Investigation of luminescence properties and the energy transfer mechanism of tunable emitting Sr3Y2(Si3O9)2:Eu2+,Tb3+ phosphors†
Mengfei
Zhang
abc,
Yujun
Liang
*ab,
Shuangyu
Xu
b,
Yingli
Zhu
b,
Xingya
Wu
b and
Shiqi
Liu
b
aEngineering Research Center of Nano-Geomaterials of Ministry of Education, China University of Geosciences, Wuhan 430074, People's Republic of China. E-mail: yujunliang@sohu.com; Fax: +86 27 67883733; Tel: +86 27 67884814
bFaculty of Materials Science and Chemistry, China University of Geosciences, Wuhan 430074, People's Republic of China
cState Key Laboratory of New Ceramics and Fine Processing, School of Materials Science and Engineering, Tsinghua University, Beijing 100084, People's Republic of China
First published on 10th November 2015
Abstract
A series of Eu2+ and Tb3+ singly-doped and co-doped Sr3Y2(Si3O9)2 (SYSO) phosphors have been synthesized via a conventional high-temperature solid-state reaction. The crystal structures, photoluminescence properties, fluorescence lifetimes, thermal properties and energy transfer of SYSO:Eu2+,Tb3+ were systematically investigated in detail. Rietveld structure refinement of the obtained phosphors indicated that the SYSO host crystallized in a monoclinic system with the space group C2/c (15) and there are three kinds of cation sites for the doped ions to occupy forming emission centers. The photoluminescence (PL) emission bands of SYSO:xEu2+ show a red-shift tendency with increasing Eu2+ content which should be attributed to more Eu2+ ions entering into Sr2/Y2 and Sr3/Y3 sites from the Sr1/Y1 site. For the co-doped SYSO:Eu2+,Tb3+ samples, tunable colors from cyan to green can be realized by varying the doping concentration of the Tb3+ ions. The intense green emission was realized in the SYSO:Eu2+,Tb3+ phosphors on the basis of the highly efficient energy transfer from Eu2+ to Tb3+ with an efficiency of over 89%. As a result, the emission intensity of SYSO:0.01Eu2+,0.21Tb3+ is about 2.5 times higher than that of SYSO:0.21Tb3+ under 340 nm UV excitation. The energy transfer mechanism from Eu2+ to Tb3+ in the SYSO host was ascribed to the quadrupole–quadrupole interactions. These results indicated that the SYSO:Eu2+,Tb3+ phosphors can act as single-phase green emitting phosphors for possible applications in ultraviolet light-based white light-emitting diodes (w-LEDs).
1. Introduction
Inorganic luminescent materials have found extensive applications in cathode ray tubes (CRTs), plasma display panels (PDPs), field emission displays (FEDs), X-ray imaging scintillators and white light-emitting diodes (w-LEDs), etc.1–6 As a new lighting source for the next generation, w-LEDs have received a lot of attention in the solid-state lighting area because of their high luminous efficiency, long lifetimes, and environmentally friendly features.1,7 At present, the commercial w-LED devices suffer from the problems of low color rendering index (Ra < 80) and high correlated color temperature (Tc > 4500 K), restricting their application. In order to overcome these disadvantages, single-phase multi-color phosphors have been introduced and have attracted much attention.8 Simultaneously, efficient energy transfers can obviously improve the luminescence efficiency and color reproducibility as well as widen the emission spectra of phosphors. The energy transfer mechanism from a sensitizer to an activator has been investigated in many hosts,9 such as Ca2Gd8(SiO4)6O2:Ce3+,Mn2+,10 Ca5(PO4)2SiO4:Ce3+/Tb3+/Mn2+,11 Na2Gd2B2O7:Ce3+,Tb3+,12 β-Na2Ca4(PO4)2(SiO4):Ce3+,Tb3+,13 BaCa2MgSi2O8:Eu2+,Tb3+/Mn2+,14 KCaGd(PO4)2:Eu2+,Tb3+,Mn2+,15 and BaMg2Al6Si9O30:Eu2+,Tb3+,Mn2+.16In the above single-phase phosphors, the Tb3+ ion has been used frequently as the green emission activator because it shows sharp lines at about 488, 543 and 582 nm due to the 4f–4f transition.3,17 However, the major problem for the Tb3+ ion is the lack of an efficient and broad excitation band from the near-ultraviolet (n-UV) region to the visible range, which limits its application in w-LEDs. Moreover, since the f–f transition is spin-forbidden, the emission intensity of the Tb3+ ion is weak.18 To enhance the Tb3+ absorption intensity in the n-UV region, the general method is to induce a sensitizer which has intense absorption in the n-UV region into Tb3+-doped materials. Because the f–d transitions of Ce3+ and Eu2+ ions are spin-allowed, Ce3+ and Eu2+ ions are usually co-doped into the host as sensitizers to transfer excitation energy to Tb3+ ions.19–21 As a common sensitizer for Tb3+ ions, Eu2+ has been widely used frequently as an activator ion for various host lattices due to the broad excitation and emission bands derived from its dipole-allowed 4f–5d electronic transitions.22,23 Strong ligand–activator bonding interactions will cause a small energy difference between the 4f65d1 and 4f7 states, thus leading to a red shift of the emission band of the Eu2+ ions.24,25 Moreover, for Eu2+ ions, the Stokes shift is relatively small and the decay time is short, making Eu2+ a popular activator.26,27 Therefore, the luminescence originating from Eu2+/Tb3+ couples means that they could act as green n-UV convertible phosphors.28,29 Furthermore, the energy transfer phenomenon of Eu2+/Tb3+ has been investigated in many hosts, including Mg2Al4Si5O18:Eu2+,Tb3+,8 Sr2LiSiO4F:Eu2+,Tb3+,21 Ba3LaNa(PO4)3F:Eu2+,Tb3+,28 Sr3.5Y6.5O2(PO4)1.5(SiO4)4.5:Eu2+,Tb3+,30 BaB2Si2O8:Eu2+,Tb3+,31 and so on.
