A study on the influence of sodium carbonate concentration on the synthesis of high Mg calcites†
Heng
Yang
,
Shiqiang
Chai
,
Yuzhe
Zhang
and
Yurong
Ma
*
College of Chemistry, Peking University, Beijing, 100871, China. E-mail: yurong.ma@pku.edu.cn; Fax: 086 1062751708; Tel: 086 1062753269
First published on 16th November 2015
Abstract
Well crystallized high Mg calcites in pure phase with Mg contents higher than 50 mol%, so-called protodolomite, have not been synthesized in the laboratories to our knowledge. In this work, the synthesis of high Mg calcites in pure phase with Mg contents controlled from 10 to 63 mol% was realized by using amorphous calcium magnesium carbonate as an intermediate precursor phase through a hydrothermal process in the absence of any organic additive. Besides the molar ratios of [Mg2+]/[Ca2+], the molar ratios of carbonate and calcium ions in the mother solutions are also very important for the Mg contents in high Mg calcites in pure phase. The higher molar ratios of [CO32−]/[Ca2+] in the mother solutions, the higher Mg contents in Mg calcites can be reached at relatively low carbonate concentrations. This is the first time that the variation of Mg contents in high Mg calcites by changing the molar ratios of [CO32−]/[Ca2+] in the mother solutions has been reported. Further increase of carbonate concentration results in the formation of other crystal phases such as Ca-magnesite, brucite, and aragonite. Reports on the structural analysis and formation mechanisms of thermodynamically unstable biogenic high-Mg calcite minerals may shed light on the preparation of functional materials with enhanced mechanical properties.
Introduction
High magnesium-bearing calcite with Mg contents higher than 10 mol% is a thermodynamically unstable phase of calcium carbonate under ambient conditions,1–3 which are normally synthesized at high temperature and high pressure in the laboratories.4–6 However, biogenic calcites with very high Mg contents (20–43 mol%) have been found in many marine organisms such as coralline algae,7 Alcyonarian corals,8 echinoids (sea urchins)9,10 and sea stars.11 How marine organisms form high Mg calcites under ambient conditions has been an enigma for a long time. Amorphous calcium magnesium carbonate (ACMC) is supposed to be an intermediate precursor phase for the formation of biogenic high Mg calcites in biominerals.12–15 It was reported that magnesium plays a key role in stabilizing the amorphous calcium carbonate (ACC) phase2,16,17 and in improving the mechanical properties of biogenic Mg calcites.18,19 Therefore, studying the formation mechanism of high Mg calcites not only has great importance in fundamental research of biomineralization but may also shed light on the design and fabrication of new functional materials.The synthesis of Mg-bearing calcites has been successful under ambient conditions in the last few years with the help of an intermediate amorphous phase,2,20,21 mixed solvents,22–25 organic/inorganic surfaces,26 and soluble additives.20,25,27–32 The Mg content in high Mg calcites is considered to be positively related to the [Mg2+]/[Ca2+] molar ratio in the reaction solution.20,30,33 Raz et al. synthesized magnesium calcite particles with 34 mol% magnesium along with aragonite in the presence of macromolecules extracted from skeletons of coralline algae.2 Calcites with at most 15 mol% magnesium content and aragonite were produced by using additives that contain alcohols22 or carboxylate groups27,30,34 that usually occur in biomineral-producing organisms in solutions. Cheng et al. produced high magnesium-bearing calcite films via a polymer induced liquid precursor process, which contain up to 26 mol% magnesium but with a low crystallization degree.29 Compared with biogenic high magnesium-bearing calcites, these synthesized Mg-bearing calcite products often contain considerable amounts of aragonite,2,21,22,25,27 as well as a certain amount of ACC.29 The synthesis of thermodynamically unstable high Mg calcites in pure phase is especially difficult under ambient conditions. It is hard for Mg2+ to be inserted into the calcite lattices because of the bigger hydration radius of Mg2+ ion than that of Ca2+ ion,35 which is attributed to the higher solvation free energy36 and the higher dehydration enthalpy34 of Mg2+ ion than those of Ca2+ ion at room temperature. There is a fundamental barrier that prevents the formation of long range ordered structures of Mg2+ and CO32− resulting from the lattice limitation on the spatial structure of the carbonate group in high Mg carbonate minerals (i.e., dolomite and magnesite).37 Our group succeeded in synthesizing high Mg calcites in pure phase with Mg contents as high as 43 mol% via polymer stabilized ACMC precursors under ambient conditions.20
Aqueous carbonate accumulation and the pH value of seawater are important for the mineralization rates of biogenic calcium carbonate in marine organisms. It was pointed out that the calcification rates for marine organisms decline along with the carbonate concentration owing to its reaction with increasing concentration of anthropogenic CO2.38 Furthermore, it was found that the aqueous carbonate concentration influences the polymorphs of calcium carbonate while the [CO32−]/[Ca2+] molar ratio is between 10−1.5–10−3.5.39 However, the influence of the [CO32−]/[Ca2+] ratio on the crystallization process of magnesium containing calcium carbonate has not been investigated as far as we know. The effect of relatively high [CO32−]/[Ca2+] molar ratio (>0.1) or relatively high carbonate concentration (>0.05 mol L−1) on the polymorph of calcium carbonate has not been studied up to now.
