In situ monitoring the role of citrate in chemical bath deposition of PbS thin films
Sucheta
Sengupta
,
Maayan
Perez
,
Alexander
Rabkin
and
Yuval
Golan
*
Department of Materials Engineering, and the Ilse Katz Institute for Nanoscale Science and Technology, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel. E-mail: ygolan@bgu.ac.il
First published on 16th November 2015
Abstract
Trisodium citrate (citrate) has been commonly used as a co-complexing agent during chemical bath deposition to improve film quality and control grain size, yet its precise role has not been elucidated to date. In this study, we have focused on the role of citrate in chemically deposited PbS thin films. Citrate effectively complexes the lead cation in solution thereby decreasing growth rate and inducing growth in the cluster mechanism. This was monitored in real time using laser light-scattering and UV-vis absorption spectroscopy which quantitatively confirmed the retarded PbS formation in the cluster mechanism in the presence of citrate. Nanocrystalline PbS films formed in the presence of citrate show quantum confinement effects with blue shifted optical properties compared to the bulk, adding an important path for controlling film properties towards future infrared optoelectronic applications.
Introduction
Chemical bath deposition (CD) is by far the most cost effective method for reproducibly producing high quality semiconductor thin films.1 Early studies focused on deposition of lead chalcogenide (PbS, PbSe) thin films by CD due to their technological importance as infrared radiation detectors and emitters predicted as early as 1904.2,3 Particularly, lead sulfide (PbS) has been the subject of considerable research due to its direct narrow bandgap energy (0.41 eV) and large exciton Bohr radius (18 nm) and useful optoelectronic properties for infrared detection and emission.4–6 Deposition of thin PbS films on GaAs provides the means for integration of PbS IR optoelectronics with GaAs based technology.We have previously showed that control over the microstructure of PbS thin films on GaAs (100) can be achieved by optimizing the deposition time, deposition temperature and the concentrations of the reactants present in the bath.7–9 PbS was deposited from a highly alkaline aqueous solution where the NaOH, besides affecting the rate of thiourea decomposition, also effectively complexes the Pb2+ metal ions. Trisodium citrate (citrate) has been commonly used as a co-complexing agent during CD especially for metal cations such as zinc (Zn2+) and tin (Sn2+) to produce high quality films with better uniformity and decrease in the grain size.10,11 In the particular case of PbS, it was observed that an increase in the concentration of citrate resulted in increased film uniformity and decrease in the crystallize size, with the citrate ions being present as the functional group in the PbS film.12–14 In the presence of citrate, the hydroxyl citrate complex of Pb [Pb(OH)(cit)]2− (with variable co-ordination number depending on the pH of the solution15) would be expected to decompose differently from the common Pb–hydroxide complex. Thus, it would be important to study the effect of the co-complexant on film growth which can provide control over the growth mechanism and result in different film morphologies and physical properties.
The mechanism involved in the CD process is quite intriguing and holds the key for controlling film morphology. Generally, the role of the complexing agent is to bind with the metal ions to prevent rapid reaction and precipitation of the metal hydroxide; hence, in the absence of a complexant there is excessive and rapid precipitation with little or no film formation. The metal complex allows the slow release of the free metal ion to react with the slowly generated anion (usually formed by the controlled hydrolysis of the precursor) directly onto the substrate, a process commonly known as the ion-by-ion mechanism. Alternatively, the film growth can also occur by migration and adsorption of colloidal particles of either the metal chalcogenide, the metal hydroxide or the insoluble metal complexes generated in the solution onto the substrate, a process commonly known as the cluster mechanism. The change in the mechanism is manifested in a shift from the formation of a highly oriented monocrystalline film to a nano-crystalline cluster. Hodes et al. have previously demonstrated that this changeover in the mechanism can be affected by a change in the metal-complex ratio.16,17 They described that there is a particular critical ratio (Rc) between the complexing agent and the metal ions below which the cluster mechanism is dominant which can be related to the presence of a metal-hydroxide, and above it, one has the direct deposition of the film onto the substrate through the ion-by-ion mechanism. The range of Rc could be more accurately defined in the case of CdSe compared to PbSe since PbSe was always found to form in the solution under all the experimental conditions. Notably, under the same conditions, PbS was always found to form directly onto the substrate. This change in the behavior can be rationalized by comparing the solubility product of the different chalcogenides formed which decreases in the order PbS > CdSe > PbSe and hence the nucleation of PbSe occurs at much lower concentrations in the solution than would be required for equivalent formation of either CdSe or PbS.
