Crystallization of linear low density polyethylene on an in situ oriented isotactic polypropylene substrate manipulated by an extensional flow field†
Ben
Niu
a,
Jing-Bin
Chen
a,
Jun
Chen
a,
Xu
Ji
b,
Gan-Ji
Zhong
*a and
Zhong-Ming
Li
*a
aCollege of Polymer Science and Engineering, State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu, 610065, Sichuan, People's Republic of China. E-mail: ganji.zhong@scu.edu.cn; zmli@scu.edu.cn; Fax: +86 28 8540 6866; Tel: +86 28 8540 0211, +86 28 8540 6866
bCollege of Chemical Engineering, Sichuan University, Chengdu, 610065, Sichuan, People's Republic of China
First published on 10th November 2015
Abstract
In this work, we demonstrate that utilization of extensional flow with different intensities can regulate the flow-induced crystallization and epitaxially surface-induced crystallization simultaneously in crystalline–crystalline immiscible blends, leading to improved interfacial adhesion and thus enhanced mechanical properties, which provides a versatile methodology to industrially achieve polymer blends with advanced performance. An accessible methodology, i.e., “extrusion–hot stretching–quenching”, was applied to fulfill the scalable achievement of an epitaxial interface for a linear low density polyethylene (LLDPE)/isotactic polypropylene (iPP) blend, where LLDPE could epitaxially grow on an oriented iPP substrate but greatly influenced by the flow field, with its chains and lamellae aligned abnormally off the flow direction revealed by wide angle X-ray diffraction and small angle X-ray scattering, respectively. Depending on the intensity of flow, the above effect of flow can be divided into two types: under a strong flow field, the LLDPE chains prefer to align along the flow direction, inducing the formation of a shish-kebab structure. For another type, i.e., under a weak flow field, the pre-oriented LLDPE chains can relax quickly and epitaxially nucleate on the surface of the oriented iPP substrate. During further growth, the epitaxial LLDPE lamellae deform and reorient along the flow direction under the mechanism of flow-induced block slips, fragmentation and reorientation. Moreover, it is believed that incomplete lamellar twist also occurs under flow. Mechanical property tests demonstrate that an epitaxial structure significantly improves the interfacial adhesion between LLDPE and iPP, showing remarkable enhancements in both strength and toughness.
Introduction
Epitaxy is generally defined as crystals of one phase (guest crystal) growing on the surface of a crystal of another phase (host crystal) in one or more strictly defined crystallographic orientations.1 Actually, as a special surface-induced crystallization process, epitaxy starts unambiguously at the interacting interface. Because of structural similarity, the epitaxial one will always show an unusual mutual orientation with respect to substrate materials, which will bring significantly improved interfacial adhesion between the two phases as well as the bulk mechanical properties of polymer blends, especially for the incompatible systems.2,3 The special orientation relationship and resultant performance improvement make the research studies in polymer epitaxy meaningful in both theory and practice. In the pioneering studies, epitaxy is usually achieved by two steps: preparation of an oriented polymer substrate and the following deposition of the epitaxial one. Generally, there are three methods for preparing an oriented polymer substrate, i.e., melt-drawing,4 friction transfer of the polymer onto the surface of a glass or mica substrate5–8 and epitaxial crystallization on the substrate of organic or inorganic single crystals.9–11 In addition, the deposition process can be realized by annealing between the melting points of the two components,3 solution crystallization,12 and vacuum deposition.6,12 Due to the above preparation methods, great progress in understanding the mechanism of polymer epitaxy at a molecular level has been well accomplished.13,14 Following the successful pioneering studies, another challenging question is how to effectively obtain this special surface-induced crystalline structure for preparing advanced polymeric materials especially in conventional processing, such as extrusion, injection and blowing.In most polymer processing operations, polymer melts are always subjected to shear or/and extensional flow fields, under which the crystallization behavior of a semicrystalline polymer is greatly changed.15–22 The effect of flow field can be summarized: the crystallization rate of a polymer melt when oriented can be significantly accelerated, and the pre-oriented polymer chains in the melt can, for certain, influence the subsequent crystallization manner: taking isotactic polypropylene (iPP) as an example, when sheared or stretched, β-iPP crystals will preferentially form instead of its thermodynamically most stable α-iPP crystals; finally, highly oriented crystalline structures, such as shish-kebab and extended-chain structures, are always formed. Until now, a clear understanding of flow-induced polymer crystallization has been achieved after decades of extensive research. Nevertheless, the history of establishing epitaxy by conventional processing methods is poor, and nearly none of the obvious opportunities to study the interplay of epitaxial crystallization and flow-induced crystallization under an external flow field have been examined, let alone a systematic study.
Inspired by the studies of in situ microfibrillar blends in our previous work,23–27 where the dispersed phase in situ deforms into microfibrils by melt stretching, which can considerably improve the mechanical properties of the matrix polymer, in the present work, the methodology, i.e., the “extrusion–hot stretching–quenching” technique, was proposed to highly orient the iPP phase, thus serving as a template to induce the following epitaxial crystallization of linear low density polyethylene (LLDPE), finally accomplishing the scalable achievement of an epitaxial interface followed by notably improved strength and ductility for the LLDPE/iPP blend. Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction results both confirm the achievement of a highly oriented iPP substrate along the stretching direction. Wide angle X-ray diffraction (WAXD) and small angle X-ray scattering (SAXS) results show that under a strong flow field, the LLDPE chains prefer to align along the flow direction and a shish-kebab structure is preferentially formed. However, under a weak flow field, the pre-oriented LLDPE chains relax quickly and turn to epitaxially crystallize on the oriented iPP substrate but in an unexpected manner, which should be ascribed to the effect of the extensional flow field. Combined with differential scanning calorimetry (DSC) analysis, the crystallization behavior of the LLDPE/iPP blend under an extensional flow field, especially the epitaxial crystallization process of LLDPE on the oriented iPP substrate under a weak flow field, is thoroughly elucidated.
