Conformational adjustment for high-pressure glass formation of 1-alkyl-3-methylimidazolium tetrafluoroborate
Takahiro
Takekiyo
*a,
Yoshihiro
Koyama
b,
Machiko
Shigemi
a,
Kiyoto
Matsuishi
b,
Hiroshi
Abe
 c,
Nozomu
Hamaya
d and
Yukihiro
Yoshimura
a
c,
Nozomu
Hamaya
d and
Yukihiro
Yoshimura
a
aDepartment of Applied Chemistry, National Defense Academy, Hashirimizu, Yokosuka, Kanagawa 239-8686, Japan. E-mail: take214@nda.ac.jp
bGraduate School of Pure and Applied Science, University of Tsukuba, Tsukuba, Ibaraki 305-8573, Japan
cDepartment of Materials Science and Technology, National Defense Academy, Hashirimizu, Yokosuka, Kanagawa 239-8686, Japan
dGraduate School of Humanities and Sciences, Ochanomizu University, 2-1-1 Otsuka, Bunkyo-ku, Tokyo 112-8610, Japan
First published on 22nd November 2016
Abstract
The conformational stability of 1-alkyl-3-methylimidazolium tetrafluoroborate ([Cnmim][BF4], n = 3–8) under high pressure was investigated using Raman spectroscopy to reveal the preferential role of the alkyl-chain length (n) in high-pressure glass transition. To evaluate this, we determined the intensity ratio (r) and differences in the partial molar volume (ΔVtrans→gauche) between the whole trans and gauche conformers of the [Cnmim] cation using Raman intensities. Interestingly, both values were classified into a two alkyl-chain length region at the border of n = 5. The coulombic interaction (cation–anion interaction) for the conformational stability is the predominant factor below n = 5 (the cation-head portion: alkyl carbon number C < 5), and the alkyl-chain packing effect (cation–cation interaction) is the predominant factor above n = 5 (the cation-tail portion: C > 5). In combination with the conformational preference of the [Cnmim] cation under a high-pressure glassy state, the alkyl chain displays a preferential role, i.e., an increase in the gauche conformer of [Cnmim][BF4] adjusts to avoid crystallization (the conformational adjustment effect). In the presence of the coulombic interaction, the preferential role of the flexible alkyl chain is an important key to elucidate the mechanism of the complicated high-pressure phase transition behavior of ionic liquids.
Introduction
The high-pressure (HP) phase transition of liquids, such as alkanes, acids, and alcohols, has been studied through the years to investigate the HP polymerization and HP metallization of liquids and the origin of the intermolecular interactions.1–9 It is well known that many molecular liquids (MLs) crystallize under high pressure, with further compression causing amorphous or disordered phases.1–9 Specific interactions, such as molecular packing, electrostatic interactions, aromatic interactions, and hydrogen bonding, in MLs have been discussed to understand the HP phase transition mechanism of liquids.1–11In contrast, ionic liquids (ILs) comprising organic cations and anions remain in the liquid state below 373 K and have attracted much attention because of their attractive properties, such as conductivity, viscosity, and thermal stability, making them suitable for use as environmentally benign solvents.12 These properties are related to the static and kinetic hierarchy structure, which produces the nanostructure, ionic portion diffusion, alkyl-chain conformation, and methyl-group rotation of ILs.13,14 In addition to the coulombic interaction, ILs can introduce various interactions, such as alkyl-chain packing (via selection of the cation and anion). Thus, information on the HP phase transition of ILs with a hierarchy structure might be expected to yield a new class of materials for HP applications, such as in HP synthesis techniques, lubrication, catalysis, and bioscience fields.