Despite the critical role of citrate as a complexing agent in CD, its exact role in affecting the film growth has rarely been systematically studied. The present work elucidates the two opposing roles of citrate depending on the active mechanism involved. Using in situ light scattering and optical absorbance spectroscopy, we have monitored the deposition solutions during the early stages of the film growth. Combined with ex situ characterization of the resulting thin films, we show that citrate has a unique and well-defined effect depending on the active growth mechanism.
Experimental
2.1 Materials and methods
PbS thin films were deposited from solutions containing Pb(NO3)2, CS(NH2)2, Na3C6H5O7 and NaOH at two different temperatures. Thiourea (TU) (Aldrich, ACS ≥99.0%), lead nitrate (Aldrich, analytical grade 99.99+%), trisodium citrate (referred to here as ‘citrate’) (Aldrich) and sodium hydroxide (Aldrich, reagent grade ≥98% pellets, anhydrous) were used without further purification. Distilled water was obtained from a Millipore Direct Q3 system. The deposition temperature was fixed at 20 and 30 °C. The deposition solutions were prepared by adding NaOH, citrate and lead nitrate in this particular order. Before the addition of TU, the last reagent, the solutions were purged with pure N2 gas for 60 min in order to reduce the levels of dissolved O2 and CO2. Single side polished, undoped GaAs (100) wafers were purchased from AXT Inc. The wafers were cleaved into 2 cm × 1 cm samples, cleaned with ethanol and water and passivated for 10 min in the deposition solution prior to the deposition. Typically, the deposition was carried out for 120 min at a particular temperature controlled by a thermostat. The resultant film morphology was studied by X-ray diffraction (XRD) and ultrahigh resolution scanning electron microscopy (UHR-SEM). For in situ measurements, the growth solutions (except for TU) were purged and thermally equilibrated for 5 min before initiating the reaction by adding the last reagent, the point from where real time monitoring was carried out using laser light scattering and UV-vis spectroscopy.2.2 Characterization methods
Results and discussion
The effect of citrate concentration on the microstructure of PbS films chemically deposited on GaAs (100) for 120 min at a temperature of 20 °C was studied using XRD (Fig. 1). The diffractogram in Fig. 1a corresponds to a PbS film grown at 20 °C in the absence of citrate. Fig. 1b–d corresponds to films grown at 20 °C with three different concentrations of citrate, 4.25 mM, 8.5 mM and 17 mM. The XRD patterns indicate that the introduction of citrate in the deposition solution induces prominent texturing in the (100) direction. Interestingly, this texturing is observed for the lowest concentration of citrate and thereafter increasing the concentration has little effect on the film texture. The HRSEM images (Fig. 1e–h) show that the films deposited in the presence of citrate are composed of highly oriented cube-shaped ∼80–100 nm grains compared to the randomly oriented grains observed in the absence of the citrate. Another prominent observation is the drastic reduction in the grain size in the presence of citrate. These cube-shaped grains are formed as the preferred morphology for all citrate concentrations.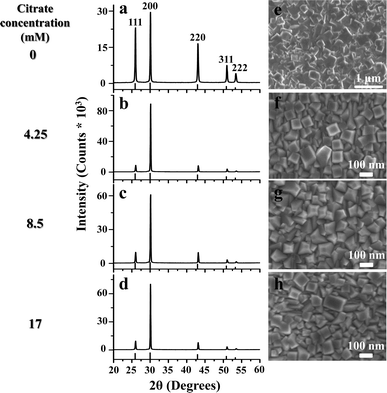 | ||