As an important phosphor host, silicate has been widely used due to its several merits, such as facile synthesis, stable crystal structures, excellent long-term stability and environment friendly characteristics.32,33 Rare earth-doped silicate phosphors have been considered in w-LEDs.34,35 As a member of the silicate family, the crystal structure of the cyclosilicate Sr3Y2(Si3O9)2 (SYSO) has been determined, which has three different cation sites for doped ions to occupy and thus attracted researchers' attention.36 In our previous work, we studied the energy transfer between the cerium ion and other rare earth ions in the cyclosilicate host.37 However, energy transfer from Eu2+ to Tb3+ has not been studied in detail yet. Therefore, in the present work, we have synthesized and studied the optical properties of SYSO:Eu2+ and SYSO:Eu2+,Tb3+ phosphors for their possible applications in w-LEDs. After co-doping with Eu2+, the emission intensity of Tb3+ was enhanced and a series of tunable cyan-green colors can be obtained by varying the relative ratio of Eu2+/Tb3+ under 350 nm irradiation. Moreover, the photoluminescence spectra, concentration quenching, fluorescence lifetimes, CIE chromaticity, thermal properties and the energy transfer mechanism between Eu2+ and Tb3+ have been investigated systematically.
2. Experimental
Chemicals and materials
SrCO3 (A.R.), Y2O3 (A.R.), SiO2 (A.R.) and H3BO3 (A.R.) were purchased from Sinopharm Chemical Reagent Co., Ltd. Eu2O3 (≥99.99%) and Tb4O7 (≥99.99%) were purchased from the Science and Technology Parent Company of Changchun Institute of Applied Chemistry. All of the initial chemicals were used without further purification. Aluminum oxide crucibles were used in the sintering process of the samples.Synthesis
A series of polycrystalline Sr3−3xY2−2y(Si3O9)2:3xEu2+,2yTb3+ powder samples were prepared by a conventional high-temperature solid-state reaction process. In order to facilitate the expression, in the following sections, Sr3−3xY2−2y(Si3O9)2:3xEu2+,2yTb3+ are abbreviated as SYSO:xEu2+,yTb3+ (x and y represent mol% in this article). For example, Sr2.97Y1.90(Si3O9)2:0.03Eu2+,0.10Tb3+ is denoted as SYSO:0.01Eu2+,0.05Tb3+. On the basis of the similar effective ionic radius and valence of the cations, we suggested that Tb3+ ions prefer to occupy Y3+ sites,38,39 while Eu2+ ions more easily replace Sr2+ ions. The doping concentrations of Eu2+ and Tb3+ were chosen to be 0–0.07 of Sr2+ and 0–0.21 of Y3+ in SYSO, respectively. Typically, stoichiometric amounts of SrCO3, Y2O3, SiO2, Eu2O3 and Tb4O7 and an excess of 5 mol% H3BO3 as a flux were thoroughly mixed for 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min in an agate mortar to form a homogeneous mixture. Then, the mixtures were transferred into alumina crucibles and calcined at 1300 °C for 8 h under a reducing atmosphere of 85% N2–15% H2 in a horizontal tube furnace. After the furnace was slowly cooled to room temperature, the sintered products were ground again, yielding the resulting phosphor powders.
min in an agate mortar to form a homogeneous mixture. Then, the mixtures were transferred into alumina crucibles and calcined at 1300 °C for 8 h under a reducing atmosphere of 85% N2–15% H2 in a horizontal tube furnace. After the furnace was slowly cooled to room temperature, the sintered products were ground again, yielding the resulting phosphor powders.