Experimental
Materials
LLDPE (DNDA-7144), supplied by Maoming Petroleum Chemical Co., China, has a melt flow rate (MFR) of 20 g per 10 min (190 °C, 2.16 kg) and a density of 0.919 g cm−3. iPP (S1003), supplied by Dushanzi Petroleum Chemical Co., China, has a MFR of 3 g per 10 min (230 °C, 2.16 kg) and a density of 0.905 g cm−3.Sample preparation
Firstly, LLDPE and iPP, with a weight ratio of 80/20, was melt-blended together using a TSSJ-20 co-rotating twin-screw extruder at 160, 180, 200, 200, 190, and 180 °C from the feed section to the die. After pelleting and drying (the crystalline information of the pellet, such as the crystalline structure obtained by WAXD and crystallinity by DSC, is shown in Fig. S1†), the extruded compound pellets were further applied in the process of “extrusion–hot stretching–quenching” (Fig. S2†). The single screw extruder used has a screw length to screw diameter of 16, and the die for the extruder is a slit die with 20 mm width and 2 mm thickness. The temperature profile from the hopper to the die of the extruder was 150, 180, 180, and 150 °C, respectively, under which the pellets are fully melted (Fig. S3†), and the screw rotation was maintained at 50 rpm (the extrusion speed was about 24.9 mm s−1). The pull rolls were applied to continuously provide the extensional flow field. Finally, the extruded tapes, with an average thickness of 180 μm, were cooled down in a cooling unit which was full of cold water and then collected. Different hot stretching ratios (HSRs), which are defined as the area of the transverse section of the die to that of the extrudate, could be obtained by adjusting the speed of the pull rolls. In this study, the HSR was fixed at 5.0 (the stretching speed of the pull rolls was about 77.8 mm s−1). The common blend of LLDPE/iPP also underwent the same procedures without “hot stretching” only for comparison.Fourier transform infrared spectroscopy (FTIR)
FTIR with a polarizer (Nioclent 6700, Thermal Scientific, USA) was employed to examine the iPP molecular orientation in the LLDPE/iPP common and stretched blends. The spectra were recorded from 400 to 4000 cm−1 by averaging 16 scans at a 2 cm−1 resolution. The molecular orientation of iPP could be characterized by the dichroic ratio, which is calculated as eqn (1):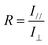 | (1) |
WAXD and SAXS measurements
WAXD and SAXS were used to characterize the crystalline structure and orientation of LLDPE and iPP. WAXD and SAXS measurements were performed at the beamlines BL15U and 16B (wavelength λ = 0.1240 nm) of Shanghai Synchrotron Radiation Facility (SSRF, Shanghai, China), respectively. An X-ray CCD detector, with a resolution of 2048 × 2048 pixels, was employed to detect 2D-WAXD and 2D-SAXS patterns. The sample to detector distances were 110 and 2024 mm for WAXD and SAXS tests, respectively. Meanwhile, the incident X-ray beam was set perpendicular to the stretching direction. To evaluate the orientation degree of stretched iPP, the orientation parameter was calculated mathematically using Herman's method from the (040) reflection of WAXD for iPP.28Moreover, in order to study the melting and crystallization behaviors of LLDPE on the oriented iPP substrate under quiescent conditions, a Linkam temperature stage was used to precisely control the thermal history.28 The experimental temperatures of non-isothermal crystallization were set as follows: (1) heating at a rate of 5 °C min−1 from room temperature to 150 °C; (2) holding at 150 °C for 5 min to eliminate the thermal history of LLDPE thoroughly; (3) cooling at a rate of 5 °C min−1 down to room temperature. In the heating and cooling processes, the 2D-WAXD and 2D-SAXS data were collected every 0.9 °C from 70 to 150 °C. For data analysis, all the measured patterns were corrected by subtraction of background reflection.
Differential scanning calorimetry (DSC)
As a complement for the time-resolved X-ray measurements, the melting and recrystallization behaviors of LLDPE on the oriented iPP substrate under quiescent conditions were further probed by a DSC Q2000 (TA Instruments, USA). For the melting behavior, a sample of the LLDPE/iPP stretched blend was heated from 40 to 150 °C. After holding at 150 °C for 5 min to eliminate the thermal history of LLDPE completely, recrystallization of LLDPE on the oriented iPP substrate was conducted by cooling the sample down to 40 °C. Meanwhile, for comparison, the same procedure was carried out for the LLDPE/iPP common blend, and DSC curves of pure LLDPE and iPP were obtained by heating the samples from 40 to 200 °C, holding at 200 °C for 5 min, and cooling down to 40 °C.Moreover, to investigate the effect of thermal history on the crystallization sequence of LLDPE and iPP, DSC cooling tests of the LLDPE/iPP stretched blend after being held at different temperatures and for different times (with a partially reserved and completely orientation erased structure) were conducted as follows. For the effect of thermal history on the crystallization of the LLDPE phase, the sample was initially heated to 135 °C, held for 2 min, and cooled to 40 °C (1st); then it was heated to 150 °C, held for 2 min, and cooled to 40 °C (2nd); finally, the sample was heated to 150 °C, held for 5 min, and cooled to 40 °C (3rd). A similar procedure was applied to study the effect of thermal history on the crystallization of the iPP phase, i.e., heated to 180 °C, held for 2 min, and cooled to 40 °C (1st); heated to 200 °C, held for 2 min, and cooled to 40 °C (2nd); heated to 200 °C, held for 5 min, and cooled to 40 °C (3rd); heated to 220 °C, held for 5 min, and cooled to 40 °C (4th). All the DSC experiments were conducted under nitrogen atmosphere, and the heating/cooling rate was constantly set as 5 °C min−1. The crystallinity of LLDPE is derived from integrating the enthalpy peak with respect to a baseline drawn as a tangent to the trace at 60 and 140 °C and normalizing it with the melting enthalpy of hypothetical 100% crystalline polyethylene, 293 J g−1.29
Mechanical property tests
To verify the improvement effect of the epitaxial interface for the LLDPE/iPP blend, samples of pure LLDPE, iPP, LLDPE/iPP common blend and stretched blend were firstly compressed at 10 MPa and 150 °C (200 °C for pure iPP) for 10 min, respectively, and then the samples were cut into a dumbbell shape for tensile testing. Note that the stretched tapes were arranged with the stretching direction parallel to the length direction of the dumbbell samples. Furthermore, for comparison, the LLDPE/iPP stretched blend (HSR = 17.1) was also applied (without hot compressing) for tensile tests to examine the effect of the shish-kebab structure on the mechanical properties. Finally, tensile properties of the dumbbell samples were measured using the Instron Instrument model 5576 at a cross-head speed of 100 mm min−1.Results and discussion
Molecular orientation of the stretched iPP substrate
Since an oriented iPP substrate is a prerequisite for the epitaxial growth of LLDPE, it is very necessary to examine the orientation degree of iPP molecules in the LLDPE/iPP blend after stretching. Here, Fig. 1 represents the polarized FTIR spectra of the LLDPE/iPP common and stretched blends with the electron vector perpendicular and parallel to the stretching direction in the wavenumber range from 800 to 1200 cm−1. Among them, 841, 998, and 1167 cm−1 represent the 3/1 helical conformation bands of iPP, while 973 cm−1 represents the isotactic band, respectively.30–32 Both the 3/1 helical conformation bands and the isotactic band are related to the critical lengths of the regular isotactic sequence. In addition, the various regularity bands of iPP can be rearranged in terms of order degree from high to low as follows: 940, 1220, 1167, 1303, 1330, 841, 998, 900, 808, 1100, and 973 cm−1.30–32 Moreover, it should be noted that the transition moments of the above 4 characteristic absorption bands are parallel to the axis of the iPP chains. In Fig. 1a, the parallel- and perpendicular-polarized FTIR spectra of the common blend are almost identical with each other; in other words, R values of the common blend are about 1 with the maximum deviation of 0.10, suggesting that the iPP chains in the common blend are randomly oriented. In clear contrast, for the stretched blend, obvious different absorbance intensities are observed in the parallel- and perpendicular-polarized FTIR spectra (Fig. 1b), and R values of the 4 characteristic absorption bands are 3.03 for 841 cm−1, 2.16 for 973 cm−1, 2.78 for 998 cm−1 and 2.48 for 1167 cm−1, respectively. This is, no doubt, indicative of highly oriented iPP molecules along the stretching direction in the LLDPE/iPP stretched blend. The orientation degree for the (040) plane of stretched iPP was calculated to be about 0.90, indicating the high orientation degree of iPP after stretching as well. Furthermore, direct SEM observations also evidence the orientation of the iPP phase after stretching, showing an obvious transformation from spherical dispersion of the common blend to the oriented microfibrillar morphology of the stretched blend (Fig. S4†). | ||
