Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of type-I/type-II hybrid dye sensitizer with both pyridyl group and catechol unit as anchoring group for type-I/type-II dye-sensitized solar cell†
Yousuke
Ooyama
*,
Kensuke
Furue
,
Toshiaki
Enoki
,
Masahiro
Kanda
,
Yohei
Adachi
and
Joji
Ohshita
*
Department of Applied Chemistry, Graduate School of Engineering, Hiroshima University, Higashi-Hiroshima 739-8527, Japan. E-mail: yooyama@hiroshima-u.ac.jp; Fax: +81 82-424-5494
First published on 21st October 2016
Abstract
A type-I/type-II hybrid dye sensitizer with a pyridyl group and a catechol unit as the anchoring group has been developed and its photovoltaic performance in dye-sensitized solar cells (DSSCs) is investigated. The sensitizer has the ability to adsorb on a TiO2 electrode through both the coordination bond at Lewis acid sites and the bidentate binuclear bridging linkage at Brønsted acid sites on the TiO2 surface, which makes it possible to inject an electron into the conduction band of the TiO2 electrode by the intramolecular charge-transfer (ICT) excitation (type-I pathway) and by the photoexcitation of the dye-to-TiO2 charge transfer (DTCT) band (type-II pathway). It was found that the type-I/type-II hybrid dye sensitizer adsorbed on TiO2 film exhibits a broad photoabsorption band originating from ICT and DTCT characteristics. Here we reveal the photophysical and electrochemical properties of the type-I/type-II hybrid dye sensitizer bearing a pyridyl group and a catechol unit, along with its adsorption modes onto TiO2 film, and its photovoltaic performance in type-I/type-II DSSC, based on optical (photoabsorption and fluorescence spectroscopy) and electrochemical measurements (cyclic voltammetry), density functional theory (DFT) calculation, FT-IR spectroscopy of the dyes adsorbed on TiO2 film, photocurrent–voltage (I–V) curves, incident photon-to-current conversion efficiency (IPCE) spectra, and electrochemical impedance spectroscopy (EIS) for DSSC.
Introduction
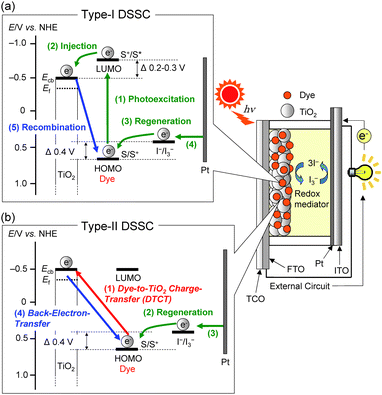
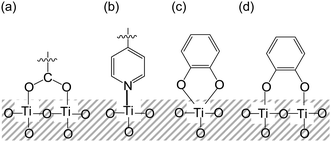
Since Grätzel and co-workers produced high-performance dye-sensitized solar cells (DSSCs) employing a Ru-complex dye-adsorbed TiO2 electrode in 1991, which showed a solar energy-to-electricity conversion yield (η) of 11%,1 DSSCs have received considerable attention as one of the most promising new renewable photovoltaic cells alternative to conventional solar cells based on silicon because they have interesting construction and operational principles, and a colorful and decorative nature.2–8 To further improve the photovoltaic performance based on the construction and operational principles of DSSC, much effort has been made towards the development of various types of organic dye sensitizers that possess the following characteristics: (i) good light-harvesting features over the wide spectral region of sunlight, (ii) high electron-injection efficiencies from photoexcited dyes to the conduction band (CB) of the TiO2 electrode, (iii) high dye loading and high surface coverage of the TiO2 electrode, (iv) efficient re-reduction of the oxidized dyes by electron transfer from I− ions in the electrolyte containing the I3−/I− redox couple, (v) reduction of charge recombination between the injected electrons in the CB of the TiO2 electrode and the oxidized dyes or I3− ions in the electrolyte, and (vi) reduction of dye aggregation on the TiO2 surface.3,4TiO2-based DSSCs can be classified into two types, I and II, depending on the electron-injection pathway from the dye to the CB of the TiO2 electrode (Fig. 1).4 The pathway for type-I DSSCs is photoexcitation of the local band of the dye adsorbed on the TiO2 surface followed by an electron injection from the photoexcited dye to the CB of the TiO2 electrode (Fig. 1a: i.e., an electron is excited from the HOMO to the LUMO level of the dye by sunlight illumination, followed by injection into the CB of TiO2 electrode). Thus, this pathway can also be called the “two-step” or “indirect” electron-injection pathway. To achieve efficient electron injection from the excited dye to the CB of the TiO2 electrode, the LUMO energy level of the dye for type-I DSSC must be higher (more negative) than the energy level (Ecb) of the CB (−0.5 V versus the normal hydrogen electrode (NHE)) of the TiO2 electrode (some researchers have proposed that an energy gap of over 0.2–0.3 V is necessary for efficient electron injection).3–5 Almost all dye sensitizers developed so far for type-I DSSCs have carboxyl groups as anchoring groups, which are usually bound to the TiO2 electrode through the bidentate bridging linkage between the carboxyl group of the dye and the Brønsted acid site (surface-bound hydroxyl groups, Ti–OH) on the TiO2 surface (Fig. 2a). Ru complexes,1,9,10 porphyrin dyes,11–13 phthalocyanine dyes,14 and organic dyes3,4,6,15–17 bearing carboxyl groups have been known to inject electrons into the TiO2 electrode, according to the type-I pathway. Among dye sensitizers bearing carboxyl groups used so far in type-I DSSCs, donor–acceptor–π-conjugated (D–π–A) dyes, which are constructed of a diphenyl- or dialkylamino group as the electron donor (D) and a carboxyl group as the electron acceptor (A) and anchor linked by π-conjugated bridges, would be especially expected to be one of the most promising classes of organic dye sensitizers for type-I DSSCs because the wide variety of structures and the facile modification provide potential for molecular design, with the introduction of substituents onto the chromophore skeletons allowing easy control not only of their photophysical and electrochemical properties (the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energy levels), but also of their stereochemical structures. As a noteworthy structural feature of D–π–A dyes, the HOMO is localized over the π-conjugated system close to the D moiety, and the LUMO is localized over the A moiety.3,4,6 Therefore, in light of the D–π–A structure concept, the expansion of π-conjugation and the increase in the electron-donating and electron-accepting abilities of D moieties and A moieties, respectively, leads to the decrease in the energy gap between the HOMO and LUMO. Moreover, the photoabsorption properties of a D–π–A dye are associated with intramolecular charge transfer (ICT) excitation from the D to the A moiety of the dye, resulting in efficient electron transfer from the excited dye through the acceptor moiety (carboxyl group) into the CB of TiO2 electrode. Moreover, D–π–A dyes can facilitate the ICT and the subsequent charge separation of the D and A moieties in the excited dye, and rapidly inject electrons into the CB of TiO2 and effectively retard the charge recombination because the cationic charge on the D moiety of the excited dye is spatially separated by the π-conjugated bridge from the TiO2 surface with the injected electrons.
On the other hand, we have reported that a new type of D–π–A dye sensitizers bearing a pyridyl group as an electron-withdrawing anchoring group are predominantly adsorbed on the TiO2 electrode through the coordination bond between the pyridyl group of the dye and the Lewis acid site (exposed Tin+ cations) on the TiO2 surface (Fig. 2b).18,19 It was revealed that the new type of D–π–A dye sensitizers can inject electrons efficiently from the pyridyl group to the CB of the TiO2 electrode through the coordination bond, compared to the bidentate bridging linkages of conventional D–π–A dye sensitizers bearing a carboxyl group. We demonstrated that the pyridyl group is a promising candidate as not only an electron-withdrawing anchoring group but also an electron-injecting group for type-I D–π–A dye sensitizers, compared to the carboxyl groups. Recently, some researchers reported that type-I DSSCs based on porphyrin dyes and D–π–A dyes bearing pyridyl groups.20,21 Consequently, much effort has been made on the development of various types of D–π–A organic dye sensitizers for type-I DSSCs and there has been a gradual accumulation of information about the relationship between the chemical structures and the photovoltaic performances of type-I DSSCs. As a result, type-I DSSCs have achieved an η value of up to 13%.12c,d However, as mentioned above, to achieve electron injection from the excited dye to the CB of the TiO2 electrode for type-I DSSCs, the LUMO levels of the type-I dye sensitizers must be higher than in the CB of the TiO2 electrode. Thus, the restriction of the LUMO levels results in low light harvesting efficiency (LHE).
In contrast, the electron-injection pathway for type-II DSSCs is direct, that is, “one-step” electron injection from the ground state of the dye to the CB of TiO2 by photoexcitation of the dye-to-TiO2 charge transfer (DTCT) bands (Fig. 1b: i.e., an electron is injected directly from the HOMO of the dye into the CB of TiO2 electrode upon photoexcitation).4b Catechol (Cat) dyes, such as dopamine and fluorine, numerous natural pigments, such as bromopyrogallol red, and anthocyanins containing Cat moieties, which are classified as type-II dye sensitizers, show strong new absorption bands in the longer wavelength region corresponding to the DTCT upon binding to the TiO2 surface.22–29 The Cat dyes have been known to bind to the TiO2 surface through a bidentate mononuclear chelating linkage and/or a bidentate binuclear bridging linkage at the Brønsted acid site on TiO2 (Fig. 2c and d). The most advantageous aspect of type-II DSSCs over type-I DSSCs is their light-harvesting capabilities over the wide spectral range of sunlight because the direct electron injection in type-II DSSCs can lead to the creation of a broad DTCT band and the easing of restrictions on the LUMO levels of the dye sensitizers relative to type-I DSSCs with an indirect electron-injection pathway, although the absorption coefficient of the DTCT band for type-II dye sensitizers is much lower than in the absorption band based on the π → π* transition for conventional dye sensitizers due to lower overlap of the ground (molecular-localized) and the excited state (Ti-localized) wave functions.30a However, the photovoltaic performance of type-II DSSCs based on Cat dyes is significantly lower than in type-I DSSCs based on D–π–A organic dye sensitizers. Fundamental studies for the interfacial electron transfer dynamics of Cat dyes adsorbed on TiO2 nanoparticles have revealed that the back-electron transfer (charge recombination) of electrons injected into the TiO2 electrode for the type-II pathway takes place in a sub-picosecond timescale, which was significantly faster than in the type-I pathway (μs–ms). Thus, type-II Cat dyes have rarely been employed as sensitizers for DSSCs and thus there have been few attempts to develop type-II Cat dye sensitizers. In fact, the η values of type-II DSSCs based on Cat dyes had never exceeded 2.5%.30b In an attempt to overcome this problem, Yoon and co-workers showed that attaching electron-donating moieties to the Cat unit can lead to retardation of the back-electron-transfer rate and a dramatic increase in electron-injection efficiency, and a red-shift of the DTCT band caused by the increase in the donor strength of the dye.27a Their work inspired us to conceive the application of the molecular design of D–π–Cat dye sensitizers with strong electron donors, such as triphenylamine, indoline, and carbazole, which is one of the most promising strategies not only to inhibit back electron transfer, but also to enhance direct electron-injection efficiency in type-II DSSCs based on Cat dyes. Thus, to provide a direction in molecular design of Cat dye sensitizers for type-II DSSCs, in our previous work, we designed and synthesized a D–π–Cat dye, which shows a broad absorption band corresponding to the DTCT upon binding to TiO2 film.29a It was found that the photocurrent for the DSSC based on the D–π–Cat dye is mainly generated by a direct electron-injection pathway from the D–π–Cat dye to the CB of the TiO2 electrode, that is, type-II DSSCs. Moreover, we have demonstrated that the stabilization of the LUMO level and the expansion of the π-conjugated system by the introduction of a long π-bridge such as terthiophene on the Cat moiety can lead to an increase in the ICT excitation based on the π–π* transition with a decrease in the DTCT characteristics, resulting in enhancement of an indirect electron injection pathway from the excited dye to the CB of the TiO2 electrode by photoexcitation of the local band of the adsorbed dye on the TiO2 surface. Interestingly, Nagata et al. reported that a DSSC based on 2-anthroic acid exhibits the type-II pathway with an η value of 2.2%.30b Thus, the combination of the two injection (direct and indirect) mechanisms with some dye sensitizers has been investigated.24,30 To study the electronic injection mechanism in more depth and to generalize the main differences between the type-I and type-II pathways, Sanz and co-workers studied five organic dyes of increasing size, Cat, 2,3-naphthalenediol, alizarin, coumarin C343 having a carboxylic acid group, and coumarin derivative NKX-2311 having a cyanoacrylic acid group, and the electronic structures and optical response of the five dyes attached to the TiO2 electrode were analyzed and compared by using time-dependent density functional theory (TDDFT).24b From their analysis, NKX-2311 and Cat show purely indirect (type-I pathway) and direct (type-II pathway) electron-injection behavior, respectively. In the sequence from Cat to 2,3-naphthalenediol, C343, alizarin, and NKX-2311, the electron injection changes from a purely direct mechanism to a purely indirect mechanism. Alizarin and C343 represent intermediate behavior in which both injection regimes are present. Consequently, the electron injection mechanisms are related to the relative position of the LUMO energy of the dye to the edge of the CB of TiO2 electrode.