| Fig. 1 (a–d) XRD patterns for films grown at 20 °C for 120 min with different concentrations of citrate, 0 mM, 4.25 mM, 8.5 mM and 17 mM, respectively. (e–h) Corresponding plan-view HRSEM images. | ||
This trend in orientation can be quantitatively evaluated by plotting the diffracted intensity ratio I(200)/I(220) as a function of citrate concentration (Fig. 2a). In the presence of citrate, this ratio increases from the powder ratio (randomly oriented grains) in the 0 mM citrate sample to about 6 for all three concentrations, reflecting the prominent (100) texture. The film thickness values were estimated from cross sectional HRSEM images and plotted as a function of citrate concentration (Fig. 2b). In the presence of the citrate, the film thickness is drastically reduced compared to the blank. With increasing citrate concentration, the film thickness slightly decreases further. In agreement with the film thickness variation, the integrated area of the XRD spectra similarly decreases with increasing citrate concentration.
Next, the deposition temperature was increased to 30 °C. The resultant XRD diffractograms for the films grown for 120 min at 30 °C with different citrate concentrations are described in Fig. 3(a–d). At 30 °C, without citrate, the resultant PbS thin film has a strong (110) texture as previously reported in the literature (Fig. 3a),7,9 while there is a gradual increase of the (200) Bragg peak with increasing citrate concentration. The corresponding plan-view HRSEM images are described in Fig. 3(e–h). For films deposited in the presence of citrate, the HRSEM images show the formation of cube-shaped grains with increasing citrate concentration along with the presence of a rectangular pyramidal topography that is typical for <110> oriented films. Although the cube-shaped grains are formed for the highest citrate concentration at both the temperatures, it is important to note that the grain size is increased quite significantly with temperature.
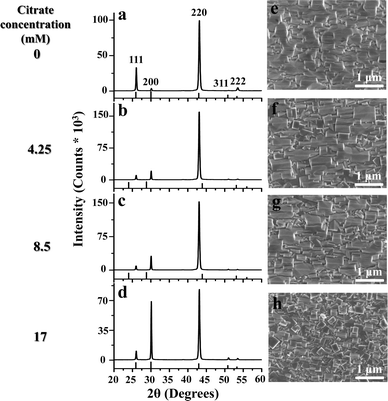 | ||
| Fig. 3 (a–d) XRD patterns for films grown at 30 °C for 120 min with different concentrations of citrate, 0 mM, 4.25 mM, 8.5 mM and 17 mM. (e–h) Corresponding plan-view HRSEM images. | ||
This increase in the preferred texturing can be quantified by plotting the ratio of Bragg peak intensities I(200)/I(220) as a function of citrate concentration (Fig. 4a). With increasing citrate concentration, the intensity ratio increases and approaches the powder ratio value from a highly (110) oriented film in the case of the blank. When the film thickness is plotted as a function of citrate concentration, it is found to initially increase compared to the blank and then remains more-or-less constant with further increase in the citrate concentration (Fig. 4b). The same trend was also observed, as expected, for the integrated peak area of the XRD spectra vs. citrate concentration. Interestingly, with the highest citrate concentration, although the film thickness increases from the blank, the total integrated peak area as calculated from the XRD spectrum decreases. This may suggest the formation of amorphous clusters resulting from the parallel growth of the cluster mechanism along with the dominant ion-by-ion mechanism induced by the presence of citrate.