| Fig. 1 Polarized FTIR spectra of the LLDPE/iPP (a) common blend and (b) stretched blend in the 800–1200 cm−1 region with the electron vector perpendicular and parallel to the stretching direction. | ||
Crystallization behavior of LLDPE induced by the oriented iPP substrate and extensional flow field
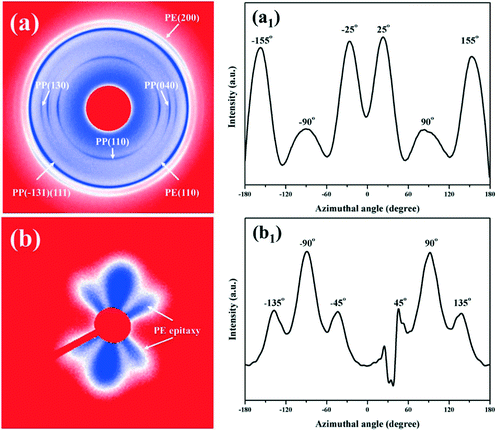
For the crystallization of LLDPE induced by the oriented iPP substrate and extensional flow field, the corresponding 2D-WAXD and 2D-SAXS patterns of the LLDPE/iPP stretched blend are shown in Fig. 2, including the results of azimuthal scans. In Fig. 2a, the diffraction circles or arcs from the inner to the outer part are originated from the (110), (040) and (130) planes of the iPP α-modification and the (110), (200) planes of orthorhombic crystallites of LLDPE. Due to a similar 2θ angle, the diffractions of the (111) and (−131) planes of iPP overlapped with the (110) plane of LLDPE. The strong diffractions of the iPP (hk0) planes at the equator suggest that the iPP chains are preferentially oriented along the stretching direction, which is in accordance with the results of FTIR. For the (110) plane of iPP, the two diffraction arcs that emerged at the meridian are indicative of a lamellar branching through homoepitaxy between iPP α-crystals.33,34In order to reveal the detailed orientation angle of LLDPE chains, the (110) intensity distribution along the azimuthal angle between −180° and 180° was integrated and is shown in Fig. 2a1. Unexpectedly, the orientation angle of LLDPE is very peculiar in contrast to that of iPP. Obviously, four intensive diffraction arcs occur at about ±25° apart from the equator (Fig. 2a1), which means that the LLDPE chains are inclined ±25° off the stretching direction, viz., the chain direction of the iPP substrate. Besides the above four arcs, it should be noted that there are still two strong diffraction peaks at ±90° (the meridian), denoting that some LLDPE chains are perpendicular to the stretching direction.35,36 Furthermore, for the results of the 2D-SAXS pattern (Fig. 2b) and the corresponding azimuthal scan (Fig. 2b1), besides the strong scattering peaks around the meridian which mainly belong to the scattering of the oriented iPP, one can also see four scattering spots which are tilted by ±45° to the meridian, i.e., the stretching direction, indicating that LLDPE lamellae are arranged ±45° apart from the stretching direction. The above results are, no doubt, indicative of the special overgrowth of LLDPE on the oriented iPP substrate. In general, for the overgrowth of PE on the oriented iPP substrate, it is believed that PE will crystallize on the surface of the iPP substrate, with the chains of PE ±50° apart from the chain direction of the iPP substrate. The peculiar arrangement of the PE lamellae can be well explained in terms of epitaxial crystallization of the (100) PE lattice planes parallel to the (010) lattice planes of iPP.9,13,37–41 In this case, LLDPE crystallizes on the oriented iPP substrate, but unexpectedly, with its chains and lamellae ±25° and ±45° apart from the stretching (or iPP chains) direction, respectively, which disagrees with the classical epitaxy theory of ±50° for the PE/iPP system.9,13,39 Considering the growth conditions of LLDPE in this case, we preliminarily speculate that it is the cooperation of the oriented iPP substrate and extensional flow that induces the above special orientation of LLDPE, for LLDPE chains will definitely align along the stretching direction under the extensional flow field only and epitaxially ±50° apart from the chain direction of iPP under the induction of the oriented iPP substrate only, respectively. Furthermore, some other LLDPE/iPP stretched blends with different HSRs, characterized by different flow intensities, were prepared to offer more information. As clearly shown in Fig. 3a, at a lower flow intensity (HSR = 5.0, 6.4 and 8.3), LLDPE is dominated by a peculiar orientation under a weak flow field, and more LLDPE chains tend to orient along the flow direction with HSR further increasing. A similar process can also be identified by SAXS in Fig. 3b, where LLDPE lamellae gradually transform from peculiar into perpendicular growth with respect to the flow direction with HSR increasing, finally leading to the formation of a shish-kebab structure (Fig. S5†). The variation of the LLDPE orientation can be well explained by the competition between the flow-induced molecular orientation and relaxation of the oriented chains. Under flow, almost all the LLDPE chains tend to align along the flow direction and a shish-kebab structure is preferentially formed instead of the peculiar one, for the former is more thermally stable. However, since the initial stretching temperature (ca. 150 °C) is much higher than the melting point of LLDPE (ca. 129 °C), the LLDPE oriented chains can easily relax, and the relaxation degree depends heavily on the flow intensity. Hence, under a weak flow field, the pre-oriented LLDPE chains relax quickly and turn to epitaxially crystallize on the surface of the oriented iPP substrate under a continuous flow field, finally giving rise to the peculiar orientation and special diffraction X-ray patterns in Fig. 2. As the extensional flow increases, the relaxation of LLDPE pre-oriented chains is gradually suppressed, immediately resulting in the formation of shish-kebab instead of the peculiar lamellae.
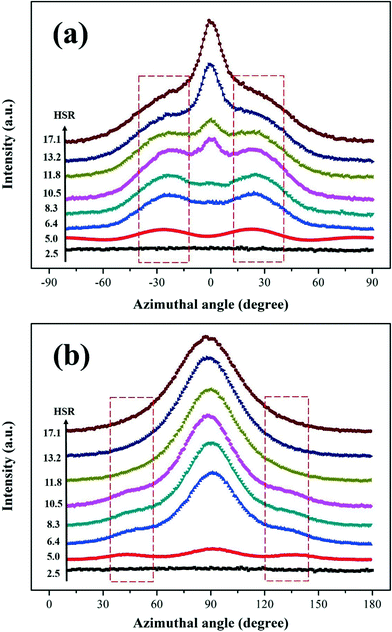 | ||
| Fig. 3 (a) Azimuthal scan of the LLDPE (110) plane; (b) azimuthal scan of the 2D-SAXS pattern of the LLDPE/iPP stretched blend with different HSRs. | ||
In brief summary, the crystallization of LLDPE on the oriented iPP substrate depends heavily on the extensional flow field. That is, under a strong flow field, the LLDPE chains prefer to align along the flow direction, and a shish-kebab structure is preferentially formed because of its high thermal stability. However, under a weak flow field, the pre-oriented LLDPE chains can relax quickly and turn to epitaxially crystallize on the surface of the oriented iPP substrate, but the lamellar growth process is greatly affected by the extensional flow during stretching, showing an obvious change in the epitaxial growth angle, which is similar to the deformation of a semicrystalline polymer when strained in the solid state. The deformational behavior of semicrystalline polymers subjected to uniaxial extension has been extensively studied over the past decades, and PE has always been selected as a model polymer material primarily due to its simple chemical structure and wide applications in daily life.42–47 As a consequence of tensile deformation, the original spherulites of PE will be transformed into the highly oriented fibrillar morphology, with the chains preferentially oriented along the extension direction. Reflected by SAXS, the characteristic scattering patterns of deformed PE lamellae can be identified as follows: at low strains, a scattering pattern with peaks perpendicular to the extension direction, a four-point scattering feature at moderate strains, and eventually a highly anisotropic scattering pattern aligned along the extension direction only if the deformation is large enough.48–50 The molecular mechanism of the above deformation behavior can be summarized as slips within the lamellae and stress-induced melting and recrystallization. Combined with the above deformation behavior of solid PE, the mechanism for the peculiar orientation of LLDPE under a weak extensional flow field will be elucidated in detail below.