Characterization
The finely ground powders were used in all of the measurements. The crystal structure and phase purity of the as-prepared samples were characterized by X-ray powder diffractometer (XRD) (Bruker D8 Focus, Bruker, Karlsruhe, Germany) with Ni-filtered Cu-Kα (λ = 1.540598 Å) radiation at 40 kV tube voltage and 40 mA tube current. The XRD data were collected in a 2θ range from 10° to 120°, with the continuous scan mode at the speed of 0.05 s per step with a step size of 0.01°. XRD Rietveld profile refinements of the structural models and texture analysis were performed with the use of Fullprof software. Photoluminescence excitation (PLE) and emission (PL) spectra were measured by using a fluorescence spectrometer (Fluoromax-4P, Horiba Jobin Yvon, New Jersey, U.S.A.) equipped with a 450 W xenon lamp as the excitation source and both excitation and emission spectra were set up to be 1.0 nm with the width of the monochromator slits adjusted to 0.50 nm. The photoluminescence decay curves were obtained from a Lecroy Wave Runner 6100 digital oscilloscope (1 GHz) using a tunable laser (pulse width = 4 ns, gate = 50 ns) as the excitation source (Continuum Sunlite OPO). All the measurements were carried out at room temperature.3. Results and discussion
3.1 Phase identification, XRD refinement and crystal structure
The phase purity and structural type of the as-prepared powder samples were first identified by XRD. Fig. 1 demonstrates the typical powder XRD patterns of representative SYSO:0.01Eu2+, SYSO:0.10Tb3+ and SYSO:0.01Eu2+,yTb3+ (y = 0.01, 0.05, 0.13, 0.14, 0.17, 0.21) samples. It is obvious that all the diffraction peaks of the samples were well indexed to the reported Sr3Y2(Si3O9)2 phase,36 indicating the formation of a single phase and that the doped ions were completely dissolved in the SYSO host without inducing significant changes in the crystal structure. This demonstrates that the crystal structure of the SYSO host has excellent stability and accommodation capacity for doped ions.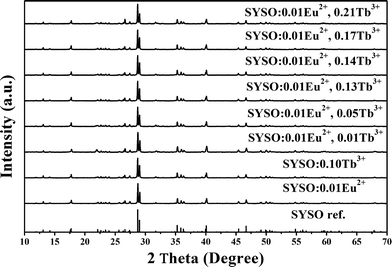 | ||
| Fig. 1 XRD patterns of SYSO:0.10Tb3+ and SYSO:0.01Eu2+,yTb3+ (y = 0, 0.01, 0.05, 0.13, 0.14, 0.17 and 0.21). The Sr3Y2(Si3O9)2 phase reported by Tyutyunnik36 is shown as a reference. | ||
To further investigate the crystal structure of the host, the XRD pattern of the representative SYSO:0.01Eu2+ sample was used to perform the Fullprof refinement. The starting model was built with crystallographic data taken from Tyutyunnik et al.36 The experimental, calculated and difference XRD profiles and Bragg positions for the Fullprof refinement of SYSO:0.01Eu2+ at room temperature are shown in Fig. 2(a). The pure SYSO:0.01Eu2+ crystallizes in a monoclinic phase with the space group C2/c (15), a = 13.512 Å, b = 7.984 Å, c = 14.815 Å, α = γ = 90.000°, β = 90.111°, V = 1598.220 Å3 and Z = 4. All atom positions, fraction factors, and thermal vibration parameters were refined by convergence and satisfied well the reflection conditions, Rp = 6.03%, Rwp = 8.47%, as shown in Table 1. Therefore, the refinement result further verifies that the generation of a single-phase pattern and the crystal structure of the SYSO host are unchanged with the doping of luminescent ions. Fig. 2(b) shows the coordination environments of the cation sites. In the SYSO host, there are three kinds of cation sites which are named Sr1/Y1, Sr2/Y2 and Sr3/Y3 here for easier identification.36 Three independent Sr/Y sites are coordinated by eight, seven and six oxygen atoms, respectively. The coordination diversity of the Sr/Y sites in the host is beneficial to the multiple 5d–4f transition emission of the activator ions and the design of energy transfer.40
 | ||
| Fig. 2 (a) Experimental (crosses) and calculated (red solid line) XRD patterns and their difference (blue solid line) for the Rietveld fit of the SYSO:0.01Eu2+ XRD pattern using the Fullprof program. The short vertical lines show the positions of Bragg reflections of the calculated pattern. (b) Crystal structure schematic diagram of Sr3Y2(Si3O9)2 (from ref. 32). | ||
| Atom | Wyckoff position | X | Y | Z | Occ |
|---|---|---|---|---|---|
| Cell parameters: a = 13.512 Å, b = 7.984 Å, c = 14.815 Å; α = γ = 90.000°, β = 90.111°; V = 1598.220 Å3; Z = 4; Space group: C2/c (15); Reliability factor: Rp = 6.03%, Rwp = 8.47% and χ2 = 14.9. | |||||
| Sr1/Eu1 | 8f | 0.16073(0) | 0.12070(0) | 0.41270(0) | 0.63 |
| Y1/Eu1 | 8f | 0.16073(0) | 0.12070(0) | 0.41270(0) | 0.37 |
| Sr2/Eu2 | 8f | 0.34103(0) | 0.12660(0) | 0.07852(0) | 0.74 |
| Y2/Eu2 | 8f | 0.34103(0) | 0.12660(0) | 0.07852(0) | 0.26 |
| Sr3/Eu3 | 4e | 0.00000(0) | 0.37730(0) | 0.25000(0) | 0.26 |
| Y3/Eu3 | 4e | 0.00000(0) | 0.37730(0) | 0.25000(0) | 0.74 |