Apart from varying the growth temperature, we have studied the effect of the pH of the growth solution at both the temperatures. At 20 °C, at pH 12.9 and 13.2 randomly oriented PbS grains are formed (Fig. 5a and b) in the absence of citrate. In the presence of citrate, cube shaped grains are formed at both pH values with the average dimensions increasing with pH from ∼20–50 nm to ∼100 nm (Fig. 5d and e). Upon increasing the pH further to 13.4, both in the presence and in the absence of citrate, the resultant film morphology is composed of a rectangular pyramidal topography (Fig. 5c and f) typical of the ion-by-ion growth mechanism.
 | ||
| Fig. 5 Plan-view HRSEM images of samples deposited at 20 °C for 120 min with three different pH values. (a–c) 0 mM citrate, (d–f) 17 mM citrate. | ||
The same trend is observed at a deposition temperature of 30 °C (Fig. 6). At pH 12.9 the presence of citrate results in the formation of nanoscale spherical particles. At pH 13.2, cube-like grains are formed in the presence of citrate and with further increase in pH to 13.4; the presence of the citrate has little effect on the orientation of the film.
 | ||
| Fig. 6 Plan-view HRSEM images of samples deposited at 30 °C for 120 min with three different pH values. (a–c) 0 mM citrate, (d–f) 17 mM citrate. | ||
The variation in film thickness vs. pH at both temperatures is presented in Fig. 7. From the plots it is evident that there is a transition between the two active deposition mechanisms with the change in temperature and pH. Citrate is found to have an opposing effect on the two deposition mechanisms. While it retards the film growth in the case of the cluster mechanism, for the ion-by-ion mechanism the film thickness increases in the presence of citrate. Interestingly, the transition between the two mechanisms was found to shift to a lower pH value with increasing temperature, at 20 °C the pH for the transition was found to be 13.2 which decreases to <13.1 at 30 °C.
 | ||
| Fig. 7 Film thickness plotted vs. pH for 0 mM and 17 mM citrate (dashed and solid lines, respectively) for two deposition temperatures: (a) 20 °C and (b) 30 °C. | ||
From the above results, it is evident that the citrate affects the growth of PbS in two completely different ways depending on the active deposition mechanism. It has been previously shown that the observed changes in film morphology upon changing the temperature or pH occur due to a transition in the active deposition mechanism.1 As discussed earlier, there are primarily two main mechanisms that are active in CD, the cluster mechanism and the ion-by-ion mechanism. It has been shown earlier that these two mechanisms result in distinct film morphologies and can easily be identified using laser light scattering (LS).18 The cluster mechanism gives rise to high scattering intensities which strongly depend on the particle size and particle concentration present in the solution. On the other hand, the scattering intensity sharply decreases once the clusters settle and the deposition proceeds via a transition to the ion-by-ion mechanism. Thus using LS one can effectively determine the transition point between the mechanisms which can be further correlated with the structural characterization of the films obtained from time interrupted growth series.18
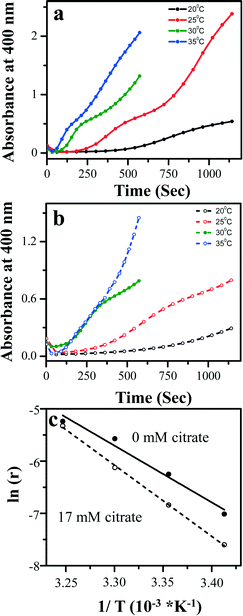
In situ LS measurements have been performed for the deposition solutions at 20 °C and 30 °C both in the absence and in the presence of citrate, and are shown in Fig. 8. It is seen that at the beginning of the reaction, the scattering intensity increases sharply, indicating the initiation of deposition in the cluster mechanism, where clusters are formed during the initial stages of the reaction giving rise to a strong scattering intensity. Subsequently, upon increasing the reaction time, most particles settle and the solution becomes transparent to light indicating a transition to an active ion-by-ion mechanism. In the absence of citrate at 20 °C, this transition time is found to be in the interval of 30–45 min after the reaction commences. However, in the presence of citrate, this transition is substantially slowed down and it now occurs in the time interval range of 75–120 min after the commencement of the reaction. Interestingly, the onset of the reaction is not only delayed but the FWHM (full-width-half-maximum) of the curves also increases from ∼9 min (in the blank) to ∼20 min in the presence of citrate. On increasing the temperature to 30 °C, we see the same effect but at a much shorter time scale. Without citrate, there is an initial formation of clusters which rapidly settle within 8–15 min. In the presence of citrate, the initial cluster formation is somewhat a bit delayed with the transition now occurring within 15–25 min. At this temperature also, the FWHM of the curves increases from ∼4 min (in the blank) to ∼10 min in the presence of citrate. Thus, the LS results indicate that the presence of citrate in the deposition solution results in a much delayed transition from the cluster to the ion-by-ion mechanism at both the temperatures.