Melting behavior of LLDPE crystallites induced by the oriented iPP substrate and extensional flow field
Limited by the experimental facilities, it is hard to trace online the epitaxial crystallization process induced by the extensional flow field in the current work. Since it is considered to be a reverse process of polymer crystallization, the melting process has attracted much attention, and fruitful research results have been achieved. By studying the melting process, it certainly offers us some information on the polymer crystallization from a different viewpoint. Hence, the melting behavior of epitaxial LLDPE crystallites induced by the extensional flow and oriented iPP substrate was investigated by time-resolved WAXD and SAXS. As shown in Fig. 4, in the whole heating process, the diffraction arcs of iPP (hk0) planes remain unchanged, which implies that the orientation of iPP does not get any damage during heating. At 150 °C, LLDPE crystallites are fully molten, showing complete disappearance of LLDPE (110) and (200) diffraction arcs. In addition, there appear four diffraction arcs which are about ±41° off the equator in the position of the LLDPE (110) plane. Combined with the results of 1D-WAXD and the corresponding azimuthal scans of the LLDPE (110) plane in Fig. 5, the above four diffraction arcs at 150 °C should be originated from the diffraction of the iPP (111) and (−131) planes, which possess a similar 2θ angle with the (110) plane of LLDPE. The highly oriented diffraction arcs of the iPP (111) and (−131) planes further evidence the reservation of highly oriented iPP molecules during heating. Furthermore, it is found that, when heating up to 129 °C, the diffraction peaks of the LLDPE (110) and (200) planes disappear in Fig. 5a, and the azimuthal angle of the LLDPE (110) plane in Fig. 5b shifts from ±25° to ±41°, viz., varying from the diffraction of the LLDPE (110) plane to the iPP (111) and (−131) planes, both indicating that the melting point of LLDPE crystallites is about 129 °C. In Fig. 5a, besides the diffraction peaks of orthorhombic LLDPE and α-iPP, there is also an extra reflection peak at about 12.9°, which is assigned to the (300) plane of β-iPP. The β-iPP is usually obtained in the flow or shear field.16,51 Since the amount of β-iPP is very small, it will not be discussed further in this work. | ||
| Fig. 4 Selected 2D-WAXD patterns of the LLDPE/iPP stretched blend during heating from room temperature to 150 °C (the stretching direction is vertical). | ||
 | ||
| Fig. 5 (a) 1D-WAXD curves and (b) azimuthal scans of the LLDPE (110) plane evaluated from Fig. 4. | ||
Some typical 2D-SAXS patterns for the LLDPE/iPP stretched blend during heating are shown in Fig. 6. Combined with WAXD results, the 2D-SAXS signals can be divided into two parts, i.e., scattering signals around the meridian which come from oriented iPP and scattering signals at ±45° which stem from the epitaxial lamellae of LLDPE. Taking a careful look at the development of 2D-SAXS, two abnormal phenomena can be evidently observed which contradict with the results of WAXD: besides the discrepancy of the LLDPE epitaxial angle on the oriented iPP substrate as mentioned above, the melting point of epitaxial LLDPE crystallites by WAXD and SAXS is also different. As shown in Fig. 5, WAXD results show that the melting point of LLDPE crystallites is about 129 °C, whereas the scattering signals of LLDPE epitaxy in 2D-SAXS can still be clearly observed at 130 °C and disappear totally at 141 °C (see Fig. 6). Beyond expectation, the gap of the disappearance temperature of LLDPE epitaxy signals between WAXD and SAXS is about 12 °C.
 | ||
| Fig. 6 Selected 2D-SAXS patterns of the LLDPE/iPP stretched blend during heating from room temperature to 150 °C (the stretching direction is vertical). | ||
The corresponding 1D-SAXS curves are shown in Fig. 7. Clearly, at low temperature, there is only one scattering peak, and the peak gradually shifts to smaller q values with temperature increasing, which arises from the melting of LLDPE lamellae. Besides the varying LLDPE scattering peaks, one can observe two separate peaks when heating up to about 120 °C, rather than one broad peak in the original curves. In addition, one of the peaks remains unchanged during the whole heating process and still survives at 150 °C, which should be attributed to the scattering of oriented iPP. Subsequently, the long period (L) is calculated using the Bragg equation, L = 2π/q, where q refers to the peak position in the scattering curves. As shown in Fig. 7b, during the whole heating process, L of LLDPE increases from 16.1 to 38.3 nm and finally disappears at about 141 °C, while that of iPP remains constant at 17.0 nm.
 | ||
| Fig. 7 (a) 1D-SAXS curves evaluated from Fig. 6 and (b) long period development of LLDPE and iPP during heating. | ||
Epitaxial crystallization behavior of LLDPE on the oriented iPP substrate under quiescent conditions
As described above, under an extensional flow field, LLDPE epitaxially grows on the oriented iPP substrate, but markedly affected by the flow, resulting in its chains and lamellae ±25° and ±45° apart from the flow direction, respectively. For comparison, Fig. 8 shows some selected 2D-WAXD patterns during the epitaxial crystallization process of LLDPE on the oriented iPP substrate under quiescent conditions. Clearly, four symmetric diffraction arcs emerge again in the position of the LLDPE (110) plane at 80 °C. According to the development of 1D-WAXD curves and azimuthal scan results (Fig. 9), it can be concluded that the epitaxial crystallization of LLDPE on the oriented iPP substrate starts at 120 °C, and LLDPE chains are ±50° apart from the stretching direction, i.e., the chain direction of the iPP substrate. The result is consistent with the classical theory,9,13,39i.e., the epitaxial crystallization of PE on the oriented iPP substrate principally lies in the chain-row matching by parallel alignment of PE chains in the (100) lattice plane along the methyl group rows in the [101] direction of iPP with a dinky mismatch of 2%, finally resulting in the chains of PE aligned ±50° apart from that of iPP. The well-defined orientation of PE has been evidenced by a lot of experiments, such as transmission electron microscopy combined with electron microscopy,2,3,13,39 and X-ray scattering35–38 just as shown in the present work. | ||
| Fig. 8 Selected 2D-WAXD patterns of the LLDPE/iPP stretched blend during cooling from 150 °C to room temperature (the stretching direction is vertical). | ||
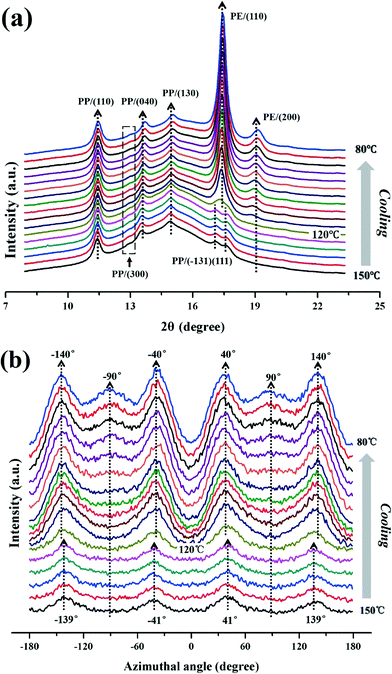 | ||
| Fig. 9 (a) 1D-WAXD curves and (b) azimuthal scans of the LLDPE (110) plane evaluated from Fig. 8. | ||
In the selected 2D-SAXS patterns during cooling shown in Fig. 10, it can be similarly observed that LLDPE epitaxially recrystallizes on the oriented iPP substrate, and the azimuthal scan result in Fig. 11a shows that the epitaxial LLDPE lamellae are inclined about ±50° to the direction of the iPP lamellae, i.e., ±40° apart from the stretching direction, which is in line with the above result of WAXD. Fig. 11b presents the development of 1D-SAXS curves during cooling, where L of iPP remains 17.0 nm and that of LLDPE shows a gradually decreasing trend as the temperature decreases. For more information about the development of LLDPE lamellae during recrystallization, the constant scattering signal of iPP is carefully deducted by the software Polar. The processed results are displayed in Fig. 11c, presenting a variation of LLDPE L from 39.2 to 21.2 nm (Fig. 11d). Additionally, it can be found that the epitaxial crystallization of LLDPE starts at about 129 °C with the appearance of LLDPE scattering peak (Fig. 11c), which is different from the above WAXD results of 120 °C; in other words, the appearance temperature of LLDPE epitaxy signals on the oriented iPP substrate by SAXS is 9 °C higher than that of WAXD.
