApoE showed higher efficiency when applied to transport other proteins.82,157 This observation may be related to ApoE binding a variety of LDLRs, including LRP1.79 Of note, altering the homeostasis of this protein entails many secondary effects,9 which could be minimized using only the moiety involved in transport. With the aim of finding the most suitable fragment of ApoE, several sequences reported to interact with LDLRs158,159 were compared.82 In this comprehensive study, ApoE fragments were expressed as a fusion protein with a lysosomal enzyme (IDUA) in the liver in order to target this enzyme to the brain (Fig. 4).
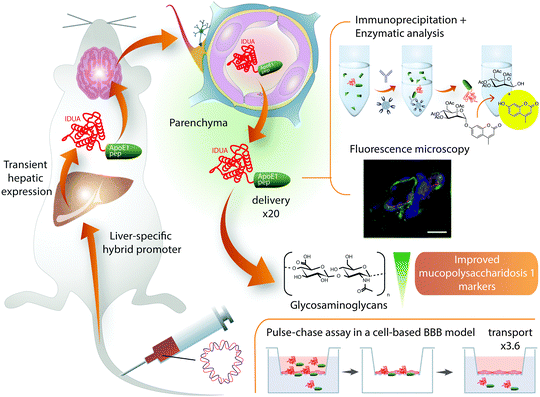 | ||
| Fig. 4 Brain delivery of IDUA–ApoE (159–167)2 expressed in the liver. Increased delivery of IDUA into the mouse brain parenchyma was achieved by expressing it as a fusion protein with ApoE (159–167)2 in mouse liver.82 The fusion of ApoE peptide increased 3.6-fold the transport of IDUA in a pulse-chase assay performed on a cell-based BBB model – in this experiment the protein is incubated and, after washing, the transport of the protein adsorbed onto or internalized by endothelial cells is measured; this strategy intends to minimize the paracellular contribution to the transport, which is still a concern in cell-based BBB models.21 Remarkably, the amount of IDUA found in the brain when expressed in mice livers fused to ApoE (159–167)2 was 20-fold higher than when expressed without the BBB shuttle. Quantification was performed after capillary-depletion. In addition, these authors provided fluorescence microscopy images, which indicate colocalization of the construct with neurons and astrocytes and show a clear pattern of diffusion from the capillary into the parenchyma. With this approach, 23% of normal brain IDUA activity was restored, which was sufficient to decrease brain glycosaminoglycan and β-hexosaminidase concentrations to normal levels in mucopolysaccharidosis type I mice. | ||
One of the two peptides with the best performance found in the aforementioned study82 was the tandem dimer sequence ApoE (159–167)2 (UniProt P02649-1) which had better transport in a cell-based BBB model than the monomer.160 This peptide was also the best-ranked BBB shuttle in a very recent comparative study157 using a mouse model for another lysosomal storage disease, namely metachromic leukodystrophy. In that publication, ApoE (159–167)2 did not show the best performance in endocytosis or in a porcine BBB cell-based model; however, it was the only shuttle to increase the in vivo brain delivery of arylsulfatase A (by 54%) when compared to Angiopep-2, ApoE (148–170), and ApoB. Surprisingly, this shuttle did not compete with the endogenous protein as suggested by the lack of increased transport efficiency in ApoE-knock out mice.
It is also worth highlighting the delivery of large proteins into the brain using a physical mixture of the cargoes with ApoE (151–170) in tandem with a 16-lysine sequence.161 However, this construct has subsequently been found to prompt a transient disruption of the BBB,162 as has been observed with other highly positively charged carriers.163
5.3. g7-Mediated PLGA-nanoparticle delivery
Although Angiopep-2 has shown the highest versatility for nanoparticle delivery, many other shuttles have proven useful for certain kinds of nanocarriers. In particular, the g7 heptapeptide is a modified analogue of the synthetic opioid MMP-2200, in which the N-terminal tyrosine was exchanged for phenylalanine in order to avoid the antinociceptive effect.100 It was also found that O-glycosylation with glucose but not xylose or lactose favoured brain uptake. This observation, together with a remarkable selectivity for this organ and a poor permeation of a scrambled version of the peptide, indicates that transport across the BBB is due to a receptor, though not necessarily opioid.Numerous in vitro and in vivo studies show that g7 is capable of delivering PEG–PLGA nanoparticles into the brain.164 The increase in brain accumulation has been assessed by whole-animal fluorescence imaging165 and also by measuring the amount of rhodamine 123 released from the nanoparticle; remarkably, 15% of the fluorophore administered intravenously was reported to reach the brain. These nanoparticles are mainly delivered to the grey matter166 and their presence in the brain parenchyma has been imaged using fluorescence and transmission electron microscopy. Further evidence of the therapeutic effect of this peptide-coated nanocarriers upon intravenous injection would certainly encourage a more widespread use.
6. Rescuing the origins
In general, CPPs and peptides undergoing passive diffusion across the BBB do not provide brain selectivity. However, their high internalizing capacity can be fine-tuned or exploited in tandem with BBB shuttles in order to enhance the delivery of cargoes to the brain.6.1. Cell-penetrating peptides
TAT is the most used CPP for brain delivery for proteins52,167–170 and nanoparticles.171–173 However, in addition to its lack of brain selectivity, very little qualitative or quantitative data are available regarding intact BBB penetration. Although some studies achieve a fast bulk brain accumulation and a few show an improved therapeutic effect, others indicate that the constructs could be trapped in brain endothelium. In this regard, one of the most perspicuous examples is the 800-fold increase in ritonavir delivery achieved two weeks after injection using TAT-coated polylactate nanoparticles.174 As it could be expected from the cell-penetrating ability of TAT, nanoparticles are efficiently internalized in brain capillary endothelial cells, probably by adsorptive-mediated endocytosis, and are slowly released as indicated by the parenchyma/capillary ratio.Nonetheless, recent studies have shown that dual-targeted liposomes with either Angiopep-2–oligoarginine, T7–TAT, THR–transportan or Tf combined with TAT, penetratin or mastoparan outperformed those with a single targeting peptide, both in vitro and in vivo.131,175–177 This strategy takes advantage of the penetrating capacity of CPPs by combining it with the higher selectivity of receptor ligands. It would certainly be interesting to study the effect of the double functionalization approach with more novel, potent and less toxic CPPs such as dNP2.178
6.2. Passive diffusion shuttles
Passive diffusion BBB-shuttle peptides dramatically enhance the transport of drugs like baicalin, dopamine, 4-aminobutanoic acid, nipecotic acid and 5-aminolevulinic acid in a BBB cell-based model and in the parallel artificial membrane permeability assay (PAMPA),30–32 which is a well-established method to measure passive diffusion.179 With the aim of further enhancing the transport capacity of prolyl oligopeptidase inhibitors, diketopiperazines have been combined with a redox chemical delivery system to avoid back transport across brain endothelium.180 Very recently, the chirality of phenylproline shuttle diastereomers has been shown to affect their permeability.68 Thus, the transport of these peptides may depend on the phospholipid composition of biological membranes, thereby suggesting a route towards cell-type and even tissue selectivity. Furthermore, phenylprolines have overcome the low solubility limitations of their forerunners.Despite the achievements described above, the applicability of BBB shuttle peptides that work through passive diffusion is still limited by the lack of selectivity and the non-negligible impact of the cargo on the efficiency of the shuttle and vice versa. On the side of the BBB transport capacity, this problem can be overcome by fine-tuning the peptide for each particular cargo.29 In order to decrease the effect of the construct on the activity of the molecule, linkers that can be cleaved inside the brain parenchyma could be incorporated.