It has previously been reported that in a polydisperse solution, the scattering intensity depends on the size of the particles and thus at low scattering angles most scattered light comes from the large particles and at higher angles most scattered light comes from the smaller particles.19 While at higher temperature, 30 °C, there is not much of a difference in the scattering intensities with the presence of citrate, at 20 °C the scattering intensity at the lower angle is much higher in the presence of citrate compared to the blank. In the presence of citrate, due to the chelating effect, it can presumably bind to more than one metal ion to form larger, chain-like clusters, increasing the extent of Pb2+ complexation and slowing the rate of the complex decomposition and hence delaying the formation of the PbS film via the cluster mechanism. Once the mechanism switches to the ion-by-ion mechanism at higher temperature, the complex directly and rapidly decomposes on the substrate and hence the presence of citrate has little effect on the rate of the PbS film formation and on the resulting film morphology.
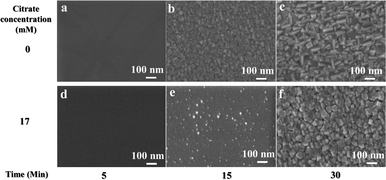
To verify this assumption, an interrupted growth series of chemically deposited PbS films in the presence and absence of citrate was deposited on GaAs (100) with the growth terminated after 5, 15 and 30 min. The resulting samples were studied using plan-view HRSEM and the results are depicted in Fig. 9. As the LS data suggested, at 20 °C, 5 min after the initiation of the reaction, there is no sign of nucleation for both with and without citrate. However, in the absence of citrate, film growth started after 15 min with uniform PbS nucleation which grows to form bigger grains with time, while in the presence of citrate, the commencement of the reaction is drastically retarded with very small signs of nucleation showing up only after 15 min of reaction, and resulting in non-uniform coverage.
For a deposition temperature of 30 °C, uniform nucleation is seen already after 5 min in the absence of citrate, which rapidly grows to larger crystallite size with time (Fig. 10a–c). In the presence of citrate, the nucleation is somewhat delayed with a uniform film forming only after 15 min of reaction (Fig. 10d–f). In general, the addition of citrate slows down the reaction, manifested by slower nucleation on the GaAs substrates. This observation is in good agreement with the data obtained from the LS experiments.
The fact that the cluster mechanism is favoured by the retarded growth rate in the presence of citrate is evidenced from the formation of cube-like PbS nanocrystals in the presence of the citrate. It has been reported that at low deposition rates (with sufficient thermal energy and a low flux of reactants), controlled growth results in the most stable shapes of the film grains such as cube-shaped grains in the rocksalt phase.20,21 The grains in the initial nucleation of the PbS film appear to have a rounded shape as can be seen in Fig. 9e and 10a, and then develop into shapes that are terminated by (111) and (100) facets, and their relative stability determines the final shape of the nanocrystal.20 The facets usually develop during the ion-by-ion growth mechanism. After the initial film formation through the cluster mechanism, there is a transition to the ion-by-ion mechanism in which these initial clusters transform into faceted nano-cubes. In rocksalt structures, the (111) surface typically has a higher surface energy and hence in a thermodynamically driven regime, there is a faster growth on the (100) facets favouring the formation of cube shaped PbS nanocrystals. In the presence of the citrate, the effective complexation of Pb2+ results in the low supply of free metal cations, resulting in the thermodynamically driven regime which in turn results in the cube shaped PbS nanocrystals.
