 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sequential detection of multiple phase transitions in model biological membranes using a red-emitting conjugated polyelectrolyte†
Judith E.
Houston
a,
Mario
Kraft
b,
Ullrich
Scherf
b and
Rachel C.
Evans
*a
aSchool of Chemistry, University of Dublin, Trinity College, College Green, Dublin 2, Ireland. E-mail: raevans@tcd.ie
bMacromolecular Chemistry Group (buwmacro) and Institute for Polymer Technology, Bergische Universität Wuppertal, D-42119, Wuppertal, Germany
First published on 21st April 2016
Abstract
The anionic conjugated polyelectrolyte, poly[3-(6-sulfothioatehexyl)thiophene] (P3Anionic), functions as a highly sensitive probe of membrane order, uniquely capable of sequentially detecting the three key phase transitions occurring within model phospholipid bilayers. The observed sensitivity is the result of charge-mediated, selective localisation of P3Anionic within the head-groups of the phospholipid bilayer.
Cell membranes are complex dynamic systems that modify their structure or phase in order to accomplish different tasks. Membrane phase transitions are crucial for the transport of materials, energy and information between the interior and exterior cell environment.1 However, membrane order is a delicate balance, and unsought changes in membrane permeability via phase transitions are thought to be behind a number of degenerative or life-threatening diseases, including age-related neurological conditions, such as Alzheimer's or Parkinson's disease,2,3 and tumour cell formation.4 As such, the development of probes that are able to accurately detect subtle changes in phase or order in model and live membranes at the nanoscale is crucial for the development of targeted diagnostic and therapeutic platforms.5,6
Cell membrane order can be probed directly using Raman,7 optical8 and atomic force microscopies (AFM).9 However, these methods often require a fixation procedure, which can introduce artefacts into the images. Indirect methods, such as X-ray and neutron scattering,10,11 differential scanning calorimetry (DSC)12,13 and computer simulations14 allow the cell membrane to be studied in solution, but are not always applicable to in vivo measurements. Fluorescent probes overcome these limitations, offering a non-invasive approach to investigate cell membrane order in vivo with high sensitivity, low concentration demands and suitable time resolution.15–21 However, while phase-sensitive fluorescence probes exist (e.g. quantum dots,22 phospholipid labels/analogues,20,23 small organic dyes16,24), their widespread use has been limited by time-consuming experimental procedures, intrinsic cytotoxicity or the tendency towards aggregation or cell internalisation.25 In contrast, conjugated oligo-/polyelectrolytes (COEs/CPEs) have recently shown considerable promise as lipid phase-transition probes due to their tunable emission, low cytotoxicity and intense fluorescence.5,26,27 The COEs/CPEs probes reported to date typically show a change in their fluorescence properties in response to a significant membrane transition. The main transition between the liquid-disordered (fluid) and the solid-ordered (gel) phases in lipid bilayers has been shown to induce a red-shift of up to 140 nm in the fluorescence spectra of twisted quarterthiophenes27 and dithienothiophenes,5 partly attributed to the planarisation of the oligopolymer backbones within the ordered gel phase. The steady-state emission intensity of the blue-emitting, cationic polyfluorene, HTMA-PFP, was found to be sensitive to two phase transitions in the phospholipids dipalmitoylphosphatidylcholine (DPPC) and 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC).26 In these examples, the COE/CPE probe is able to monitor one, or at most two, membrane transitions via its optical properties. Moreover, to the best of our knowledge, there is no COE/CPE probe reported to date that is able to detect the less energetically demanding liquid crystal to lamellar gel transformation, known as the sub-transition, Ts.28
Here, we report for the first time a red-emitting, anionic poly(thiophene) CPE, poly[3-(6-sulfothioatehexyl)thiophene], that can be used to sequentially monitor the three primary phase transitions in model lipid cell membranes using a single probe. P3Anionic (Mn = 4990 g mol−1, PDI = 1.13) was synthesised as previously reported (Fig. 1a).29,30 The CPE contains one charge per repeat unit (r.u.) and is soluble in water at pH = 7.3. The zwitterionic phospholipid DPPC (Fig. 1a) was prepared as large unilamellar vesicles (LUVs) in HEPES buffer solution (pH = 7.3, 30 mM NaCl) as a model cell membrane. DPPC was selected as it undergoes three distinct phase transitions, as shown in Fig. 1b: (i) sub-transition between the liquid crystal, Lc, and lamellar gel, Lβ′, phases (∼19 °C),31 (ii) pre-transition between the Lβ′ and gel, Pβ′, phases (32–35 °C)13 and (iii) main transition between the Pβ′ and fluid, Lα, phases (∼42 °C).31 For each measurement, stock solutions of the CPE were mixed with stock solutions of the DPPC LUVs in HEPES buffer to obtain the required charge ratio between the negative P3Anionic monomer units and the zwitterionic DPPC molecules.