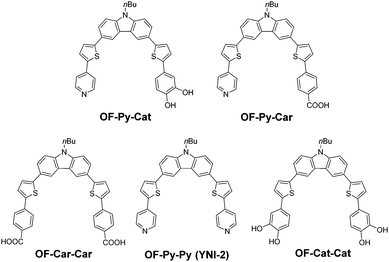
Therefore, on the basis of the useful knowledge about the molecular design of D–π–A dye sensitizers with a pyridyl group for type-I DSSC and D–π–Cat dye sensitizers with a Cat unit for type-II DSSC obtained from the our previous study,18,19,29 in this work, we have designed and developed a type-I/type-II hybrid dye sensitizer OF-Py-Cat with a pyridyl group and a catechol unit as the anchoring group possessing the ability to adsorb on the TiO2 electrode through both a coordination bond at the Lewis acid sites and a bidentate binuclear bridging linkage at the Brønsted acid sites on the TiO2 surface (Fig. 3). It would be expected that the type-I/type-II hybrid dye sensitizer makes it possible to inject electrons into the CB of the TiO2 electrode by the ICT excitation (type-I pathway) and by the photoexcitation of the DTCT band (type-II pathway). Furthermore, not only to gain insight into the influence of the molecular structure of type-I/type-II hybrid dye sensitizer on the appearance of ICT and DTCT bands and the electron-injection mechanism, but also to investigate the impacts of the ICT and DTCT characteristics of the type-I/type-II hybrid dye sensitizer on the photovoltaic performances of DSSCs, we also synthesized a type-I dye sensitizer OF-Car-Car with two carboxyl groups, a type-I dye sensitizer OF-Py-Car with a pyridyl group and a carboxyl group, a type-II dye sensitizer OF-Cat-Cat with two catechol units, as well as a type-I dye sensitizer OF-Py-Py (YNI-2) with two pyridyl groups.18d It was found that the type-I/type-II hybrid dye sensitizer adsorbed on TiO2 film exhibits a broad photoabsorption band originating from the ICT and DTCT characteristics. Here we reveal the photophysical and electrochemical properties of the type-I/type-II hybrid dye sensitizers bearing a pyridyl group and a Cat unit, along with their adsorption mode onto TiO2 film, and their photovoltaic performances in type-I/type-II DSSC, based on optical (photoabsorption and fluorescence spectroscopy) and electrochemical measurements (cyclic voltammetry), density functional theory (DFT) calculation, FT-IR spectroscopy of the dyes adsorbed on TiO2 film, photocurrent–voltage (I–V) curves, incident photon-to-current conversion efficiency (IPCE) spectra, and electrochemical impedance spectroscopy (EIS) for DSSC.
Results and discussion
Synthesis
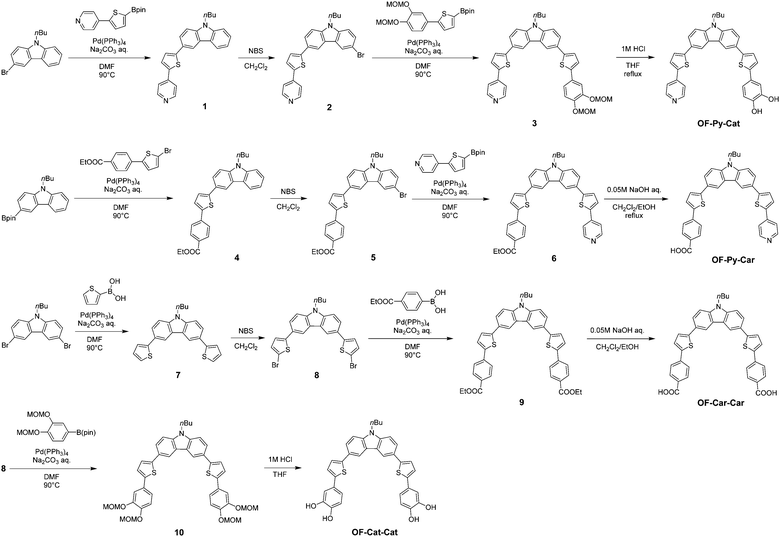
The type-I dye sensitizer OF-Py-Py (YNI-2)18d with two pyridyl groups was prepared by Stile coupling of 3,6-dibromo-9-butyl-9H-carbazole with 4-(5-tributylstannanyl-thiophen-2-yl)-pyridine. In this work, to develop a new synthetic route to D–π–A dye sensitizers with two anchoring groups, we adopted a synthetic strategy utilizing Suzuki coupling for the synthesis of the D–π–A dye sensitizers employed (Scheme 1). Indeed, the type-I/type-II hybrid dye sensitizer OF-Py-Cat and the type-I dye sensitizer OF-Py-Car with two different anchoring groups were successfully prepared by using Suzuki coupling twice to introduce the anchoring (electron acceptor) moiety, where 3-bromo-9-butyl-9H-carbazole31 and (9-butyl-9H-carbazol-3-yl)boronic acid pinacol ester,31 respectively, as starting materials were employed. Moreover, 3,6-bis(5-bromothiophen-2-yl)-9-butyl-9H-carbazole 8 was synthesized as a useful intermediate capable of introducing anchoring groups, that is, the corresponding anchoring unit was introduced by Suzuki coupling of 8 with 4-ethoxycarbonylphenylboronic acid and (3,4-bis(methoxymethoxy)phenyl)boronic acid pinacol ester to give 9 and 10, respectively, which is then treated with the appropriate alkali and acid, respectively, to provide the type-I dye sensitizer OF-Car-Car with two carboxyl groups and the type-II dye sensitizer OF-Cat-Cat with two catechol units.Photophysical properties in solution
The UV/vis absorption and fluorescence spectra of OF-Car-Car, OF-Py-Py, OF-Cat-Cat, OF-Py-Car and OF-Py-Cat in THF are shown in Fig. 4 and their spectral data are summarized in Table 1. The five dyes show a bimodal absorption band with peaks at around 350 and 380 nm (Fig. 4a). Interestingly, for OF-Car-Car, OF-Py-Py and OF-Py-Car with two electron-withdrawing anchoring groups the molar extinction coefficient (ε) of the absorption band at around 380 nm is higher than that of the absorption band at around 350 nm, but for OF-Cat-Cat and OF-Py-Cat with a catechol unit as the relatively electron-donating anchoring group, the ε of the absorption band at around 380 nm is lower than in the absorption band at around 350 nm. Moreover, the absorption band at around 380 nm for OF-Cat-Cat is blue-shifted by ca. 10 nm compared with that of OF-Car-Car, OF-Py-Py and OF-Py-Car. Thus, for the four dyes OF-Car-Car, OF-Py-Py, OF-Py-Car and OF-Py-Cat with an electron-withdrawing anchoring group except OF-Cat-Cat, this result shows that the intense absorption band at the longest wavelength corresponds to the intramolecular charge transfer (ICT) excitation from the carbazole moiety for OF-Car-Car, OF-Py-Py and OF-Py-Car and the carbazole containing a thienylcatechol moiety for OF-Py-Cat, respectively, to the electron-withdrawing anchoring group. The corresponding fluorescence spectra (Fig. 4b) show that only the dye OF-Cat-Cat exhibits a vibronic-structured fluorescence band (λem = 412 nm), which occurs at a shorter wavelength by ca. 35–55 nm than in the other four dyes (λfl = ca. 445–465 nm). Moreover, the dye OF-Cat-Cat (Φf = 0.16) exhibits a lower fluorescence quantum yield (Φf) than those of the other four dyes (Φf = ca. 0.5–0.7).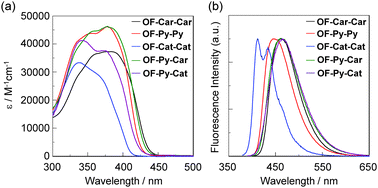 | ||
| Fig. 4 (a) UV/vis absorption and (b) fluorescence spectra of OF-Car-Car, OF-Py-Py, OF-Cat-Cat, OF-Py-Car and OF-Py-Cat in THF. | ||
| Dye | λ absmax/nm (ε/M−1 cm−1)a | λ fl/nm (Φf)b | E ox1/2 or Eoxonset/Vc | HOMO/Vd | LUMO/Vd | Molecules/cm−2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
J
sc/mA cm−2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f f |
V
oc/mV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f f |
ff![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f f |
η (%)f |
|---|---|---|---|---|---|---|---|---|---|---|
| a In 1,4-dioxane. b Fluorescence quantum yields (Φf) were determined by using a calibrated integrating sphere system (λex = 383, 378, 370, 378 and 373 nm for OF-Car-Car, OF-Py-Py, OF-Cat-Cat, OF-Py-Car, and OF-Py-Cat, respectively). c Half-wave potentials versus Fc/Fc+ of oxidation (Eox1/2) for OF-Py-Py were recorded in THF/Bu4NClO4 (0.1 M) solution. Onset (Eoxonset) versus Fc/Fc+ of the oxidation potential for OF-Car-Car, OF-Cat-Cat, OF-Py-Car, and OF-Py-Cat were recorded in DMF/Bu4NClO4 (0.1 M) solution. d Versus normal hydrogen electrode (NHE). e Adsorption amount of dye molecules per unit area of the TiO2 electrode, when the 9 μm thick TiO2 electrode was immersed into 0.1 mM dye solution in THF. f Under a simulated solar light (AM 1.5, 100 mW cm−2). | ||||||||||
| OF-Car-Car | 383 (37300) | 461 (0.56) | 0.55 | 1.27 | −1.63 | 9.1 × 1016 | 4.60 | 480 | 0.62 | 1.37 |
| OF-Py-Py | 378 (46100) | 448 (0.67) | 0.53 | 1.22 | −1.75 | 1.0 × 1017 | 5.88 | 542 | 0.64 | 2.04 |
| OF-Cat-Cat | 370 (27700) | 412 (0.16) | 0.28 | 1.00 | −2.12 | 9.2 × 1016 | 0.47 | 304 | 0.49 | 0.07 |
| OF-Py-Car | 378 (46200) | 464 (0.69) | 0.55 | 1.27 | −1.65 | 1.1 × 1017 | 5.28 | 485 | 0.62 | 1.51 |
| OF-Py-Cat | 373 (37600) | 465 (0.51) | 0.15 | 0.87 | −2.05 | 1.2 × 1017 | 0.40 | 299 | 0.48 | 0.06 |
Photophysical properties of dye-adsorbed TiO2 film
The photoabsorption spectra of the dyes adsorbed on the TiO2 film are shown in Fig. 5a. It is worth noting that in addition to the ICT band observed in THF, OF-Cat-Cat and OF-Py-Cat adsorbed on the TiO2 film show a broad absorption band corresponding to the DTCT upon binding to the TiO2 film, that is, the DTCT band appears in the region of 430 to 650 nm for OF-Cat-Cat and 450 to 650 nm for OF-Py-Cat. The red-shift of the absorption peak wavelengths for dyes with a catechol unit upon binding to the TiO2 film can be attributed to the stabilization of the LUMO level of the Ti–Cat dye complexes, which has been demonstrated by DFT calculations in our previous work.29a As shown in Fig. 5b, the colors of the dyes in THF are light yellow or yellow for OF-Car-Car, OF-Py-Py, OF-Py-Car and OF-Py-Cat, and light purple for OF-Cat-Cat. For OF-Car-Car, OF-Py-Py and OF-Py-Car, the colors of dyes adsorbed on the TiO2 film are similar to those in THF. In contrast, the colors of OF-Cat-Cat and OF-Py-Cat adsorbed on the TiO2 film are almost black and dark-orange, respectively, which indicates a decrease in the transition energy by the appearance of the DTCT band associated with the formation of the surface-bound Ti–Cat complex. Thus, this result reveals that the dye OF-Py-Cat adsorbed on the TiO2 film exhibits a broad photoabsorption band originating from the ICT and DTCT characteristics, that is, the dye OF-Py-Cat can act as a type-I/type-II hybrid dye sensitizer.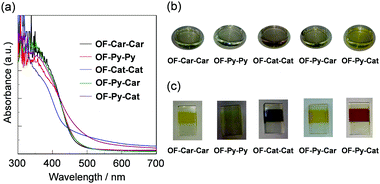 | ||
| Fig. 5 (a) Photoabsorption spectra of OF-Car-Car, OF-Py-Py, OF-Cat-Cat, OF-Py-Car and OF-Py-Cat adsorbed on TiO2 film. Photographs of the dyes (b) in THF and (c) adsorbed on TiO2 film. | ||
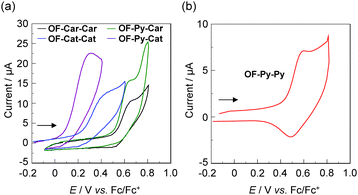
Electrochemical properties