7. Toward protease-resistant shuttles
Most of the sequences reported for the delivery of large cargoes are linear and made of L-amino acid residues. Both of these features make peptides susceptible to degradation by proteases, a process that decreases their efficiency, especially in vivo. Notwithstanding, many strategies can be applied to overcome this limitation such as the use of non-natural amino acids, N-methylation, and cyclization.50 Very recently, several publications have revealed the great potential of increasing the metabolic resistance of BBB shuttle peptides.7.1. The retro-enantio approach
The retro-enantio or retro-inverso sequence of a peptide is obtained by changing the stereochemistry of all the amino acid residues (from L- to D-amino acids) and reversing the order of the sequence. In this way, the topochemical features and the structure of the peptide are often preserved despite the inversion of the amide bond, yielding highly protease-resistant analogues. It has recently been shown that this approach yields more efficient BBB shuttles.94,181,182 In order to illustrate this point, we will focus on the retro-enantio THR and DCDX peptides.THR is a dodecapeptide obtained by phage display that interacts with TfR but does not compete with Tf.88 This peptide shuttle enhances the in vitro and in vivo transport of gold NPs coated with a peptide (LPFFD) capable of binding amyloid-β in order to disrupt aggregates upon microwave irradiation.183 TEM micrographs confirmed the presence of NPs in the parenchyma. Remarkably, it has recently been shown that the retro-enantio version of THR (THRre) transports a variety of cargoes with higher efficiency than the parent peptide in a cell-based BBB model and in vivo.182 Moreover, THRre was capable of delivering quantum dots to the brain parenchyma as shown by two-photon intravital microscopy (Fig. 5).
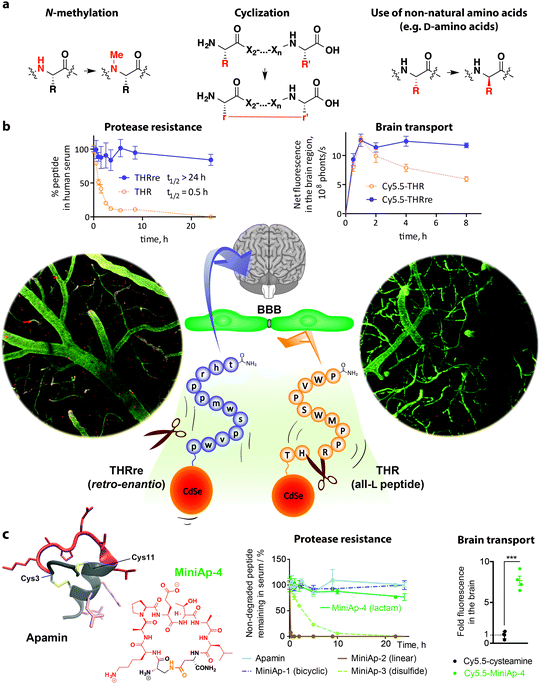 | ||
| Fig. 5 Protease-resistance provides efficient BBB shuttle peptides. (a) Structural modifications to achieve protease-resistance that have been applied to BBB shuttle peptides. (b) The half-life of THR peptide is 30 min, whereas that of the retro-enantio version (THRre) is over 24 h.182 As a result, THRre transcytosed in a cell-based BBB model and accumulated in the brain more efficiently. Furthermore, this peptide was capable of delivering quantum dots across the BBB as shown by intracranial two-photon microscopy (bottom; capillaries are shown in green and quantum dots in red. The image is reproduced with permission from ref. 182. Copyright 2015 Wiley); this technique avoids artefacts introduced during perfusion, necropsy and tissue preparation for ex vivo microscopy imaging. (c) Cyclization of peptides also results in increased protease-resistance as illustrated in the case of apamin derivatives.97 In addition, introducing non-natural elements such as substitution of a disulfide bond by a lactam bridge to produce peptidomimetics like MiniAp-4 further reinforces metabolic stability. MiniAp-4 enhanced the transport of a variety of cargoes in mice and in a human cell-based BBB model. | ||
In contrast to THR, LCDX is a peptide of natural origin. This linear fragment of snake neurotoxin candotoxin, which interacts with nAchRs, was reported following the success of RVG29.93LCDX increased nanoparticle accumulation in mouse brain and enhanced the survival of tumour-bearing mice. Recently, the retro-enantio analogue has been developed and shown to retain the capacity to interact with the same receptor.94 Although the affinity of this analogue is 5-fold lower than the original peptide, its transport capacity is enhanced because of its superior resistance to proteases in serum and in the lysosome.
7.2. A venom-inspired peptidomimetic shuttle
The retro-enantio approach has proved highly efficient. However, this transformation decreases the affinity for the transporter and requires D-amino acids, thereby significantly raising production costs. Hence, it would be of interest to identify alternative sources of protease-resistant shuttles. Although the capacity of peptides found in animal venoms has already been exploited in this field, the relevance of preserving their knotted structure has been overlooked.Apamin is a bicyclic neurotoxin that binds KCa2.2 channels, which are found in neural cells and the vasculature, and has long been known to reach the CNS.184,185 This peptide is highly resistant to proteases95 and is capable of targeting nanoparticles and proteins to the brain.96 However, high toxicity and immunogenicity, as well as a relatively complex structure, have discouraged its extended application as a shuttle. Recently, MiniAp-4, which is a safer and minimized version of apamin cyclized through a lactam bridge, has been reported.97 Importantly, this cyclic peptidomimetic preserves the high protease-resistance and brain targeting ability of apamin and has reduced toxicity and immunogenicity. MiniAp-4 is more permeable than the natural peptide and can transport nanoparticles and proteins in a human cell-based model of the BBB. Furthermore, this shuttle can carry a cargo across the BBB in mice and displays remarkable selectivity for the brain.
8. Conclusions and outlook
Although the BBB remains a formidable obstacle, since the Trojan horse concept was coined in the 1980s, the field of drug delivery to the brain has made remarkable progress. In the last few years, a plethora of new BBB shuttle peptides have emerged and hold great promise to overcome the limitations of the first generation of shuttles dominated by large proteins. Peptides are more affordable, easier to characterize and to link to nanocarriers or proteins. Moreover, they have lower immunogenicity and often have a reduced effect on the activity of the cargo than their larger counterparts. Furthermore, many peptide shuttles do not compete with endogenous substrates in contrast to endogenous proteins, nor stay bound to the receptor unlike some antibodies. BBB shuttle peptides have so far provided promising results in terms of brain delivery in preclinical settings. In addition, a relevant increase in the therapeutic effect has been proven in a wide variety of animal disease models, with a focus on brain tumours but also including neurodegenerative and lysosomal diseases as well as epilepsy among others.Despite the considerable achievements described, new shuttles with higher transport capacity and selectivity are required. Approaches like phage display and natural sources of peptides that reach the CNS offer an excellent opportunity to explore the multitude of poorly characterized or still unknown routes into the brain. These strategies should be complemented with additional efforts in the characterization of the transport mechanisms and in global proteomic approaches to identify new receptors. Also, further comparative studies between shuttles and a more accurate quantification of the free drug in the brain parenchyma would enable a more efficient identification and optimization of BBB shuttles. The next generation of BBB shuttle peptides should aim for an enhanced metabolic stability, a higher transendothelial transport and an improved selectivity for the brain – even for particular regions of this organ – possibly through yet uncharacterized transctytotic pathways.