The aim of this work is to study the influence of the molar ratios of [CO32−]/[Ca2+] and [Mg2+]/[Ca2+] on the crystal phases of carbonate minerals and the Mg contents in Mg containing calcites. A hydrothermal process was applied for the synthesis of very high Mg calcites in pure phase by using ACMC as an intermediate precursor.
Results and discussion
The amorphous precursor phase
White precipitates were quickly formed after mixing the metal chloride solution and sodium carbonate solution. These white precipitates were separated from the mother solution by filtration and dried for further characterizations. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images indicate that the white precipitates are composed of nanoparticles with size in the range of 25–60 nm (Fig. 1a and b). The selected area electron diffraction (SAED) pattern in Fig. 1b shows that the nanoparticles are in the amorphous phase, which is further confirmed by the X-ray diffraction (XRD) pattern in Fig. 1c. Keeping in mind that calcium ions, magnesium ions and carbonate were present in the aqueous solutions, we assume that the white precipitates are composed of the ACMC phase. The aqueous suspensions including the white nanoparticles were further treated with a hydrothermal process.XRD characterizations
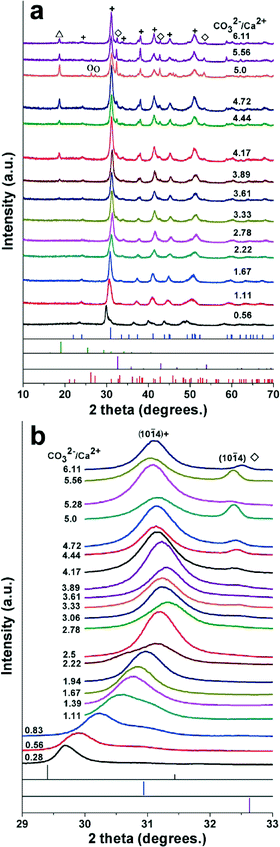
In a typical synthesis, the freshly prepared cloudy suspensions with different molar ratios of [CO32−]/[Ca2+] were kept at 150 °C for 24 hours. The precipitates were separated from the mother solutions by filtration, washed with double distilled water and ethanol, and then dried at 70 °C for 24 hours. The final products were characterized in detail by using XRD, SEM and Fourier transform infrared (FTIR) spectroscopy. The XRD patterns for the final products indicate that the primary phase of the products is Mg-containing calcite while the molar ratios of [CO32−]/[Ca2+] in the mother solution change from 0.56 to 6.11 at an [Mg2+]/[Ca2+] ratio of 2 (Fig. 2). With the increase of [CO32−]/[Ca2+] ratios from 0.28 to 2.78, the final products are in the pure phase of Mg-containing calcites and the diffraction peaks shift to higher 2 theta values (Fig. 2), which is more clearly seen from the (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4) peak movement at 2 theta values in the range of 29–33° scanned with a slow rate (0.2° min−1). The lattice spacings of Mg containing calcites decrease when Mg2+ ion replaces the position of Ca2+ ion in the lattice of the calcite crystal because the radius of Mg2+ is smaller than that of Ca2+.4 Thus the diffraction peaks of Mg containing calcites shift to higher 2 theta values and the Mg contents in the Mg-calcites can be calculated by using the shifts of the 2 theta values of the (10
4) peak movement at 2 theta values in the range of 29–33° scanned with a slow rate (0.2° min−1). The lattice spacings of Mg containing calcites decrease when Mg2+ ion replaces the position of Ca2+ ion in the lattice of the calcite crystal because the radius of Mg2+ is smaller than that of Ca2+.4 Thus the diffraction peaks of Mg containing calcites shift to higher 2 theta values and the Mg contents in the Mg-calcites can be calculated by using the shifts of the 2 theta values of the (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4) peak according to formula (1) found in the literature:4
4) peak according to formula (1) found in the literature:4 | (1) |
The Mg content is given as a molar percentage relative to the number of Ca2+ ions in the calcite lattice such that the sum of Ca mol% and Mg mol% in the samples is 100%. The Mg contents of the obtained Mg-calcite products in pure phase increase from 8.7 mol% to 58 mol% while the molar ratios of [CO32−]/[Ca2+] increase from 0.56 to 2.78 according to the above mentioned formula. High Mg calcite mesocrystals with Mg contents as high as 53 mol% mixed with aragonite were synthesized in an organic solvent/water mixture by Lenders and collaborators.25 However, this is the first time that well crystallized pure phase high Mg calcites with Mg contents higher than 50 mol% have been obtained, as far as we know. More importantly, no organic additive was applied to tune the polymorphs in the reaction solutions during the synthesis of pure phase high Mg-calcites. Recently, our group reported the synthesis of high Mg calcites with controlled magnesium contents from 15 to 40 mol% via polymer stabilized amorphous precursors under ambient conditions.20