| Si1 | 8f | 0.09850(0) | 0.06570(0) | 0.10990(0) | 1.000 |
| Si2 | 8f | 0.27160(0) | 0.37040(0) | 0.26110(0) | 1.000 |
| Si3 | 8f | 0.40620(0) | 0.17870(0) | 0.39690(0) | 1.000 |
| O1 | 8f | 0.00610(0) | 0.17740(0) | 0.13660(0) | 1.000 |
| O2 | 8f | 0.05290(0) | 0.12960(0) | 0.58320(0) | 1.000 |
| O3 | 8f | 0.15770(0) | 0.37890(0) | 0.30150(0) | 1.000 |
| O4 | 8f | 0.15990(0) | 0.10220(0) | 0.02120(0) | 1.000 |
| O5 | 8f | 0.17870(0) | 0.03510(0) | 0.18950(0) | 1.000 |
| O6 | 8f | 0.29390(0) | 0.37960(0) | 0.16590(0) | 1.000 |
| O7 | 8f | 0.32150(0) | 0.20790(0) | 0.31390(0) | 1.000 |
| O8 | 8f | 0.34040(0) | 0.10560(0) | 0.48670(0) | 1.000 |
| O9 | 8f | 0.50990(0) | 0.06590(0) | 0.13180(0) | 1.000 |
3.2 Luminescence properties of Eu2+-doped phosphors
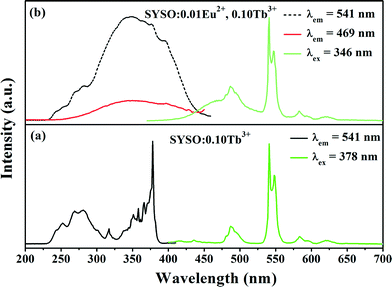
The PL and PLE spectra of the as-prepared SYSO:0.01Eu2+ at room temperature are shown in Fig. 3. Monitored at 474 nm, the sample shows a broad intense band from 250 to 450 nm with a maximum at 346 nm due to the 4f7 → 4f65d1 transition of the Eu2+ ions. Under 346 nm UV excitation, SYSO:0.01Eu2+ shows an asymmetric emission band extending from 400 to 620 nm with the maximum at 474 nm, indicating a possible spectral overlap originating from different luminescence centers. To investigate the relationship between the coordinative environments and PL properties of the Eu2+ ion, the PL spectrum of SYSO:0.01Eu2+ is deconvoluted into at least three Gaussian components with peaks at 463, 489 and 521 nm, as shown in Fig. 3. These components can be ascribed to the contributions of the transitions from the lowest excited states to the ground states of Eu2+ ions at three different Eu2+ luminescence centers, which is in accordance with the number of Sr/Y sites in the host. The excitation spectra of 463, 489 and 521 nm emission bands are shown in Fig. S1 (ESI†). The excitation spectra of the three emission bands have a noticeable difference. Furthermore, similar phenomena have been observed by Seo H. J. and the results are in accordance with our experimental conclusions.41 Therefore, the doped Eu2+ ions can randomly enter into three kinds of Sr/Y cation sites. | ||
| Fig. 3 The typical PLE (black solid line) and PL spectra (blue solid line) of SYSO:0.01Eu2+, together with the Gaussian peak fitting (cyan dashed lines) of the PL spectrum. | ||
Fig. 4(a) exhibits the dependence of the Eu2+ luminescence intensity on its doping concentration. It can be observed that the emission intensity of the Eu2+ ions first increases with increasing doping concentration, reaching a maximum at x = 0.01, and then decreases sharply with enhancing doping content due to the concentration quenching effect. In general, the concentration quenching of luminescence is due to the energy migration among the activator ions at high concentrations. The critical distance RC between Eu2+ ions can be estimated using the equation given by Blasse:42
 | (1) |
As we know, non-radiative energy transfer usually occurs as a result of exchange interaction or multipole–multipole interaction. Since the exchange interaction comes into effect only when the distance between activators is shorter than 4 Å, the concentration quenching mechanism of Eu2+ in the phosphor is dominated by the multipole–multipole interaction. According to Van Uiter's report, the emission intensity (I) per activator ion follows the equation:
| I/x = k[1 + β(x)θ/3]−1 | (2) |
3.3. Luminescence properties and energy transfer in SYSO:Eu2+,Tb3+
The Tb3+ ion as an important activator can be well incorporated into many host lattices giving intense emission. It is known to us that Tb3+ has a simple 4f-configurational energy level structure with low-energy state 7FJ (J = 6, 5, 4, 3) and excited states 5D3 and 5D4. Generally, the blue emissions resulting from 5D3 → 7FJ transitions will dominate at a very low doping concentration of the Tb3+ ion. As the Tb3+ concentration increases, the cross-relaxation between 5D3—5D4 and 7F0—7F6 occurs, which can increase the population of the 5D4 energy level, correspondingly enhancing the transition of 5D4 to 7FJ and producing green light. Fig. 6(a) presents the PLE and PL spectra of the SYSO:0.10Tb3+ sample. The PLE spectrum shows a broad band from 230 to 300 nm and a series of peaks in the range of 310–400 nm with a maximum at 378 nm. Upon excitation at 378 nm, several narrow emission peaks of SYSO:0.10Tb3+ appear in the range of 400–650 nm. The highest peak at 541 nm is ascribed to the 5D4 → 7F5 transition. The other peaks at 487, 584 and 622 nm correspond to the traditional 5D4 → 7FJ (J = 6, 4, 3) transitions of Tb3+ ions, respectively.37,45,46Fig. 6(b) demonstrates the PLE and PL spectra of the Eu2+ and Tb3+ co-doped phosphor. It is observed that the PLE spectrum monitoring the emission of Tb3+ at 543 nm is similar to that monitoring the cyan emission of Eu2+, demonstrating the existence of energy transfer from Eu2+ to Tb3+.In order to explain the energy transfer process from Eu2+ to Tb3+ in the SYSO host, the corresponding energy level diagrams and the possible optical transition involved in the energy transfer processes from Eu2+ to Tb3+ are presented in Fig. 7. From this diagram, it is necessary to note that the energy level of the lowest excited state (4f65d1 ~23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 529 cm−1) of the Eu2+ ion is 2953 cm−1 higher than that of the excited state (5D4 ~20