Acknowledgements
IRB Barcelona is the recipient of a Severo Ochoa Award of Excellence from MINECO (Government of Spain). We appreciate financial support from MINECO-FEDER (Bio2013-40716-R and CTQ2013-49462-EXP), MINECO (PCIN-2015-051 Cure2DIPG), RecerCaixa-2014-Gate2Brain, Generalitat de Catalunya (XRB and 2014-SGR-521), FARA and GENEFA. B.O.-S. and M.S.-N. are grateful for “La Caixa”/IRB Barcelona and Juan de la Cierva fellowships, respectively.References
- C. J. L. Murray, T. Vos, R. Lozano, M. Naghavi, A. D. Flaxman, C. Michaud, M. Ezzati, K. Shibuya, J. A. Salomon and S. Abdalla, et al. , Lancet, 2012, 380, 2197–2223 CrossRef.
- A. Gustavsson, M. Svensson, F. Jacobi, C. Allgulander, J. Alonso, E. Beghi, R. Dodel, M. Ekman, C. Faravelli and L. Fratiglioni, et al. , Eur. Neuropsychopharmacol., 2011, 21, 718–779 CrossRef CAS PubMed.
- W. M. Pardridge, J. Cereb. Blood Flow Metab., 2012, 32, 1959–1972 CrossRef CAS PubMed.
- M. Malakoutikhah, M. Teixidó and E. Giralt, Angew. Chem., Int. Ed., 2011, 50, 7998–8014 CrossRef CAS PubMed.
- W. M. Pardridge, Nat. Rev. Drug Discovery, 2002, 1, 131–139 CrossRef CAS PubMed.
- N. J. Abbott, A. A. Patabendige, D. E. Dolman, S. R. Yusof and D. J. Begley, Neurobiol. Dis., 2010, 37, 13–25 CrossRef CAS PubMed.
- R. Daneman and A. Prat, Cold Spring Harbor Perspect. Biol., 2015, 7, a020412 CrossRef PubMed.
- Z. Zhao, A. R. Nelson, C. Betsholtz and B. V. Zlokovic, Cell, 2015, 163, 1064–1078 CrossRef CAS PubMed.
- K. Nagpal, S. K. Singh and D. N. Mishra, Expert Opin. Drug Delivery, 2013, 10, 927–955 CrossRef CAS PubMed.
- S. Mitragotri, P. A. Burke and R. Langer, Nat. Rev. Drug Discovery, 2014, 13, 655–672 CrossRef CAS PubMed.
- A. G. de Boer and P. J. Gaillard, Annu. Rev. Pharmacol. Toxicol., 2007, 47, 323–355 CrossRef CAS PubMed.
- C. T. Lu, Y. Z. Zhao, H. L. Wong, J. Cai, L. Peng and X. Q. Tian, Int. J. Nanomed., 2014, 9, 2241–2257 CrossRef PubMed.
- D. J. Wolak and R. G. Thorne, Mol. Pharmaceutics, 2013, 10, 1492–1504 CrossRef CAS PubMed.
- B. Obermeier, R. Daneman and R. M. Ransohoff, Nat. Med., 2013, 19, 1584–1596 CrossRef CAS PubMed.
- J. Lichota, T. Skjørringe, L. B. Thomsen and T. Moos, J. Neurochem., 2010, 113, 1–13 CrossRef CAS PubMed.
- J. Rautio, K. Laine, M. Gynther and J. Savolainen, AAPS J., 2008, 10, 92–102 CrossRef CAS PubMed.
- T. B. Lentz, S. J. Gray and R. J. Samulski, Neurobiol. Dis., 2012, 48, 179–188 CrossRef CAS PubMed.
- G. F. Woodworth, G. P. Dunn, E. A. Nance, J. Hanes and H. Brem, Front. Oncol., 2014, 4, 126 Search PubMed.
- E. V. Batrakova, H. E. Gendelman and A. V. Kabanov, Expert Opin. Drug Delivery, 2011, 8, 415–433 CrossRef CAS PubMed.
- M. Bourdenx, N. Dutheil, E. Bezard and B. Dehay, Front. Mol. Neurosci., 2014, 7, 1–8 Search PubMed.
- J. Niewoehner, B. Bohrmann, L. Collin, E. Urich, H. Sade, P. Maier, P. Rueger, J. O. Stracke, W. Lau, A. C. Tissot, H. Loetscher, A. Ghosh and P. O. Freskgård, Neuron, 2014, 81, 49–60 CrossRef CAS PubMed.
- H. Liu, W. Zhang, L. Ma, L. Fan, F. Gao, J. Ni and R. Wang, Int. J. Pharm., 2014, 476, 1–8 CrossRef CAS PubMed.
- B. Zhang, X. Sun, H. Mei, Y. Wang, Z. Liao, J. Chen, Q. Zhang, Y. Hu, Z. Pang and X. Jiang, Biomaterials, 2013, 34, 9171–9182 CrossRef CAS PubMed.
- D. Guarnieri, A. Falanga, O. Muscetti, R. Tarallo, S. Fusco, M. Galdiero, S. Galdiero and P. A. Netti, Small, 2013, 9, 853–862 CrossRef CAS PubMed.
- H. Xin, X. Sha, X. Jiang, L. Chen, K. Law, J. Gu, Y. Chen, X. Wang and X. Fang, Biomaterials, 2012, 33, 1673–1681 CrossRef CAS PubMed.
- R. Prades, M. Teixidó and E. Giralt, in Nanostructured Biomaterials for Overcoming Biological Barriers, ed. M. J. Alonso and N. S. Csaba, Royal Soc Chemistry, Cambridge, 2012, pp. 364–391 Search PubMed.
- J. D. Malcor, N. Payrot, M. David, A. Faucon, K. Abouzid, G. Jacquot, N. Floquet, F. Debarbieux, G. Rougon, J. Martinez, M. Khrestchatisky, P. Vlieghe and V. Lisowski, J. Med. Chem., 2012, 55, 2227–2241 CrossRef CAS PubMed.
- F. C. Thomas, K. Taskar, V. Rudraraju, S. Goda, H. R. Thorsheim, J. A. Gaasch, R. K. Mittapalli, D. Palmieri, P. S. Steeg, P. R. Lockman and Q. R. Smith, Pharm. Res., 2009, 26, 2486–2494 CrossRef CAS PubMed.
- M. Malakoutikhah, B. Guixer, P. Arranz-Gibert, M. Teixidó and E. Giralt, ChemMedChem, 2014, 9, 1594–1601 CrossRef CAS PubMed.