In recent years, there have been studies on the HP phase transition of ILs using optical spectroscopy, X-ray scattering, and molecular dynamics (MD) simulations.15–29 On the whole, high pressure (up to 3 GPa) causes at least three IL phase transition patterns: the HP glassy state, compression crystallization, and decompression crystallization. These complicated phase transitions in this pressure range are characteristic of ILs and are hardly observed for most MLs, e.g., alkanes and haloalkanes.1,5–7 According to our Raman and X-ray studies,18,23 1-alkyl-3-methylimidazolium tetrafluoroborate ([Cnmim][BF4], n = 2–8) with a short alkyl chain (n = 2) and a long alkyl chain (n > 8) tends to exhibit compression or decompression crystallization.18,23 On the other hand, [Cnmim][BF4] with an intermediate alkyl chain (n = 3–7) tends to form a HP glassy state.23
Related to these effects, a conformational change of the alkyl chain of [Cnmim][BF4] occurred, accompanying the phase transitions under high pressure.15–19,21,23–25,28,29 For instance, it is well known that [Cnmim][PF6] with a hydrophobic anion and [Cnmim][BF4] with a hydrophilic anion exhibit differing HP phase transitions: the former tends to crystallize under high pressure15,16,21 and the latter tends to form a HP glassy state.23,30 The difference in these phase transitions is due to the cation–anion affinity (such as the position of the anion for the [Cnmim] cation) and cation–anion interaction (hydrophobicity and hydrophilicity). In addition to these transitions, the previous Raman results have revealed that the single conformer (trans or gauche conformer) for the NCCC angles of the [C4mim] cation of [C4mim][PF6] is predominant in compression crystallization.15,16,21,31 A predominance of the single conformer of [C4mim][PF6] in compression crystallization has also been observed in many MLs and can be explained by the molecular packing order effect.1,5–7 In contrast, the HP glassy state of [C4mim][BF4] induced a higher gauche conformer population than the liquid state.30
Here it is intriguing as to why the higher gauche conformer population of [C4mim][BF4] is needed to form a glassy state under high pressure. Although it has been noted that the conformational change of the alkyl chain is strongly related to phase transition,15–19,21,23–25,28,29 the preferential role (increase of the gauche conformer) of the alkyl-chain length of [C4mim][BF4] in HP glassy formation is still unclear. Elucidation of the preferential role of the alkyl-chain length under high pressure is related to the origin of the complicated high pressure phase transition of ILs such as the decompression crystallization mechanism.18,19,23
In this study, to deepen the previous study,23 we investigated the pressure effect on the conformational equilibrium of [Cnmim][BF4] (n = 3–8) using Raman spectroscopy to elucidate the preferential role of the alkyl-chain length in glass formation under high pressure. Fig. 1 shows (a) the chemical structures of [Cnmim][BF4] and (b) the representative trans and gauche conformers of the [Cnmim] cation. The present finding is that the pressure-induced conformational stability of the [Cnmim] cation is related to competition between the coulombic interaction and the alkyl-chain packing. In combination with the conformational preference of the [Cnmim] cation under a high-pressure glassy state, the alkyl-chain length of the [Cnmim] cation in [Cnmim][BF4] contributes to the maintenance of the liquid structure, and its adjustment (increase of the gauche conformer) induced glass formation under high pressure, thereby avoiding compression crystallization.
Experiments
1-Alkyl-3-methylimidazolium tetrafluoroborate ([Cnmim][BF4], n = 3–10) was investigated in this study (Kanto Chemical Co. for n = 4; Ionic Liquids Technologies Inc. for n = 3, 5, 6, and 7; and Wako Pure Chemical Co. for n = 8, 9, and 10). Raman spectra were measured using a JASCO NR-1800 Raman spectrophotometer equipped with a single monochromator and a liquid nitrogen-cooled charge-coupled device detector. The spectra were excited by the 514.5 nm line of an Ar+ laser (Lexel) with a power of 350 mW. The pressure-loading device was a screw-type diamond anvil cell (DAC, SR-DAC-KYO3-3d, Kyowa) with anvil cutlet 0.6 mm in size. We used a SUS301 gasket with a 0.3 mm hole. The pressure was determined from the spectral shift of the R1 fluorescence line of several ruby balls (0.015 mm diameter) placed in the sample chamber of the DAC.32 The error of the estimated pressure was ±0.05 GPa. The sample was prepared in a glove box filled with argon gas to mitigate contamination from water in air. All Raman spectral measurements of [Cnmim][BF4] were performed in the liquid (298 K and 0.1 MPa) and glassy (77 K and 0.1 MPa or 298 K and high pressure) states. High-pressure experiments for [C9mim][BF4] and [C10mim][BF4] were not performed because both ILs were crystallized at low pressure (ca. 0.4–0.7 GPa). Each sample was normally compressed in steps of approximately 1 GPa h−1, including the duration time. To improve the data quality, all spectral measurements were repeated at least three times. All of the spectral lines were fitted with Gaussian–Lorentzian mixing functions provided by GRAMS/386 software (Galactic Industries Co.).Results and discussion
Conformational stability of [Cnmim][BF4] under high pressure
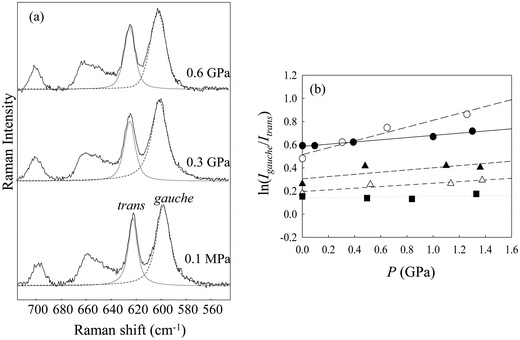
Fig. 2(a) shows the Raman spectra of [Cnmim][BF4] in the region from 560 to 680 cm−1 at several alkyl-chain lengths at 298 K and 0.1 MPa. The vibrational assignments of the Raman band in this region of [Cnmim][BF4] were well established by Hamaguchi et al.33 Two peaks at ∼600 cm−1 and ∼625 cm−1, assigned to the CH2 rocking bands, correspond to the whole gauche and trans conformers of the alkyl chain in the [Cnmim] cation, respectively. The Raman intensity at ∼600 cm−1 (the gauche conformer) decreases, and that at ∼625 cm−1 (the trans conformer) increases, with increasing alkyl-chain length. To further investigate the effect of the alkyl-chain length on the conformational equilibrium of the [Cnmim] cation, Fig. 2(b) shows the intensity ratio (r = Igauche/Itrans) between the conformers as a function of alkyl-chain length (n). The r-value using the same vibrational mode corresponds to the conformational free energy change (ΔGtrans→gauche = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(Igauche/Itrans), where R and T are the gas constant and temperature).34,35 A drastic decrease in the r-value was observed in the range from n = 5 to 6, and this value is almost constant above n = 6. This indicates that the conformational stability of the [Cnmim] cation below n = 5 is different from that above n = 5.