To quantify the slower growth rate in the presence of citrate, the initial formation of the PbS clusters in solution was monitored in real time using UV-vis absorption under different deposition conditions and the resulting spectra are shown in Fig. 11. Interestingly, before the addition of TU, the last reagent to initiate the reaction, there is a peak at ∼300 nm for solutions containing Pb2+ ions and NaOH (similar with or without citrate). This peak corresponds to the formation of various Pb–OH complexes as described by Hodes et al.17 However, the peak intensity and position are unaffected by the presence of citrate, indicating that –OH remains the dominating complexing agent and citrate is acting as a co-complexant at this relatively high pH. This well-defined peak at 300 nm is replaced by a strong absorption edge with an onset at ∼330 nm immediately after thiourea addition and initiation of the reaction. At lower temperature, 20 °C, with time there is a new peak emerging at ∼400 nm which is further red shifted with time to ∼600 nm, indicating the growth of the initial PbS clusters in suspension. The walls of the absorption cuvette were found to be not absorbing in this region, indicating that film formation on the quartz cuvette is negligible and that these results are due to absorption of the PbS clusters formed in the solution itself. Estimating the possible sizes from the band gap calculation22 reveals the formation of ultra-small PbS nanoclusters with sizes ranging from ∼2–3 nm which ripen in size with time. In the presence of citrate at the same temperature, the peak growth is much slower, showing that within the same time interval of 20 min, the peak remains at 400 nm without further red shift. At 30 °C, in the absence of citrate, the peak appears much faster (after 1 min) and rapidly red-shifts towards the near infrared region. Interestingly, after 10 min from initiation of the reaction, the peak at 400 nm re-appears and again, red-shifts with time, probably indicating a second phase of nucleation in solution. In the presence of citrate at 30 °C, the red shifting of the peak is much slower, with the peak shifting to 700 nm for the same interval of time (10 min).
This rate of formation of PbS can be quantified by plotting the rate of change in absorbance at a particular wavelength with time at different temperatures (Fig. 12a and b). This rate of change can be fitted with a straight line indicating a zero-order reaction. For growth through the cluster mechanism, the rate determining step is supposed to be the decomposition of the metal-complex on the substrate with the rates independent of the concentrations of both Pb and TU while for the ion-by-ion mechanism, the rate is found to be directly dependent on the reaction between the metal ion and the sulfide ions onto the substrate. Thus, the occurrence of a zero-order reaction indicates the presence of the cluster mechanism. The line fitting provides the corresponding growth rate (r) which can be subsequently used for constructing Arrhenius plots, showing an inverse exponential dependence of the growth rate with temperature (Fig. 12c). The Arrhenius plot presents an evaluation of the activation energies of the reaction in the presence and absence of citrate. The activation energy for the formation of PbS in the absence of citrate has been found to be 19 ± 2 kcal mol−1. In the presence of citrate, the activation energy was found to substantially increase to 30 ± 3 kcal mol−1. This quantitatively reflects the much slower formation of PbS clusters in the presence of citrate in solution, due to the effective complexation of the Pb2+ ion.
From the results obtained so far it has been shown that the introduction of citrate results in a significant decrease in particle size which can be expected to affect the optical properties of the films.23–25 The ability to tune the optical properties of lead chalcogenide thin films has been of considerable interest for advanced optoelectronic applications such as short wave infrared night vision systems, etc. We note that band gap values obtained from the straightforward PL measurements are accurate compared to those obtained from IR transmission measurements which must be corrected for reflectance and depend on surface quality. The previous HRSEM images show the formation of three different sizes of PbS under different experimental conditions. At 30 °C and pH 13.2 bulk PbS is formed in the absence of citrate. Decreasing the temperature to 20 °C, in the presence of 17 mM citrate, PbS nanocubes with dimensions of ∼100 nm are formed and further decreasing the pH to 12.9 results in the formation of nanocubes with dimensions of ∼20–50 nm. The PL spectra obtained for these three different PbS films are depicted in Fig. 13d.