529 cm−1) of the Eu2+ ion is 2953 cm−1 higher than that of the excited state (5D4 ~20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 576 cm−1) of the Tb3+ ion, which indicates a small mismatch.47 Hence, it is predicated that the energy transfer process from Eu2+ to Tb3+ can be divided into two processes.48 One is the resonant process in which the electrons of Eu2+ non-radiatively or radiatively relax from the lowest excited state (4f65d1) to the ground state (4f7(8S7/2)) and simultaneously the electrons of Tb3+ migrate from the ground state to the excited state (5D4). The other is the temperature-dependent process in which the energy mismatch between the excited state (4f65d1) of Eu2+ and the excited state (5D4) of Tb3+ is overcome by a phonon in the host.
576 cm−1) of the Tb3+ ion, which indicates a small mismatch.47 Hence, it is predicated that the energy transfer process from Eu2+ to Tb3+ can be divided into two processes.48 One is the resonant process in which the electrons of Eu2+ non-radiatively or radiatively relax from the lowest excited state (4f65d1) to the ground state (4f7(8S7/2)) and simultaneously the electrons of Tb3+ migrate from the ground state to the excited state (5D4). The other is the temperature-dependent process in which the energy mismatch between the excited state (4f65d1) of Eu2+ and the excited state (5D4) of Tb3+ is overcome by a phonon in the host.
In order to further optimize the green emission of Tb3+ ions, the concentration-dependent emission intensity of SYSO:0.01Eu2+,yTb3+ is studied. Fig. 8 gives the PL spectra of selected SYSO:0.01Eu2+,yTb3+ phosphors (y = 0, 0.01, 0.05, 0.13, 0.14, 0.17 and 0.21). Upon excitation into the Eu2+ absorption band, it is clearly observed that the emission intensity of Eu2+ gradually decreases while the emission intensity of Tb3+ gradually increases with the increase of Tb3+ concentration. When the y value is higher than 0.13, the Tb3+ and Eu2+ emission intensities decrease remarkably. This can be attributed to the Tb3+–Tb3+ internal concentration quenching effect, which occurs when the activator concentration is high.49,50 These results also confirm the existence of Eu2+ → Tb3+ energy transfer in SYSO:Eu2+,Tb3+. Fig. S2 (ESI†) shows the emission intensity of SYSO:0.01Eu2+,0.21Tb3+ is about 2.5 times higher than that of SYSO:0.21Tb3+ under 340 nm UV excitation. This not only proves again that energy transfer from Eu2+ to Tb3+ really occurs but also demonstrates that the green emission of SYSO:Eu2+,Tb3+ should mainly originate from the energy transfer of Eu2+ → Tb3+.
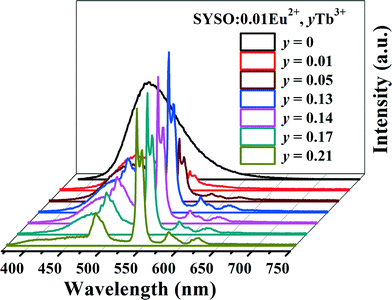 | ||
| Fig. 8 The PL spectra of SYSO:0.01Eu2+,yTb3+ phosphors (y = 0, 0.01, 0.05, 0.13, 0.14, 0.17 and 0.21). | ||
The decay curves of SYSO:0.01Eu2+,yTb3+ phosphors (y = 0, 0.01, 0.05, 0.13, 0.14, 0.17 and 0.21) excited at 340 nm and monitored at 474 nm are shown in Fig. 9. Since there are three Eu2+ luminescence centers in the SYSO host, the decay curves are successfully fitted using the following three-exponential equation:37,43
I(t) = I0 + A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) + A3 exp(−t/τ2) + A3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ3) exp(−t/τ3) | (3) |
 | (4) |
The average decay times (τ*) were calculated to be 153.5, 144.5, 51.9, 49.6, 40.4, 38.7 and 16.3 ns for SYSO:0.01Eu2+,yTb3+ with y = 0, 0.01, 0.05, 0.13, 0.14, 0.17 and 0.21, respectively. Obviously, the decay times of Eu2+ emission gradually decrease with increasing Tb3+ doping content, indicating the existence of the Eu2+ → Tb3+ energy transfer process in the SYSO host lattice. ηT can be calculated using the following equation derived by Paulose et al.52
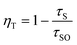 | (5) |
As a consequence, the ηT values for SYSO:0.01Eu2+,yTb3+ were calculated to be 6.2%, 66.2%, 67.8%, 73.7%, 74.9% and 89.4% as a function of y (y = 0.01, 0.05, 0.13, 0.14, 0.17 and 0.21), respectively, as shown in the inset of Fig. 9. The above results indicate that the energy transfer from Eu2+ to Tb3+ in the SYSO:Eu2+,Tb3+ system is very efficient.