ln(Igauche/Itrans), where R and T are the gas constant and temperature).34,35 A drastic decrease in the r-value was observed in the range from n = 5 to 6, and this value is almost constant above n = 6. This indicates that the conformational stability of the [Cnmim] cation below n = 5 is different from that above n = 5.
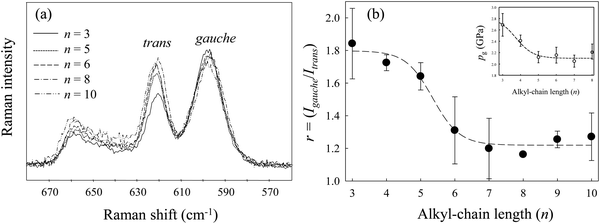 | ||
| Fig. 2 (a) Raman spectral changes in the CH2 rocking region of [Cnmim][BF4] at several alkyl-chain lengths (n). (b) Changes in the intensity ratio (r) between the conformers of the [Cnmim] cation as a function of n. The inset figure in (b) shows changes in pg of [Cnmim][BF4] as a function of n. Data were re-plotted from ref. 23. | ||
Remarkably, the r-value shows similar behavior to the change in the glass transition pressure (pg) of [Cnmim][BF4] in relation to n, as reported by Yoshimura et al.23 (the inset in Fig. 2(b)), although the r-value shifts to higher n rather than pg. The similar behavior of r-values and pg-values indicates that the conformational stability of the [Cnmim] cation at 0.1 MPa and 298 K is related to the glass transition behavior of [Cnmim][BF4]. Related to these results, Yamamuro et al.36 used X-ray and neutron scattering methods to show that the structure of low-temperature (LT) glassy state of [Cnmim]-based ILs is similar to that in the liquid state. Moreover, Endo et al.37 used Raman spectroscopy to demonstrate that the gauche conformer of [C4mim][PF6] increases when [C4mim][PF6] forms the LT glassy state. Considering their results, we suggest that the investigation of local structural stability, such as the conformational change of the [Cnmim] cation in the liquid state, is an important aspect of the HP glass formation mechanism of [Cnmim][BF4]. In view of the pressure effect on the conformational equilibrium of the cation, we discuss changes in the conformational stability of the [Cnmim] cation.
As a representative result, Fig. 3(a) shows the Raman spectra in the CH2 rocking region of [C5mim][BF4] at several pressures. The peak at 600 cm−1 (gauche conformer) increases with increasing pressure, whereas that at 625 cm−1 (trans conformer) decreases. The increase of the gauche conformer under high pressure was observed in other [Cnmim][BF4]. Similarly, previous Raman studies showed that the gauche conformer of MLs such as alkanes and haloalkanes increases with increasing pressure until crystallization occurs.1,5–7,38 Pressure-induced conformational preferences of the [Cnmim] cation prior to phase transition are consistent with those of alkanes and haloalkanes.
To further investigate the pressure effect on the conformational equilibrium of the [Cnmim] cation, we determined the difference in the partial molar volume (PMV) (ΔVtrans→gauche) between the whole trans and gauche conformers on the basis of the pressure dependence of the relative Raman intensities, as shown in Fig. 3(b). The relationship between the Raman intensities for the conformers and pressure has been well established.38–41 Assuming that the ratio of the Raman scattering cross sections between the conformers is independent of pressure, ΔVtrans→gauche is given by
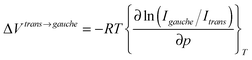 | (1) |
Fig. 4 shows the changes in the ΔVtrans→gauche value of [Cnmim][BF4] versus n; the data are combined with the previous results for alkanes.8,9,44,45 Remarkably, the negative values of ΔVtrans→gauche became larger when n = 3–5 and became smaller above n = 5. Whereas ΔVtrans→gauche of alkanes became larger above n = 5. Although the conformational preference of [Cnmim][BF4] until high pressure phase transition is similar to that of alkanes, for ILs that construct cations and anions, the absolute value and inversion of the slope in ΔVtrans→gaucheversus n are quite different from those of alkanes that construct only neutral alkyl chains. We suppose that the abrupt change in the conformational stability at n = 5 is related to the pressure effect on the nanostructure of [Cnmim][BF4], because it is well known that the formation of a nanostructure of [Cnmim]-based IL with a shorter alkyl-chain length (n < 5) is weak, and the increase of the alkyl-chain length of the cation (n > 5) induced enhancement of the nanostructure.12 In fact, our recent studies demonstrated that an abrupt conformational change occurs in the [C8mim] cation of [C8mim][BF4] having the enhanced nanostructure after the disruption of the nanostructure by the applied pressure (as we discuss below).23 Based on these results, as the formation of a nanostructure of [Cnmim][BF4] at n < 5 is relatively weak, the conformational stability of the [Cnmim] cation might be largely affected by the pressure. On the other hand, the enhanced nanostructure of [Cnmim][BF4] at n > 5 is disrupted by the pressure before the conformational change of the [Cnmim] cation occurs.