 | ||
| Fig. 10 Selected 2D-SAXS patterns of the LLDPE/iPP stretched blend during cooling from 150 °C to room temperature (the stretching direction is vertical). | ||
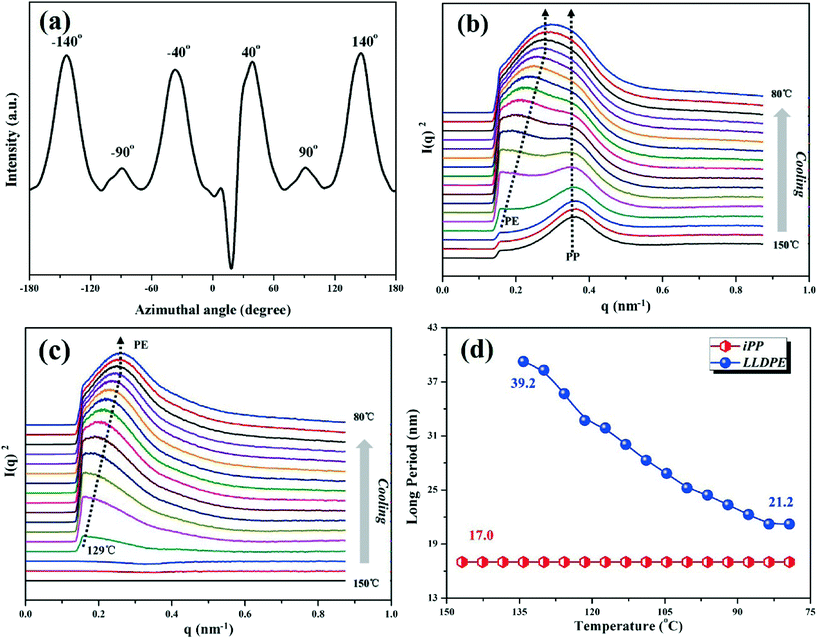 | ||
| Fig. 11 (a) Azimuthal scan of the LLDPE/iPP stretched blend after recrystallization under quiescent conditions; (b) 1D-SAXS curves evaluated from Fig. 10; (c) 1D-SAXS curves after deducting the scattering signal of iPP; (d) long period development of LLDPE and iPP during cooling. | ||
Mechanical properties of the LLDPE/iPP blend with an interface of epitaxial crystallites
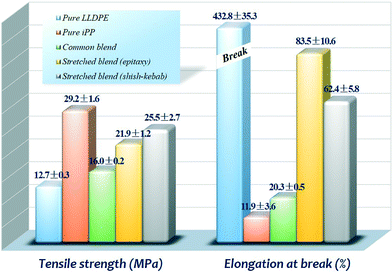
Since an epitaxial structure has been successfully created in the LLDPE/iPP blend by hot stretching, it is imperative to verify whether epitaxy can lead to the improvement in mechanical properties for the blend. Fig. 12 shows the detailed tensile properties with regard to the tensile strength and elongation at break of the samples.Compared to the initial values of 12.7 MPa and 432.8% of pure LLDPE, iPP is characterized by higher strength (29.2 MPa) but poorer ductility (11.9%). When incorporated by 20 wt% iPP, the tensile strength of LLDPE gets very limited improvement of only 3.3 MPa, and it is unfortunate to observe the heavily deteriorated ductility, showing a dramatical drop of elongation at break to 20.3%. The above phenomena arise from, in principle, the excessive phase separation caused by inherent immiscibility between LLDPE and iPP. In clear contrast, the formation of the oriented iPP phase and attendant improved interfacial adhesion with epitaxial crystallites, enormously benefit the mechanical properties of the LLDPE/iPP blend in terms of strength and ductility. As shown in Fig. 12, the tensile strength of LLDPE loaded by 20 wt% oriented iPP increases from 12.7 to 21.9 MPa, showing an amplification of 72.4%. The considerable enhancement of tensile strength, in principle, should be attributed to the excellent performance of the substantially strengthened interfacial adhesion and highly oriented iPP phase. Additionally, it is of great interest to observe the remarkable promotion of ductility, presenting 83.5% for the elongation at break, which is about 3-fold higher than that of the LLDPE/iPP common blend (20.3%), although lower than that of pure LLDPE (432.8%). The improvement in ductility clearly demonstrates the improved effective transfer of applied stress and external deformation through the greatly enhanced interfacial adhesion between LLDPE and iPP. Moreover, compared with the stretched blend with an exclusive shish-kebab structure, although the highly oriented shish-kebab structure endows the blend with superior tensile strength (25.5 MPa), its elongation at break decreases to 62.4%, which seems that the exclusive shish-kebab structure in the vicinity of the oriented iPP phase is not as effective as that of interfacial epitaxy for ductile deformation. Therefore, it can be concluded that the preferred generation of a strong interfacial interaction is of crucial significance, bridging the stress transfer from the LLDPE matrix to the highly oriented iPP phase. When encountered, the external stress deformation, the oriented iPP phase, and the interface of epitaxial crystallites show strong retardation of crack propagation, and the applied stress is prone to traverse along their length direction quite easily rather than the conventional stress concentration, finally giving rise to the notable increment of strength and ductility. In fact, the mechanical improvement in epitaxy can be explained as that the mechanical soft amorphous interlamellar regions of iPP are bridged by the crystalline lamellae of LLDPE, which desirably permits the effective transfer of applied stress and impact load from the LLDPE matrix to the oriented iPP phase, consequently resulting in a lot of plastic deformation and energy dissipation.2,39,52 In contrast with other approaches to obtain epitaxy, such as vacuum deposition or casting film crystallization onto some signal crystals or oriented film substrates, the methodology of “extrusion–hot stretching–quenching” is readily extendable for large-scale practical processing, desirably allowing the industrially feasible achievement of an epitaxial structure and expanding the application of PE in the future, which may be applied in other epitaxial polymer systems.