The electrochemical properties of the five dyes were determined by cyclic voltammetry (CV) in DMF containing 0.1 M tetrabutylammonium perchlorate (Bu4NClO4) for OF-Car-Car, OF-Cat-Cat, OF-Py-Car and OF-Py-Cat and THF containing 0.1 M Bu4NClO4 for OF-Py-Py18d (Fig. 6). The potentials were referenced to ferrocene/ferrocenium (Fc/Fc+) as the internal reference. The oxidation waves for OF-Car-Car, OF-Py-Py and OF-Py-Car were observed at around 0.6 V versus Fc/Fc+, whereas the oxidation waves for OF-Cat-Cat and OF-Py-Cat were observed at around 0.4 V and 0.3 V, respectively, which are cathodically shifted by 0.2 V and 0.3 V, respectively, compared with those of OF-Car-Car, OF-Py-Py and OF-Py-Car. The corresponding reduction wave was clearly observed at 0.48 V for OF-Py-Py, whereas a clear reduction wave was not observed for OF-Car-Car, OF-Cat-Cat, OF-Py-Car and OF-Py-Cat. These results show that the redox process of OF-Py-Py is electrochemically reversible, but those of OF-Car-Car, OF-Cat-Cat, OF-Py-Car and OF-Py-Cat are electrochemically irreversible. The HOMO energy levels of the dyes were estimated from the onset (Eoxonset) of the oxidation potential for OF-Car-Car (0.55 V), OF-Cat-Cat (0.28 V), OF-Py-Car (0.55 V) and OF-Py-Cat (0.15 V), and the half-wave potential (Eox1/2 = 0.53 V) for OF-Py-Py (Table 1). The HOMO energy levels (versus NHE) of OF-Cat-Cat (1.00 V) and OF-Py-Cat (0.87 V) are higher than in OF-Car-Car, OF-Py-Py and OF-Py-Car (ca. 1.25 V). This result shows that the HOMO energy levels are more positive than the I3−/I− redox potential (0.4 V), and thus this ensures an efficient regeneration of the oxidized dyes by electron transfer from the I3−/I− redox couple in the electrolyte. The LUMO energy levels of the dyes were estimated from the Eoxonset or the Eox1/2 and an intersection of the UV/vis absorption and fluorescence spectra (428 nm; 2.90 eV for OF-Car-Car, 417 nm; 2.97 eV for OF-Py-Py, 398 nm; 3.12 eV for OF-Cat-Cat, 424 nm; 2.92 eV for OF-Py-Car and 418 nm; 2.97 eV for OF-Py-Cat). The LUMO energy levels of OF-Cat-Cat (−2.12 V) and OF-Py-Cat (−2.05 V) are also higher than in OF-Car-Car, OF-Py-Py and OF-Py-Car (ca. −1.70 V). Therefore, this result shows that from OF-Car-Car, OF-Py-Py and OF-Py-Car to OF-Cat-Cat, that is, by replacing the electron-withdrawing carboxyl and pyridyl groups with the electron-donating catechol unit as the anchoring group, the elevation of the LUMO energy level is larger than that of the HOMO energy level. Consequently, it was revealed that the blue-shift of the ICT absorption band for OF-Cat-Cat relative to OF-Car-Car, OF-Py-Py and OF-Py-Car is attributed to the destabilization of the LUMO energy level by the introduction of two catechol units with electron-donating ability, resulting in an increase in the HOMO–LUMO band gap. Evidently, the LUMO energy levels of the five dyes are higher than the Ecb of the CB of TiO2 (−0.5 V) electrode, suggesting that an electron injection from the photoexcited dye to the CB of TiO2 electrode for type-I DSSCs is thermodynamically feasible.Theoretical calculations
To examine the electronic structures of OF-Car-Car, OF-Py-Py, OF-Cat-Cat, OF-Py-Car and OF-Py-Cat, the molecular orbitals of these dyes were calculated using density functional theory (DFT) at the B3LYP/6-31G(d,p) level.33 The DFT calculations indicate that for OF-Car-Car, OF-Py-Py and OF-Py-Car the HOMOs are mostly localized on the carbazole moiety containing a thiophene ring (Fig. 7). The LUMOs for OF-Car-Car and OF-Py-Py are mostly localized on the two thienylbenzoic acid moieties and the two thienylpyridine moieties, respectively. On the other hand, for OF-Py-Car the LUMO is mostly localized on the thienylbenzoic acid moiety, and the LUMO+1 is mostly localized on the thienylpyridine moiety. Interestingly, for OF-Py-Cat the HOMO is mostly localized on the carbazole containing a thienylcatechol moiety and a thiophene ring, and the LUMO is mostly localized on the thienylpyridine moiety. Moreover, for OF-Cat-Cat the HOMO is delocalized over the whole molecule but the LUMO is mostly localized on the carbazole moiety containing the two thiophene rings. Accordingly, the DFT calculations reveal that for the four dyes with an electron-withdrawing anchoring group, except OF-Cat-Cat, the dye excitations upon light irradiation induce a strong ICT from the carbazole moiety to the electron-withdrawing anchoring groups.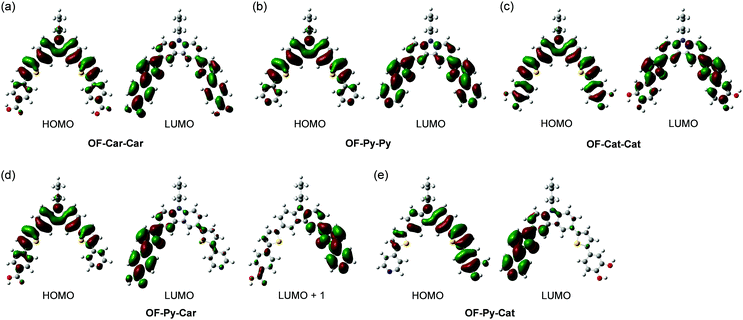 | ||
| Fig. 7 HOMO and LUMO of (a) OF-Car-Car, (b) OF-Py-Py, (c) OF-Cat-Cat, (d) OF-Py-Car and (e) OF-Py-Cat derived from the DFT calculations at the B3LYP/6-31G(d,p) level. | ||
FTIR spectra
To elucidate the adsorption states of the five dyes on TiO2 nanoparticles, we measured the FTIR spectra of the dye powders and the dyes adsorbed on TiO2 film. For the dye powders of OF-Car-Car and OF-Py-Car (Fig. 8a and d), the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band of the carboxyl group was observed at 1678 cm−1 for OF-Car-Car and 1695 cm−1 for OF-Py-Car. When the two dyes were adsorbed on the TiO2 surface, the C
O stretching band of the carboxyl group was observed at 1678 cm−1 for OF-Car-Car and 1695 cm−1 for OF-Py-Car. When the two dyes were adsorbed on the TiO2 surface, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching bands of the carboxyl group disappeared completely; this indicates the formation of a bidentate bridging linkage between the carboxyl group of the dye and the Brønsted acid site on the TiO2 surface. In addition, for the dye powder of OF-Py-Car the C
O stretching bands of the carboxyl group disappeared completely; this indicates the formation of a bidentate bridging linkage between the carboxyl group of the dye and the Brønsted acid site on the TiO2 surface. In addition, for the dye powder of OF-Py-Car the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching band of the pyridyl group was clearly observed at 1601 cm−1. In the FTIR spectrum of OF-Py-Car adsorbed on the TiO2 film, a new band appeared at around 1612 cm−1, which can be assigned to the pyridyl group coordinated to the Lewis acid sites of the TiO2 surface. As reported previously, the FTIR spectrum of OF-Py-Py adsorbed on TiO2 films demonstrated that the dye OF-Py-Py is predominantly adsorbed on the TiO2 surface through the coordination bond between the pyridyl group of the dye and the Lewis acid site on the TiO2 surface, that is, the fact is based on a new band at around 1614 cm−1 in the FTIR spectrum of OF-Py-Py adsorbed on the TiO2 film (Fig. 8b).18d Consequently, the dye OF-Py-Car with a pyridyl group and a carboxyl group was adsorbed on the TiO2 surface through both the bidentate bridging linkage between the carboxyl group of the dye and the Brønsted acid site of the TiO2 surface and the coordination bond between the pyridyl group of the dye and the Lewis acid site of the TiO2 surface. On the other hand, for the dye powders of OF-Cat-Cat and OF-Py-Cat (Fig. 8c and e), the stretching vibrations of the phenolic group (C–OH) for the catechol unit was observed at 1280, 1256 and 1242 cm−1 for OF-Cat-Cat and 1306, 1292, 1260 and 1242 cm−1 for OF-Py-Cat. In the FTIR spectra of dyes adsorbed on TiO2 film, the stretching vibrations merge to one prominent signal or lose their hyperfine structure. Thus, these observations indicate that the two dyes are adsorbed on the TiO2 surface through the formation of a bidentate mononuclear chelating linkage and/or a bidentate binuclear bridging linkage between the Cat unit of dye and the TiO2 surface.28 In addition, for the FTIR spectrum of OF-Py-Cat adsorbed on the TiO2 film, a new band appeared at around 1612 cm−1, which can be assigned to the pyridyl group coordinated to the Lewis acid sites of the TiO2 surface. Therefore, the dye OF-Py-Cat with a pyridyl group and a catechol unit was adsorbed on the TiO2 surface through both the bidentate linkage between the catechol unit of the dye and the Brønsted acid site of the TiO2 surface and the coordination bond between the pyridyl group of the dye and the Lewis acid site of the TiO2 surface.
N stretching band of the pyridyl group was clearly observed at 1601 cm−1. In the FTIR spectrum of OF-Py-Car adsorbed on the TiO2 film, a new band appeared at around 1612 cm−1, which can be assigned to the pyridyl group coordinated to the Lewis acid sites of the TiO2 surface. As reported previously, the FTIR spectrum of OF-Py-Py adsorbed on TiO2 films demonstrated that the dye OF-Py-Py is predominantly adsorbed on the TiO2 surface through the coordination bond between the pyridyl group of the dye and the Lewis acid site on the TiO2 surface, that is, the fact is based on a new band at around 1614 cm−1 in the FTIR spectrum of OF-Py-Py adsorbed on the TiO2 film (Fig. 8b).18d Consequently, the dye OF-Py-Car with a pyridyl group and a carboxyl group was adsorbed on the TiO2 surface through both the bidentate bridging linkage between the carboxyl group of the dye and the Brønsted acid site of the TiO2 surface and the coordination bond between the pyridyl group of the dye and the Lewis acid site of the TiO2 surface. On the other hand, for the dye powders of OF-Cat-Cat and OF-Py-Cat (Fig. 8c and e), the stretching vibrations of the phenolic group (C–OH) for the catechol unit was observed at 1280, 1256 and 1242 cm−1 for OF-Cat-Cat and 1306, 1292, 1260 and 1242 cm−1 for OF-Py-Cat. In the FTIR spectra of dyes adsorbed on TiO2 film, the stretching vibrations merge to one prominent signal or lose their hyperfine structure. Thus, these observations indicate that the two dyes are adsorbed on the TiO2 surface through the formation of a bidentate mononuclear chelating linkage and/or a bidentate binuclear bridging linkage between the Cat unit of dye and the TiO2 surface.28 In addition, for the FTIR spectrum of OF-Py-Cat adsorbed on the TiO2 film, a new band appeared at around 1612 cm−1, which can be assigned to the pyridyl group coordinated to the Lewis acid sites of the TiO2 surface. Therefore, the dye OF-Py-Cat with a pyridyl group and a catechol unit was adsorbed on the TiO2 surface through both the bidentate linkage between the catechol unit of the dye and the Brønsted acid site of the TiO2 surface and the coordination bond between the pyridyl group of the dye and the Lewis acid site of the TiO2 surface.