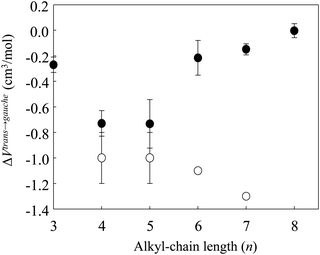 | ||
| Fig. 4 Volume changes (ΔVtrans→gauche) between the conformers of the [Cnmim] cation of [Cnmim][BF4] as a function of n (combined with the results for [C4mim][BF4]41 and alkanes8,9,45,46). Closed and open circles represent [Cnmim][BF4] and alkanes, respectively. | ||
Here, we discuss the origin of the inversion of slope in ΔVtrans→gauche for [Cnmim][BF4]. Considering the coulombic interaction and the alkyl-chain packing effect in [Cnmim][BF4], the crystallization of [Cnmim][BF4] with a shorter alkyl-chain length is plausible to be caused by the larger contribution of the coulombic interaction rather than by the alkyl-chain packing effect, whereas that with a longer alkyl-chain length is due to the larger contribution of the alkyl-chain packing effect rather than that of the coulombic interaction. If the competition between the coulombic interaction and the alkyl-chain packing effect is reflected in the inversion of slope in ΔVtrans→gauche for [Cnmim][BF4], the corresponding pressure-induced frequency shifts for the BF4 stretching (νBF4) band (observed at ∼750 cm−1) suggesting the coulombic interaction and the CH2 rocking (νCH2) band (which indicates the alkyl-chain packing effect) should occur in response to n. Thus, we checked the pressure-induced frequency shifts of νBF4 and νCH2 of [Cnmim][BF4]. νBF4 and two νCH2 bands (the gauche and trans conformers) shifted to higher frequencies with increasing pressure. These higher frequency shifts in response to pressure show the repulsion effect of the cation/anion species, and they are consistent with the general trend of the pressure-induced frequency shift of MLs.1,40,50
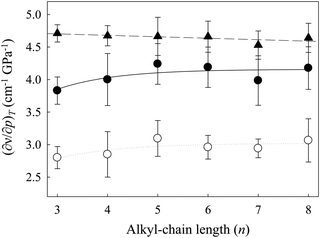
Fig. 5 shows the changes in the values for the pressure dependency of νBF4 ((∂νBF4/∂p)T) and νCH2 ((∂νCH2/∂p)T) of [Cnmim][BF4] as a function of n. The values of (∂νBF4/∂p)T (▲) are almost constant throughout the studied n range. On the other hand, the values of (∂νCH2/∂p)T show different behavior from (∂νBF4/∂p)T. On the whole, the (∂νCH2/∂p)T value (●) of the gauche conformer with the smaller PMV is larger than that for the trans conformer (○) throughout the studied n range. Remarkably, the values of (∂νCH2/∂p)T for both conformers increase up to n = 5 and become constant above n = 5. Related to this, as stated above, the increase in the alkyl-chain length induced the enhancement of the alkyl-chain packing effect of [Cnmim]-based ILs (nanoheterogeneity enhancement),46–49 although the coulombic interaction arising from the imidazolium ring and the anion is independent of n. These indicate that the pressure-induced environmental change around the gauche conformer is larger than that around the trans conformer throughout the studied n range. In addition, changes in (∂νCH2/∂p)T of both conformers versus n are in good agreement with the inversion of slope in ΔVtrans→gauche.
Based on these results, and because the (∂νBF4/∂p)T values are almost constant in relation to n and the (∂νCH2/∂p)T values of both conformers increase up to n = 5, the alkyl-chain packing effect (cation–cation interaction) of [Cnmim][BF4] at n = 3–5 is shielded by the coulombic interaction (cation–anion interaction). In contrast, with a further increase of the alkyl-chain length above n = 5, the alkyl-chain packing effect of [Cnmim][BF4] above n = 5 might be stronger than the coulombic interaction. According to the recent MD simulations of [C8mim][BF4] by Russina et al.,25 the coulombic correlation (cation–anion interaction) in the pair distribution functions is relatively unchanged up to 1 GPa. They noted that the coulombic interaction of [C8mim][BF4] is independent of pressure; pressure changes will largely affect the conformation of the alkyl chain. Their results are consistent with the present results of the frequency shifts versus n.