Formation mechanism of the oriented crystallites and epitaxial interface under an extensional flow field
The above experimental results clearly demonstrate that the epitaxial interface can be successfully achieved by an “extrusion–hot stretching–quenching” process, which endows the LLDPE/iPP blend with greatly improved strength and ductility. However, by analyzing the data of WAXD and SAXS, there arise some interesting phenomena, for example, the prominent influence of the extensional flow field on the epitaxial crystallization process, which is characterized by peculiar orientation angles for LLDPE chains and lamellae, respectively; nevertheless, the detailed mechanism still remains unclear. Besides that, it is also of great interest to observe the desynchrony of WAXD signal development with that of SAXS during melting and recrystallization, respectively, a compelling evidence of the existence of some ordered LLDPE chains in the contact surface with the iPP substrate, which play an important role in initiating the subsequent epitaxial crystallization during cooling. In this part, we will try to understand how the extensional flow field affects the unusual epitaxial crystallization process of LLDPE on the oriented iPP substrate and disclose the molecular dynamics of LLDPE chains in the molten state and the early stage of crystallization.Since, for the epitaxial growth of LLDPE lamellae on the oriented iPP substrate, earlier crystallization of iPP prior to that of LLDPE is necessary, thus providing a template for the following epitaxial crystallization of LLDPE, the most important thing that needs to be considered is the crystallization sequence of LLDPE and iPP under an extensional flow field. As depicted in Fig. 13a, under quiescent conditions, indeed iPP starts to crystallize around 124.0 °C and the peak temperature is about 115.8 °C, whereas those of LLDPE are 115.9 and 110.5 °C, respectively. So it is clear that under quiescent conditions iPP crystallizes prior to LLDPE and then serves as a nucleating agent to accelerate the subsequent crystallization of LLDPE, resulting in an increment of 4.0 °C for the onset crystallization temperature of LLDPE irrespective of iPP orientation (Fig. 13a). Then what about the situation when subjected to an extensional flow field? In general, for the effect of flow field, besides the change in crystalline structure and morphology, it is well believed that the flow will produce a chain orientation and thus reduce the energy barrier for crystallization, finally leading to the increased crystallization kinetics. In other words, the accelerating effect of flow on the crystallization process should be attributed to the pre-oriented chains. Therefore, by choosing different heating temperatures and holding times, the pre-oriented structure can be partially reserved or completely erased. This offers a good way to study the effect of thermal history on the crystallization process, reflecting the effect of flow from another side. As shown in Fig. 13c and d, the crystallization temperature of LLDPE changes marginally under flow compared with iPP, while that of iPP increases markedly when oriented. The above different behavior is mainly due to the great disparity of the melting points between LLDPE and iPP, presenting a gap of 44.1 °C as manifested in Fig. 13b.37,38 Moreover, one can find that iPP crystallizes firstly and LLDPE follows in all cases. In brief summary, under flow, the crystallization temperature of LLDPE varies slightly, while that of iPP increases substantially when oriented. In addition, iPP always crystallizes prior to LLDPE whether there is a flow or not, therewith serving as a template to induce the epitaxial crystallization of LLDPE on the highly oriented iPP substrate.
Moreover, it is observed that the intensity development of WAXD is completely out of sync with that of SAXS, showing a gap of 12 °C and 9 °C during melting and crystallization processes, respectively. For a direct comparison, Table 1 lists the disappearance and appearance temperatures of LLDPE epitaxy signals reflected by DSC, WAXD and SAXS during melting and recrystallization, respectively. Obviously, the two temperatures of DSC are nearly identical with that of WAXD, suggesting that the appearance temperature of LLDPE epitaxial crystallites is about 128 °C during crystallization, and the crystallites are totally melted at 120 °C during melting. In general, for explaining the above gaps between WAXD and SAXS, two major explanations have often been adopted: one is the different sensitivities between WAXD and SAXS detectors,53,54 and the other is that the difference is a signal of spinodal-like ordering structures.55,56 Based on the experimental results, we believe that it is the ordered LLDPE chains that cause the above gaps, because the gap during melting is 12 °C and the WAXD signals disappear prior to that of SAXS, while the gap during recrystallization is 9 °C and the development of WAXD signals lags behind that of SAXS. Moreover, considering the different detecting mechanisms between WAXD and SAXS, it can be speculated that the periodic structure composed of ordered LLDPE chains are responsible for the SAXS signals of LLDPE epitaxy in the temperature range of 129–141 °C during melting (or 120–129 °C during recrystallization), while no measurable signals are detected in WAXD and DSC. In addition, the structure shows higher thermal stability than crystals and takes shape in advance of crystals. For the melting process, it was reported that an ordered stable layer of alkanes could form above the melting point of the bulk crystal on flat solid substrates,57,58 and Yan's work59,60 on the polycarprolactone (PCL)/PE epitaxial system also denotes that the PCL chains can align along the chain direction of the highly oriented PE film above its bulk melting point, i.e., the occurrence of soft epitaxy, and with sufficient time, all of the PCL chains can be organized into a similar ordered structure, showing an extremely broad lamellae thickness. Hence, the SAXS signals above the bulk melting temperature by DSC or WAXD are, in essence, some ordered LLDPE chains between the melt and crystals, which originate from the strong interaction between LLDPE and iPP substrate, and can be detected by SAXS because of density fluctuation. For the recrystallization process, the intensity of SAXS develops prior to that of WAXD, implying that a preordered structure is actually formed before crystals, i.e., the formation of the so-called precursor at the early stage of crystallization. In fact, the existence of a crystallization precursor in the induction period has been well demonstrated by many experiments.55,56,61
| Disappearance temperature | Appearance temperature | |
|---|---|---|
| Notes: TW represents the temperature of WAXD, while TS refers to that of SAXS. | ||
| DSC | 128.2 °C | 120.1 °C |
| WAXD | 129 °C | 120 °C |
| SAXS | 141 °C | 129 °C |
| T W–TS | −12 °C | −9 °C |
In brief summary, the extensional flow field causes high orientation of iPP chains, accompanied with a markedly enhanced crystallization temperature at the early stage of crystallization. No doubt, the flow field will further affect the epitaxial growth process of LLDPE on the substrate of oriented iPP, leading to different crystalline morphologies. In this case, it is known that the LLDPE chains in its contact layer with the iPP substrate have first been arranged into an ordered structure before the formation of crystals, initiating the following epitaxial crystallization process. Then how does the extensional flow field affect the growth of LLDPE lamellae during crystallization? According to the deformation mechanism of solid PE based on “true stress–strain” experiments, it is known that upon stretching, intralamellar slipping of crystalline blocks proceeds first, followed by the stress-induced fragmentation and recrystallization of the freed chains at larger strain.50,62 In this work, the block slips within the lamellae of LLDPE can be well identified. As depicted in Fig. 13, owing to the nucleating effect of iPP, not only the onset crystallization temperature of LLDPE is increased by 4.1 °C, but also the crystallinity of LLDPE is enhanced from 38.8 to 39.9%. However, when stretched, one can observe that the melting point of LLDPE decreases from 125.1 to 123.8 °C, accompanied with a drop in crystallinity from 39.9 to 36.3%. The reduction of melting point and crystallinity should be ascribed to the destruction of the original lamellae and formation of defective crystals caused by lamellar slips during stretching. A similar conclusion can be drawn by comparing the long period of LLDPE lamellae in the common and stretched blends. Under quiescent conditions, the final long period of LLDPE epitaxial lamellae is 21.2 nm (Fig. 11d). When stretched, the above value decreases to 16.1 nm (Fig. 7b). The drop in the LLDPE long period of 5.1 nm can be explained as follows: the initial slips within the lamellae cause reorientation of both the LLDPE chain direction and the lamellae normal, and consequently substantial thinning of LLDPE lamellae and reduction of the long period take place; such a thinning effect leads to unstable lamellae and then to heavy fragmentation of lamellae into small crystalline blocks; in the end, the newly-generated blocks are free of restraint imposed previously by the lamellae and then rotate to produce a new orientation and long period along the stretching direction. Furthermore, it is considered that no flow-induced recrystallization occurs or the effect is negligible. On the one hand, the recrystallization will cause thickening and perfection of LLDPE lamellae, definitely leading to the increment of LLDPE melting point and crystallinity, which is not in line with the experimental results. On the other hand, according to the deformation experiments of PE at elevated temperatures by Men,48,49 the thermal stability of the crystalline blocks composing the lamellae is only dependent on the crystallization temperature; hence, the blocks generated at high crystallization temperature before quenching in this work are thermally stable and can be preserved at intermediate strains, and before the formation of a highly oriented fibrillar structure by further deformation, the extensional flow field stops and the as-formed structure of LLDPE is immediately preserved by quenching. To obtain more information on LLDPE epitaxial lamellae, an azimuthal scan of the LLDPE (200) plane in the 2D-WAXD pattern was further analyzed (Fig. S6†). Obviously, the profile is split into six parts. As reported by Keller and coworkers, this special diffraction implies that the lamellae are incompletely twisted.63–65 That is, under intermediate stress, the lamellae have the possibility of twisting along the growth direction (the b axis) as they do in spherulites, which should be the reason for the difference in the LLDPE chains' orientation between WAXD and SAXS (Fig. 2).