- M. Malakoutikhah, R. Prades, M. Teixidó and E. Giralt, J. Med. Chem., 2010, 53, 2354–2363 CrossRef CAS PubMed.
- M. Malakoutikhah, M. Teixidó and E. Giralt, J. Med. Chem., 2008, 51, 4881–4889 CrossRef CAS PubMed.
- M. Teixidó, E. Zurita, M. Malakoutikhah, T. Tarragó and E. Giralt, J. Am. Chem. Soc., 2007, 129, 11802–11813 CrossRef PubMed.
- E. Soddu, G. Rassu, P. Giunchedi, B. Sarmento and E. Gavini, Eur. J. Pharm. Sci., 2015, 74, 63–76 CrossRef CAS PubMed.
- W. M. Pardridge, Endocr. Rev., 1986, 7, 314–330 CrossRef CAS PubMed.
- A. K. Kumagai, J. B. Eisenberg and W. M. Pardridge, J. Biol. Chem., 1987, 262, 15214–15219 CAS.
- P. M. Friden, L. R. Walus, G. F. Musso, M. A. Taylor, B. Malfroy and R. M. Starzyk, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 4771–4775 CrossRef CAS.
- Y. J. Yu, Y. Zhang, M. Kenrick, K. Hoyte, W. Luk, Y. Lu, J. Atwal, J. M. Elliott, S. Prabhu, R. J. Watts and M. S. Dennis, Sci. Transl. Med., 2011, 3, 84ra44 Search PubMed.
- J. Kreuter, T. Hekmatara, S. Dreis, T. Vogel, S. Gelperina and K. Langer, J. Controlled Release, 2007, 118, 54–58 CrossRef CAS PubMed.
- W. Pan, A. J. Kastin, T. C. Zankel, P. van Kerkhof, T. Terasaki and G. Bu, J. Cell Sci., 2004, 117, 5071–5078 CrossRef CAS PubMed.
- Z. M. Qian, H. Li, H. Sun and K. Ho, Pharmacol. Rev., 2002, 54, 561–587 CrossRef CAS PubMed.
- C. Fillebeen, L. Descamps, M. P. Dehouck, L. Fenart, M. Benaissa, G. Spik, R. Cecchelli and A. Pierce, J. Biol. Chem., 1999, 274, 7011–7017 CrossRef CAS PubMed.
- M. Demeule, J. Poirier, J. Jodoin, Y. Bertrand, R. R. Desrosiers, C. Dagenais, T. Nguyen, J. Lanthier, R. Gabathuler, M. Kennard, W. A. Jefferies, D. Karkan, S. Tsai, L. Fenart, R. Cecchelli and R. Beliveau, J. Neurochem., 2002, 83, 924–933 CrossRef CAS PubMed.
- W. A. Banks, A. J. Kastin, W. Huang, J. B. Jaspan and L. M. Maness, Peptides, 1996, 17, 305–311 CrossRef CAS.
- W. A. Banks and R. D. Broadwell, J. Neurochem., 1994, 62, 2404–2419 CrossRef CAS PubMed.
- P. J. Gaillard, A. Brink and A. G. de Boer, Int. Cong. Ser., 2005, 1277, 185–198 CrossRef CAS.
- Y. J. Yu, J. K. Atwal, Y. Zhang, R. K. Tong, K. R. Wildsmith, C. Tan, N. Bien-Ly, M. Hersom, J. A. Maloney and W. J. Meilandt, et al. , Sci. Transl. Med., 2014, 6, 261ra154 CrossRef PubMed.
- A. Abulrob, J. Zhang, J. Tanha, R. MacKenzie and D. Stanimirovic, Int. Congr. Ser., 2005, 1277, 212–223 CrossRef CAS.
- T. Uhlig, T. Kyprianou, F. G. Martinelli, C. A. Oppici, D. Heiligers, D. Hills, X. R. Calvo and P. Verhaert, EuPa Open Proteomics, 2014, 4, 58–69 CrossRef CAS.
- C. J. Camacho, Y. Katsumata and D. P. Ascherman, PLoS Comput. Biol., 2008, 4, e1000231 Search PubMed.
- I. R. Kumarasinghe and V. J. Hruby, in Peptide chemistry and drug design, ed. B. M. Dunn, Wiley, 2015, pp. 247–264 Search PubMed.
- W. A. Banks, Peptides, 2015, 72, 16–19 CrossRef CAS PubMed.
- S. R. Schwarze, A. Ho, A. Vocero-Akbani and S. F. Dowdy, Science, 1999, 285, 1569–1572 CrossRef CAS PubMed.
- P. Kumar, H. Wu, J. L. McBride, K. E. Jung, M. H. Kim, B. L. Davidson, S. K. Lee, P. Shankar and N. Manjunath, Nature, 2007, 448, 39–43 CrossRef CAS PubMed.
- M. Demeule, A. Régina, C. Ché, J. Poirier, T. Nguyen, R. Gabathuler, J. P. Castaigne and R. Béliveau, J. Pharmacol. Exp. Ther., 2008, 324, 1064–1072 CrossRef CAS PubMed.
- P. J. Gaillard, C. C. Appeldoorn, J. Rip, R. Dorland, S. M. van der Pol, G. Kooij, H. E. de Vries and A. Reijerkerk, J. Controlled Release, 2012, 164, 364–369 CrossRef CAS PubMed.
- H. F. Liang, Y. C. Chen, T. F. Yang, L. W. Chang, A. J. Wang, J. M. Lu, C. H. Jian, Y. F. Lin and S. J. Liu, US7704956 B2, 2010.
- F. Hervé, N. Ghinea and J. M. Scherrmann, AAPS J., 2008, 10, 455–472 CrossRef PubMed.
- I. Martín, M. Teixidó and E. Giralt, Curr. Pharm. Des., 2013, 19, 2924–2942 CrossRef.
- F. Madani, S. Lindberg, Ü. Langel, S. Futaki and A. Gräslund, J. Biophys., 2011, 2011, 414729 Search PubMed.
- A. D. Frankel and C. O. Pabo, Cell, 1988, 55, 1189–1193 CrossRef CAS PubMed.
- A. Joliot, C. Pernelle, H. Deagostini-Bazin and A. Prochiantz, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 1864–1868 CrossRef CAS.
- C. Rousselle, P. Clair, J. M. Lefauconnier, M. Kaczorek, J. M. Scherrmann and J. Temsamani, Mol. Pharmacol., 2000, 57, 679–686 CAS.
- G. Drin, S. Cottin, E. Blanc, A. R. Rees and J. Temsamani, J. Biol. Chem., 2003, 278, 31192–31201 CrossRef CAS PubMed.
- M. Pooga, M. Hallbrink, M. Zorko and U. Langel, FASEB J., 1998, 12, 67–77 CAS.
- S. Futaki, T. Suzuki, W. Ohashi, T. Yagami, S. Tanaka, K. Ueda and Y. Sugiura, J. Biol. Chem., 2001, 276, 5836–5840 CrossRef CAS PubMed.