Based on these results, the inversion of slope in ΔVtrans→gauche for [Cnmim][BF4] might be related to the competition between the coulombic interaction and the alkyl-chain packing effect of the conformers together with BF4 anions. Here, ΔVtrans→gauche was simply decomposed into two contributions:
| ΔVtrans→gauche = ΔVC + ΔVP | (2) |
Consequently, we can conclude that the inversion of slope in ΔVtrans→gauche for [Cnmim][BF4] is due to the competition between ΔVC and ΔVP: the former is predominant for ΔVtrans→gauche below n = 5, and the latter is predominant above n = 5. These results indicate that this competition is reflected in the r-values, which correspond to ΔGtrans→gaucheversus n.
Preferential role of the alkyl-chain length in glassy formation of [Cnmim][BF4] under high pressure
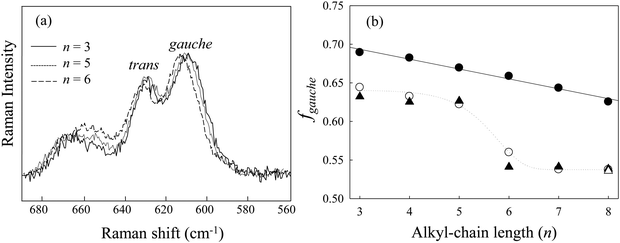
As seen in the previous section, the conformational stability of [Cnmim][BF4] versus n under high pressure has been discussed, and changes in the conformational stability of the [Cnmim] cation are due to the competition between the coulombic interaction and the alkyl-chain packing effect. In this section, we discuss the preferential role of the alkyl-chain length in conjunction with the glass formation of [Cnmim][BF4] under high pressure.Fig. 6(a) shows the Raman spectra in the CH2 rocking region of [Cnmim][BF4] in the HP glassy state at several n values. The spectra showed similar spectral shapes throughout the studied n range. To further investigate the conformational preference of the [Cnmim] cation, we determined the fraction of the gauche conformer (fgauche); in combination with the liquid state and retrieved results, this is shown in Fig. 6(b). In the liquid state, a drastic decrease in fgauche was observed from n = 5 to 6. The fgauche values (fgauche = 0.65–0.68) in the HP glassy state are higher than those in the liquid state (fgauche = 0.53–0.65) and linearly decrease with increasing n. Moreover, the retrieved fgauche values are almost consistent with the fgauche values before compression.
Related to these results, our recent high-pressure small-angle X-ray scattering (HP-SAXS) results demonstrated that the intensity of a low-Q peak (∼2.8 Å−1) that shows the nanostructure in the SAXS profiles of [C8mim][BF4] drastically decreases up to 2.0 GPa.23 In response to the HP-SAXS results, Russia et al.25 used MD simulations to demonstrate that the simulated low-Q peak (∼2.8 Å−1) of [C8mim][BF4] vanished below 1 GPa. Similar MD simulation results were also obtained in the case of ammonium-based ILs.51 These results indicate that the nanostructure of ILs is disrupted with increasing pressure. Moreover, our Raman results showed that [C8mim][BF4] forms the glassy state above 2.2 GPa, and that the gauche conformer of the [C8mim] cation increases above 1 GPa.23 Combined with these results, after the nanostructure of [Cnmim]-based ILs is disrupted under high pressure, further compression will induce an increase in the gauche conformer of the [Cnmim] cation; [Cnmim][BF4] then formed the glassy state. On the other hand, the pressure release caused the reversal of the conformational preference in the liquid state. It is evident that an increase in the disordered conformer (gauche conformer) of the glassy state showing a metastable phase is necessary to avoid crystallization of the highly ordered conformer (trans conformer). Thus, it is intriguing which alkyl-chain part in the [Cnmim] cation contributes the HP glassy state.
A remarkable result is the difference in the fgauche between the ambient pressure and the HP glassy state depending n, when n is classified into two regions: (1) n = 3–5 and (2) n = 6–8. Since the second region is consistent with the region of ΔVtrans→gauche inversion, the increase in fgauche from 0.1 MPa to HP in the former region is due to the coulombic interaction and that in the latter region is due to the alkyl-chain packing effect. Recent MD simulations demonstrated the difference in the conformational preference of the alkyl-chain length of ILs under high pressure.25,52 Russina et al.25 showed that the increase in the gauche conformer occurred for the cation-tail portion above C = 5 (C is the carbon number of the alkyl chain) in [C8mim][BF4] rather than for the cation-head portion below C = 5. On the other hand, Sharma et al.52 reported that the conformational preference of the cation in 1-alkyl-methylpyrrolidinium bis(trifluoromethylsulfonyl) imide ([Pyrr1,n][NTf2], n = 8 and 10) under high pressure is opposite to that of [C8mim][BF4]; the increase in the gauche-kink conformer of [Pyrr1,n] cations occurred below C = 3–5 (the cation-head portion) under high pressure. They reported that the different pressure dependency of the cation-head and cation-tail portions of these ILs is due to the difference in the conformational flexibility between the imidazolium ring-BF4 and the pyrrolidinium ring-NTf2. These results indicate that the conformational stability of the cation-head portion under high pressure is different from that of the cation-tail portion.