In order to establish the sensitivity of P3Anionic to membrane order, the temperature dependence of the fluorescence properties of the P3Anionic–DPPC complex were monitored (Fig. 1c). Dilute samples of the CPE and CPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPC mixture at a 1
DPPC mixture at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 charge ratio were measured at 2.5 °C intervals between 10–60 °C. Three distinct changes were noted in the emission spectra in response to the temperature: (i) an increase in the emission maximum, λmax, and a decrease in emission intensity between 17.5–20 °C, corresponding to the sub-transition temperature (Ts) at 18.8 °C;31 (ii) an increase in the emission intensity above 32.5 °C, which corresponds to the pre-transition temperature (Tp) of DPPC (at 32–35 °C);13 (iii) the λmax blue-shifts rapidly above 40 °C, which corresponds to the main phase transition temperature (Tm) of 41.6 °C between the gel and fluid phase.31 The observed emission intensity enhancement and blue-shift in the λmax are indicative of higher interchain disorder due to distortion of the thienylene building blocks, which leads to a corresponding decreased conjugative interaction in the deaggregated state.32–34 This suggests that P3Anionic is located in a motionally restricted environment within the lipid bilayer.35,36 This response mode contrasts that of some other microenvironment fluorescent probes, which distinguish phospholipid phases by the penetration depth of water molecules into the lipid bilayer and the degree of lipid packing.13,15,20,35 It should be noted that after equilibrating the CPE
1 charge ratio were measured at 2.5 °C intervals between 10–60 °C. Three distinct changes were noted in the emission spectra in response to the temperature: (i) an increase in the emission maximum, λmax, and a decrease in emission intensity between 17.5–20 °C, corresponding to the sub-transition temperature (Ts) at 18.8 °C;31 (ii) an increase in the emission intensity above 32.5 °C, which corresponds to the pre-transition temperature (Tp) of DPPC (at 32–35 °C);13 (iii) the λmax blue-shifts rapidly above 40 °C, which corresponds to the main phase transition temperature (Tm) of 41.6 °C between the gel and fluid phase.31 The observed emission intensity enhancement and blue-shift in the λmax are indicative of higher interchain disorder due to distortion of the thienylene building blocks, which leads to a corresponding decreased conjugative interaction in the deaggregated state.32–34 This suggests that P3Anionic is located in a motionally restricted environment within the lipid bilayer.35,36 This response mode contrasts that of some other microenvironment fluorescent probes, which distinguish phospholipid phases by the penetration depth of water molecules into the lipid bilayer and the degree of lipid packing.13,15,20,35 It should be noted that after equilibrating the CPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPC mixture at 4 °C for a few days, the initial emission intensity and λmax were recovered. In contrast, the emission intensity of pure P3Anionic in HEPES buffer decreased proportionately as the temperature was increased over this range (Fig. S3, ESI†).
DPPC mixture at 4 °C for a few days, the initial emission intensity and λmax were recovered. In contrast, the emission intensity of pure P3Anionic in HEPES buffer decreased proportionately as the temperature was increased over this range (Fig. S3, ESI†).