The optical properties of PbS have been previously studied by various groups which suggested a hyberbolic model to explain the band gap dependence on particle size.26–29 Our PL spectra indicate a significant blue shift from the bulk value depending on the size of the PbS nanocrystals, showing a clear trend of increasing band gap energy with decreasing grain size. We note that it is quite surprising that quantum size effects are observed already for the 100 nm nanocube sample, suggesting either that (i) the bulk of the film is composed of grains that are substantially smaller than those observed with HRSEM on the sample surface or that (ii) the individual nanocubes are composed of sub-domains that are separated by confining boundaries. These results indicate that realization of size tunable band gap properties of the PbS nanocrystals can be easily achieved by using citrate bath additives under moderate growth temperatures and relatively short deposition periods. Earlier studies reported that nanocrystalline PbS could only be achieved at extremely low bath temperatures (close to 0 °C) and for significantly long deposition times (over 24 hours).30
Conclusions
We conclude that the citrate acting as a co-complexant for the Pb2+ cations has a prominent effect on the CD of PbS thin films. For the conditions favouring film growth via the cluster mechanism, the presence of citrate slows down the reaction of PbS formation, with strong texturing along the (100) direction and the formation of cube-shaped PbS nanocrystals. The slower growth rate in the presence of citrate has been confirmed using HRSEM and XRD coupled with in situ light scattering and UV-vis absorption spectroscopy for real time monitoring of deposition. Calculations indicate a substantial increase of ∼10 kcal mol−1 in activation energy in the presence of citrate which results in retardation of the rate of cluster formation in solution. Upon increasing the temperature and pH, the ion-by-ion mechanism becomes dominant and citrate has less effect on the deposition and on the resulting film morphology. The slower growth rate during film growth via the cluster mechanism leads to a significant decrease in grain size of the films, and subsequently, to a blue shift in the film bandgap. Apart from acting as a co-complexant, the citrate additive appears to act also as a surface active agent, stabilizing the smaller PbS grain size which can be obtained at moderate growth conditions. This trend in decreasing PbS nanocrystal size and its manifestation in a corresponding increase in band gap energy, provides additional means of control for future use of size quantized films in advanced optoelectronic applications.Acknowledgements
We thank Dr. Dmitry Mogilyansky for expert assistance in XRD, Dr. Sharon Hazan for expert assistance in LS measurements and Prof. Ira Weinstock for providing access to their UV-vis spectrometer. This work was funded by the Israel Science Foundation under Grant #156/14, and by the Focal Technological Area (FTA) program of the Israeli National Nanotechnology Initiative (INNI). S. S. G acknowledges a postdoctoral fellowship from the Planning and Budgeting Committee of the Israeli Council for Higher Education.Notes and references
- G. Hodes, Chemical Solution Deposition of Semiconductor Films, Marcel Dekker, Inc., New York, 2002 Search PubMed.
- J. C. Bose, US Pat., 755,840, 1904 Search PubMed.
- P. K. Nair, M. T. S. Nair, A. Fernandez and M. Ocampo, J. Phys. D: Appl. Phys., 1989, 22, 829 CrossRef CAS.
- P. K. Nair and M. T. S. Nair, J. Phys. D: Appl. Phys., 1990, 23, 150 CrossRef CAS.
- D. Kumar, G. Agarwal, B. Tripathi, D. Vyas and V. Kulshrestha, J. Alloys Compd., 2009, 484, 463 CrossRef CAS.
- S. Seghaier, N. Kamoun, R. Brini and A. B. Amara, Mater. Chem. Phys., 2006, 97, 71 CrossRef CAS.