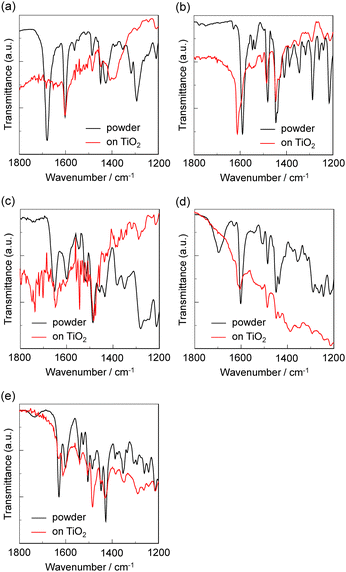 | ||
| Fig. 8 FTIR spectra of the powders and the dyes adsorbed on TiO2 nanoparticles. (a) OF-Car-Car, (b) OF-Py-Py, (c) OF-Cat-Cat, (d) OF-Py-Car and (e) OF-Py-Cat. | ||
Photovoltaic performances
The DSSCs based on OF-Car-Car, OF-Py-Py, OF-Cat-Cat, OF-Py-Car and OF-Py-Cat were fabricated by using the dye-adsorbed TiO2 electrode (9 mm), Pt-coated glass as a counter electrode, and an acetonitrile solution with iodine (0.05 M), lithium iodide (0.1 M), and 1,2-dimethyl-3-propylimidazolium iodide (0.6 M) as the electrolyte. The photocurrent–voltage (I–V) characteristics were measured under simulated solar light (AM 1.5, 100 mW cm−2). The IPCE spectra and the I–V curves are shown in Fig. 9. The photovoltaic performance parameters are collected in Table 1. It is worth mentioning here that the adsorption amount of all the five dyes bearing two anchoring groups adsorbed on the TiO2 electrode is ca. 1.0 × 1017 molecules per cm2, which is comparable to that of conventional dye sensitizers bearing one anchoring group. The IPCE spectra of the DSSCs based on these dyes demonstrated that the IPCE of OF-Car-Car, OF-Py-Py and OF-Py-Car are much higher than those of OF-Cat-Cat and OF-Py-Cat (Fig. 9a). Interestingly, the maximum IPCE values of OF-Py-Py (66% at 426 nm) and OF-Py-Car (66% at 428 nm) are somewhat higher than in OF-Car-Car (59% at 423 nm). For the DSSCs based on OF-Cat-Cat and OF-Py-Cat the DTCT bands make surprisingly little contribution to the IPCE spectra, that is, the IPCE originating from the DTCT band, which is below 10% in the region of 450 to 600 nm, is observed. The I–V curves show that the short circuit photocurrent density (Jsc) and η increase in the order of OF-Py-Cat (0.40 mA cm−2, 0.06%) ≈ OF-Cat-Cat (0.47 mA cm−2, 0.07%) < OF-Car-Car (4.60 mA cm−2, 1.37%) < OF-Py-Car (5.28 mA cm−2, 1.51%) < OF-Py-Py (5.88 mA cm−2, 2.04%) (Fig. 9b). Thus, this result demonstrates that the photovoltaic performances of DSSC based on OF-Py-Car and OF-Py-Py with at least a pyridyl group are higher than in OF-Car-Car with only carboxyl group, which is attributed to efficient electron injection from the pyridyl group to the CB of the TiO2 electrode through the coordination bond at the Lewis acid sites on the TiO2 surface, compared to the carboxyl group through the bidentate bridging linkages at the Brønsted acid sites on the TiO2 surface. On the other hand, the significantly low photovoltaic performance of DSSC based on OF-Cat-Cat with the two catechol units is due to the facilitation of the back-electron transfer from the electrons injected into TiO2 to the oxidized dye. Moreover, the most important finding to be emphasized is that the totally unexpected result for DSSC based on OF-Py-Cat with both a pyridyl group and a catechol unit may be due to significantly faster back-electron transfer, in spite of the efficient electron injection from the pyridyl group to the CB of the TiO2 electrode.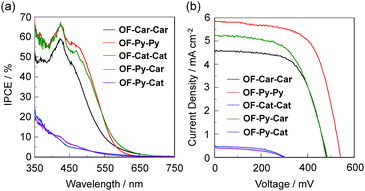 | ||
| Fig. 9 (a) IPCE spectra and (b) I–V curves of DSSCs based on OF-Car-Car, OF-Py-Py, OF-Cat-Cat, OF-Py-Car and OF-Py-Cat. | ||
On the other hand, it is also worth mentioning here that the open-circuit photovoltage (Voc) values of DSSC based on OF-Cat-Cat (304 mV) and OF-Py-Cat (299 mV) with a catechol unit are much lower than in OF-Car-Car (480 mV), OF-Py-Py (542 mV) and OF-Py-Car (485 mV). Thus, electrochemical impedance spectroscopy (EIS) analysis was performed to study the electron recombination process in DSSCs based on the dyes in the dark under a forward bias of −0.35 or −0.55 V with a frequency range of 1 Hz to 100 kHz. The large semicircle in the Nyquist plot (Fig. 10a), which corresponds to the midfrequency peaks in the Bode phase plots, represents the charge recombination between the injected electrons into the TiO2 electrode and I3− ions in the electrolyte, that is, the charge-transfer resistances at the TiO2/dye/electrolyte interface. The Nyquist plots show that the electron resistance (Rrec) value of the large semicircle for OF-Car-Car (64 Ω), OF-Py-Py (68 Ω) and OF-Py-Car (50 Ω) is higher than in OF-Cat-Cat (16 Ω) and OF-Py-Cat (20 Ω), indicating that the DSSCs with high Voc values have a tendency to exhibit a large Rrec value. The electron recombination lifetimes (τe), which are an expression of the electron recombination between the injected electrons into TiO2 and I3− ions in the electrolyte, are extracted from the angular frequency (ωre) at the mid-frequency peak in the Bode phase plot (Fig. 10b) using τe = 1/ωrec. The τe value (8 ms) for OF-Car-Car, OF-Py-Py and OF-Py-Car is higher than in OF-Cat-Cat (4 ms) and OF-Py-Cat (6 ms), that is, the τe values have the same tendency as the Rrec values. This result revealed that the charge recombination between the injected electrons into the TiO2 electrode and I3− ions in the electrolyte may be one of the major reasons for the difference in Voc value between the DSSC based on the five dyes, that is, the dyes OF-Car-Car, OF-Py-Py and OF-Py-Car can effectively retard the charge recombination compared with OF-Cat-Cat and OF-Py-Cat, but the decrease in the number of electrons injected into the CB of the TiO2 electrode results in a lower Voc value for the DSSC based on OF-Cat-Cat and OF-Py-Cat.3,4 Consequently, on the basis of the photophysical and electrochemical properties, the dye-adsorption modes onto TiO2 film and the photovoltaic performances for OF-Car-Car, OF-Py-Py, OF-Cat-Cat, OF-Py-Car and OF-Py-Cat, this work develops a new concept of the molecular design and synthesis of a type-I/type-II hybrid dye sensitizer capable of efficiently injecting electrons and with good light-harvesting features, although the photovoltaic performance of the DSSC based on the type-I/type-II hybrid dye sensitizer with a pyridyl group and a catechol unit that has been developed in this current stage is much lower than in DSSCs based on type-I dye sensitizers with two carboxyl groups, two pyridyl groups, or both a pyridyl group and a carboxyl group.
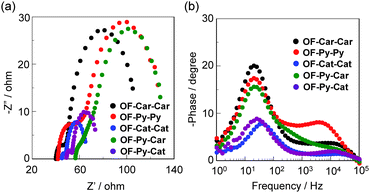 | ||
| Fig. 10 (a) Nyquist plots and (b) Bode phase plots of DSSCs based on OF-Car-Car, OF-Py-Py, OF-Cat-Cat, OF-Py-Car and OF-Py-Cat. | ||
Conclusions
Working toward the creation of a type-I/type-II hybrid dye sensitizer for dye-sensitized solar cells (DSSCs) that makes it possible to inject electron into the conduction band (CB) of the TiO2 electrode by intramolecular charge-transfer (ICT) excitation (type-I pathway) and by the photoexcitation of the dye-to-TiO2 charge transfer (DTCT) band (type-II pathway), we have designed and developed the dye sensitizer OF-Py-Cat with a pyridyl group and a catechol unit as the anchoring group possessing the ability to adsorb on the TiO2 electrode through both the coordination bond at the Lewis acid sites and the bidentate binuclear bridging linkage at the Brønsted acid sites on the TiO2 surface. Furthermore, not only to gain insight into the influence of the molecular structure of the type-I/type-II hybrid dye sensitizer on the appearance of the ICT and DTCT bands and the electron-injection mechanism, but also to investigate the impacts of the ICT and DTCT characteristics of type-I/type-II hybrid dye sensitizer on the photovoltaic performances of DSSCs, type-I dye sensitizer OF-Car-Car with two carboxyl groups, type-I dye sensitizer OF-Py-Py with two pyridyl groups, type-I dye sensitizer OF-Py-Car with a pyridyl group and a carboxyl group, and type-II dye sensitizer OF-Cat-Cat with two catechol units have been also synthesized. It was found that the dye OF-Py-Cat adsorbed on the TiO2 film exhibits a broad photoabsorption band originating from the ICT and DTCT characteristics, that is, the dye OF-Py-Cat can act as a type-I/type-II hybrid dye sensitizer. However, the photovoltaic performance of the type-I/type-II DSSC based on OF-Py-Cat is lower than in the type-I DSSC based on the dye sensitizers (OF-Car-Car, OF-Py-Py, and OF-Py-Car) with a carboxyl group or a pyridyl group, but is equivalent to that of type-II DSSC based on the dye sensitizer (OF-Cat-Cat) with a catechol unit. On the basis of the photophysical and electrochemical properties, the dye-adsorption modes onto TiO2 film and the photovoltaic performances for OF-Car-Car, OF-Py-Py, OF-Cat-Cat, OF-Py-Car and OF-Py-Cat, we revealed that the low photovoltaic performance of the DSSC based on OF-Py-Cat may be due to significantly faster back-electron transfer from the electrons injected into TiO2 electrode to the oxidized dye, in spite of the efficient electron injection from the pyridyl group to the CB of TiO2 electrode, which is the most important finding to be emphasized in this work. Consequently, this work develops a new concept of the molecular design and synthesis of a type-I/type-II hybrid dye sensitizer capable of efficiently injecting electrons and good light-harvesting feature, and thus in order to provide a direction in the molecular design of the high performance type-I/type-II hybrid dye sensitizer, further study to gain greater insight into the effects of the molecular structure of type-I/type-II hybrid dye sensitizer on the appearance of the ICT and DTCT characteristics, and the photovoltaic performances is now in progress by developing dye sensitizers bearing the substituted-pyridyl group and -catechol unit.Experimental
General
Melting points were measured with a Yanaco micro melting point apparatus MP model. IR spectra were recorded on a PerkinElmer Spectrum One FT-IR spectrometer by ATR method. High-resolution mass spectral data were acquired on a Thermo Fisher Scientific LTQ Orbitrap XL. High-resolution gas chromatography-time of flight mass spectral data (GC-TOFMS) were acquired on a JMS-T100 GCV 4G (JEOL). 1H NMR and 13C NMR spectra were recorded on a Varian-400 (400 MHz) or a Varian-500 (500 MHz) FT NMR spectrometer. Absorption spectra were observed with a Shimadzu UV-3150 spectrophotometer and fluorescence spectra were measured with a HORIBA FluoroMax-4 spectrofluorometer. The fluorescence quantum yields in solution were determined by a HORIBA FluoroMax-4 spectrofluorometer by using a calibrated integrating sphere system (λex = 383, 378, 370, 378 and 373 nm for OF-Car-Car, OF-Py-Py, OF-Cat-Cat, OF-Py-Car, and OF-Py-Cat, respectively). Cyclic voltammetry (CV) curves were recorded in THF/Bu4NClO4 (0.1 M) solution for OF-Py-Py and DMF/Bu4NClO4 (0.1 M) solution for OF-Car-Car and OF-Cat-Cat, OF-Py-Car, and OF-Py-Cat with a three-electrode system consisting of Ag/Ag+ as the reference electrode, a Pt plate as the working electrode, and Pt wire as the counter electrode by using an Electrochemical Measurement System HZ-7000 (HOKUTO DENKO). The HOMO and LUMO energy levels of OF-Car-Car and OF-Py-Py, OF-Cat-Cat, OF-Py-Car, and OF-Py-Cat were evaluated from the spectral analyses and the CV data (the HOMO energy level was evaluated from the Eoxonset of the oxidation potential or the Eox1/2). The LUMO energy level was estimated from the Eoxonset or the Eox1/2 and an intersection of absorption and fluorescence spectra (428 nm; 2.90 eV for OF-Car-Car, 417 nm; 2.97 eV for OF-Py-Py, 398 nm; 3.12 eV for OF-Cat-Cat, 424 nm; 2.92 eV for OF-Py-Car and 418 nm; 2.97 eV for OF-Py-Cat) in THF, that is, the LUMO energy level was obtained through the equation −[E0–0 − HOMO], where the E0–0 transition energy is an intersection of the absorption and fluorescence spectra corresponding to the energy gap between the HOMO and the LUMO. Electrochemical impedance spectroscopy (EIS) for DSSCs in the dark under a forward bias of −0.35 or −0.55 V with a frequency range of 1 Hz to 100 kHz was measured with an AMETEK Versa STAT 3.Synthesis
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1590, 1445, 1206 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.94 (t, J = 7.6 Hz, 3H), 1.40–1.43 (m, 2H), 1.86–1.90 (m, 2H), 4.47 (t, J = 6.8 Hz, 2H), 7.22–7.27 (m, 1H), 7.47–7.51 (m, 1H), 7.56 (d, J = 4.0 Hz, 1H), 7.60 (d, J = 8.2 Hz, 1H), 7.64–7.67 (m, 3H), 7.76 (d, J = 3.8 Hz, 1H), 7.85 (dd, J = 1.9 and 8.6 Hz, 1H), 8.25 (d, J = 7.4 Hz, 1H), 8.54 (d, J = 1.3 Hz, 1H), 8.58 (dd, J = 1.7 and 4.4 Hz, 2H) ppm; HRMS (ESI): m/z (%): [M + H+] calcd for C25H23N2S, 383.15765; found 383.15845.
= 1590, 1445, 1206 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.94 (t, J = 7.6 Hz, 3H), 1.40–1.43 (m, 2H), 1.86–1.90 (m, 2H), 4.47 (t, J = 6.8 Hz, 2H), 7.22–7.27 (m, 1H), 7.47–7.51 (m, 1H), 7.56 (d, J = 4.0 Hz, 1H), 7.60 (d, J = 8.2 Hz, 1H), 7.64–7.67 (m, 3H), 7.76 (d, J = 3.8 Hz, 1H), 7.85 (dd, J = 1.9 and 8.6 Hz, 1H), 8.25 (d, J = 7.4 Hz, 1H), 8.54 (d, J = 1.3 Hz, 1H), 8.58 (dd, J = 1.7 and 4.4 Hz, 2H) ppm; HRMS (ESI): m/z (%): [M + H+] calcd for C25H23N2S, 383.15765; found 383.15845.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1590, 1453, 1214 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.93 (t, J = 7.6 Hz, 3H), 1.34–1.40 (m, 2H), 1.79–1.85 (m, 2H), 4.27 (t, J = 7.2 Hz, 2H), 7.27 (d, J = 8.6 Hz, 1H), 7.33 (d, J = 3.8 Hz, 1H), 7.39 (d, J = 8.6 Hz, 1H), 7.47–7.51 (m, 3H), 7.54 (dd, J = 1.9 and 8.6 Hz, 1H), 7.74 (dd, J = 1.9 and 8.6 Hz, 1H), 8.23 (d, J = 1.7 Hz, 1H), 8.26 (d, J = 1.4 Hz, 1H), 8.58 (d, J = 4.4 Hz, 2H) ppm; HRMS (ESI): m/z (%): [M + H+] calcd for C25H32N2BrS, 461.06816; found 461.06839.