Besides Mg-calcite being the primary crystalline phase, three small diffraction peaks at 32.55, 42.92, 53.73 degrees appeared when the molar ratios of [CO32−]/[Ca2+] increased from 3.33 to 6.11. The above 2 theta values were shifted by about 0.05–0.1 degrees to lower 2 theta values, in comparison with the diffraction peaks 32.6, 43.0, 53.7 degrees for (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4), (11
4), (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 3), and (11
3), and (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 6) peaks of magnesite, respectively (JCPDS 080479). It is deduced that the above mentioned diffraction peaks in Fig. 2 are attributed to the presence of a small amount of Ca2+ ions occluded in magnesite. The lattice spacings of Ca-magnesite increased slightly when Mg ion was replaced in magnesite with Ca2+ ion with a slightly larger atomic size. The Mg contents in the Ca-magnesite were also calculated by using the (10
6) peaks of magnesite, respectively (JCPDS 080479). It is deduced that the above mentioned diffraction peaks in Fig. 2 are attributed to the presence of a small amount of Ca2+ ions occluded in magnesite. The lattice spacings of Ca-magnesite increased slightly when Mg ion was replaced in magnesite with Ca2+ ion with a slightly larger atomic size. The Mg contents in the Ca-magnesite were also calculated by using the (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4) peak shift according to formula (1). With the appearance of Ca-magnesite, the (10
4) peak shift according to formula (1). With the appearance of Ca-magnesite, the (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4) peak of Mg-calcite did not shift along with the increase of [CO32−]/[Ca2+] ratios in the range of 3.33–6.11 (Fig. 2b). Besides Mg calcite and Ca-magnesite, a small diffraction peak (001) of brucite (Mg(OH)2) (JCPDS 7-239) appeared when the molar ratios of [CO32−]/[Ca2+] were between 5.00 and 6.11. The product includes the primary phase of Mg-calcite and a small amount of Ca-magnesite, brucite, and aragonite at a [CO32−]/[Ca2+] ratio of 5.00, which is the only case when aragonite appeared in this system (Fig. 2a), even though aragonite appears very often together with Mg-bearing calcites while Mg2+ is present in the reaction solutions.22,25
4) peak of Mg-calcite did not shift along with the increase of [CO32−]/[Ca2+] ratios in the range of 3.33–6.11 (Fig. 2b). Besides Mg calcite and Ca-magnesite, a small diffraction peak (001) of brucite (Mg(OH)2) (JCPDS 7-239) appeared when the molar ratios of [CO32−]/[Ca2+] were between 5.00 and 6.11. The product includes the primary phase of Mg-calcite and a small amount of Ca-magnesite, brucite, and aragonite at a [CO32−]/[Ca2+] ratio of 5.00, which is the only case when aragonite appeared in this system (Fig. 2a), even though aragonite appears very often together with Mg-bearing calcites while Mg2+ is present in the reaction solutions.22,25
SEM analysis
SEM characterizations of the samples indicate that the high Mg calcites are short rod-like aggregates of nanoparticles when the molar ratios of [CO32−]/[Ca2+] are less than 1.39 (Fig. 3a–d). Further increasing the [CO32−]/[Ca2+] ratio to 2.5, the morphology of the samples transforms to spindle-like aggregates composed of nanoparticles (Fig. 3e and f), which is similar to the high Mg calcites obtained in the literature.37 All of the above mentioned products are high Mg calcites in pure phase according to their XRD patterns (Fig. 2), even though their morphologies vary with the change of [CO32−]/[Ca2+] ratios (Fig. 3a–f). The spindle shaped aggregates were transformed to spherical aggregates mixed together with a small amount of nanoplates when the molar ratios of [CO32−]/[Ca2+] were further increased from 3.33 to 4.72 (Fig. 3g–i).The chemical components of the different carbonate samples were characterized by using energy-dispersive X-ray spectroscopy (EDS). Two examples were given herein (Fig. S1†). The short rod-like aggregates obtained at a [CO32−]/[Ca2+] ratio of 1.39 are composed of elements such as Ca, Mg, C and O according to the EDS analysis (Fig. S1a†). EDS analysis indicates that the nanoplates obtained at a [CO32−]/[Ca2+] ratio of 3.89 were composed of Mg, C and O (Fig. S1b†). Based on the XRD (Fig. 2), SEM (Fig. 3) and EDS (Fig. S1†) characterizations, we propose that the spindle-shaped and spherical shaped aggregates are high Mg calcites while the nanoplates are brucite.