Based on all the results, we propose the preferential role of the alkyl-chain length in glass formation of [Cnmim][BF4] under high pressure. As mentioned in the Introduction, high pressure causes glass formation of [Cnmim][BF4] in the range n = 3–8 and the gauche conformer increases. Moreover, the inversion of slope in ΔVtrans→gauche occurred at n = 5 due to competition between the coulombic interaction and the alkyl-chain packing effect. Considering these results, it is apparent that the alkyl-chain length plays an important role in glass formation; i.e., if the alkyl chain carbon number is longer than 5, in addition to the cation-head portion curling, the cation-tail curling is necessary to inhibit the crystallization due to the alkyl-chain packing effect (Fig. 7). This adjustment is a likely consequence, because in the case of [Cnmim][BF4] the BF4 anion is positioned around the top of the imidazolium-ring.54 Since such a conformational role was not observed for alkanes under crystallization at high pressure, the role of the alkyl chain of ILs is quite different from that of alkanes in the absence of the coulombic interaction. Moreover, this cation-tail (conformational) adjustment effect can explain the decompression crystallization, as seen in [C2mim][BF4] and [C8mim][BF4] at around 1 GPa.18,23 The decompression crystallization is related to the relaxation of the restricted terminal alkyl-chain group under high pressure, i.e., the cation-tail portion via the cation-tail adjustment effect. Very recently, Faria et al.53 reported that the oligomerization of 1-allyl-3-methylimidazolium dicyanamide ([allylC1im][N(CN)2]) occurred above 8.0 GPa. They mentioned that the terminal double bond of the allyl-group directly involves the HP oligomerization reaction. The terminal double bond in their study corresponds to the cation-tail part of the [Cnmim] cation in our study. This result ensures that the cation-tail portion of imidazolium-based ILs is an important key of the HP phase transition of ILs after glass transition (>3.0 GPa).
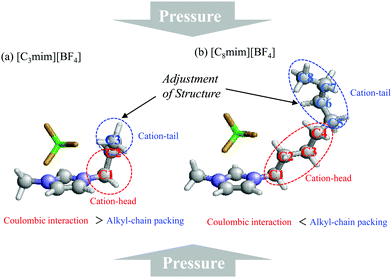 | ||
| Fig. 7 Schematic representation for the preferential role of the alkyl-chain length of (a) [C3mim][BF4] and (b) [C8mim][BF4]. | ||
On the other hand, in the case of supercooled glass, the situation is quite different. There is no need to make a conformational adjustment under no compression. The fgauche value is comparable to that at the liquid state at 0.1 MPa, as seen in Fig. 6(b).
It is difficult to mention more about the relationship between the cation-head and cation-tail portions under high pressure since the direct conformational probability for each dihedral angle of the [Cnmim] cation is still unclear. Further theoretical investigations, such as MD simulations under high pressure, e.g., the systematic conformational probability of the cation-head and cation-tail portions and the pressure dependence of the cation–anion configuration, have provided us with further knowledge for understanding the glass formation and crystallization mechanism of [Cnmim]-based ILs under high pressure.
Conclusions
The preferential role of the alkyl-chain in glass formation under high pressure was provided in view of the conformational stability of 1-alkyl-3-methylimidazolium tetrafluoroborate ([Cnmim][BF4], n = 3–8). To evaluate the results, we determined the intensity ratio (r) and the differences in the partial molar volume (ΔVtrans→gauche) between the conformers of the [Cnmim] cation using Raman intensities, and both values were classified into two alkyl-chain length groups at the border of n = 5. The conformational stability of the [Cnmim] cation is due to the competition between the coulombic interaction and the alkyl-chain packing effect of the conformers together with BF4 anions. In combination with the conformational preference under HP glassy states, the alkyl-chain part plays an important role in glass formation; i.e., the cation-tail portion (above C = 5) adjusts to inhibit the crystallization (the conformational adjustment effect).However, the present findings on the preferential role of the alkyl-chain part is only the case for [Cnmim][BF4]; it is unclear whether the cation-tail adjustment effect can adapt other ILs. A recent MD simulation study of the ester functionalized imidazolium-based ILs with various anions indicated that the nanoscale organization of ILs is affected by the cation–anion interaction, the cation–anion configuration, and the alkyl-side chain terminal of cations.55 In fact, the increase in the gauche conformer of the cation-tail or cation-head portion is largely dependent on the cationic and anionic species (as in the case of other imidazolium-based and pyrrolidinium-based ILs with NTf2).56 Related to this, the conformational preference (trans > gauche) of [C4mim][I] at 0.1 MPa is different from that of [C4mim][BF4] (trans < gauche) by the difference in the cation–anion configuration.54 Based on these results, studies of the counter anion effect on the conformational stability of ILs under high pressure are clearly important for further elucidation of the role of alkyl-chain flexibility in the phase transition behavior. We are currently investigating the topics which will be published elsewhere.
References
- G. Kavitha and C. Narayana, J. Phys. Chem. B, 2007, 111, 14130–14135 CrossRef CAS PubMed.
- C. Murli and Y. Song, J. Phys. Chem. B, 2010, 114, 9744–9750 CrossRef CAS PubMed.
- C. Murli, N. Lu, Z. Dong and Y. Song, J. Phys. Chem. B, 2012, 116, 12574–12580 CrossRef CAS PubMed.
- Z. Dong, N. G. Beilby, Y. Huang and Y. Song, J. Chem. Phys., 2008, 128, 074501 CrossRef PubMed.
- G. Kavitha and C. Narayana, J. Phys. Chem. B, 2006, 110, 8777–8781 CrossRef CAS PubMed.
- G. Kavitha and C. Narayana, J. Phys. Chem. B, 2007, 111, 7003–7008 CrossRef CAS PubMed.
- R. Sabharwal, Y. Huang and Y. Song, J. Phys. Chem. B, 2007, 111, 7267–7273 CrossRef CAS PubMed.
- P. T. T. Wong and H. H. Mantsch, J. Chem. Phys., 1983, 79, 2369–2374 CrossRef CAS.
- M. Kato and Y. Taniguchi, J. Chem. Phys., 1991, 94, 4440–4445 CrossRef CAS.
- K. K. Zhuravlev, K. Traikov, Z. Dong, S. Xie and Y. Song, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 064116 CrossRef.
- M. Pravica, D. Sneed, Y. Wang, Q. Simith and M. White, J. Phys. Chem. B, 2016, 120, 2854–2858 CrossRef CAS PubMed.
- R. Hayes, G. G. Warr and R. Atkin, Chem. Rev., 2015, 115, 6357–6426 CrossRef CAS PubMed.
- O. Yamamuro, T. Yamada, M. Kofu, M. Nakakoshi and M. Nagao, J. Chem. Phys., 2011, 135, 054508 CrossRef PubMed.
- M. Kofu, M. Tyagi, Y. Inamura, K. Miyazaki and O. Yamamuro, J. Chem. Phys., 2015, 143, 234502 CrossRef PubMed.
- L. Su, L. Li, Y. Hu, C. Yuan, C. Shao and S. Hong, J. Chem. Phys., 2009, 130, 184503 CrossRef PubMed.
- O. Russina, B. Fazio, C. Schmidt and A. Triolo, Phys. Chem. Chem. Phys., 2011, 13, 12067–12074 RSC.
- Y. Yoshimura, T. Takekiyo, Y. Imai and H. Abe, J. Phys. Chem. C, 2012, 116, 2097–2101 CAS.
- Y. Yoshimura, H. Abe, T. Takekiyo, M. Shigemi, N. Hamaya, R. Wada and M. Kato, J. Phys. Chem. B, 2013, 117, 12296–12302 CrossRef CAS PubMed.
- Y. Yoshimura, H. Abe, Y. Imai, T. Takekiyo and N. Hamaya, J. Phys. Chem. B, 2013, 117, 3264–3269 CrossRef CAS PubMed.
- L. Pison, M. F. Costa Gomes, A. A. H. Pádua, D. Andrault, S. Norrman, C. Hardacre and M. C. C. Ribeiro, J. Chem. Phys., 2013, 139, 054510 CrossRef CAS PubMed.
- H. Abe, T. Takekiyo, N. Hatano, M. Shigemi, N. Hamaya and Y. Yoshimura, J. Phys. Chem. B, 2014, 118, 1138–1145 CrossRef CAS PubMed.
- M. C. C. Ribeiro, A. A. H. Pádua and M. F. Costa Gomes, J. Chem. Phys., 2014, 140, 244514 CrossRef PubMed.
- Y. Yoshimura, M. Shigemi, M. Takaku, M. Yamamura, T. Takekiyo, H. Abe, N. Hamaya, D. Wakabayashi, N. Funamori, T. Sato and T. Kikegawa, J. Phys. Chem. B, 2015, 119, 8143–8156 CrossRef PubMed.
- Y. Yoshimura, T. Takekiyo, H. Abe and N. Hamaya, J. Mol. Liq., 2015, 206, 89–94 CrossRef CAS.
- O. Russina, F. Le Celso and A. Triolo, Phys. Chem. Chem. Phys., 2015, 17, 29496–29500 RSC.
- L. F. O. Faria and M. C. C. Ribeiro, J. Phys. Chem. B, 2015, 119, 14315–14372 CrossRef CAS PubMed.
- T. A. Lima, V. H. Paschoal, L. F. O. Faria, M. C. C. Ribeiro, F. F. Ferreira, F. N. Costa and C. Giles, J. Chem. Phys., 2016, 144, 244505 CrossRef PubMed.