= 1590, 1453, 1214 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.93 (t, J = 7.6 Hz, 3H), 1.34–1.40 (m, 2H), 1.79–1.85 (m, 2H), 4.27 (t, J = 7.2 Hz, 2H), 7.27 (d, J = 8.6 Hz, 1H), 7.33 (d, J = 3.8 Hz, 1H), 7.39 (d, J = 8.6 Hz, 1H), 7.47–7.51 (m, 3H), 7.54 (dd, J = 1.9 and 8.6 Hz, 1H), 7.74 (dd, J = 1.9 and 8.6 Hz, 1H), 8.23 (d, J = 1.7 Hz, 1H), 8.26 (d, J = 1.4 Hz, 1H), 8.58 (d, J = 4.4 Hz, 2H) ppm; HRMS (ESI): m/z (%): [M + H+] calcd for C25H32N2BrS, 461.06816; found 461.06839.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1590, 1483, 1241, 1071 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.95 (t, J = 7.6 Hz, 3H), 1.39–1.48 (m, 2H), 1.87–1.95 (m, 2H), 3.49 (s, 3H), 3.53 (s, 3H), 4.50 (t, J = 7.5 Hz, 2H), 5.24 (s, 2H), 5.30 (s, 2H), 7.19 (d, J = 8.4 Hz, 1H), 7.33 (dd, J = 2.2 and 8.4 Hz, 1H), 7.38 (d, J = 4.0 Hz, 1H), 7.47–7.49 (m, 2H), 7.60 (d, J = 3.8 Hz, 1H), 7.65–7.69 (m, 4H), 7.78 (d, J = 3.8 Hz, 1H), 7.84–7.89 (m, 2H), 8.58 (dd, J = 1.6 and 4.4 Hz, 2H), 8.61 (d, J = 1.2 Hz, 1H), 8.68 (d, J = 1.4 Hz, 1H) ppm; HRMS (ESI): m/z (%): [M + H+] calcd for C39H37O4N2S2, 661.21893; found 661.21930.
= 1590, 1483, 1241, 1071 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.95 (t, J = 7.6 Hz, 3H), 1.39–1.48 (m, 2H), 1.87–1.95 (m, 2H), 3.49 (s, 3H), 3.53 (s, 3H), 4.50 (t, J = 7.5 Hz, 2H), 5.24 (s, 2H), 5.30 (s, 2H), 7.19 (d, J = 8.4 Hz, 1H), 7.33 (dd, J = 2.2 and 8.4 Hz, 1H), 7.38 (d, J = 4.0 Hz, 1H), 7.47–7.49 (m, 2H), 7.60 (d, J = 3.8 Hz, 1H), 7.65–7.69 (m, 4H), 7.78 (d, J = 3.8 Hz, 1H), 7.84–7.89 (m, 2H), 8.58 (dd, J = 1.6 and 4.4 Hz, 2H), 8.61 (d, J = 1.2 Hz, 1H), 8.68 (d, J = 1.4 Hz, 1H) ppm; HRMS (ESI): m/z (%): [M + H+] calcd for C39H37O4N2S2, 661.21893; found 661.21930.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1629, 1600, 1427 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.95 (t, J = 7.4 Hz, 3H), 1.39–1.48 (m, 2H), 1.87–1.89 (m, 2H, overlapping peak of residual proton in acetone-d6), 4.50 (t, J = 7.8 Hz, 2H), 6.89 (d, J = 8.2 Hz, 1H), 7.07 (dd, J = 2.2 and 8.2 Hz, 1H), 7.19 (d, J = 2.2 Hz, 1H), 7.26 (d, J = 3.8 Hz, 1H), 7.43 (d, J = 3.8 Hz, 1H), 7.60 (d, J = 3.8 Hz, 1H), 7.64–7.69 (m, 4H), 7.78 (d, J = 3.8 Hz, 1H), 7.83 (dd, J = 1.9 and 8.6 Hz, 1H), 7.87 (dd, J = 1.9 and 8.6 Hz, 1H), 8.58–8.59 (m, 3H), 8.67 (d, J = 1.6 Hz, 1H) ppm; HRMS (ESI): m/z (%): [M + H+] calcd for C35H29O2N2S2, 573.16650; found 573.16656.
= 1629, 1600, 1427 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.95 (t, J = 7.4 Hz, 3H), 1.39–1.48 (m, 2H), 1.87–1.89 (m, 2H, overlapping peak of residual proton in acetone-d6), 4.50 (t, J = 7.8 Hz, 2H), 6.89 (d, J = 8.2 Hz, 1H), 7.07 (dd, J = 2.2 and 8.2 Hz, 1H), 7.19 (d, J = 2.2 Hz, 1H), 7.26 (d, J = 3.8 Hz, 1H), 7.43 (d, J = 3.8 Hz, 1H), 7.60 (d, J = 3.8 Hz, 1H), 7.64–7.69 (m, 4H), 7.78 (d, J = 3.8 Hz, 1H), 7.83 (dd, J = 1.9 and 8.6 Hz, 1H), 7.87 (dd, J = 1.9 and 8.6 Hz, 1H), 8.58–8.59 (m, 3H), 8.67 (d, J = 1.6 Hz, 1H) ppm; HRMS (ESI): m/z (%): [M + H+] calcd for C35H29O2N2S2, 573.16650; found 573.16656.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1710, 1595, 1443, 1275 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.94 (t, J = 7.2 Hz, 3H), 1.36–1.45 (m, 5H), 1.86–1.90 (m, 2H), 4.36 (q, J = 7.2 Hz, 2H), 4.47 (t, J = 7.2 Hz, 2H), 7.22-7.26 (m, 1H), 7.47–7.51 (m, 1H), 7.54 (d, J = 3.8 Hz, 1H), 7.61 (d, J = 8.3 Hz, 1H), 7.64–7.66 (m, 2H), 7.83–7.86 (m, 3H), 8.05 (dd, J = 2.0 and 6.7 Hz, 2H), 8.25 (d, J = 7.5 Hz, 1H), 8.53 (d, J = 1.3 Hz, 1H) ppm; HRMS (ESI): m/z (%): [M + H+] calcd for C29H28O2NS, 454.18353; found 454.18335.
= 1710, 1595, 1443, 1275 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.94 (t, J = 7.2 Hz, 3H), 1.36–1.45 (m, 5H), 1.86–1.90 (m, 2H), 4.36 (q, J = 7.2 Hz, 2H), 4.47 (t, J = 7.2 Hz, 2H), 7.22-7.26 (m, 1H), 7.47–7.51 (m, 1H), 7.54 (d, J = 3.8 Hz, 1H), 7.61 (d, J = 8.3 Hz, 1H), 7.64–7.66 (m, 2H), 7.83–7.86 (m, 3H), 8.05 (dd, J = 2.0 and 6.7 Hz, 2H), 8.25 (d, J = 7.5 Hz, 1H), 8.53 (d, J = 1.3 Hz, 1H) ppm; HRMS (ESI): m/z (%): [M + H+] calcd for C29H28O2NS, 454.18353; found 454.18335.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1701, 1604, 1454, 1274 cm−1; 1H NMR (400 MHz, dichloromethane-d2) δ = 0.95 (t, J = 7.2 Hz, 3H), 1.35–1.42 (m, 5H), 1.81–1.88 (m, 2H), 4.29 (t, J = 7.2 Hz, 2H), 4.37 (q, J = 7.2 Hz, 2H), 7.33 (d, J = 8.6 Hz, 1H), 7.37 (d, J = 3.8 Hz, 1H), 7.44–7.47 (m, 2H), 7.57 (dd, J = 2.0 and 8.6 Hz, 1H), 7.71–7.74 (m, 2H), 7.79 (dd, J = 1.9 and 8.4 Hz, 1H), 8.03–8.06 (m, 2H), 8.25 (d, J = 2.4 Hz, 1H), 8.31 (d, J = 1.8 Hz, 1H) ppm; HRMS (APCI): m/z (%): [M+˙] calcd for C29H26O2NBrS, 531.08621; found 531.08643.
= 1701, 1604, 1454, 1274 cm−1; 1H NMR (400 MHz, dichloromethane-d2) δ = 0.95 (t, J = 7.2 Hz, 3H), 1.35–1.42 (m, 5H), 1.81–1.88 (m, 2H), 4.29 (t, J = 7.2 Hz, 2H), 4.37 (q, J = 7.2 Hz, 2H), 7.33 (d, J = 8.6 Hz, 1H), 7.37 (d, J = 3.8 Hz, 1H), 7.44–7.47 (m, 2H), 7.57 (dd, J = 2.0 and 8.6 Hz, 1H), 7.71–7.74 (m, 2H), 7.79 (dd, J = 1.9 and 8.4 Hz, 1H), 8.03–8.06 (m, 2H), 8.25 (d, J = 2.4 Hz, 1H), 8.31 (d, J = 1.8 Hz, 1H) ppm; HRMS (APCI): m/z (%): [M+˙] calcd for C29H26O2NBrS, 531.08621; found 531.08643.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1710, 1590, 1440, 1271 cm−1; 1H NMR (400 MHz, dichloromethane-d2) δ = 0.95 (t, J = 7.3 Hz, 3H), 1.38–1.45 (m, 5H), 1.86–1.93 (m, 2H), 4.33–4.40 (m, 4H), 7.42 (d, J = 3.7 Hz, 1H), 7.43 (d, J = 3.8 Hz, 1H), 7.47–7.50 (m, 2H), 7.53–7.54 (m, 2H), 7.58 (d, J = 3.8 Hz, 1H), 7.71–7.76 (m, 3H), 7.80–7.83 (m, 2H), 8.05–8.07 (m, 2H), 8.43–8.44 (m, 2H), 8.58 (d, J = 4.6 Hz, 2H) ppm; HRMS (ESI): m/z (%): [M + H+] calcd for C38H33O2N2S2, 613.19780; found 613.19824.
= 1710, 1590, 1440, 1271 cm−1; 1H NMR (400 MHz, dichloromethane-d2) δ = 0.95 (t, J = 7.3 Hz, 3H), 1.38–1.45 (m, 5H), 1.86–1.93 (m, 2H), 4.33–4.40 (m, 4H), 7.42 (d, J = 3.7 Hz, 1H), 7.43 (d, J = 3.8 Hz, 1H), 7.47–7.50 (m, 2H), 7.53–7.54 (m, 2H), 7.58 (d, J = 3.8 Hz, 1H), 7.71–7.76 (m, 3H), 7.80–7.83 (m, 2H), 8.05–8.07 (m, 2H), 8.43–8.44 (m, 2H), 8.58 (d, J = 4.6 Hz, 2H) ppm; HRMS (ESI): m/z (%): [M + H+] calcd for C38H33O2N2S2, 613.19780; found 613.19824.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1695, 1601, 1448, 1288 cm−1; 1H NMR (500 MHz, tetrahydrofuran-d8) δ = 0.96 (t, J = 7.0 Hz, 3H), 1.39–1.44 (m, 2H), 1.55–1.88 (m, 2H, overlapping peak of residual proton in tetrahydrofuran-d8), 4.44 (t, J = 7.0 Hz, 2H), 7.48 (d, J = 3.9 Hz, 1H), 7.51 (d, J = 3.9 Hz, 1H), 7.56–7.58 (m, 4H), 7.68 (d, J = 4.0 Hz, 1H), 7.74–7.79 (m, 3H), 7.81–7.84 (m, 2H), 8.03–8.05 (m, 2H), 8.54–8.56 (m, 4H) ppm; HRMS (ESI): m/z (%): [M + H+] calcd for C36H29O2N2S2, 585.16650; found 585.16685.
= 1695, 1601, 1448, 1288 cm−1; 1H NMR (500 MHz, tetrahydrofuran-d8) δ = 0.96 (t, J = 7.0 Hz, 3H), 1.39–1.44 (m, 2H), 1.55–1.88 (m, 2H, overlapping peak of residual proton in tetrahydrofuran-d8), 4.44 (t, J = 7.0 Hz, 2H), 7.48 (d, J = 3.9 Hz, 1H), 7.51 (d, J = 3.9 Hz, 1H), 7.56–7.58 (m, 4H), 7.68 (d, J = 4.0 Hz, 1H), 7.74–7.79 (m, 3H), 7.81–7.84 (m, 2H), 8.03–8.05 (m, 2H), 8.54–8.56 (m, 4H) ppm; HRMS (ESI): m/z (%): [M + H+] calcd for C36H29O2N2S2, 585.16650; found 585.16685.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane 1
hexane 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as the eluent) to give 7 (1.6 g, yield 40%) as a white solid; 1H NMR (400 MHz, acetone-d6) δ = 0.94 (t, J = 7.2 Hz, 3H), 1.39–1.45 (m, 2H), 1.85–1.93 (m, 2H), 4.47 (t, J = 7.2 Hz, 2H), 7.13 (dd, J = 3.5 and 5.1 Hz, 2H), 7.39 (dd, J = 1.2 and 5.1 Hz, 2H), 7.48 (d, J = 1.2 and 3.5 Hz, 2H), 7.62 (d, J = 8.6 Hz, 2H), 7.79 (dd, J = 1.9 and 8.6 Hz, 2H), 8.56 (d, J = 1.4 Hz, 2H) ppm.