To confirm that the observed changes in the fluorescence spectrum as a function of temperature correspond to the phase transitions of pure DPPC, DSC measurements were performed on the LUVs before and after the addition of P3Anionic (Fig. S4, ESI†). The Tm gave rise to an intense, sharp peak at ∼42 °C,13 the Tp is a less intense broad peak at 31–33 °C,13 whilst the Ts is not observed in the thermogram, which is common for lipid bilayers.28 This confirms that the phase transitions specific to DPPC are retained upon addition of the CPE and correlate well with the observed spectral changes. In addition, the negligible effect on the shape or position of the Tm profile at ∼41 °C upon addition of P3Anionic signifies the absence of any substantial lipid reorganisation due to CPE binding.12 However, an increase in the width of the peaks may suggest a decrease in cooperativity among the acyl chains of the DPPC bilayers,37 whilst the modest decrease in the pre-transition temperature (ΔTp ≈ −2 °C) can be attributed to slight destabilisation of the ordered bilayers.38 One possible explanation for this effect is that CPE incorporation disrupts the van der Waals forces between DPPC molecules.26 Although subtle, these apparent structural rearrangements may confer an undesirable increase in the permeability of the membrane bilayer39 or limit the potential resolution of the phase transition temperatures acquired using this method to within a few degrees Celsius.
Phase transition probes require specific localisation of the probe within the phospholipid hydrophobic tails and/or hydrophilic head-groups.5,35 Considering the chemical structures, electrostatic association between the anionic CPE and the positive-charge on the zwitterionic phospholipid head-group is expected.40 The UV/vis absorption and fluorescence spectra of P3Anionic were studied as a function of the concentration of DPPC vesicles at 25 °C. Titration of DPPC LUVs into a dilute solution of P3Anionic (1.93 × 10−5 M (r.u.)) resulted in a significant blue-shift and increased absorbance of the CPE (Δλabs = 435–425 nm), which is accompanied by a red-edge broadening, as shown in Fig. 2a. Red-edge broadening has previously been attributed to an increase in the conjugation length of the CPE;34,41–43 however scattering effects arising from the addition of nanometre sized vesicles to the solution cannot be excluded. P3Anionic exhibits a single broad emission band (λmax = 607 nm) which is quenched upon the addition of DPPC LUVs (Fig. 2b). The amplified quenching of the P3Anionic emission by DPPC vesicles could be the result of the CPE embedding within the membrane and forming non-emissive ground-state complexes with the charged phospholipid head-groups.42
The partition coefficient, Kp, provides information about the partitioning of the CPE between the lipid and water phases of the phospholipid vesicles, and can be determined from the quenching of the fluorescence intensity, from:44
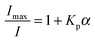 | (1) |
Dynamic light scattering (DLS) has previously been used to confirm that the integrity of phospholipid vesicles is retained upon the addition of a CPE.46 The z-average hydrodynamic diameter (Dh) of the DPPC vesicles was measured before and after addition of P3Anionic.47 The Dh of the DPPC vesicles was 115.3 (±2.0) nm with a narrow polydispersity (PDI) of 0.12 (±0.01). The Dh of the pure CPE was 244.7 (±2.5) nm. The DPPC vesicle size increased to 130.0 (±4.5) nm for DPPC–P3Anionic (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 charge ratio), indicating that the vesicle structure is not disrupted into smaller fragments,46 and that no polymer aggregates are formed.48 In addition, the PDI only increases slightly to 0.16 (±0.02), significantly lower than that of the pure CPE at ∼0.3. Zeta potential (ζ) measurements can be used to determine the effective charge of the vesicle surface, which will change depending on whether electrostatic association between the CPE and DPPC occur at the vesicle surface or within the lipid bilayer.49 In HEPES buffer (pH 7.3) P3Anionic exhibits a negative ζ of −28.1 (±5.2) mV, whilst the ζ of the DPPC LUVs was −3.3 (±0.1) mV, which is close to the literature value of ∼−4.4 mV.50 Upon addition of P3Anionic to a solution of DPPC LUVs (1