Based on the above discussion, the effect mechanism of the extensional flow field on the crystallization of the LLDPE/iPP blend can be clearly illustrated in Fig. 14. Firstly, under flow, the crystallization of iPP is notably accelerated, and a highly oriented iPP phase is formed, thus serving as an effective substrate to induce the following crystallization of LLDPE. According to the intensity of flow, the crystallization of LLDPE can be divided into two types. Under a strong flow field, the LLDPE chains prefer to align along the flow direction, and a shish-kebab structure is preferentially formed because of its high thermal stability (Fig. 14a–c). For another type, i.e., under a weak flow field, the pre-oriented LLDPE chains can relax quickly and epitaxially nucleate on the surface of the oriented iPP substrate. During further growth, the epitaxial LLDPE lamellae deform and reorient along the flow direction under the mechanism of flow-induced block slips, fragmentation and reorientation (Fig. 14d–f). Moreover, it is believed that incomplete lamellar twist also occurs under flow.
Conclusion
In this work, we show an accessible method, i.e., “extrusion–hot stretching–quenching”, to accomplish the large-scale achievement of an epitaxial interface for the LLDPE/iPP blend. It is firstly found that, under an extensional flow field, LLDPE could epitaxially grow on an oriented iPP substrate but showing an abnormal molecular and lamellar orientation angle of ±25° and ±45° apart from the flow direction reflected by WAXD and SAXS, respectively. In contrast with the situation under quiescent conditions, it is concluded that iPP always crystallizes prior to LLDPE under flow, and the extensional flow field can significantly affect the following crystallization process of LLDPE on the oriented iPP substrate. Additionally, the above effect depends heavily on the intensity of flow field. That is, under a strong flow field, the LLDPE chains prefer to align along the flow direction, and a shish-kebab structure is preferentially formed because of its high thermal stability, while under a weak flow field, the pre-oriented LLDPE chains can relax quickly and turn to epitaxially crystallize on the oriented iPP substrate. During further growth, the epitaxial LLDPE lamellae deform and reorient along the flow direction under the mechanism of flow-induced block slips, fragmentation and reorientation, finally giving rise to the above peculiar WAXD and SAXS patterns. Moreover, it is considered that incomplete twist also occurs in the deformed epitaxial LLDPE lamellae. Finally, mechanical property tests demonstrate that an epitaxial structure greatly enhances the interfacial adhesion between LLDPE and iPP, showing a significant improvement for the LLDPE/iPP blend in both strength and toughness. This work provides the first understanding of epitaxial crystallization under a flow field, and of particular significance is the methodology based on practical processing of extrusion in the present work, making it promising to industrially achieve an epitaxial structure in polymer blends with advanced properties.Acknowledgements
The authors gratefully acknowledge the financial support to this work by the National Natural Science Foundation of China (No. 21276168, 51273131, 51227801, and 51473101), the China Postdoctoral Science Foundation (Grant No. 2014 T70868), the State Key Laboratory of Polymer Materials Engineering (sklpme2014-3-08), the Specialized Research Fund for the Doctoral Program of Higher Education (Grant No. 20120181120101) and the Fundamental Research Funds for the Central Universities. We would also like to express our heartfelt thanks to the Shanghai Synchrotron Radiation Facility (SSRF, Shanghai, China), for the kind help on WAXD and SAXS measurements.Notes and references
- I. Bonev, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1972, 28, 508–512 CrossRef CAS
.
- B. Gross and J. Peterman, J. Mater. Sci., 1984, 19, 105–112 CrossRef CAS
.
- J. Petermann, G. Broza, U. Rieck and A. Kawaguchi, J. Mater. Sci., 1987, 22, 1477–1481 CrossRef CAS
.
- J. Petermann and R. Gohil, J. Mater. Sci., 1979, 14, 2260–2264 CrossRef CAS
.
- C. Plummer and H.-H. Kausch, Polym. Bull., 1996, 37, 393–397 CrossRef CAS
.
- D. Fenwick, P. Smith and J. Wittmann, J. Mater. Sci., 1996, 31, 128–131 CrossRef CAS
.
- F. Motamedi, K. J. Ihn, D. Fenwick, J. C. Wittmann and P. Smith, J. Polym. Sci., Part B: Polym. Phys., 1994, 32, 453–457 CrossRef CAS
.
- D. Fenwick, K. J. Ihn, F. Motamedi, J. C. Wittmann and P. Smith, J. Appl. Polym. Sci., 1993, 50, 1151–1157 CrossRef CAS
.
- B. Lotz and J. Wittmann, J. Polym. Sci., Part B: Polym. Phys., 1986, 24, 1559–1575 CrossRef CAS
.
- J. Wittmann and B. Lotz, J. Polym. Sci., Polym. Phys. Ed., 1981, 19, 1837–1851 CrossRef CAS
.
- J. Wittmann, A. Hodge and B. Lotz, J. Polym. Sci., Polym. Phys. Ed., 1983, 21, 2495–2509 CrossRef CAS
.
- M. Ashida, Y. Ueda and T. Watanabe, J. Polym. Sci., Polym. Phys. Ed., 1978, 16, 179–188 CrossRef CAS
.
- J. Wittmann and B. Lotz, Prog. Polym. Sci., 1990, 15, 909–948 CrossRef CAS
.
- J. Wittmann and B. Lotz, J. Polym. Sci., Polym. Phys. Ed., 1985, 23, 205–226 CrossRef CAS
.
- Q. Liu, X. Sun, H. Li and S. Yan, Polymer, 2013, 54, 4404–4421 CrossRef CAS
.
- F. Jay, J. M. Haudin and B. Monasse, J. Mater. Sci., 1999, 34, 2089–2102 CrossRef CAS
.
- G. Kumaraswamy, A. M. Issaian and J. A. Kornfield, Macromolecules, 1999, 32, 7537–7547 CrossRef CAS
.
- J. Varga and J. Karger-Kocsis, J. Polym. Sci., Polym. Phys. Ed., 1996, 34, 657–670 CrossRef CAS
.
- C.-M. Wu, M. Chen and J. Karger-Kocsis, Polymer, 1999, 40, 4195–4203 CrossRef CAS
.
- R. H. Somani, L. Yang, L. Zhu and B. S. Hsiao, Polymer, 2005, 46, 8587–8623 CrossRef CAS
.
- A. Peterlin, Colloid Polym. Sci., 1987, 265, 357–382 CAS
.