- S. Stalmans, N. Bracke, E. Wynendaele, B. Gevaert, K. Peremans, C. Burvenich, I. Polis and B. De Spiegeleer, PLoS One, 2015, 10, e0139652 Search PubMed.
- E. G. Chikhale, K. Y. Ng, P. S. Burton and R. T. Borchardt, Pharm. Res., 1994, 11, 412–419 CrossRef CAS.
- P. Arranz-Gibert, B. Guixer, M. Malakoutikhah, M. Muttenthaler, F. Guzmán, M. Teixidó and E. Giralt, J. Am. Chem. Soc., 2015, 137, 7357–7364 CrossRef CAS PubMed.
- C. Zhan, C. Li, X. Wei, W. Lu and W. Lu, Adv. Drug Delivery Rev., 2015, 90, 101–118 CrossRef CAS PubMed.
- G. P. Smith, Science, 1985, 228, 1315–1317 CAS.
- S. Cabilly, Mol. Biotechnol., 1999, 12, 143–148 CrossRef CAS PubMed.
- K. K. Jain, Drug Delviery in Central Nervous System Disorders – Technologies, Markets and Companies, Jain PharmaBiotech, Basel, Switzerland, 2012 Search PubMed.
- B. Dehouck, L. Fenart, M. P. Dehouck, A. Pierce, G. Torpier and R. Cecchelli, J. Cell Biol., 1997, 138, 877–889 CrossRef CAS PubMed.
- K. R. Duffy and W. M. Pardridge, Brain Res., 1987, 420, 32–38 CrossRef CAS PubMed.
- H. Tu, W. Pan, L. Feucht and A. J. Kastin, J. Cell. Physiol., 2007, 212, 215–222 CrossRef CAS PubMed.
- G. L. Barrett, J. Trieu and T. Naim, Regul. Pept., 2009, 155, 55–61 CrossRef CAS PubMed.
- Y. Liu, J. Li, K. Shao, R. Huang, L. Ye, J. Lou and C. Jiang, Biomaterials, 2010, 31, 5246–5257 CrossRef CAS PubMed.
- A. P. Sagare, R. Deane and B. V. Zlokovic, Pharmacol. Ther., 2012, 136, 94–105 CrossRef CAS PubMed.
- N. S. Chung and K. M. Wasan, Adv. Drug Delivery Rev., 2004, 56, 1315–1334 CrossRef CAS PubMed.
- M. Notarnicola, M. Linsalata, M. Caruso, A. Cavallini and A. Di Leo, J. Gastroenterol., 1995, 30, 705–709 CrossRef CAS PubMed.
- B. J. Spencer and I. M. Verma, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 7594–7599 CrossRef CAS PubMed.
- D. Wang, S. S. El-Amouri, M. Dai, C. Y. Kuan, D. Y. Hui, R. O. Brady and D. Pan, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 2999–3004 CrossRef CAS PubMed.
- Y. Uchida, S. Ohtsuki, Y. Katsukura, C. Ikeda, T. Suzuki, J. Kamiie and T. Terasaki, J. Neurochem., 2011, 117, 333–345 CrossRef CAS PubMed.
- R. Daneman, L. Zhou, D. Agalliu, J. D. Cahoy, A. Kaushal and B. A. Barres, PLoS One, 2010, 5, e13741 Search PubMed.
- Y. Uchida, M. Tachikawa, W. Obuchi, Y. Hoshi, Y. Tomioka, S. Ohtsuki and T. Terasaki, Fluids Barriers CNS, 2013, 10, 21 CrossRef CAS PubMed.
- A. R. Jones and E. V. Shusta, Pharm. Res., 2007, 24, 1759–1771 CrossRef CAS PubMed.
- H. Xia, B. Anderson, Q. Mao and B. L. Davidson, J. Virol., 2000, 74, 11359–11366 CrossRef CAS PubMed.
- J. H. Lee, J. A. Engler, J. F. Collawn and B. A. Moore, Eur. J. Biochem., 2001, 268, 2004–2012 CrossRef CAS PubMed.
- F. I. Staquicini, M. G. Ozawa, C. A. Moya, W. H. Driessen, E. M. Barbu, H. Nishimori, S. Soghomonyan, L. G. Flores, 2nd, X. Liang and V. Paolillo, et al. , J. Clin. Invest., 2011, 121, 161–173 CAS.
- L. Xiang, R. Zhou, A. Fu, X. Xu, Y. Huang and C. Hu, J. Drug Targeting, 2011, 19, 632–636 CrossRef CAS PubMed.
- A. Fu, Y. Wang, L. Zhan and R. Zhou, Pharm. Res., 2012, 29, 1562–1569 CrossRef CAS PubMed.
- C. Zhan, Z. Yan, C. Xie and W. Lu, Mol. Pharmaceutics, 2010, 7, 1940–1947 CrossRef CAS PubMed.
- C. Zhan, B. Li, L. Hu, X. Wei, L. Feng, W. Fu and W. Lu, Angew. Chem., Int. Ed., 2011, 50, 5482–5485 CrossRef CAS PubMed.
- X. Wei, C. Zhan, Q. Shen, W. Fu, C. Xie, J. Gao, C. Peng, P. Zheng and W. Lu, Angew. Chem., Int. Ed., 2015, 54, 3023–3027 CrossRef CAS PubMed.
- B. Oller-Salvia, M. Teixidó and E. Giralt, Biopolymers, 2013, 100, 675–686 CrossRef CAS PubMed.
- J. Wu, H. Jiang, Q. Bi, Q. Luo, J. Li, Y. Zhang, Z. Chen and C. Li, Mol. Pharmaceutics, 2014, 11, 3210–3222 CrossRef CAS PubMed.
- B. Oller-Salvia, M. Sánchez-Navarro, S. Ciudad, S. Guiu, P. Arranz-Gibert, C. Garcia, R. Gomis, R. Cecchelli, J. Garcia, E. Giralt and M. Teixidó, Angew. Chem., Int. Ed., 2016, 55, 572–575 CrossRef CAS PubMed.
- J. Rip, L. Chen, R. Hartman, A. van den Heuvel, A. Reijerkerk, J. van Kregten, B. van der Boom, C. Appeldoorn, M. de Boer, D. Maussang, E. C. de Lange and P. J. Gaillard, J. Drug Targeting, 2014, 22, 460–467 CrossRef CAS PubMed.
- A. K. Bachhawat, A. Thakur, J. Kaur and M. Zulkifli, Biochim. Biophys. Acta, 2013, 1830, 3154–3164 CrossRef CAS PubMed.
- L. Costantino, F. Gandolfi, G. Tosi, F. Rivasi, M. A. Vandelli and F. Forni, J. Controlled Release, 2005, 108, 84–96 CrossRef CAS PubMed.
- K. N. Sugahara, T. Teesalu, P. P. Karmali, V. R. Kotamraju, L. Agemy, D. R. Greenwald and E. Ruoslahti, Science, 2010, 328, 1031–1035 CrossRef CAS PubMed.
- D. Hauzenberger, J. Klominek and K. G. Sundqvist, J. Immunol., 1994, 153, 960–971 CAS.