As we know, there are two main aspects that are responsible for the resonant energy transfer mechanism: one is exchange interactions and the other is multipolar interactions.53 The critical distance RC for energy transfer from the Eu2+ to Tb3+ ions can also be calculated using eqn (1). Here, Xc is the total concentration of Eu2+ and Tb3+ ions, and the critical concentration (Xc) is that at which the luminescence intensity of the sensitizer (Eu2+) is half that in the sample in the absence of an activator (Tb3+). Therefore, the critical distance (RC) of energy transfer was calculated to be about 20.39 Å. Because the calculated RC value is much longer than 4 Å, there is little possibility of energy transfer via the exchange interaction mechanism. Thus, the electric multipolar interaction is responsible for energy transfer between the Eu2+ and Tb3+ ions. According to Dexter's energy transfer formula of multipolar interaction and Reisfeld's approximation, the following relation can be obtained:
| ηSO/ηS ∝ yn/3 | (6) |
| ISO/IS ∝ yn/3 | (7) |
Energy transfer makes it possible to obtain both the cyan emission of the Eu2+ ions and the green emission of the Tb3+ ions in a single host, which is a feasible route to realize the color-tunable emission under excitation of UV radiation. This result is also confirmed by the CIE chromaticity coordinates shown in Fig. 11. Fig. 11 depicts the chromaticity coordinates for SYSO:0.01Eu2+,yTb3+ (y = 0, 0.01, 0.05, 0.13, 0.14, 0.17 and 0.21) calculated based on the corresponding emission spectra excited at 346 nm. We can find that the color tone of the phosphors shifts gradually from cyan (0.1603, 0.2432) to green (0.2662, 0.4699) with an increase in the doping content of Tb3+, confirming that the CIE chromaticity coordinates are tunable. It is found that the quantum efficiency of the as-prepared SYSO:0.01Eu2+,0.17Tb3+ sample is 25.6%. Moreover, it can be further improved by process optimization. Based on these results, it is clear that these SYSO:Eu2+,Tb3+ phosphors can be efficiently excited in the UV range. This indicates that SYSO:Eu2+,Tb3+ can act as potential cyan-green tunable phosphors for their possible applications in w-LEDs.
3.4. Thermal quenching properties
Aside from high luminescence, the thermal stability of phosphors is also one of the important issues to be considered in high-power LEDs. Fig. 12(a) depicts the temperature dependence of the luminescence intensity of SYSO:0.01Eu2+,0.17Tb3+ excited at 350 nm in the temperature range of 298 K to 473 K. The maximum peak is constantly located at 541 nm even at higher temperature. The emission intensity progressively decreased as the temperature increased due to thermal quenching and it showed poor thermal stability. Similar phenomena have been observed by other researchers, and it may be accounted for the fact that deficiencies formed due to the radii mismatch of rare earth ions and cation ions.54 As a result, the probability of a non-radiative process increased, leading to worse thermal stability. The temperature quenching mechanism could be explained by the configuration coordinate diagram shown in Fig. 12(b). With the increase in temperature, the phonon vibration is strengthened and more electrons located at the lowest excited state B can absorb the phonon energy and are excited to state C. Electrons at state C can go back to ground state A by relaxation and give no emission, decreasing the number of electrons which can go back to the ground state through radiation. Thus, the emission intensity of the phosphor will decrease with the increment of temperature.4. Conclusions
In summary, a series of Eu2+ single-doped phosphors (SYSO:xEu2+) and Eu2+–Tb3+ co-doped phosphors (SYSO:0.01Eu2+,yTb3+) were synthesized and investigated for the first time via solid-state reactions. By structure refinement, it is found that there are three kinds of cation sites in the SYSO host lattice, which are coordinated by eight, seven and six O atoms for Sr1/Y1, Sr2/Y2 and Sr3/Y3 sites, respectively. The SYSO:0.01Eu2+ sample presents a broad emission band centered at 474 nm, which can be resolved into three symmetrical bands peaking at 463, 489 and 521 nm, attributed to Eu2+ occupying three kinds of Sr/Y sites under UV excitation. A red-shifted emission at the higher Eu2+ concentration should be attributed to more Eu2+ ions entering into Sr2/Y2 and Sr3/Y3 sites from the Sr1/Y1 site. The critical distance for Eu2+ has been calculated to be 24.81 Å and the concentration quenching is dominated by dipole–dipole interactions. For the co-doped SYSO:0.01Eu2+,yTb3+ samples, tunable colors from cyan to green can be realized by singly varying the doping concentration of the Tb3+ ions. The energy transfer mechanism from Eu2+ to Tb3+ in the SYSO host has been demonstrated to be quadrupole–quadrupole interactions, and the energy transfer efficiency obtained through the decay curves gradually increased from 6.2% to 89.4% by increasing the Tb3+ doping content from 0.01 to 0.21. In conclusion, these results indicate that the color-tunable SYSO:Eu2+,Tb3+ phosphor can be a promising candidate to serve as a potential phosphor for w-LEDs.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21571162, 21171152) and the Guangdong Province Enterprise-University-Academy Collaborative Project (No. 2012B091100474).References
- H. A. Hoppe, Angew. Chem., Int. Ed., 2009, 48, 3572–3582 CrossRef PubMed.