1 as the eluent) to give 7 (1.6 g, yield 40%) as a white solid; 1H NMR (400 MHz, acetone-d6) δ = 0.94 (t, J = 7.2 Hz, 3H), 1.39–1.45 (m, 2H), 1.85–1.93 (m, 2H), 4.47 (t, J = 7.2 Hz, 2H), 7.13 (dd, J = 3.5 and 5.1 Hz, 2H), 7.39 (dd, J = 1.2 and 5.1 Hz, 2H), 7.48 (d, J = 1.2 and 3.5 Hz, 2H), 7.62 (d, J = 8.6 Hz, 2H), 7.79 (dd, J = 1.9 and 8.6 Hz, 2H), 8.56 (d, J = 1.4 Hz, 2H) ppm.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1601, 1482, 1430 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.93 (t, J = 7.3 Hz, 3H), 1.37–1.44 (m, 2H), 1.84–1.90 (m, 2H), 4.47 (t, J = 7.2 Hz, 2H), 7.18 (d, J = 3.9 Hz, 2H), 7.30 (d, J = 3.9 Hz, 2H), 7.64 (d, J = 8.4 Hz, 2H), 7.74 (dd, J = 1.8 and 8.4 Hz, 2H), 8.52 (d, J = 1.3 Hz, 2H) ppm; HRMS (APCI): m/z (%): [M + H+] calcd for C24H20NBr2S2, 545.93780; found 545.93811.
= 1601, 1482, 1430 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.93 (t, J = 7.3 Hz, 3H), 1.37–1.44 (m, 2H), 1.84–1.90 (m, 2H), 4.47 (t, J = 7.2 Hz, 2H), 7.18 (d, J = 3.9 Hz, 2H), 7.30 (d, J = 3.9 Hz, 2H), 7.64 (d, J = 8.4 Hz, 2H), 7.74 (dd, J = 1.8 and 8.4 Hz, 2H), 8.52 (d, J = 1.3 Hz, 2H) ppm; HRMS (APCI): m/z (%): [M + H+] calcd for C24H20NBr2S2, 545.93780; found 545.93811.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane 3
hexane 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as eluent) to give 9 (0.65 g, yield 50%) as a yellow solid; m.p. 172–174 °C; IR (ATR):
1 as eluent) to give 9 (0.65 g, yield 50%) as a yellow solid; m.p. 172–174 °C; IR (ATR): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1705, 1601, 1444, 1270 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.96 (t, J = 7.6 Hz, 3H), 1.36–1.47 (m, 8H), 1.88–1.93 (m, 2H), 4.36 (q, J = 7.2 Hz, 4H), 4.51 (t, J = 7.2 Hz, 2H), 7.58 (d, J = 3.8 Hz, 2H), 7.67–7.70 (m, 4H), 7.85–7.90 (m, 6H), 8.05–8.08 (m, 4H), 8.67 (d, J = 1.3 Hz, 2H) ppm; HRMS (APCI): m/z (%): [M + H+] calcd for C42H38O4NS2, 684.22368; found 684.22290.
= 1705, 1601, 1444, 1270 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.96 (t, J = 7.6 Hz, 3H), 1.36–1.47 (m, 8H), 1.88–1.93 (m, 2H), 4.36 (q, J = 7.2 Hz, 4H), 4.51 (t, J = 7.2 Hz, 2H), 7.58 (d, J = 3.8 Hz, 2H), 7.67–7.70 (m, 4H), 7.85–7.90 (m, 6H), 8.05–8.08 (m, 4H), 8.67 (d, J = 1.3 Hz, 2H) ppm; HRMS (APCI): m/z (%): [M + H+] calcd for C42H38O4NS2, 684.22368; found 684.22290.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1678, 1601, 1425, 1292 cm−1; 1H NMR (400 MHz, tetrahydrofuran-d8) δ = 0.96 (t, J = 7.5 Hz, 3H), 1.36–1.48 (m, 2H), 1.56–1.89 (m, 2H, overlapping peak of residual proton in tetrahydrofuran-d8), 4.43 (t, J = 7.0 Hz, 2H), 7.48 (d, J = 3.5 Hz, 2H), 7.56–7.58 (m, 4H), 7.78–7.83 (m, 6H), 8.04 (d, J = 8.5 Hz, 4H), 8.55 (d, J = 1.5 Hz, 2H) ppm; 13C NMR (125 MHz, tetrahydrofuran-d8) δ = 14.03, 21.09, 31.96, 43.01, 110.29, 118.26, 123.89, 124.13, 124.76, 125.29, 126.42, 127.65, 130.07, 130.90, 131.07, 139.24, 141.59, 147.19, 167.01 ppm; TOFMS (FD): m/z (%): [M+˙] calcd for C38H29O4NS2, 627.15380; found 627.15430.
= 1678, 1601, 1425, 1292 cm−1; 1H NMR (400 MHz, tetrahydrofuran-d8) δ = 0.96 (t, J = 7.5 Hz, 3H), 1.36–1.48 (m, 2H), 1.56–1.89 (m, 2H, overlapping peak of residual proton in tetrahydrofuran-d8), 4.43 (t, J = 7.0 Hz, 2H), 7.48 (d, J = 3.5 Hz, 2H), 7.56–7.58 (m, 4H), 7.78–7.83 (m, 6H), 8.04 (d, J = 8.5 Hz, 4H), 8.55 (d, J = 1.5 Hz, 2H) ppm; 13C NMR (125 MHz, tetrahydrofuran-d8) δ = 14.03, 21.09, 31.96, 43.01, 110.29, 118.26, 123.89, 124.13, 124.76, 125.29, 126.42, 127.65, 130.07, 130.90, 131.07, 139.24, 141.59, 147.19, 167.01 ppm; TOFMS (FD): m/z (%): [M+˙] calcd for C38H29O4NS2, 627.15380; found 627.15430.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane 1
hexane 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as eluent) and then recycling GPC (toluene as eluent) was performed to give 10 (0.15 g, yield 52%) as a yellow solid; m.p. 41–43 °C; IR (ATR):
1 as eluent) and then recycling GPC (toluene as eluent) was performed to give 10 (0.15 g, yield 52%) as a yellow solid; m.p. 41–43 °C; IR (ATR): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1600, 1484, 1241, 1151, 1071 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.82 (t, J = 7.2 Hz, 3H), 1.23–1.29 (m, 2H), 1.70–1.75 (m, 2H), 3.48 (s, 6H), 3.51 (s, 6H), 4.23 (t, J = 7.8 Hz, 2H), 5.21 (s, 4H), 5.28 (s, 4H), 7.14 (d, J = 8.4 Hz, 2H), 7.25–7.29 (m, 4H), 7.37 (d, J = 3.7 Hz, 2H), 7.41 (d, J = 8.6 Hz, 2H), 7.48 (d, J = 2.2 Hz, 2H), 7.70 (dd, J = 1.8 and 8.6 Hz, 2H), 8.53 (d, J = 1.8 Hz, 2H) ppm; HRMS (ESI): m/z (%): [M + Na+] calcd for C44H45O8NS2Na, 802.24788; found 802.24664.
= 1600, 1484, 1241, 1151, 1071 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 0.82 (t, J = 7.2 Hz, 3H), 1.23–1.29 (m, 2H), 1.70–1.75 (m, 2H), 3.48 (s, 6H), 3.51 (s, 6H), 4.23 (t, J = 7.8 Hz, 2H), 5.21 (s, 4H), 5.28 (s, 4H), 7.14 (d, J = 8.4 Hz, 2H), 7.25–7.29 (m, 4H), 7.37 (d, J = 3.7 Hz, 2H), 7.41 (d, J = 8.6 Hz, 2H), 7.48 (d, J = 2.2 Hz, 2H), 7.70 (dd, J = 1.8 and 8.6 Hz, 2H), 8.53 (d, J = 1.8 Hz, 2H) ppm; HRMS (ESI): m/z (%): [M + Na+] calcd for C44H45O8NS2Na, 802.24788; found 802.24664.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1651, 1599, 1486 cm−1; 1H NMR (500 MHz, acetonitrile-d3) δ = 0.94 (t, J = 7.5 Hz, 3H), 1.36–1.41 (m, 2H), 1.79–2.00 (m, 2H, overlapping peak of residual proton in acetonitrile-d3), 4.39 (t, J = 7.0 Hz, 2H), 6.87 (d, J = 8.0 Hz, 2H), 7.09 (dd, J = 2.5 and 8.0 Hz, 2H), 7.17 (d, J = 2.5 Hz, 2H), 7.26 (d, J = 3.5 Hz, 2H), 7.39 (d, J = 3.5 Hz, 2H), 7.57 (d, J = 8.5 Hz, 2H), 7.80 (dd, J = 1.5 and 8.5 Hz, 2H), 8.49 (d, J = 2.0 Hz, 2H) ppm; TOFMS (FD): m/z (%): [M+˙] calcd for C36H29O4NS2, 603.15380; found 603.15521.
= 1651, 1599, 1486 cm−1; 1H NMR (500 MHz, acetonitrile-d3) δ = 0.94 (t, J = 7.5 Hz, 3H), 1.36–1.41 (m, 2H), 1.79–2.00 (m, 2H, overlapping peak of residual proton in acetonitrile-d3), 4.39 (t, J = 7.0 Hz, 2H), 6.87 (d, J = 8.0 Hz, 2H), 7.09 (dd, J = 2.5 and 8.0 Hz, 2H), 7.17 (d, J = 2.5 Hz, 2H), 7.26 (d, J = 3.5 Hz, 2H), 7.39 (d, J = 3.5 Hz, 2H), 7.57 (d, J = 8.5 Hz, 2H), 7.80 (dd, J = 1.5 and 8.5 Hz, 2H), 8.49 (d, J = 2.0 Hz, 2H) ppm; TOFMS (FD): m/z (%): [M+˙] calcd for C36H29O4NS2, 603.15380; found 603.15521.
Preparation of DSSCs
The TiO2 paste (JGC Catalysts and Chemicals Ltd, PST-18NR) was deposited on a fluorine-doped-tin-oxide (FTO) substrate by doctor-blading, and sintered for 50 min at 450 °C. The 9 μm thick TiO2 electrode was immersed into 0.1 mM dye solution in THF for 15 hours, which was enough to adsorb the dye sensitizers. The DSSCs were fabricated by using the TiO2 electrode (0.5 × 0.5 cm2 in the photoactive area) thus prepared, Pt-coated glass as the counter electrode, and a solution of 0.05 M iodine, 0.1 M lithium iodide, and 0.6 M 1,2-dimethyl-3-propylimidazolium iodide in acetonitrile as the electrolyte. The photocurrent–voltage characteristics were measured using a potentiostat under simulated solar light (AM 1.5, 100 mW cm−2). IPCE spectra were measured under monochromatic irradiation with a tungsten-halogen lamp and a monochromator. The amount of dye molecules adsorbed on TiO2 film was determined from the calibration curve by absorption spectral measurement of the concentration change of the dye solution before and after adsorption. The quantification of dye was made based on the molar extinction coefficient for λabsmax of dye in the above solution. Absorption spectra of the dyes adsorbed on TiO2 film were recorded on the dyes-adsorbed TiO2 film in the transmission mode with a calibrated integrating sphere system.Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 15H03859, by Matching Planner Program (MP27115659061) from the Japan Science and Technology Agency (JST) and by the Yazaki Memorial Foundation for Science and Technology.Notes and references
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef.
- A. Mishra, M. K. R. Fischer and P. Bäuerle, Angew. Chem., Int. Ed., 2009, 48, 2474 CrossRef CAS PubMed.
- (a) Z. Ning and H. Tian, Chem. Commun., 2009, 5483 RSC; (b) Z. Ning, Y. Fu and H. Tian, Energy Environ. Sci., 2010, 3, 1170 RSC.
- (a) Y. Ooyama and Y. Harima, Eur. J. Org. Chem., 2009, 2903 CrossRef CAS; (b) Y. Ooyama and Y. Harima, ChemPhysChem, 2012, 13, 4032 CrossRef CAS PubMed.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595 CrossRef CAS PubMed.
- N. Manfredi, B. Cecconi and A. Abbotto, Eur. J. Org. Chem., 2014, 7069 CrossRef CAS.
- L. Zhang and J. M. Cole, ACS Appl. Mater. Interfaces, 2015, 7, 3427 CAS.
- C.-P. Lee, R. Y.-Y. Lin, L.-Y. Lin, C.-T. Li, T.-C. Chu, S.-S. Sun, J. T. Lin and K.-C. Ho, RSC Adv., 2015, 5, 23810 RSC.
- B. Pashaei, H. Shahroosvand, M. Graetzel and M. K. Nazeeruddin, Chem. Rev., 2016, 116, 9485 CrossRef CAS PubMed.