- Y. Miura, T. Takenaka, K. Toh, S. Wu, H. Nishihara, M. R. Kano, Y. Ino, T. Nomoto, Y. Matsumoto, H. Koyama, H. Cabral, N. Nishiyama and K. Kataoka, ACS Nano, 2013, 7, 8583–8592 CrossRef CAS PubMed.
- D. E. Saslowsky, Y. M. te Welscher, D. J. Chinnapen, J. S. Wagner, J. Wan, E. Kern and W. I. Lencer, J. Biol. Chem., 2013, 288, 25804–25809 CrossRef CAS PubMed.
- G. Tettamanti, R. Bassi, P. Viani and L. Riboni, Biochimie, 2003, 85, 423–437 CrossRef CAS PubMed.
- J. K. Liu, Q. Teng, M. Garrity-Moses, T. Federici, D. Tanase, M. J. Imperiale and N. M. Boulis, Neurobiol. Dis., 2005, 19, 407–418 CrossRef CAS PubMed.
- J. V. Georgieva, R. P. Brinkhuis, K. Stojanov, C. A. Weijers, H. Zuilhof, F. P. Rutjes, D. Hoekstra, J. C. van Hest and I. S. Zuhorn, Angew. Chem., Int. Ed., 2012, 51, 8339–8342 CrossRef CAS PubMed.
- K. Stojanov, J. V. Georgieva, R. P. Brinkhuis, J. C. van Hest, F. P. Rutjes, R. A. Dierckx, E. F. de Vries and I. S. Zuhorn, Mol. Pharmaceutics, 2012, 9, 1620–1627 CrossRef CAS PubMed.
- J. Li, L. Feng, L. Fan, Y. Zha, L. Guo, Q. Zhang, J. Chen, Z. Pang, Y. Wang, X. Jiang, V. C. Yang and L. Wen, Biomaterials, 2011, 32, 4943–4950 CrossRef CAS PubMed.
- H. Gao, J. Qian, S. Cao, Z. Yang, Z. Pang, S. Pan, L. Fan, Z. Xi, X. Jiang and Q. Zhang, Biomaterials, 2012, 33, 5115–5123 CrossRef CAS PubMed.
- C. Zhang, X. Wan, X. Zheng, X. Shao, Q. Liu, Q. Zhang and Y. Qian, Biomaterials, 2014, 35, 456–465 CrossRef CAS PubMed.
- T. Vitalis and R. Gabathuler, WO2014160438 A1, 2014.
- M. Demeule, J. C. Currie, Y. Bertrand, C. Ché, T. Nguyen, A. Régina, R. Gabathuler, J. P. Castaigne and R. Béliveau, J. Neurochem., 2008, 106, 1534–1544 CrossRef CAS PubMed.
- C. Ché, G. Yang, C. Thiot, M. C. Lacoste, J. C. Currie, M. Demeule, A. Régina, R. Béliveau and J. P. Castaigne, J. Med. Chem., 2010, 53, 2814–2824 CrossRef PubMed.
- A. Régina, M. Demeule, C. Ché, I. Lavallée, J. Poirier, R. Gabathuler, R. Béliveau and J. P. Castaigne, Br. J. Pharmacol., 2008, 155, 185–197 CrossRef PubMed.
- M. Demeule, N. Beaudet, A. Reégina, É. Besserer-Offroy, A. Murza, P. Tétreault, K. Belleville, C. Ché, A. Larocque, C. Thiot, R. Béliveau, J. M. Longpré, É. Marsault, R. Leduc, J. E. Lachowicz, S. L. Gonias, J. P. Castaigne and P. Sarret, J. Clin. Invest., 2014, 124, 1199–1213 CAS.
- Y. Bertrand, J. C. Currie, J. Poirier, M. Demeule, A. Abulrob, D. Fatehi, D. Stanimirovic, H. Sartelet, J. P. Castaigne and R. Béliveau, Br. J. Cancer, 2011, 105, 1697–1707 CrossRef CAS PubMed.
- R. Gabathuler, in Drug Delivery to the Central Nervous System, ed. K. K. Jain, Humana Press, 2010, vol. 45, pp. 249–260 Search PubMed.
- J. Drappatz, A. Brenner, E. T. Wong, A. Eichler, D. Schiff, M. D. Groves, T. Mikkelsen, S. Rosenfeld, J. Sarantopoulos, C. A. Meyers, R. M. Fielding, K. Elian, X. Wang, B. Lawrence, M. Shing, S. Kelsey, J. P. Castaigne and P. Y. Wen, Clin. Cancer Res., 2013, 19, 1567–1576 CrossRef CAS PubMed.
- R. Kurzrock, N. Gabrail, C. Chandhasin, S. Moulder, C. Smith, A. Brenner, K. Sankhala, A. Mita, K. Elian, D. Bouchard and J. Sarantopoulos, Mol. Cancer Ther., 2012, 11, 308–316 CrossRef CAS PubMed.
- S. E. Bates, M. L. Lindenberg, C. Bryla, M. E. Burotto Pichun, N. Patronas, E. Mena Gonzalez, L. Amiri-Kordestani, T. Fojo, S. Balasubramaniam and P. L. Choyke, J. Clin. Oncol., 2015, 33(suppl. 15), 2552 Search PubMed.
- Z. Z. Yang, J. Q. Li, Z. Z. Wang, D. W. Dong and X. R. Qi, Biomaterials, 2014, 35, 5226–5239 CrossRef CAS PubMed.
- X. Sun, Z. Pang, H. Ye, B. Qiu, L. Guo, J. Li, J. Ren, Y. Qian, Q. Zhang, J. Chen and X. Jiang, Biomaterials, 2012, 33, 916–924 CAS.
- J. Ren, S. Shen, D. Wang, Z. Xi, L. Guo, Z. Pang, Y. Qian, X. Sun and X. Jiang, Biomaterials, 2012, 33, 3324–3333 CrossRef CAS PubMed.
- H. Yan, L. Wang, J. Wang, X. Weng, H. Lei, X. Wang, L. Jiang, J. Zhu, W. Lu, X. Wei and C. Li, ACS Nano, 2012, 6, 410–420 CrossRef CAS PubMed.
- H. Yan, J. Wang, P. Yi, H. Lei, C. Zhan, C. Xie, L. Feng, J. Qian, J. Zhu, W. Lu and C. Li, Chem. Commun., 2011, 47, 8130–8132 RSC.
- X. Gao, J. Qian, S. Zheng, Y. Xiong, J. Man, B. Cao, L. Wang, S. Ju and C. Li, Pharm. Res., 2013, 30, 2538–2548 CrossRef CAS PubMed.
- S. Huang, J. Li, L. Han, S. Liu, H. Ma, R. Huang and C. Jiang, Biomaterials, 2011, 32, 6832–6838 CrossRef CAS PubMed.
- R. Huang, H. Ma, Y. Guo, S. Liu, Y. Kuang, K. Shao, J. Li, Y. Liu, L. Han, S. Huang, S. An, L. Ye, J. Lou and C. Jiang, Pharm. Res., 2013, 30, 2549–2559 CrossRef CAS PubMed.