- A. Osherov, V. Ezersky and Y. Golan, J. Cryst. Growth, 2007, 308, 334 CrossRef CAS.
- A. Osherov and Y. Golan, MRS Bull., 2010, 35, 790 CrossRef CAS.
- A. Osherov, V. Ezersky and Y. Golan, Eur. Phys. J.: Appl. Phys., 2007, 37, 39 CrossRef CAS.
- F. Gode, E. Guneri and O. Baglayan, Appl. Surf. Sci., 2014, 318, 227 CrossRef CAS.
- G. L. Agawane, S. W. Shin, A. V. Moholkar, K. V. Gurav, J. H. Yun, J. Y. Lee and J. H. Kim, J. Alloys Compd., 2012, 535, 53 CrossRef CAS.
- E. Yucel, Y. Yucel and B. Beleli, J. Alloys Compd., 2015, 642, 63 CrossRef CAS.
- S. I. Sadovnikov and A. I. Gusev, J. Alloys Compd., 2014, 586, 105 CrossRef CAS.
- S. B. Pawar, J. S. Shaikh, R. S. Devan, Y. R. Ma, D. Haranath, P. N. Bhosale and P. S. Patil, Appl. Surf. Sci., 2011, 258, 1869 CrossRef CAS.
- M. Bobtelsky and B. Graus, J. Am. Chem. Soc., 1953, 75, 4172 CrossRef CAS.
- S. Gorer and G. Hodes, J. Phys. Chem., 1994, 98, 5338 CrossRef CAS.
- S. Gorer, A. Albu-Yaron and G. Hodes, Chem. Mater., 1995, 7, 1243 CrossRef CAS.
- M. Shandalov and Y. Golan, Chem. Mater., 2006, 18, 3593 CrossRef CAS.
- C. Tanford, Physical Chemistry of Macromolecules, John Wiley & Sons, Inc., New York, 1961 Search PubMed.
- S. M. Lee, S. N. Cho and J. Cheon, Adv. Mater., 2003, 15, 441 CrossRef CAS.
- Y. Xiong and Y. Xia, Adv. Mater., 2007, 19, 3385 CrossRef CAS.
- I. Moreels, K. Lambert, D. Smeets, D. De Muynck, T. Nollet, J. C. Martins, F. Vanhaecke, A. Vantomme, C. Delerue, G. Allan and Z. Hens, ACS Nano, 2009, 3, 3023 CrossRef CAS PubMed.
- N. Gutman, A. Armon, A. Sa'ar, A. Osherov and Y. Golan, Appl. Phys. Lett., 2008, 93, 073111 CrossRef.
- N. Gutman, A. Armon, M. Shandalov, A. Osherov, Y. Golan and A. Sa'ar, J. Nanosci. Nanotechnol., 2009, 9, 3648 CrossRef CAS PubMed.
- N. Gutman, A. Armon, A. Osherov, Y. Golan and A. Sa'ar, Phys. Status Solidi A, 2009, 206, 1290 CrossRef CAS.
- Y. Nosaka, J. Phys. Chem., 1991, 95, 5054 CrossRef CAS.
- Y. Wang, A. Suna, W. Mahler and R. Kasowski, J. Chem. Phys., 1987, 87, 7315 CrossRef CAS.
- J. J. Valenzuela-Jauregui, R. Rami rez-Bon, A. Mendoza-Galvan and M. Sotelo-Lerma, Thin Solid Films, 2003, 441, 1104 CrossRef.
- R. K. Joshi, A. Kanjilal and H. K. Sehgal, Appl. Surf. Sci., 2004, 221, 143 CrossRef.
- T. Safrani, T. A. Kumar, M. Klebanov, N. Arad-Vosk, R. Beach, A. Sa'ar, I. Abdulhalim, G. Sarusi and Y. Golan, J. Mater. Chem. C, 2014, 2, 9132 RSC.
| This journal is © The Royal Society of Chemistry 2016 |