1 charge ratio), indicating that the vesicle structure is not disrupted into smaller fragments,46 and that no polymer aggregates are formed.48 In addition, the PDI only increases slightly to 0.16 (±0.02), significantly lower than that of the pure CPE at ∼0.3. Zeta potential (ζ) measurements can be used to determine the effective charge of the vesicle surface, which will change depending on whether electrostatic association between the CPE and DPPC occur at the vesicle surface or within the lipid bilayer.49 In HEPES buffer (pH 7.3) P3Anionic exhibits a negative ζ of −28.1 (±5.2) mV, whilst the ζ of the DPPC LUVs was −3.3 (±0.1) mV, which is close to the literature value of ∼−4.4 mV.50 Upon addition of P3Anionic to a solution of DPPC LUVs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the ζ decreased significantly to −25.4 (±1.0) mV. Since the DPPC vesicles do not completely adopt the ζ of P3Anionic, this suggests that the CPE may be partially penetrating the lipid bilayer, which shields some of the charge, whilst some chains protrude from the outer surface.51
1), the ζ decreased significantly to −25.4 (±1.0) mV. Since the DPPC vesicles do not completely adopt the ζ of P3Anionic, this suggests that the CPE may be partially penetrating the lipid bilayer, which shields some of the charge, whilst some chains protrude from the outer surface.51
Epi-fluorescence microscopy was performed on a mixture of P3Anionic/DPPC multilamellar vesicles (MLVs)52 to demonstrate whether P3Anionic is an effective fluorescent membrane marker (Fig. 3a). The vesicle structure is clearly observable, with the CPE emission localising on the outer layers of the MLVs, demonstrating that the CPE is not internalised within the vesicle. AFM was then used to study the surface morphology of P3Anionic–DPPC LUVs. Samples were found to contain features of two population sizes.53 Small spherical objects, ∼70 nm in diameter, were observed, which are assigned to undoped vesicles (Fig. S7, ESI†). There is no evidence of the pure CPE aggregates which formed large, amorphous aggregates (Fig. S8, ESI†). The second population of objects in the P3Anionic–DPPC samples were 158 (±78) nm in diameter (Fig. 3b). The inset in Fig. 3b clearly shows a multilayer structure in these objects. The inner spheres have a diameter of ∼120 nm, while the outer shell could be CPE protruding from the vesicle surface as suggested by ζ measurements.
The global results indicate a distinct assembly pattern for the P3Anionic–DPPC associations, as shown by the scheme in Fig. 3c. The changes in the UV/vis absorption and fluorescence spectra confirm electrostatic interaction between P3Anionic and DPPC vesicles. AFM, Epi-fluorescence and zeta potential measurements suggest that P3Anionic penetrates within the head-groups of the lipid bilayer, with some chains protruding from the surface. We propose that the net negative charge on P3Anionic works two-fold to control the localisation of the poly(thiophene) within the phospholipid bilayer. Firstly, electrostatic attraction with the external positive ammonium ion of the DPPC may draw the CPE within the head-group region of the bilayer. However, concomitantly electrostatic and hydrophobic–hydrophilic repulsive forces will exist between the negatively charged P3Anionic and the sulfonate group on DPPC and the hydrophobic phospholipid tails, respectively, preventing P3Anionic from burying deeper within the DPPC bilayer. The specific localisation within the head-group region where the CPE will be motionally restricted35 and thus sensitive to the phase of the lipid bilayer, as we have observed.
Recently, drug delivery systems based on phospholipid unilamellar vesicles have been realised which undergo thermally-triggered phase transformations from the gel to the more permeable liquid phase, stimulating the release of the encapsulated drug.54 The unique ability of P3Anionic to monitor phase transitions in real time thus presents a significant opportunity for the development of targeted theranostic platforms based on synthetic zwitterionic phospholipids. Furthermore, as zwitterionic phospholipids form the major component of real cell membranes, we anticipate the observed localisation of P3Anionic, and its sensitivity to phase transitions, to be reflected in real cell membrane studies. However, it should be noted that this study utilises a simplified membrane system, that does not include the multitude of other lipid molecules present in membranes including sphingolipids, cholesterol and membrane proteins.1 These molecules will affect the localisation of the poly(thiophene) within the lipid bilayers to an unknown extent. Thus, whilst this preliminary study has focused on a model membrane system to highlight the effectiveness of P3Anionic as a membrane order probe, future efforts will determine whether P3Anionic can be implanted successfully in live cells.