- T. Jüstel, J. C. Krupa and D. U. Wiechert, J. Lumin., 2001, 93, 179–189 CrossRef.
- M. F. Zhang, Y. J. Liang, M. H. Tong, Q. Wang, D. Y. Yu, J. W. Zhao and J. M. Wu, Ceram. Int., 2014, 40, 10407–10413 CrossRef CAS.
- G. G. Li and J. Lin, Chem. Soc. Rev., 2014, 43, 7099–7131 RSC.
- Y. Chen, J. Wang, C. M. Liu, X. J. Kuang and Q. Su, Appl. Phys. Lett., 2011, 98, 081917 CrossRef.
- W. L. Zhou, R. Zou, X. F. Yang, N. Y. Huang, J. J. Huang, H. B. Liang and J. Wang, Nanoscale, 2015, 7, 13715–13722 RSC.
- C. Feldmann, T. Jüstel, C. R. Ronda and P. J. Schmidt, Adv. Funct. Mater., 2003, 13, 511–516 CrossRef CAS.
- W. Lü, Y. C. Jia, W. Z. Lv, Q. Zhao and H. P. You, New J. Chem., 2014, 38, 2884–2889 RSC.
- X. J. Zhang, L. Huang, F. J. Pan, M. M. Wu, J. Wang, Y. Chen and Q. Su, ACS Appl. Mater. Interfaces, 2014, 6, 2709–2717 CAS.
- G. G. Li, D. L. Geng, M. M. Shang, C. Peng, Z. Cheng and J. Lin, J. Mater. Chem., 2011, 21, 13334–13344 RSC.
- D. L. Geng, M. M. Shang, Y. Zhang, H. Z. Lian, Z. Y. Cheng and J. Lin, J. Mater. Chem. C, 2013, 1, 2345–2353 RSC.
- C. F. Guo, H. Jing and T. Li, RSC Adv., 2012, 2, 2119–2122 RSC.
- K. Li, M. M. Shang, D. L. Geng, H. Z. Lian, Y. Zhang, J. Fan and J. Lin, Inorg. Chem., 2014, 53, 6743–6751 CrossRef CAS PubMed.
- W. Lü, Y. C. Jia, W. Z. Lv, Q. Zhao and H. P. You, RSC Adv., 2013, 3, 20619–20624 RSC.
- W. R. Liu, C. H. Huang, C. W. Yeh, Y. C. Chiu, Y. T. Yeh and R. S. Liu, RSC Adv., 2013, 3, 9023–9028 RSC.
- W. Lu, Z. D. Hao, X. Zhang, Y. S. Luo, X. J. Wang and J. H. Zhang, Inorg. Chem., 2011, 50, 7846–7851 CrossRef CAS PubMed.
- H. Y. Jiao and Y. H. Wang, J. Electrochem. Soc., 2009, 156, J117–J120 CrossRef CAS.
- C. F. Guo, X. Ding, H. Jin Seo, Z. Y. Ren and J. T. Bai, Opt. Laser Technol., 2011, 43, 1351–1354 CrossRef CAS.
- H. P. You, G. Y. Hong, X. Y. Wu, J. K. Tang and H. P. Hu, Chem. Mater., 2003, 15, 2000–2004 CrossRef CAS.
- D. Jia, R. S. Meltzer, W. M. Yen, W. Jia and X. Wang, Appl. Phys. Lett., 2002, 80, 1535–1537 CrossRef CAS.
- M. B. Xie, D. Y. Li, R. K. Pan, X. P. Zhou and G. X. Zhu, RSC Adv., 2015, 5, 22856–22862 RSC.
- W. R. Liu and P. C. Lin, Opt. Express, 2014, 22, A446–A451 CrossRef PubMed.
- Y. Q. Zhang, X. J. Liu, Z. R. Huang, J. Chen and Y. Yang, J. Lumin., 2012, 132, 2561–2565 CrossRef CAS.
- D. J. Hou, C. M. Liu, X. M. Ding, X. J. Kuang, H. B. Liang, S. S. Sun, Y. Huang and Y. Tao, J. Mater. Chem. C, 2013, 1, 493–499 RSC.
- S. Lizzo, A. H. Velders, A. Meijerink, G. J. Dirksen and G. Blasse, J. Lumin., 1995, 65, 303–311 CrossRef CAS.
- M. M. Shang, C. X. Li and J. Lin, Chem. Soc. Rev., 2014, 43, 1372–1386 RSC.