- (a) K.-L. Wu, A. J. Huckaba, J. N. Clifford, Y.-W. Yang, A. Yella and E. Palomares, Inorg. Chem., 2016, 55, 7388 CrossRef CAS PubMed; (b) H. Ozawa, T. Sugiura, T. Kuroda, K. Nozawa and H. Arakawa, J. Mater. Chem. A, 2016, 4, 1762 RSC.
- (a) H. Imahori, T. Umeyama and S. Ito, Acc. Chem. Res., 2009, 42, 1809 CrossRef CAS PubMed; (b) L.-L. Li and E. W.-G. Diau, Chem. Soc. Rev., 2013, 42, 291 RSC; (c) K. Ladomenou, T. N. Kitsopoulos, G. D. Sharma and A. G. Coutsolelos, RSC Adv., 2014, 4, 21379 RSC; (d) T. Higashino and H. Imahori, Dalton Trans., 2015, 44, 448 RSC.
- (a) T. Bessho, S. M. Zakeeruddin, C.-Y. Yeh, E. W.-G. Diau and M. Grätzel, Angew. Chem., Int. Ed., 2010, 49, 6646 CrossRef CAS PubMed; (b) A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629 CrossRef CAS PubMed; (c) S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, Md. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242 CrossRef CAS PubMed; (d) A. Yella, C.-L. Mai, S. M. Zakeeruddin, S.-N. Chang, C.-H. Hsieh, C.-Y. Yeh and M. Grätzel, Angew. Chem., Int. Ed., 2014, 53, 2973 CrossRef CAS PubMed; (e) T. Higashino, Y. Fujimori, K. Sugiura, Y. Tsuji, S. Ito and H. Imahori, Angew. Chem., Int. Ed., 2015, 54, 9052 CrossRef CAS PubMed; (f) J. P. Hill, Angew. Chem., Int. Ed., 2016, 55, 2976 CrossRef CAS PubMed; (g) F. Lodermeyer, R. D. Costa, J. Malig, N. Jux and D. M. Guldi, Chem. – Eur. J., 2016, 22, 7851 CrossRef CAS PubMed; (h) G. Copley, D. Hwang, D. Kim and A. Osuka, Angew. Chem., Int. Ed., 2016, 55, 10287 CrossRef CAS PubMed.
- (a) T. Zhang, X. Qian, P. Zhang, Y.-Z. Zhu and J.-Y. Zheng, Chem. Commun., 2015, 51, 3782 RSC; (b) Y. Xie, Y. Tang, W. Wu, Y. Wang, J. Liu, X. Li, H. Tian and W.-H. Zhu, J. Am. Chem. Soc., 2015, 137, 14055 CrossRef CAS PubMed; (c) K. Sirithip, N. Prachumrak, R. Rattanawan, T. Keawin, T. Sudyoadsuk, S. Namuangruk, S. Jungsuttiwong and V. Promarak, Chem. – Asian J., 2015, 10, 882 CrossRef CAS PubMed; (d) S. Mathew, N. A. Astani, B. F. E. Curchod, J. H. Delcamp, M. Marszalek, J. Frey, U. Rothlisberger, M. K. Nazeeruddina and M. Grätzel, J. Mater. Chem. A, 2016, 4, 2332 RSC; (e) L. Zeininger, F. Lodermeyer, R. D. Costa, D. M. Guldi and A. Hirsch, Chem. Commun., 2016, 52, 8842 RSC; (f) J. Luo, J. Zhang, K.-W. Huang, Q. Qi, S. Dong, J. Zhang, P. Wang and J. Wu, J. Mater. Chem. A, 2016, 4, 8428 RSC; (g) C. Li, L. Luo, D. Wu, R. Jiang, J. Lan, R. Wang, L. Huang, S. Yang and J. You, J. Mater. Chem. A, 2016, 4, 11829 RSC; (h) Y.-C. Liu, H.-H. Chou, F.-Y. Ho, H.-J. Wei, T.-C. Wei and C.-Y. Yeh, J. Mater. Chem. A, 2016, 4, 11878 RSC.
- (a) J.-J. Cid, J.-H. Yum, S.-R. Jang, M. K. Nazeeruddin, E. Martínez-Ferrero, E. Palomares, J. Ko, M. Grätzel and T. Torres, Angew. Chem., Int. Ed., 2007, 46, 8358 CrossRef CAS PubMed; (b) M. Kimura, H. Nomoto, N. Masaki and S. Mori, Angew. Chem., Int. Ed., 2012, 51, 4371 CrossRef CAS PubMed; (c) M.-E. Ragoussi, J.-J. Cid, J.-H. Yum, G. De La Torre, D. D. Censo, M. Grätzel, M. K. Nazeeruddin and T. Torres, Angew. Chem., Int. Ed., 2012, 51, 4375 CrossRef CAS PubMed; (d) L. Yu, K. Fan, T. Duan, X. Chen, R. Li and T. Peng, ACS Sustainable Chem. Eng., 2014, 2, 718 CrossRef CAS; (e) T. Ikeuchi, H. Nomoto, N. Masaki, M. J. Griffith, S. Mori and M. Kimura, Chem. Commun., 2014, 50, 1941 RSC; (f) T. Ikeuchi, S. Agrawal, M. Ezoe, S. Mori and M. Kimura, Chem. – Asian J., 2015, 10, 2347 CrossRef CAS PubMed; (g) L. Tejerina, M. V. Martínez-Díaz, M. K. Nazeeruddin and T. Torres, Chem. – Eur. J., 2016, 22, 4369 CrossRef CAS PubMed.
- (a) X. Wang, J. Yang, H. Yu, F. Li, L. Fan, W. Sun, Y. Liu, Z. Y. Koh, J. Pan, W.-L. Yim, L. Yan and Q. Wang, Chem. Commun., 2014, 50, 3965 RSC; (b) S.-G. Li, K.-J. Jiang, J.-H. Huang, L.-M. Yang and Y.-L. Song, Chem. Commun., 2014, 50, 4309 RSC; (c) D. K. Panda, F. S. Goodson, S. Ray and S. Saha, Chem. Commun., 2014, 50, 5358 RSC; (d) K. Kakiage, Y. Aoyama, T. Yano, T. Otsuka, T. Kyomen, M. Unno and M. Hanaya, Chem. Commun., 2014, 50, 6379 RSC; (e) A. Amacher, C. Yi, J. Yang, M. P. Bircher, Y. Fu, M. Cascella, M. Grätzel, S. Decurtins and S.-X. Liu, Chem. Commun., 2014, 50, 6540 RSC; (f) W.-I. Hung, Y.-Y. Liao, T.-H. Lee, Y.-C. Ting, J.-S. Ni, W.-S. Kao, J. T. Lin, T.-C. Wei and Y.-S. Yen, Chem. Commun., 2015, 51, 2152 RSC; (g) X. Li, Z. Zheng, W. Jiang, W. Wu, Z. Wang and H. Tian, Chem. Commun., 2015, 51, 3590 RSC; (h) K. Kakiage, Y.i Aoyama, T. Yano, K. Oya, T. Kyomen and M. Hanaya, Chem. Commun., 2015, 51, 6315 RSC; (i) L. Yang, Z. Zheng, Y. Li, W. Wu, H. Tian and Z. Wang, Chem. Commun., 2015, 51, 4842 RSC; (j) N. Shibayama, Y. Inoue, M. Abe, S. Kajiyama, H. Ozawa, H. Miura and H. Arakawa, Chem. Commun., 2015, 51, 12795 RSC; (k) K. Kakiage, Y. Aoyama, T. Yano, K. Oya, J. Fujisawa and M. Hanaya, Chem. Commun., 2015, 51, 15894 RSC.
- (a) J.-S. Ni, Y.-C. Yen and J. T. Lin, Chem. Commun., 2015, 51, 17080 RSC; (b) Y.-D. Lin, B.-Y. Ke, Y. J. Chang, P.-T. Chou, K.-L. Liau, C.-Y. Liu and T. J. Chow, J. Mater. Chem. A, 2015, 3, 16831 RSC; (c) S. S. Soni, K. B. Fadadu, J. V. Vaghasiya, B. G. Solanki, K. K. Sonigara, A. Singh, D. Das and P. K. Iyer, J. Mater. Chem. A, 2015, 3, 21664 RSC; (d) X. Li, Y. Hu, I. Sanchez-Molina, Y. Zhou, F. Yu, S. A. Haque, W. Wu, J. Hua, H. Tian and N. Robertson, J. Mater. Chem. A, 2015, 3, 2173 Search PubMed; (e) Y. Hu, A. Ivaturi, M. Planells, C. L. Boldrini, A. O. Biroli and N. Robertson, J. Mater. Chem. A, 2016, 4, 2509 RSC; (f) H. Wu, L. Yang, Y. Li, M. Zhang, J. Zhang, Y. Guo and P. Wang, J. Mater. Chem. A, 2016, 4, 519 RSC; (g) J. Wu, G. Li, L. Zhang, G. Zhou and Z.-S. Wang, J. Mater. Chem. A, 2016, 4, 3342 RSC; (h) J.-S. Ni, Y.-C. Yen and J. T. Lin, J. Mater. Chem. A, 2016, 4, 6553 RSC; (i) A. J. Huckaba, A. Yella, P. Brogdon, J. S. Murphy, M. K. Nazeeruddin, M. Grätzel and J. H. Delcamp, Chem. Commun., 2016, 52, 8424 RSC; (j) T.-Y. Li, C. Su, S. B. Akula, W.-G. Sun, H.-M. Chien and W.-R. Li, Org. Lett., 2016, 18, 3386 CrossRef CAS PubMed; (k) Y. Gao, X. Li, Y. Hu, Y. Fan, J. Yuan, N. Robertson, J. Hua and S. R. Marder, J. Mater. Chem. A, 2016, 4, 12865 RSC.
- (a) Z. Yao, M. Zhang, H. Wu, L. Yang, R. Li and P. Wang, J. Am. Chem. Soc., 2015, 137, 3799 CrossRef CAS PubMed; (b) N. Zhou, K. Prabakaran, B. Lee, S. H. Chang, B. Harutyunyan, P. Guo, M. R. Butler, A. Timalsina, M. J. Bedzyk, M. A. Ratner, S. Vegiraju, S. Yau, C.-G. Wu, R. P. H. Chang, A. Facchetti, M.-C. Chen and T. J. Marks, J. Am. Chem. Soc., 2015, 137, 4414 CrossRef CAS PubMed; (c) Y.-S. Yen, J.-S. Ni, T.-Y. Lin, W.-I. Hung, J. T. Lin and M.-C. P. Yeh, Eur. J. Org. Chem., 2015, 7367 CrossRef CAS; (d) H. Jiang, G. Ferrara, X. Zhang, K. Oniwa, A. Islam, L. Han, Y.-J. Sun, M. Bao, N. Asao, Y. Yamamoto and T. Jin, Chem. – Eur. J., 2015, 21, 4065 CrossRef CAS PubMed; (e) K. Matsumura, S. Yoshizaki, M. M. Maitani, Y. Wada, Y. Ogomi, S. Hayase, T. Kaiho, S. Fuse, H. Tanaka and T. Takahashi, Chem. – Eur. J., 2015, 21, 9742 CrossRef CAS PubMed; (f) K. D. Seo, I. T. Choi and H. K. Kim, Chem. – Eur. J., 2015, 21, 14804 CrossRef CAS PubMed; (g) B. Liu, F. Giordano, K. Pei, J.-D. Decoppet, W.-H. Zhu, S. M. Zakeeruddin and M. Grätzel, Chem. – Eur. J., 2015, 21, 18654 CrossRef CAS PubMed; (h) Z. Yao, M. Zhang, R. Li, L. Yang, Y. Qiao and P. Wang, Angew. Chem., Int. Ed., 2015, 54, 5994 CrossRef CAS PubMed; (i) P. Brogdon, F. Giordano, G. A. Puneky, A. Dass, S. M. Zakeeruddin, M. K. Nazeeruddin, M. Grätzel, G. S. Tschumper and J. H. Delcamp, Chem. – Eur. J., 2016, 22, 694 CrossRef CAS PubMed; (j) S. Irie, S. Fuse, M. M. Maitani, Y. Wada, Y. Ogomi, S. Hayase, T. Kaiho, H. Masui, H. Tanaka and T. Takahashi, Chem. – Eur. J., 2016, 22, 2507 CrossRef CAS PubMed; (k) F.-L. Guo, Z.-Q. Li, X.-P. Liu, L. Zhou, F.-T. Kong, W.-C. Chen and S.-Y. Dai, Adv. Funct. Mater., 2016, 26, 5733 CrossRef CAS.