- G. Huile, P. Shuaiqi, Y. Zhi, C. Shijie, C. Chen, J. Xinguo, S. Shun, P. Zhiqing and H. Yu, Biomaterials, 2011, 32, 8669–8675 CrossRef PubMed.
- H. Gao, S. Zhang, S. Cao, Z. Yang, Z. Pang and X. Jiang, Mol. Pharmaceutics, 2014, 11, 2755–2763 CrossRef CAS PubMed.
- H. Xin, X. Jiang, J. Gu, X. Sha, L. Chen, K. Law, Y. Chen, X. Wang, Y. Jiang and X. Fang, Biomaterials, 2011, 32, 4293–4305 CrossRef CAS PubMed.
- H. Xin, X. Sha, X. Jiang, W. Zhang, L. Chen and X. Fang, Biomaterials, 2012, 33, 8167–8176 CrossRef CAS PubMed.
- J. Shen, C. Zhan, C. Xie, Q. Meng, B. Gu, C. Li, Y. Zhang and W. Lu, J. Drug Targeting, 2011, 19, 197–203 CrossRef CAS PubMed.
- X. Ying, Y. Wang, J. Liang, J. Yue, C. Xu, L. Lu, Z. Xu, J. Gao, Y. Du and Z. Chen, Angew. Chem., Int. Ed., 2014, 53, 12436–12440 CAS.
- D. Ni, J. Zhang, W. Bu, H. Xing, F. Han, Q. Xiao, Z. Yao, F. Chen, Q. He, J. Liu, S. Zhang, W. Fan, L. Zhou, W. Peng and J. Shi, ACS Nano, 2014, 8, 1231–1242 CrossRef CAS PubMed.
- S. Ruan, M. Yuan, L. Zhang, G. Hu, J. Chen, X. Cun, Q. Zhang, Y. Yang, Q. He and H. Gao, Biomaterials, 2015, 37, 425–435 CrossRef CAS PubMed.
- A. Regina, M. Demeule, S. Tripathy, S. Lord-Dufour, J. C. Currie, M. Iddir, B. Annabi, J. P. Castaigne and J. E. Lachowicz, Mol. Cancer Ther., 2015, 14, 129–140 CrossRef CAS PubMed.
- K. Shao, J. Wu, Z. Chen, S. Huang, J. Li, L. Ye, J. Lou, L. Zhu and C. Jiang, Biomaterials, 2012, 33, 6898–6907 CrossRef CAS PubMed.
- K. Shao, R. Huang, J. Li, L. Han, L. Ye, J. Lou and C. Jiang, J. Controlled Release, 2010, 147, 118–126 CrossRef CAS PubMed.
- A. Mdzinarishvili, V. Sutariya, P. K. Talasila, W. J. Geldenhuys and P. Sadana, Drug Delivery Transl. Res., 2013, 3, 309–317 CrossRef CAS PubMed.
- D. H. Lee, C. Rötger, C. C. Appeldoorn, A. Reijerkerk, W. Gladdines, P. J. Gaillard and R. A. Linker, J. Neuroimmunol., 2014, 274, 96–101 CrossRef CAS PubMed.
- W. Geldenhuys, T. Mbimba, T. Bui, K. Harrison and V. Sutariya, J. Drug Targeting, 2011, 19, 837–845 CrossRef CAS PubMed.
- A. Lindqvist, J. Rip, P. J. Gaillard, S. Björkman and M. Hammarlund-Udenaes, Mol. Pharmaceutics, 2013, 10, 1533–1541 CrossRef CAS PubMed.
- M. Rotman, M. M. Welling, A. Bunschoten, M. E. de Backer, J. Rip, R. J. Nabuurs, P. J. Gaillard, M. A. van Buchem, S. M. van der Maarel and L. van der Weerd, J. Controlled Release, 2015, 203, 40–50 CrossRef CAS PubMed.
- S. S. Kim, C. Ye, P. Kumar, I. Chiu, S. Subramanya, H. Wu, P. Shankar and N. Manjunath, Mol. Ther., 2010, 18, 993–1001 CrossRef CAS PubMed.
- C. Gong, X. Li, L. Xu and Y. H. Zhang, Biomaterials, 2012, 33, 3456–3463 CrossRef CAS PubMed.
- S. Zadran, G. Akopian, H. Zadran, J. Walsh and M. Baudry, NeuroMol. Med., 2013, 15, 74–81 CrossRef CAS PubMed.
- Y. Liu, Y. Hu, Y. Guo, H. Ma, J. Li and C. Jiang, J. Controlled Release, 2012, 163, 203–210 CrossRef CAS PubMed.
- W. Hwang do, S. Son, J. Jang, H. Youn, S. Lee, D. Lee, Y. S. Lee, J. M. Jeong, W. J. Kim and D. S. Lee, Biomaterials, 2011, 32, 4968–4975 CrossRef PubMed.
- H. Huo, Y. Gao, Y. Wang, J. Zhang, Z. Y. Wang, T. Jiang and S. Wang, J. Colloid Interface Sci., 2015, 447, 8–15 CrossRef CAS PubMed.
- Y. Liu, R. Huang, L. Han, W. Ke, K. Shao, L. Ye, J. Lou and C. Jiang, Biomaterials, 2009, 30, 4195–4202 CrossRef CAS PubMed.
- L. Alvarez-Erviti, Y. Seow, H. Yin, C. Betts, S. Lakhal and M. J. A. Wood, Nat. Biotechnol., 2011, 29, 341–345 CrossRef CAS PubMed.
- Y. Liu, Y. Guo, S. An, Y. Kuang, X. He, H. Ma, J. Li, J. Lu, N. Zhang and C. Jiang, PLoS One, 2013, 8, e62905 CAS.
- H. L. Wong, X. Y. Wu and R. Bendayan, Adv. Drug Delivery Rev., 2012, 64, 686–700 CrossRef CAS PubMed.
- N. C. Sorrentino, L. D'Orsi, I. Sambri, E. Nusco, C. Monaco, C. Spampanato, E. Polishchuk, P. Saccone, E. De Leonibus, A. Ballabio and A. Fraldi, EMBO Mol. Med., 2013, 5, 675–690 CrossRef CAS PubMed.
- A. Böckenhoff, S. Cramer, P. Wölte, S. Knieling, C. Wohlenberg, V. Gieselmann, H. J. Galla and U. Matzner, J. Neurosci., 2014, 34, 3122–3129 CrossRef PubMed.
- C. A. Dyer, D. P. Cistola, G. C. Parry and L. K. Curtiss, J. Lipid Res., 1995, 36, 80–88 CAS.
- D. Clayton, I. M. Brereton, P. A. Kroon and R. Smith, Protein Sci., 1999, 8, 1797–1805 CrossRef CAS PubMed.
- F. Re, I. Cambianica, C. Zona, S. Sesana, M. Gregori, R. Rigolio, B. La Ferla, F. Nicotra, G. Forloni, A. Cagnotto, M. Salmona, M. Masserini and G. Sancini, Nanomedicine, 2011, 7, 551–559 CAS.
- G. Sarkar, G. L. Curran, E. Mahlum, T. Decklever, T. M. Wengenack, A. Blahnik, B. Hoesley, V. J. Lowe, J. F. Poduslo and R. B. Jenkins, PLoS One, 2011, 6, e28881 CAS.