The Mg contents of the Mg calcites in pure phase obtained at a [Mg2+]/[Ca2+] ratio of 2 were further characterized in detail by using an inductively coupled plasma-atomic emission spectrometer (ICP-AES). The Mg contents in the pure phase of Mg-calcites were calculated to be as high as 58 mol% according to the XRD, ICP and EDS characterizations (Fig. S2†). The Mg contents in the Mg-calcites characterized via ICP were generally higher than the values obtained via XRD and EDS. It is known that the Mg mol% calculated from ICP-AES is the average value of the samples which includes the amorphous phase, ACMC, and crystallized Mg-containing calcites, while the XRD results only calculated the Mg percentage in the crystallized Mg-calcites. Thus we propose that the Mg content in the ACMC phase is higher than that in Mg-calcite crystals in the products.
FTIR analysis of the high Mg calcites in pure phase
The high Mg calcites in pure phase obtained at a [Mg2+]/[Ca2+] molar ratio of 2 were further characterized by using Fourier transform infrared (FTIR) spectroscopy. The absorption band of out-of-plane bending of carbonate, ν4, shifts from 716 cm−1 to 737 cm−1 while the molar ratios of [CO32−]/[Ca2+] increase from 0.28 to 2.5 (Fig. 4). It was known that the absorption band of ν4 shifts to high wavenumbers with the increase of Mg contents in the Mg-calcites.40 Thus, the higher [CO32−]/[Ca2+] molar ratios, the higher wavenumbers for the ν4 absorption band and the higher the Mg contents in Mg calcites, consistent with the XRD results (Fig. 2). The intensity ratios of the ν2/ν4 peaks for the obtained Mg calcites in pure phase are higher than 7 (Fig. 4), much higher than that the ratio of the ν2/ν4 peak (2.7) for geological calcite.41,42 The high intensity ratios of the ν2/ν4 peaks indicate that a certain amount of ACMC exists in the high Mg calcite samples,20,43 consistent with the ICP and XRD results.The influences of hydrothermal temperature and time
The influences of the experimental conditions such as the hydrothermal temperature and time on the synthesis of high Mg calcites were investigated while keeping the molar ratio of [CO32−]/[Ca2+] at 1.67 and the molar ratio of [Mg2+]/[Ca2+] at 4. The diffraction peaks for the (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4) peak of Mg-calcites shift slightly to higher 2 theta values and the Mg contents in these samples increase from ∼47 mol% to ∼48 mol% with the increase of hydrothermal temperature from 100 to 150 °C for 24 hours (Fig. S3†). The Mg contents and standard deviation data in Fig. S3b† were calculated according to at least three samples synthesized under the same reaction conditions. Therefore, the hydrothermal temperature variation from 100 to 150 °C does not have much influence on the Mg contents in the final high Mg calcites.
4) peak of Mg-calcites shift slightly to higher 2 theta values and the Mg contents in these samples increase from ∼47 mol% to ∼48 mol% with the increase of hydrothermal temperature from 100 to 150 °C for 24 hours (Fig. S3†). The Mg contents and standard deviation data in Fig. S3b† were calculated according to at least three samples synthesized under the same reaction conditions. Therefore, the hydrothermal temperature variation from 100 to 150 °C does not have much influence on the Mg contents in the final high Mg calcites.
To understand the influence of reaction time on the Mg contents in the final Mg calcites, the hydrothermal time was varied from 2 to 48 hours while keeping the hydrothermal temperature at 150 °C (Fig. S4†). The Mg contents of the obtained Mg calcites increase slowly from ∼45 mol% to ∼48 mol% as the heating time increases from 2 to 24 hours (Fig. S4†). Further extending the hydrothermal time to 48 hours did not influence the Mg content in the Mg calcites. No phase transformation occurred even when the hydrothermal time was extended to 5 days in our system, unlike the phase transformation from magnesium calcite to aragonite in a travertine crust specimen.44 We propose that the hydrothermal process at 150 °C for 24 hours is appropriate for the synthesis of well crystallized high Mg calcites in this work. The hydrothermal process for the synthesis of high Mg calcites was kept at 150 °C for 24 hours while the molar ratios of [CO32−]/[Ca2+] and [Mg2+]/[Ca2+] were changed in this work.