- X. J. Zhang, J. Wang, L. Huang, F. J. Pan, Y. Chen, B. F. Lei, M. Y. Peng and M. M. Wu, ACS Appl. Mater. Interfaces, 2015, 7, 10044–10054 CAS.
- M. M. Jiao, N. Guo, W. Lu, Y. C. Jia, W. Z. Lv, Q. Zhao, B. Q. Shao and H. P. You, Inorg. Chem., 2013, 52, 10340–10346 CrossRef CAS PubMed.
- J. Wang, Z. Y. Zhang, M. Zhang, Q. H. Zhang, Q. Su and J. K. Tang, J. Alloys Compd., 2009, 488, 582–585 CrossRef CAS.
- H. K. Liu, Y. Y. Zhang, L. B. Liao and Z. G. Xia, J. Lumin., 2014, 156, 49–54 CrossRef CAS.
- M. P. Saradhi, S. Boudin, U. V. Varadaraju and B. Raveau, J. Solid State Chem., 2010, 183, 2496–2500 CrossRef CAS.
- M. P. Saradhi and U. V. Varadaraju, Chem. Mater., 2006, 18, 5267–5272 CrossRef CAS.
- W. Z. Lv, N. Guo, Y. C. Jia, Q. Zhao and H. P. You, Opt. Mater., 2013, 35, 1013–1018 CrossRef CAS.
- Z. G. Xia, J. Zhou and Z. Y. Mao, J. Mater. Chem. C, 2013, 1, 5917–5924 RSC.
- D. X. Wei, Y. Sun, L. Z. Jiang, S. X. Hu and D. Li, New J. Chem., 2015, 39, 4753–4758 RSC.
- A. P. Tyutyunnik, I. I. Leonidov, L. L. Surat, I. F. Berger and V. G. Zubkov, J. Solid State Chem., 2013, 197, 447–455 CrossRef CAS.
- M. F. Zhang, Y. J. Liang, R. Tang, D. Y. Yu, M. H. Tong, Q. Wang, Y. L. Zhu, X. Y. Wu and G. G. Li, RSC Adv., 2014, 4, 40626–40637 RSC.
- D. L. Geng, G. G. Li, M. M. Shang, D. M. Yang, Y. Zhang, Z. Y. Cheng and J. Lin, J. Mater. Chem., 2012, 22, 14262–14271 RSC.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- C. M. Zhang, S. S. Huang, D. M. Yang, X. J. Kang, M. M. Shang, C. Peng and J. Lin, J. Mater. Chem., 2010, 20, 6674–6680 RSC.
- W. Zhang, D. Wei and H. Jin Seo, Mater. Lett., 2013, 94, 140–142 CrossRef CAS.
- G. Blasse, Philips Res. Rep., 1969, 24, 131 CAS.
- G. Zhu, S. Y. Xin, Y. Wen, Q. Wang, M. D. Que and Y. H. Wang, RSC Adv., 2013, 3, 9311–9318 RSC.
- M. M. Shang, D. L. Geng, D. M. Yang, X. Kang, Y. Zhang and J. Lin, Inorg. Chem., 2013, 52, 3102–3112 CrossRef CAS PubMed.
- W. Lu, N. Guo, Y. C. Jia, Q. Zhao, W. Z. Lv, M. M. Jiao, B. Q. Shao and H. P. You, Inorg. Chem., 2013, 52, 3007–3012 CrossRef CAS PubMed.
- K. S. Sohn, Y. G. Choi, Y. Y. Choi and H. D. Park, J. Electrochem. Soc., 2000, 147, 3552–3558 CrossRef CAS.
- Y. Chen, J. Wang, X. G. Zhang, G. G. Zhang, M. L. Gong and Q. Su, Sens. Actuators, B, 2010, 148, 259–263 CrossRef CAS.
- Z. Zhang, J. Wang, M. Zhang, Q. Zhang and Q. Su, Appl. Phys. B: Lasers Opt., 2008, 91, 529–537 CrossRef CAS.
- Z. G. Xia and R. S. Liu, J. Phys. Chem. C, 2012, 116, 15604–15609 CAS.
- P. P. Dai, X. T. Zhang, L. L. Bian, S. Lu, Y. C. Liu and X. J. Wang, J. Mater. Chem. C, 2013, 1, 4570–4576 RSC.
- M. M. Shang, G. G. Li, D. L. Geng, D. M. Yang, X. J. Kang, Y. Zhang, H. Z. Lian and J. Lin, J. Phys. Chem. C, 2012, 116, 10222–10231 CAS.
- P. I. Paulose, G. Jose, V. Thomas, N. V. Unnikrishnan and M. K. R. Warrier, J. Phys. Chem. Solids, 2003, 64, 841–846 CrossRef CAS.
- R. Reisfeld, E. Greenberg, R. Velapoldi and B. Barnett, J. Chem. Phys., 1972, 56, 1698–1705 CrossRef CAS.
- Y. Y. Li, Q. S. Wu, X. C. Wang, J. Y. Ding, Q. Long and Y. H. Wang, RSC Adv., 2014, 4, 63569–63575 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ce01814e |
| This journal is © The Royal Society of Chemistry 2016 |