- (a) Y. Ooyama, S. Inoue, R. Asada, G. Ito, K. Kushimoto, K. Komaguchi, I. Imae and Y. Harima, Eur. J. Org. Chem., 2010, 92 CrossRef CAS; (b) Y. Ooyama, S. Inoue, T. Nagano, K. Kushimoto, J. Ohshita, I. Imae, K. Komaguchi and Y. Harima, Angew. Chem., Int. Ed., 2011, 50, 7429 CrossRef CAS PubMed; (c) Y. Ooyama, T. Nagano, S. Inoue, I. Imae, K. Komaguchi, J. Ohshita and Y. Harima, Chem. – Eur. J., 2011, 17, 14837 CrossRef CAS PubMed; (d) Y. Ooyama, N. Yamaguchi, I. Imae, K. Komaguchi, J. Ohshita and Y. Harima, Chem. Commun., 2013, 49, 2548 RSC; (e) Y. Ooyama, Y. Hagiwara, T. Mizumo, Y. Harima and J. Ohshita, New J. Chem., 2013, 37, 2479 RSC; (f) Y. Ooyama, T. Sato, Y. Harima and J. Ohshita, J. Mater. Chem. A, 2014, 2, 3293 RSC; (g) Y. Ooyama, K. Uenaka and J. Ohshita, Eur. J. Org. Chem., 2015, 3713 CrossRef CAS; (h) Y. Ooyama, K. Uenaka, T. Kamimura, S. Ozako, M. Kanda, T. Koide and F. Tani, RSC Adv., 2016, 6, 16150 RSC.
- (a) Y. Harima, T. Fujita, Y. Kano, I. Imae, K. Komaguchi, Y. Ooyama and J. Ohshita, J. Phys. Chem. C, 2013, 117, 16364 CrossRef CAS; (b) N. Shibayama, H. Ozawa, M. Abe, Y. Ooyama and H. Arakawa, Chem. Commun., 2014, 50, 6398 RSC; (c) Y. Ooyama, K. Uenaka, T. Sato, N. Shibayama and J. Ohshita, RSC Adv., 2015, 5, 2531 RSC; (d) J. Ohshita, Y. Adachi, D. Tanaka, M. Nakashima and Y. Ooyama, RSC Adv., 2015, 5, 36673 RSC; (e) Y. Harima, Y. Kano, T. Fujita, I. Imae, Y. Ooyama and J. Ohshita, RSC Adv., 2015, 5, 71387 RSC; (f) Y. Adachi, Y. Ooyama, N. Shibayama and J. Ohshita, Dalton Trans., 2016, 45, 13817 RSC.
- (a) D. Daphnomili, G. D. Sharma, S. Biswas, T. K. R. Justin and A. G. Goutsolelos, J. Photochem. Photobiol., A, 2013, 253, 88 CrossRef CAS; (b) J. Lu, X. Xu, Z. Li, K. Cao, J. Cui, Y. Zhang, Y. Shen, Y. Li, J. Zhu, S. Dai, W. Chjen, Y. Cheng and M. Wang, Chem. – Asian J., 2013, 8, 956 CrossRef CAS PubMed; (c) M.-D. Zhang, H.-X. Xie, X.-H. Ju, L. Qin, Q.-X. Yang, H.-G. Zheng and X.-F. Zhou, Phys. Chem. Chem. Phys., 2013, 15, 634–661 RSC; (d) D. Daphnomili, G. Landrou, P. Singh, A. Thomas, K. Yesudas, B. K. G. D. Sharma and A. G. Goutsolelos, RSC Adv., 2012, 2, 12899 RSC; (e) L. Wang, X. Yang, S. Li, M. Cheng and L. Sun, RSC Adv., 2013, 3, 13677 RSC; (f) T. Sakurada, Y. Arai and H. Segawa, RSC Adv., 2014, 4, 13201 RSC; (g) J. Mao, D. Wang, S.-H. Liu, Y. Hang, Y. Xu, Q. Zhang, W. Wu, P.-T. Chou and J. Hua, Asian J. Org. Chem., 2014, 3, 153 CrossRef CAS; (h) T. Ikeuchi, S. Agrawal, M. Ezoe, S. Mori and M. Kimura, Chem. – Asian J., 2015, 10, 2347 CrossRef CAS PubMed.
- (a) C. Stangel, A. Bagaki, P. A. Angaridis, G. Charalambidis, G. D. Sharma and A. G. Coutsolelos, Inorg. Chem., 2014, 53, 11871 CrossRef CAS PubMed; (b) M. N. K. P. Bolisetty, C.-T. Li, K. R. J. Thomas, G. B. Bodedla and K.-C. Ho, Tetrahedron, 2014, 70, 4203 Search PubMed; (c) D. Franchi, M. Calamante, G. Reginato, L. Zani, M. Peruzzini, M. Taddei, F. F. de Biani, R. Basosi, A. Sinicropi, D. Colonna, A. Di Carlo and A. Mordin, Tetrahedron, 2014, 70, 6285 CrossRef CAS; (d) E. V. Verbitskiy, E. M. Cheprakova, J. O. Subbotina, A. V. Schepochkin, P. A. Slepukhin, G. L. Rusinov, V. N. Charushin, O. N. Chupakhin, N. I. Makarova, A. V. Metelitsa and V. I. Minkin, Dyes Pigm., 2014, 100, 201 CrossRef CAS; (e) C.-L. Mai, T. Moehl, C.-H. Hsieh, J.-D. Décoppet, S. M. Zakeeruddin, M. Grätzel and C.-Y. Yeh, ACS Appl. Mater. Interfaces, 2015, 7, 14975 CrossRef CAS PubMed; (f) H. Jia, K. Shen, X. I. Ju, M. Zhanga and H. Zheng, New J. Chem., 2016, 40, 2799 RSC; (g) P. A. Angaridis, E. Ferentinos, G. Charalambidis, K. Ladomenou, V. Nikolaou, S. Biswas, G. D. Sharma and A. G. Coutsolelos, RSC Adv., 2016, 6, 22187 RSC; (h) U. Meinhardt, F. Lodermeyer, T. A. Schaub, A. Kunzmann, P. O. Dral, A. C. Sale, F. Hampel, D. M. Guldi, R. D. Costa and M. Kivala, RSC Adv., 2016, 6, 67372 RSC.
- (a) H. Frei, D. J. Fitzmaurice and M. Grätzel, Langmuir, 1990, 6, 198 CrossRef CAS; (b) J. Moser, S. Punchihewa, P. P. Infelta and M. Grätzel, Langmuir, 1991, 7, 3012 CrossRef CAS; (c) N. J. Cherepy, G. P. Smestad, M. Grätzel and J. Z. Zhang, J. Phys. Chem. B, 1997, 101, 9342 CrossRef CAS; (d) R. Huber, S. Spcrlein, J. E. Moser, M. Grätzel and J. Wachtveitl, J. Phys. Chem. B, 2000, 104, 8995 CrossRef CAS; (e) P. Persson, R. Bergström and S. Lunell, J. Phys. Chem. B, 2000, 104, 10348 CrossRef CAS; (f) E. Hao, N. A. Anderson, J. B. Asbury and T. Lianl, J. Phys. Chem. B, 2002, 106, 10191 CrossRef CAS; (g) Y. Wang, K. Hang, N. A. Anderson and T. Lian, J. Phys. Chem. B, 2003, 107, 9434 CrossRef CAS.
- (a) S.-C. Li, J.-G. Wang, P. Jacobson, X.-Q. Gong, A. Selloni and U. Dieboldi, J. Am. Chem. Soc., 2009, 131, 980 CrossRef CAS PubMed; (b) L.-M. Liu, S.-C. Li, H. Cheng, U. Diebold and A. Selloni, J. Am. Chem. Soc., 2011, 133, 7816 CrossRef CAS PubMed; (c) S. A. Agarkar, R. R. Kulkarni, V. V. Dhas, A. A. Chinchansure, P. Hazra, S. P. Joshi and S. B. Ogale, ACS Appl. Mater. Interfaces, 2011, 3, 2440 CrossRef CAS PubMed; (d) S. Li, X. Yang, D. Qu, W. Wang, Y. Wang and L. Sun, Chin. J. Chem., 2012, 30, 2315 CrossRef CAS.
- (a) R. Sánchez-de-Armas, J. O. López, M. A. San-Miguel and J. F. Sanz, J. Chem. Theory Comput., 2010, 6, 2856 CrossRef PubMed; (b) R. Sánchez-de-Armas, J. Oviedo, M. A. San-Miguel and J. F. Sanz, J. Phys. Chem. C, 2011, 115, 11293 CrossRef; (c) R. Sánchez-de-Armas, M. A. San-Miguel, J. Oviedo, A. Márquez and J. F. Sanz, Phys. Chem. Chem. Phys., 2011, 13, 1506 RSC; (d) R. Sánchez-de-Armas, M. A. San-Miguel, J. Oviedo and J. F. Sanz, Comput. Theor. Chem., 2011, 975, 99 CrossRef.
- (a) L. G. C. Rego and V. S. Batista, J. Am. Chem. Soc., 2003, 125, 7989 CrossRef CAS PubMed; (b) S. G. Abusabara, L. G. C. Rego and V. S. Batista, J. Am. Chem. Soc., 2005, 127, 18234 CrossRef PubMed; (c) S. G. Abuabara, G. W. Cady, J. B. Baxter, C. A. Schmuttenmaer, R. H. Crabtree, G. W. Brudvig and V. S. Batsista, J. Phys. Chem. C, 2007, 111, 11982 CrossRef CAS; (d) L. G. C. Rego, R. de Silva, J. A. Freire, R. C. Snoeberger and V. S. Batsista, J. Phys. Chem. C, 2010, 114, 1317 CrossRef CAS; (e) W. Macyk, K. Szaciłowski, G. Stochel, M. Buchalska, J. Kuncewicz and P. Łabuz, Coord. Chem. Rev., 2010, 254, 2687 CrossRef CAS; (f) H. J. Nam, B. Kim, M. J. Ko, M. Jin, J. M. Kim and D.-Y. Jung, Chem. – Eur. J., 2012, 18, 14000 CrossRef CAS PubMed.
- (a) W. R. Duncan, W. M. Stier and O. V. Prezhdo, J. Am. Chem. Soc., 2005, 127, 7941 CrossRef CAS PubMed; (b) T. Lana-Villarreal, A. Rodes, J. M. Pérez and R. Gómez, J. Am. Chem. Soc., 2005, 127, 12601 CrossRef CAS PubMed; (c) W. R. Duncan, W. M. Stier and O. V. Prezhdo, J. Phys. Chem. B, 2005, 109, 365 CrossRef CAS PubMed; (d) W. R. Duncan, C. F. Craig and O. V. Prezhdo, J. Am. Chem. Soc., 2007, 129, 8528 CrossRef CAS PubMed; (e) S. Verma, P. Kar, A. Das, D. K. Palit and H. N. Ghosh, Chem. – Eur. J., 2010, 16, 611 CrossRef CAS PubMed; (f) S. Verma, P. Kar, A. Das and H. N. Ghosh, Chem. – Eur. J., 2011, 17, 1561 CrossRef CAS PubMed.
- (a) E. L. Tae, S. H. Lee, J. K. Lee, S. S. Yoo, E. J. Kang and K. B. Yoon, J. Phys. Chem. B, 2005, 109, 22513 CrossRef CAS PubMed; (b) B.-K. An, W. Hu, P. L. Burn and P. Meredith, J. Phys. Chem. C, 2010, 114, 17964 CrossRef CAS; (c) M. Adineh, P. Tahay, M. Ameri, N. Safari and E. Mohajerani, RSC Adv., 2016, 6, 14512 RSC.
- I. A. Janković, Z. V. Šaponjic, M. I. Čomor and J. M. Nedeljković, J. Phys. Chem. C, 2009, 113, 12645 Search PubMed.
- (a) Y. Ooyama, T. Yamada, T. Fujita, Y. Harima and J. Ohshita, J. Mater. Chem. A, 2014, 2, 8500 RSC; (b) Y. Ooyama, M. Kanda, K. Uenaka and J. Ohshita, ChemPhysChem, 2015, 16, 3049 CrossRef CAS PubMed.
- (a) S. Manzhos and K. Kotsis, Chem. Phys. Lett., 2016, 660, 69 CrossRef CAS; (b) J. Fujiwara and M. Nagata, Chem. Phys. Lett., 2015, 619, 180 CrossRef; (c) G. Ramakrishna, D. A. Jose, D. K. Kumar, A. Das, D. K. Palit and H. N. Ghosh, J. Phys. Chem. B, 2005, 109, 15445 CrossRef CAS PubMed; (d) F. de Angelis, Chem. Phys. Lett., 2010, 493, 323 CrossRef CAS; (e) S. Namuangruk, R. Fukuda, M. Ehara, J. Meeprasert, T. Khanasa, S. Morada, T. Kaewin, S. Jungsuttiwong, T. Sudyoadsuk and V. Promarak, J. Phys. Chem. C, 2012, 116, 25653 CrossRef CAS.
- Q. Zeng, Z. Li, Y. Dong, C. Di, A. Qin, Y. Hong, Z. Zhu, C. K. W. Jim, G. Yu, Q. Li, Z. Li, Y. Liu, J. Qin and B. Z. Tang, Chem. Commun., 2007, 70 RSC.
- D. Gudeika, J. V. Grazulevicius, D. Volyniuk, R. Butkute, G. Juska, A. Misaojedovas, A. Gruodis and S. Jursenas, Dyes Pigm., 2015, 114, 239 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Both the geometry optimization and energy calculation were performed by employing the density functional theory (DFT), at the level of B3LYP/6-31G(d, p) on the Gaussian09 program package (Gaussian 09, Revision A.02), Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- J. Li, B. Zhang, H. Yuan, X. Xu, K. Cao, J. Cui, S. Liu, Y. Shen, Y. Cheng, J. Xu and M. Wang, J. Phys. Chem. C, 2014, 118, 14739 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cp06513a |
| This journal is © the Owner Societies 2016 |