- G. Kumaraswamy, R. K. Verma and J. A. Kornfield, Rev. Sci. Instrum., 1999, 70, 2097–2104 CrossRef CAS
.
- G. J. Zhong, L. Li, E. Mendes, D. Byelov, Q. Fu and Z. M. Li, Macromolecules, 2006, 39, 6771–6775 CrossRef CAS
.
- Z. M. Li, L. Li, K. Z. Shen, M. B. Yang and R. Huang, Polymer, 2005, 46, 5358–5367 CrossRef CAS
.
- L. Xie, H. Xu, B. Niu, X. Ji, J. Chen, Z. M. Li, B. S. Hsiao and G. J. Zhong, Biomacromolecules, 2014, 15, 4054–4064 CrossRef CAS PubMed
.
- Z. M. Li, L. B. Li, K. Z. Shen, W. Yang, R. Huang and M. B. Yang, Macromol. Rapid
Commun., 2004, 25, 553–558 CrossRef CAS
.
- X. Yi, C. Chen, G. J. Zhong, L. Xu, J. H. Tang, X. Ji, B. S. Hsiao and Z. M. Li, J. Phys. Chem. B, 2011, 115, 7497–7504 CrossRef CAS PubMed
.
- Y. H. Chen, G. J. Zhong, J. Lie, Z. M. Li and B. S. Hsiao, Macromolecules, 2011, 44, 8080–8092 CrossRef CAS
.
-
B. Wunderlich, Macromolecular physics, Academic, New York, 1973 Search PubMed
.
- X. Zhu, D. Yan and Y. Fang, J. Phys. Chem. B, 2001, 105, 12461–12463 CrossRef CAS
.
- T. Konishi, K. Nishida, T. Kanaya and K. Kaji, Macromolecules, 2005, 38, 8749–8754 CrossRef CAS
.
- Y. V. Kissin and L. Rishina, Eur. Polym. J., 1976, 12, 757–759 CrossRef CAS
.
- B. Lotz and J. Wittmann, J. Polym. Sci., Part B: Polym. Phys., 1986, 24, 1541–1558 CrossRef CAS
.
- F. J. Padden Jr. and H. D. Keith, J. Appl. Phys., 1973, 44, 1217–1223 CrossRef
.
- B. Na, K. Wang, P. Zhao, Q. Zhang, R. Du, Q. Fu, Z. Yu and E. Chen, Polymer, 2005, 46, 5258–5267 CrossRef CAS
.
- R. Su, K. Wang, P. Zhao, Q. Zhang, R. Du, Q. Fu, L. Li and L. Li, Polymer, 2007, 48, 4529–4536 CrossRef CAS
.
- B. Na, Q. Zhang, K. Wang, L. Li and Q. Fu, Polymer, 2005, 46, 819–825 CrossRef CAS
.
- R. Su, K. Wang, Q. Zhang, F. Chen, Q. Fu, N. Hu and E. Chen, Polym. Adv. Technol., 2011, 22, 225–231 CrossRef CAS
.
- H. Li and S. Yan, Macromolecules, 2011, 44, 417–428 CrossRef CAS
.
- S. Yan, J. Lin, D. Yang and J. Petermann, Macromol. Chem. Phys., 1994, 195, 195–201 CrossRef CAS
.
- R. Gohil, J. Polym. Sci., Polym. Phys. Ed., 1985, 23, 1713–1722 CrossRef CAS
.
- R. Séguéla, Macromol. Mater. Eng., 2007, 292, 235–244 CrossRef
.
- H. Song, A. Argon and R. E. Cohen, Macromolecules, 1990, 23, 870–876 CrossRef CAS
.
- B. Xiong, O. Lame, J. M. Chenal, C. Rochas, R. Seguela and G. Vigier, Polymer, 2014, 55, 1223–1227 CrossRef CAS
.
- Z. Jiang, Y. Tang, J. Rieger, H.-F. Enderle, D. Lilge, S. V. Roth, R. Gehrke, W. Heckmann and Y. Men, Macromolecules, 2010, 43, 4727–4732 CrossRef CAS
.
- J. M. Kim, R. Locker and G. C. Rutledge, Macromolecules, 2014, 47, 2515–2528 CrossRef CAS
.
- J. Che, C. R. Locker, S. Lee, G. C. Rutledge, B. S. Hsiao and A. H. Tsou, Macromolecules, 2013, 46, 5279–5289 CrossRef CAS
.
- Z. Jiang, Y. Tang, Y. Men, H. F. Enderle, D. Lilge, S. V. Roth, R. Gehrke and J. Rieger, Macromolecules, 2007, 40, 7263–7269 CrossRef CAS
.
- Z. Jiang, Y. Tang, J. Rieger, H. F. Enderle, D. Lilge, S. V. Roth, R. Gehrke, Z. Wu, Z. Li and Y. Men, Polymer, 2009, 50, 4101–4111 CrossRef CAS
.
- R. Hiss, S. Hobeika, C. Lynn and G. Strobl, Macromolecules, 1999, 32, 4390–4403 CrossRef CAS
.
- R. H. Somani, B. S. Hsiao, A. Nogales, H. Fruitwala, S. Srinivas and A. H. Tsou, Macromolecules, 2001, 34, 5902–5909 CrossRef CAS
.
- A. Jaballah, U. Rieck and J. D. Petermann, J. Mater. Sci., 1990, 25, 3105–3110 CrossRef CAS
.
- Z.-G. Wang, B. S. Hsiao, E. B. Sirota, P. Agarwal and S. Srinivas, Macromolecules, 2000, 33, 978–989 CrossRef CAS
.
- P. Panine, V. Urban, P. Boesecke and T. Narayanan, J. Appl. Crystallogr., 2003, 36, 991–994 CrossRef CAS
.
- E. Heeley, A. Maidens, P. Olmsted, W. Bras, I. Dolbnya, J. Fairclough, N. Terrill and A. Ryan, Macromolecules, 2003, 36, 3656–3665 CrossRef CAS
.
- M. Imai, K. Kaji and T. Kanaya, Phys. Rev. Lett., 1993, 71, 4162 CrossRef CAS PubMed
.
- A. Tracz, I. Kucinska and J. Jeszka, Macromolecules, 2003, 36, 10130–10132 CrossRef CAS
.
- Y. Takenaka, H. Miyaji, A. Hoshino, A. Tracz, J. K. Jeszka and I. Kucinska, Macromolecules, 2004, 37, 9667–9669 CrossRef CAS
.
- C. Yan, H. Li, J. Zhang, Y. Ozaki, D. Shen, D. Yan, A. C. Shi and S. Yan, Macromolecules, 2006, 39, 8041–8048 CrossRef CAS
.
- H. Chang, J. Zhang, L. Li, Z. Wang, C. Yang, I. Takahashi, Y. Ozaki and S. Yan, Macromolecules, 2009, 43, 362–366 CrossRef
.
- M. Soccio, A. Nogales, N. Lotti, A. Munari and T. A. Ezquerra, Phys. Rev. Lett., 2007, 98, 037801 CrossRef CAS PubMed
.
- Y. Men and G. Strobl, J. Macromol. Sci., Part B: Phys., 2001, 40, 775–796 CrossRef
.
- A. Keller, J. Polym. Sci., 1955, 15, 31–49 CrossRef CAS
.
- A. Keller and M. Machin, J. Macromol. Sci., Part B: Phys., 1967, 1, 41–91 CrossRef CAS
.
- M. Hill and A. Keller, J. Macromol. Sci., Part B: Phys., 1971, 5, 591–616 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ce01433f |
| This journal is © The Royal Society of Chemistry 2016 |