Variation of [Mg2+]/[Ca2+] ratios in the mother solutions
The Mg containing calcite samples were further synthesized by changing the molar ratios of [Mg2+]/[Ca2+] to 4 and 6 while keeping the other experimental conditions similar to the above mentioned system (Fig. S5 and S6†). The XRD patterns for the final products indicate that the primary phases of the products are Mg-containing calcites and Ca-containing magnesites while the molar ratios of [CO32−]/[Ca2+] increase from 0.28 to 6.11 (Fig. S5 and S6†). With the increase of [CO32−]/[Ca2+] molar ratios from 0.28 to 3.06, the final products obtained at a [Mg2+]/[Ca2+] of 4 are in the pure phase of Mg-containing calcites and the Mg contents in the Mg-calcites increase from 24 mol% to 63 mol% according to the 2 theta values of the (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4) peak (Fig. S5†). An obvious shoulder peak can be seen from the (10
4) peak (Fig. S5†). An obvious shoulder peak can be seen from the (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4) peak when the molar ratios of [CO32−]/[Ca2+] were 0.28 and 0.56, which indicates that the d-spacings and the Mg contents in these samples are in a wide range (Fig. S5†). Diffraction peaks (10
4) peak when the molar ratios of [CO32−]/[Ca2+] were 0.28 and 0.56, which indicates that the d-spacings and the Mg contents in these samples are in a wide range (Fig. S5†). Diffraction peaks (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4), (11
4), (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 3) and (11
3) and (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 6) of calcium-bearing magnesites are very sharp and strong, indicating there are well crystallized Ca-magnesite crystals mixed together with high Mg calcites when the molar ratios of [Mg2+]/[Ca2+] are 4 and 6 at relatively high molar ratios of [CO32−]/[Ca2+] (Fig. S5 and S6†). The diffraction peak intensities of Ca-magnesite in the samples obtained at [Mg2+]/[Ca2+] ratios of 4 and 6 are much higher than those of samples obtained at a [Mg2+]/[Ca2+] ratio of 2 (Fig. 2, S5 and S6†). Thus the high [Mg2+/Ca2+] ratios in the reaction solutions are beneficial for the formation of Ca-containing magnesite. A small diffraction peak, (001) of brucite (JCPDS 7-239), appears in the XRD patterns when the molar ratios of [CO32−]/[Ca2+] were higher than 5.56 at a [Mg2+]/[Ca2+] of 4.
6) of calcium-bearing magnesites are very sharp and strong, indicating there are well crystallized Ca-magnesite crystals mixed together with high Mg calcites when the molar ratios of [Mg2+]/[Ca2+] are 4 and 6 at relatively high molar ratios of [CO32−]/[Ca2+] (Fig. S5 and S6†). The diffraction peak intensities of Ca-magnesite in the samples obtained at [Mg2+]/[Ca2+] ratios of 4 and 6 are much higher than those of samples obtained at a [Mg2+]/[Ca2+] ratio of 2 (Fig. 2, S5 and S6†). Thus the high [Mg2+/Ca2+] ratios in the reaction solutions are beneficial for the formation of Ca-containing magnesite. A small diffraction peak, (001) of brucite (JCPDS 7-239), appears in the XRD patterns when the molar ratios of [CO32−]/[Ca2+] were higher than 5.56 at a [Mg2+]/[Ca2+] of 4.
Fig. 5 summarizes the polymorphs of the samples obtained while changing the molar ratios of [Mg2+]/[Ca2+] and the molar ratios of [CO32−]/[Ca2+] according to the XRD patterns of these samples (Fig. 2, S5 and S6†). Hydrothermally unstable phase, high Mg calcites in pure phase can be obtained at relatively low molar ratios of [CO32−]/[Ca2+] as the molar ratios of [Mg2+]/[Ca2+] increase from 2 to 6. It is known that the [Mg2+]/[Ca2+] ratio is important for the Mg contents in the final Mg calcites.20 In this work, the Mg contents in the Mg calcites in pure phase generally increase with the increase of molar ratios of [Mg2+]/[Ca2+] under defined [CO32−]/[Ca2+] ratios. The effect of the [Mg2+]/[Ca2+] ratios on the Mg contents in Mg-calcites is more obvious at very low molar ratios of [CO32−]/[Ca2+]. Under a defined [Mg2+]/[Ca2+] ratio, the Mg contents in the high Mg calcites in pure phase increase with the increase of molar ratios of [CO32−]/[Ca2+]. The concentration of CO32− in the mother solution varied from 0.08 mol L−1 to 0.10 mol L−1 while the molar ratios of [CO32−]/[Ca2+] increased from 0.28 to 6.11, along with the volume variation of the Na2CO3 solution added to the metal chloride solution. This is the first time that the influence of CO32− concentration and molar ratios of [CO32−]/[Ca2+] on the synthesis of very high Mg calcites in pure phase in the laboratory has been reported.