In summary, we have demonstrated that the red-emitting poly(thiophene), P3Anionic, functions as a fluorescent probe for the sequential identification of the three key phase transitions occurring within DPPC bilayers. In particular, we have shown a facile method to determine the illusive sub-transition temperature of phospholipid membranes. The anionic CPE undergoes charge-mediated localisation within the zwitterionic head-group region of the lipid bilayers where the sensitivity to membrane order is believed to be the greatest.35 Moreover, the large polymer size is known to inhibit cell internalisation,37 which is a common problem for small molecule fluorescent dyes.25 Whilst at only 8 nm in length, P3Anionic remains small enough to accurately probe the raft-like nanodomains in cell membranes (10–200 nm), whose role and impact in cell function is yet to be fully understood.2,4,55 Finally, due its emission in the far-red region P3Anionic should be an ideal probe for in vivo/in vitro studies, since interference from tissue auto-fluorescence should be minimal.
Acknowledgements
This work was supported by a Trinity Award postgraduate scholarship (J. E. H.). The authors would like to thank Dr Gavin McManus for assistance with epi-fluorescence microscopy.Notes and references
- R. B. Gennis, Biomembranes: Molecular Structure and Function, Springer-Verlag New York, Inc., 1989, pp. 1 Search PubMed.
- R. Marin, J. A. Rojo, N. Fabelo, C. E. Fernandez and M. Diaz, Neuroscience, 2013, 245, 26 CrossRef CAS PubMed.
- N. Fabelo, V. Martín, G. Santpere, R. Marín, L. Torrent, I. Ferrer and M. Díaz, Mol. Med., 2011, 17, 1107 CAS.
- W. Stillwell and S. R. Wassall, Chem. Phys. Lipids, 2003, 126, 1 CrossRef CAS PubMed.
- M. Dal Molin, Q. Verolet, A. Colom, R. Letrun, E. Derivery, M. Gonzalez-Gaitan, E. Vauthey, A. Roux, N. Sakai and S. Matile, J. Am. Chem. Soc., 2015, 137, 568 CrossRef CAS PubMed.
- A. S. Klymchenko and R. Kreder, Chem. Biol., 2014, 21, 97 CrossRef CAS PubMed.
- J. Ando, M. Kinoshita, J. Cui, H. Yamakoshi, K. Dodo, K. Fujita, M. Murata and M. Sodeoka, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 4558 CrossRef CAS PubMed.
- R. Dimova, S. Aranda, N. Bezlyepkina, V. Nikolov, K. A. Riske and R. Lipowsky, J. Phys.: Condens. Matter, 2006, 18, S1151 CrossRef CAS PubMed.
- A. Alessandrini and P. Facci, Soft Matter, 2014, 10, 7145 RSC.
- F. A. Heberle, R. S. Petruzielo, J. Pan, P. Drazba, N. Kučerka, R. F. Standaert, G. W. Feigenson and J. Katsaras, J. Am. Chem. Soc., 2013, 135, 6853 CrossRef CAS PubMed.
- J. Pan, F. A. Heberle, S. Tristram-Nagle, M. Szymanski, M. Koepfinger, J. Katsaras and N. Kučerka, Biochim. Biophys. Acta, 2012, 1818, 2135 CrossRef CAS PubMed.
- A. A. Yaroslavov, T. A. Sitnikova, A. A. Rakhnyanskaya, E. G. Yaroslavova, D. A. Davydov, T. V. Burova, V. Y. Grinberg, L. Shi and F. M. Menger, J. Am. Chem. Soc., 2009, 131, 1666 CrossRef CAS PubMed.
- K. A. Riske, R. P. Barroso, C. C. Vequi-Suplicy, R. Germano, V. B. Henriques and M. T. Lamy, Biochim. Biophys. Acta, 2009, 1788, 954 CrossRef CAS PubMed.
- S. Baoukina, E. Mendez-Villuendas, W. F. D. Bennett and D. P. Tieleman, Faraday Discuss., 2013, 161, 63 RSC.