- Y. Meng, I. Sohar, D. E. Sleat, J. R. Richardson, K. R. Reuhl, R. B. Jenkins, G. Sarkar and P. Lobel, Mol. Ther., 2014, 22, 547–553 CrossRef CAS PubMed.
- I. Westergren and B. B. Johansson, Acta Physiol. Scand., 1993, 149, 99–104 CrossRef CAS PubMed.
- G. Tosi, B. Bortot, B. Ruozi, D. Dolcetta, M. A. Vandelli, F. Forni and G. M. Severini, Curr. Med. Chem., 2013, 20, 2212–2225 CrossRef CAS PubMed.
- G. Tosi, L. Bondioli, B. Ruozi, L. Badiali, G. M. Severini, S. Biffi, A. De Vita, B. Bortot, D. Dolcetta, F. Forni and M. A. Vandelli, J. Neural Transm., 2011, 118, 145–153 CrossRef CAS PubMed.
- A. Vilella, G. Tosi, A. M. Grabrucker, B. Ruozi, D. Belletti, M. A. Vandelli, T. M. Boeckers, F. Forni and M. Zoli, J. Controlled Release, 2014, 174, 195–201 CrossRef CAS PubMed.
- E. Kilic, G. P. Dietz, D. M. Hermann and M. Bähr, Ann. Neurol., 2002, 52, 617–622 CrossRef CAS PubMed.
- M. Aarts, Y. Liu, L. Liu, S. Besshoh, M. Arundine, J. W. Gurd, Y. T. Wang, M. W. Salter and M. Tymianski, Science, 2002, 298, 846–850 CrossRef CAS PubMed.
- S. S. Elliger, C. A. Elliger, C. Lang and G. L. Watson, Mol. Ther., 2002, 5, 617–626 CrossRef CAS PubMed.
- E. Kilic, U. Kilic and D. M. Hermann, CNS Drug Rev., 2005, 11, 369–378 CrossRef CAS PubMed.
- Y. Qin, H. Chen, Q. Zhang, X. Wang, W. Yuan, R. Kuai, J. Tang, L. Zhang, Z. Zhang, Q. Zhang, J. Liu and Q. He, Int. J. Pharm., 2011, 420, 304–312 CrossRef CAS PubMed.
- H. Wang, K. Xu, L. Liu, J. P. Tan, Y. Chen, Y. Li, W. Fan, Z. Wei, J. Sheng, Y. Y. Yang and L. Li, Biomaterials, 2010, 31, 2874–2881 CrossRef CAS PubMed.
- X.-H. Tian, Z.-G. Wang, H. Meng, Y.-H. Wang, W. Feng, F. Wei, Z.-C. Huang, X.-N. Lin and L. Ren, Int. J. Nanomed., 2013, 8, 865–876 CrossRef PubMed.
- K. S. Rao, M. K. Reddy, J. L. Horning and V. Labhasetwar, Biomaterials, 2008, 29, 4429–4438 CrossRef CAS PubMed.
- G. Sharma, A. Modgil, T. Zhong, C. Sun and J. Singh, Pharm. Res., 2014, 31, 1194–1209 CrossRef CAS PubMed.
- T. Zong, L. Mei, H. Gao, W. Cai, P. Zhu, K. Shi, J. Chen, Y. Wang, F. Gao and Q. He, Mol. Pharmaceutics, 2014, 11, 2346–2357 CrossRef CAS PubMed.
- P. Youn, Y. Chen and D. Y. Furgeson, Mol. Pharmaceutics, 2014, 11, 486–495 CrossRef CAS PubMed.
- S. Lim, W.-J. Kim, Y.-H. Kim, S. Lee, J.-H. Koo, J.-A. Lee, H. Yoon, D.-H. Kim, H.-J. Park and H.-M. Kim, et al. , Nat. Commun., 2015, 6, 8244 CrossRef CAS PubMed.
- J. Mensch, J. Oyarzabal, C. Mackie and P. Augustijns, J. Pharm. Sci., 2009, 98, 4429–4468 CrossRef CAS PubMed.
- M. Teixidó, E. Zurita, L. Mendieta, B. Oller-Salvia, R. Prades, T. Tarragó and E. Giralt, Biopolymers, 2013, 100, 662–674 CrossRef PubMed.
- X. Wei, C. Zhan, X. Chen, J. Hou, C. Xie and W. Lu, Mol. Pharmaceutics, 2014, 11, 3261–3268 CrossRef CAS PubMed.
- R. Prades, B. Oller-Salvia, S. M. Schwarzmaier, J. Selva, M. Moros, M. Balbi, V. Grazú, J. M. de La Fuente, G. Egea, N. Plesnila, M. Teixidó and E. Giralt, Angew. Chem., Int. Ed., 2015, 54, 3967–3972 CrossRef CAS PubMed.
- R. Prades, S. Guerrero, E. Araya, C. Molina, E. Salas, E. Zurita, J. Selva, G. Egea, C. López-Iglesias, M. Teixidó, M. J. Kogan and E. Giralt, Biomaterials, 2012, 33, 7194–7205 CrossRef CAS PubMed.
- J. P. Vincent, H. Schweitz and M. Lazdunski, Biochemistry, 1975, 14, 2521–2525 CrossRef CAS PubMed.
- E. Habermann and K. G. Reiz, Biochem. Z., 1965, 343, 192–203 CAS.
- L. Di, H. Rong and B. Feng, J. Med. Chem., 2013, 56, 2–12 CrossRef CAS PubMed.
- M. Guttman, J. H. Prieto, T. M. Handel, P. J. Domaille and E. A. Komives, J. Mol. Biol., 2010, 398, 306–319 CrossRef CAS PubMed.
- J. Spengler, J. C. Jiménez, K. Burger, E. Giralt and F. Albericio, J. Pept. Res., 2005, 65, 550–555 CrossRef CAS PubMed.
- R. Cecchelli, V. Berezowski, S. Lundquist, M. Culot, M. Renftel, M. P. Dehouck and L. Fenart, Nat. Rev. Drug Discovery, 2007, 6, 650–661 CrossRef CAS PubMed.
- I. van Rooy, S. Cakir-Tascioglu, W. E. Hennink, G. Storm, R. M. Schiffelers and E. Mastrobattista, Pharm. Res., 2011, 28, 456–471 CrossRef CAS PubMed.
- C. D. Kuhnline Sloan, P. Nandi, T. H. Linz, J. V. Aldrich, K. L. Audus and S. M. Lunte, Annu. Rev. Anal. Chem., 2012, 5, 505–531 CrossRef PubMed.
- J. Bicker, G. Alves, A. Fortuna and A. Falcão, Eur. J. Pharm. Biopharm., 2014, 87, 409–432 CrossRef CAS PubMed.
- I. Wilhelm and I. A. Krizbai, Mol. Pharmaceutics, 2014, 11, 1949–1963 CrossRef CAS PubMed.
- U. Bickel, NeuroRx, 2005, 2, 15–26 CrossRef PubMed.
- R. Gabathuler, Neurobiol. Dis., 2010, 37, 48–57 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |