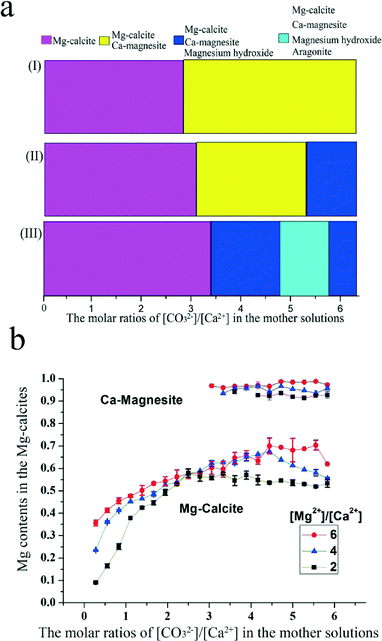 | ||
| Fig. 5 Summary of the polymorphs (a) and the Mg contents (b) in carbonate crystals formed from mother solutions with varying [CO32−]/[Ca2+] ratios and [Mg2+]/[Ca2+] ratios. | ||
Mixtures of Mg-calcite and Ca-magnesite were formed at relatively high molar ratios of [CO32−]/[Ca2+] when the molar ratios of [Mg2+]/[Ca2+] are 4 and 6. Furthermore, brucite appeared together with Mg-calcite and Ca-magnesite at relatively high molar ratios of [CO32−]/[Ca2+], 3.61–6.11 for [Mg2+]/[Ca2+] = 2, and 5.56–6.11 for [Mg2+]/[Ca2+] = 4. The pH values of the supernatant solutions for the above reaction systems after the hydrothermal process are higher than 9.0 (Table S1†). No brucite was formed when the pH values of the supernatant reaction solutions were all below 7.4 at a [Mg2+]/[Ca2+] of 6. Representative results for the pH values of the supernatant solutions after the hydrothermal process are shown in Table S1.† Based on the above experimental results, it can be concluded that a certain amount of redundant Na2CO3 and an alkaline environment are necessary for the formation of brucite in this system.
It is known from the literature that the Ksp values of calcite and magnesite in water are 4.49 × 10−9 (ref. 45 and 46) and 10−9,47 respectively. The Ksp for ACC is about 4 × 10−6.46 The concentration of free Ca2+ and CO32− in the solution is about 2 × 10−3 mol L−1. Herein, we assume that the concentrations of the free Ca2+, Mg2+ and CO32− ions are 2 × 10−3 mol L−1 at maximum. To reach a balance for the carbonate group and the metal ions in the reaction solutions, the molar ratios of [CO32−]/[Ca2+] should be 3 and 5 while the molar ratios of [Mg2+]/[Ca2+] are 2 and 4, respectively. In our system, brucite was formed under an alkaline environment only when the [CO32−]/[Ca2+] ratios are higher than 3 and 5 (3.61 and 5.56) at [Mg2+]/[Ca2+] ratios of 2 and 4, respectively.
Conclusion
We studied the influences of the carbonate concentration and the [CO32−]/[Ca2+] molar ratios on the Mg contents in high Mg calcites in detail in this work. High Mg calcites in pure phase with controlled magnesium contents were synthesized for the first time by using ACMC as an intermediate precursor through a hydrothermal process in the absence of organic additives. Besides the molar ratios of [Mg2+]/[Ca2+], our study shows that the molar ratio of [CO32−]/[Ca2+] in the mother solutions is also a very important factor for the Mg contents occluded in the high Mg-calcites. The Mg contents in the Mg-containing calcites increase from 10 to 63 mol% by increasing the CO32− concentration and the molar ratios of [CO32−]/[Ca2+] in the mother solutions in this work. The higher the molar ratios of [CO32−]/[Ca2+] in the mother solutions, the higher the Mg contents in Mg calcites in pure phase, at relatively low carbonate concentrations. Further increase of the [CO32−]/[Ca2+] ratios in the mother solutions results in the formation of other polymorphs such as Ca-magnesite, brucite, and aragonite. Studies on the formation process of thermodynamically unstable biogenic high-Mg calcites in the laboratories may shed light on the preparation of functional materials with enhanced mechanical properties.Experimental section
Materials and methods
Magnesium chloride hexahydrate (MgCl2·6H2O), calcium chloride dehydrate (CaCl2·2H2O) and sodium carbonate (Na2CO3) were bought from Alfa Aesar.Preparation of intermediate powder precursor
Typically, a precursor suspension was prepared by quickly mixing Na2CO3 solution (2 mol L−1) with 9 mL of mixed solution of MgCl2 and CaCl2 (0.1 mol L−1) ([Mg2+]/[Ca2+] = 2; 4; 6) at 4 °C. The volume of Na2CO3 solution was defined by the molar ratios of [CO32−] and [Ca2+]. A cloudy suspension formed immediately, indicating the formation of colloidal particles in the solution. For characterization, the freshly prepared suspension was quickly filtered under vacuum and washed with double distilled water and alcohol in sequence. The obtained powder precursor was later used for characterization by XRD, SEM and TEM.Hydrothermal process for the synthesis of high Mg calcites
In a typical synthesis, the freshly prepared cloudy suspension was kept at 150 °C for 24 hours in a Teflon lined autoclave with a capacity of 20 mL, if not mentioned specifically. The supernatant was poured and the product at the bottom was centrifuged and washed with double distilled water three times and then washed with ethanol once. The final products were dried in a drying oven under vacuum at 70 °C for 24 h.Characterizations
The X-ray diffraction (XRD) patterns of the products were determined using a Rigaku Dmax-2000. The XRD patterns of the products were generally recorded with a step size of 4° min−1 from 10 to 70°. To get more quantitative data for the 2 theta values of the (104) peak of Mg-calcite and Ca-magnesite, diffraction patterns were further recorded in the 2 theta range of 29–33° with a step size of 0.2° min−1.The samples were characterized by scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) using a FEI Nano 430 at an accelerating voltage of 8.0 kV and 14.0 kV, respectively. Transmission electron microscopy (TEM) images and electron diffraction patterns were recorded on a JEOL JEM-200CX at 160 kV.
The final products were further characterized in detail with a Fourier transform infrared spectrometer (FTIR, VECTOR22) and an inductively coupled plasma-atomic emission spectrometer (ICP-AES, PROFILE SPEC, Leeman).
The pH values of the reaction suspensions after the hydrothermal process were recorded by a Mettler pH meter (PE20).
Acknowledgements
Financial support from the National Natural Science Foundation of China (Grant no. 51272298, 21073005, and 51121091) is gratefully acknowledged.Notes and references
- K. E. Chave, K. S. Deffeyes, P. K. Weil, R. M. Garrels and M. E. Thompson, Science, 1962, 157, 33 Search PubMed.
- S. Raz, S. Weiner and L. Addadi, Adv. Mater., 2000, 12, 38 CrossRef CAS.
- X. Long, Y. R. Ma and L. M. Qi, J. Struct. Biol., 2014, 185, 1 CrossRef CAS PubMed.
- J. R. Goldsmith, D. L. Graf and H. C. Heard, Am. Mineral., 1961, 46, 453 CAS.
- S. E. Kaczmarek and D. F. Sibley, Sediment. Geol., 2011, 240, 30 CrossRef CAS.
- G. Montes-Hernandez, N. Findling, F. Renard and A. L. Auzende, Cryst. Growth Des., 2014, 14, 671 CAS.
- S. M. Stanley, J. B. Ries and L. A. Hardie, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 15323 CrossRef CAS PubMed.
- K. E. Chave, J. Geol., 1954, 62, 266 CAS.
- J. H. Schroeder, E. J. Dwornik and J. J. Papike, Geol. Soc. Am. Bull., 1969, 80, 1613 CrossRef CAS.
- Y. R. Ma, B. Aichmayer, O. Paris, P. Fratzl, A. Meibom, R. A. Metzler, Y. Politi, L. Addadi, P. Gilbert and S. Weiner, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 6048 CrossRef CAS PubMed.
- S. Gayathri, R. Lakshminarayanan, J. C. Weaver, D. E. Morse, R. M. Kini and S. Valiyaveettil, Chem. – Eur. J., 2007, 13, 3262 CrossRef CAS PubMed.
- Y. Ma, S. Weiner and L. Addadi, Adv. Funct. Mater., 2007, 17, 2693 CrossRef CAS.
- C. E. Killian, R. A. Metzler, Y. U. T. Gong, I. C. Olson, J. Aizenberg, Y. Politi, F. H. Wilt, A. Scholl, A. Young, A. Doran, M. Kunz, N. Tamura, S. N. Coppersmith and P. U. P. A. Gilbert, J. Am. Chem. Soc., 2009, 131, 18404 CrossRef CAS PubMed.
- X. Long, M. J. Nasse, Y. R. Ma and L. M. Qi, Phys. Chem. Chem. Phys., 2012, 14, 2255 RSC.
- J. Seto, Y. R. Ma, S. A. Davis, F. Meldrum, A. Gourrier, Y. Y. Kim, U. Schilde, M. Sztucki, M. Burghammer, S. Maltsev, C. Jager and H. Colfen, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 3699 CrossRef CAS PubMed.
- L. Addadi, S. Raz and S. Weiner, Adv. Mater., 2003, 15, 959 CrossRef CAS.
- J. H. Tao, D. M. Zhou, Z. S. Zhang, X. R. Xu and R. K. Tang, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 22096 CrossRef CAS PubMed.
- Y. Ma, S. R. Cohen, L. Addadi and S. Weiner, Adv. Mater., 2008, 20, 1555 CrossRef CAS.
- M. E. Kunitake, S. P. Baker and L. A. Estroff, MRS Commun., 2012, 2, 113 CrossRef CAS.
- X. Long, Y. Ma and L. Qi, Cryst. Growth Des., 2011, 11, 2866 CAS.
- J. Jiang, M.-R. Gao, Y.-H. Qiu, G.-S. Wang, L. Liu, G.-B. Cai and S.-H. Yu, CrystEngComm, 2011, 13, 952 RSC.
- G. Falini, M. Gazzano and A. Ripamonti, Chem. Commun., 1996, 1037 RSC.
- G. Falini, S. Fermani, M. Gazzano and A. Ripamonti, J. Mater. Chem., 1998, 8, 1061 RSC.
- Y.-Y. Liu, J. Jiang, M.-R. Gao, B. Yu, L.-B. Mao and S.-H. Yu, Cryst. Growth Des., 2012, 13, 59 Search PubMed.
- J. J. Lenders, A. Dey, P. H. Bomans, J. Spielmann, M. M. Hendrix, G. de With, F. C. Meldrum, S. Harder and N. A. Sommerdijk, J. Am. Chem. Soc., 2012, 134, 1367 CrossRef CAS PubMed.
- I. Sethmann, J. Wang, U. Becker and A. Putnis, Cryst. Growth Des., 2010, 10, 4319 CAS.
- N. Wada, K. Yamashita and T. Umegaki, J. Colloid Interface Sci., 1999, 212, 357 CrossRef CAS PubMed.
- Y.-J. Han and J. Aizenberg, J. Am. Chem. Soc., 2003, 125, 4032 CrossRef CAS PubMed.
- X. Cheng, P. L. Varona, M. J. Olszta and L. B. Gower, J. Cryst. Growth, 2007, 307, 395 CrossRef CAS.
- D. Wang, A. F. Wallace, J. J. De Yoreo and P. M. Dove, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 21511 CrossRef CAS PubMed.
- J. Xiao and S. Yang, CrystEngComm, 2011, 13, 2472 RSC.
- F. Zhang, H. Xu, H. Konishi, E. S. Shelobolina and E. E. Roden, Am. Mineral., 2012, 97, 556 CrossRef CAS.
- N. Han, C. R. Blue, J. De Yoreo and P. M. Dove, Proceedings of the Fourteenth International Symposium on Water-Rock Interaction, Wri 14, 2013, vol. 7, p. 223 Search PubMed.
- A. Stephenson, J. DeYoreo, L. Wu, K. Wu, J. Hoyer and P. Dove, Science, 2008, 322, 724 CrossRef CAS PubMed.
- M. E. Maguire and J. A. Cowan, BioMetals, 2002, 15, 203 CrossRef CAS PubMed.
- M. Pavlov, P. E. Siegbahn and M. Sandström, J. Phys. Chem. A, 1998, 102, 219 CrossRef CAS.
- J. Xu, C. Yan, F. Zhang, H. Konishi, H. Xu and H. H. Teng, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 17750 CrossRef CAS PubMed.
- J. C. Orr, V. J. Fabry, O. Aumont, L. Bopp, S. C. Doney, R. A. Feely, A. Gnanadesikan, N. Gruber, A. Ishida, F. Joos, R. M. Key, K. Lindsay, E. Maier-Reimer, R. Matear, P. Monfray, A. Mouchet, R. G. Najjar, G. K. Plattner, K. B. Rodgers, C. L. Sabine, J. L. Sarmiento, R. Schlitzer, R. D. Slater, I. J. Totterdell, M. F. Weirig, Y. Yamanaka and A. Yool, Nature, 2005, 437, 681 CrossRef CAS PubMed.
- A. Niedermayr, S. J. Kohler and M. Dietzel, Chem. Geol., 2013, 340, 105 CrossRef CAS.
- Y. Dauphin, Appl. Spectrosc., 1999, 53, 184 CrossRef CAS.
- E. Beniash, J. Aizenberg, L. Addadi and S. Weiner, Proc. R. Soc. London, Ser. B, 1997, 264, 461 CrossRef CAS.
- Y. Politi, T. Arad, E. Klein, S. Weiner and L. Addadi, Science, 2004, 306, 1161 CrossRef CAS PubMed.
- Y. Politi, Y. Levi-Kalisman, S. Raz, F. Wilt, L. Addadi, S. Weiner and I. Sagi, Adv. Funct. Mater., 2006, 16, 1289 CrossRef CAS.
- H. F. Greer, W. Z. Zhou and L. Guo, Mineral. Petrol., 2015, 109, 453 CrossRef CAS.
- F. Lippmann, Sedimentary Carbonate Minerals, Berlin, 1973 Search PubMed.
- D. Gebauer, A. Volkel and H. Colfen, Science, 2008, 322, 1819 CrossRef CAS PubMed.
- P. Benezeth, G. D. Saldi, J. L. Dandurand and J. Schott, Chem. Geol., 2011, 286, 21 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ce01821h |
| This journal is © The Royal Society of Chemistry 2016 |