- V. Kilin, O. Glushonkov, L. Herdly, A. Klymchenko, L. Richert and Y. Mely, Biophys. J., 2015, 108, 2521 CrossRef CAS PubMed.
- M. R. Dent, I. López-Duarte, C. J. Dickson, N. D. Geoghegan, J. M. Cooper, I. R. Gould, R. Krams, J. A. Bull, N. J. Brooks and M. K. Kuimova, Phys. Chem. Chem. Phys., 2015, 17, 18393 RSC.
- I.-H. Lee, S. Saha, A. Polley, H. Huang, S. Mayor, M. Rao and J. T. Groves, J. Phys. Chem. B, 2015, 119, 4450 CrossRef CAS PubMed.
- R. Kreder, K. A. Pyrshev, Z. Darwich, O. A. Kucherak, Y. Mély and A. S. Klymchenko, ACS Chem. Biol., 2015, 10, 1435 CrossRef CAS PubMed.
- A. P. Demchenko, Y. Mély, G. Duportail and A. S. Klymchenko, Biophys. J., 2009, 96, 3461 CrossRef CAS PubMed.
- R. Saxena, S. Shrivastava, S. Haldar, A. S. Klymchenko and A. Chattopadhyay, Chem. Phys. Lipids, 2014, 183, 1 CrossRef CAS PubMed.
- M. Nazari, M. Kurdi and H. Heerklotz, Biophys. J., 2012, 102, 498 CrossRef CAS PubMed.
- W. Zheng, Y. Liu, A. West, E. E. Schuler, K. Yehl, R. B. Dyer, J. T. Kindt and K. Salaita, J. Am. Chem. Soc., 2014, 136, 1992 CrossRef CAS PubMed.
- H. Sasaki and S. H. White, Biophys. J., 2009, 96, 4631 CrossRef CAS PubMed.
- J. Seeliger, N. Erwin, C. Rosin, M. Kahse, K. Weise and R. Winter, Phys. Chem. Chem. Phys., 2015, 17, 7507 RSC.
- H.-Y. Wang, H.-R. Jia, X. Lu, B. Chen, G. Zhou, N. He, Z. Chen and F.-G. Wu, J. Mater. Chem. B, 2015, 3, 6165 RSC.
- Z. Kahveci, M. J. Martínez-Tomé, R. Esquembre, R. Mallavia and C. R. Mateo, Materials, 2014, 7, 2120 CrossRef CAS.
- D. A. Doval, M. Dal Molin, S. Ward, A. Fin, N. Sakai and S. Matile, Chem. Sci., 2014, 5, 2819 RSC.
- M. Kinoshita, K. Ito and S. Kato, Chem. Phys. Lipids, 2010, 163, 712 CrossRef CAS PubMed.
- M. Kraft, S. Adamczyk, A. Polywka, K. Zilberberg, C. Weijtens, J. Meyer, P. Görrn, T. Riedl and U. Scherf, ACS Appl. Mater. Interfaces, 2014, 6, 11758 CAS.
- For full synthetic procedure of P3Anionic see the ESI†.
- R. Koynova and M. Caffrey, Biochim. Biophys. Acta, 1998, 1376, 91 CrossRef CAS.
- K. K. Stokes, K. Heuzé and R. D. McCullough, Macromolecules, 2003, 36, 7114 CrossRef CAS.
- C. Tan, E. Atas, J. G. Müller, M. R. Pinto, V. D. Kleiman and K. S. Schanze, J. Am. Chem. Soc., 2004, 126, 13685 CrossRef CAS PubMed.
- R. C. Evans, M. Knaapila, N. Willis-Fox, M. Kraft, A. Terry, H. D. Burrows and U. Scherf, Langmuir, 2012, 28, 12348 CrossRef CAS PubMed.
- S. Shrivastava, S. Haldar, G. Gimpl and A. Chattopadhyay, J. Phys. Chem. B, 2009, 113, 4475 CrossRef CAS PubMed.