- F. Capitani, S. Gatto, P. Postorino, O. Palumbo, F. Trequattrini, M. Deutsch, J.-B. Brubach, P. Roy and A. Paolone, J. Phys. Chem. B, 2016, 120, 1312–1318 CrossRef CAS PubMed.
- F. Capitani, F. Trequattrini, O. Palumbo, A. Paolone and P. Postorino, J. Phys. Chem. B, 2016, 120, 2921–2928 CrossRef CAS PubMed.
- M. Shigemi, T. Takekiyo, H. Abe, N. Hamaya and Y. Yoshimura, J. Solution Chem., 2014, 43, 1614–1624 CrossRef CAS.
- T. Takekiyo, N. Hatano, Y. Imai, H. Abe and Y. Yoshimura, High Pressure Res., 2011, 31, 35–38 CrossRef CAS.
- H. K. Mao, P. M. Bell, J. W. Shaner and D. J. Steinberg, J. Appl. Phys., 1978, 49, 3276–3283 CrossRef CAS.
- H. Hamaguchi and R. Ozawa, Adv. Chem. Phys., 2005, 131, 85–104 CrossRef CAS.
- N. Hatano, T. Takekiyo, H. Abe and Y. Yoshimura, Int. J. Spectrosc., 2011, 2011, 648245 CrossRef.
- R. Wada and M. Kato, Chem. Phys. Lett., 2015, 641, 74–79 CrossRef CAS.
- O. Yamamuro, Y. Minamimoto, Y. Inamura, S. Hayashi and H. Hamaguchi, Chem. Phys. Lett., 2006, 423, 371–375 CrossRef CAS.
- T. Endo, T. Kato, K. Tozaki and K. Nishikawa, J. Phys. Chem. B, 2010, 114, 407–411 CrossRef CAS PubMed.
- M. Kato and Y. Taniguchi, J. Phys. Chem., 1994, 98, 2688–2693 CrossRef CAS.
- T. Takekiyo and Y. Yoshimura, J. Phys. Chem. A, 2006, 110, 10829–10833 CrossRef CAS PubMed.
- K. Kasezawa and M. Kato, J. Phys. Chem. B, 2009, 113, 8607–8612 CrossRef CAS PubMed.
- T. Takekiyo, Y. Imai, N. Hatano, H. Abe and Y. Yoshimura, Chem. Phys. Lett., 2011, 511, 241–246 CrossRef CAS.
- M. Kato and Y. Taniguchi, J. Chem. Phys., 1991, 94, 4440–4445 CrossRef CAS.
- Y. Taniguchi, H. Takaya, P. T. T. Wong and E. Whalley, J. Chem. Phys., 1981, 75, 4815–4822 CrossRef CAS.
- J. Devaure and J. Lacombe, Nouv. J. Chem., 1979, 3, 579–581 CAS.
- P. E. Schoen, R. G. Priest, J. P. Sherida and J. M. Schur, J. Chem. Phys., 1979, 71, 317–323 CrossRef CAS.
- A. Triolo, O. Russina, B. Fazio, R. Triolo and E. Di Cola, Chem. Phys. Lett., 2008, 457, 362–365 CrossRef CAS.
- J. N. A. C. Lopes and A. A. H. Pádua, J. Phys. Chem. B, 2006, 110, 3330–3335 CrossRef PubMed.
- A. Triolo, O. Russina, C. Hardacre, M. Nieuwenhuyzen, M. A. Gonzalez and H. Grimm, J. Phys. Chem. B, 2005, 109, 22061–22066 CrossRef CAS PubMed.
- A. Triolo, O. Russina, H.-J. Bluif and E. D. Cola, J. Phys. Chem. B, 2007, 111, 4641–4644 CrossRef CAS PubMed.
- S. Ikawa and E. Whally, J. Chem. Phys., 1984, 81, 1620–1625 CrossRef CAS.
- A. Mariani, R. Caminiti, M. Campetella and L. Gontrani, Phys. Chem. Chem. Phys., 2016, 18, 2297–2302 RSC.
- S. Sharma, A. Gupta and H. K. Kashyap, J. Phys. Chem. B, 2016, 120, 3206–3214 CrossRef CAS PubMed.
- L. F. O. Faria, M. M. Nobrega, M. Laudelina, A. Temperini, R. Bini and M. C. C. Ribeiro, J. Phys. Chem. B, 2016, 120, 9097–9102 CrossRef CAS PubMed.
- Y. Jeon, J. Sung, C. Seo, H. Lim, H. Cheong, M. Kang, B. Moon, Y. Ouchi and D. Kim, J. Phys. Chem. B, 2008, 112, 4735–4740 CrossRef CAS PubMed.
- M. Fakhraee and M. R. Gholami, J. Phys. Chem. B, 2016, 120, 11539–11555 CrossRef CAS PubMed.
- A. R. Choudhury, N. Winterson, A. Steiner, A. I. Cooper and K. A. Johnson, J. Am. Chem. Soc., 2005, 127, 16792–16793 CrossRef CAS PubMed.
| This journal is © the Owner Societies 2017 |