- It is known that the labile sulfur–sulfur bonds of the anionic thiosulfate groups can undergo decomposition under thiol formation followed by further chemical reactions. It can't be fully excluded that the CPEs are to some extent anchored into the lipid bilayers by covalent chemical bonding.
- M. J. Tapia, M. Monteserín, H. D. Burrows, J. A. S. Almeida, A. A. C. C. Pais, J. Pina, J. S. Seixas de Melo, S. Jarmelo and J. Estelrich, Soft Matter, 2015, 11, 303 RSC.
- V. Librando, M. G. Sarpietro and F. Castelli, Environ. Toxicol. Pharmacol., 2003, 14, 25 CrossRef CAS PubMed.
- S. Drori, G. D. Eytan and Y. G. Assaraf, Eur. J. Biochem., 1995, 228, 1020 CrossRef CAS PubMed.
- T. Costa, D. de Azevedo, B. Stewart, M. Knaapila, A. J. M. Valente, M. Kraft, U. Scherf and H. D. Burrows, Polym. Chem., 2015, 6, 8036 RSC.
- T. M. Swager, C. J. Gil and M. S. Wrighton, J. Phys. Chem., 1995, 99, 4886 CrossRef CAS.
- S. M. Fonseca, R. P. Galvão, H. D. Burrows, A. Gutacker, U. Scherf and G. C. Bazan, Macromol. Rapid Commun., 2013, 34, 717 CrossRef CAS PubMed.
- M. Chevrier, J. E. Houston, J. Kesters, N. Van den Brande, A. E. Terry, S. Richeter, A. Mehdi, O. Coulembier, P. Dubois, R. Lazzaroni, B. Van Mele, W. Maes, R. C. Evans and S. Clément, J. Mater. Chem. A, 2015, 3, 23905 CAS.
- M. Vermeir, N. Boens and K. P. Heirwegh, Biochem. J., 1992, 284, 483 CrossRef CAS PubMed.
- A. I. Greenwood, S. Tristram-Nagle and J. F. Nagle, Chem. Phys. Lipids, 2006, 143, 1 CrossRef CAS PubMed.
- L. Ding, E. Y. Chi, S. Chemburu, E. Ji, K. S. Schanze, G. P. Lopez and D. G. Whitten, Langmuir, 2009, 25, 13742 CrossRef CAS PubMed.
- A representative correlogram and phase diagram for the DLS and zeta potential measurements, respectively, are available in the ESI†.
- Z. Kahveci, R. Vázquez-Guilló, M. J. Martínez-Tomé, R. Mallavia and C. R. Mateo, ACS Appl. Mater. Interfaces, 2016, 8, 1958 CAS.
- H. Y. Fan, M. Nazari, G. Raval, Z. Khan, H. Patel and H. Heerklotz, Biochim. Biophys. Acta, 2014, 1838, 2306 CrossRef CAS PubMed.
- A. E. Wiącek, Appl. Surf. Sci., 2011, 257, 4495 CrossRef.
- M. Kepczynski, D. Jamróz, M. Wytrwal, J. Bednar, E. Rzad and M. Nowakowska, Langmuir, 2012, 28, 676 CrossRef CAS PubMed.
- Larger MLVs (5–20 μm in diameter) were used for Epi-fluorescence microscopy measurements due to the limited size resolution of this technique. The preparation procedure for MLVs is in the ESI†.
- Doped and undoped vesicles were assigned according to their relative diameters. Small spheres (∼70 nm) were assigned to ‘undoped’ DPPC vesicles based on the average vesicle diameter determined from pure DPPC samples, see Fig. S7a, ESI.† Larger spheres (>100 nm) were assigned as P3Anionic-doped DPPC vesicles.
- D. Needham, G. Anyarambhatla, G. Kong and M. W. Dewhirst, Cancer Res., 2000, 60, 1197 CAS.
- D. Lingwood and K. Simons, Science, 2010, 327, 46 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of P3Anionic, preparation of DPPC vesicles, instrumental methods, fluorescence, representative correlogram and phase diagram for DLS and ζ, respectively, DLS data, DSC thermograms and AFM images. See DOI: 10.1039/c6cp01553k |
| This journal is © the Owner Societies 2016 |



