Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Alignment of paired molecules of C60 within a hexagonal platform networked through hydrogen-bonds†
Ichiro
Hisaki
 *a,
Shoichi
Nakagawa
a,
Hiroyasu
Sato
b and
Norimitsu
Tohnai
a
*a,
Shoichi
Nakagawa
a,
Hiroyasu
Sato
b and
Norimitsu
Tohnai
a
aDepartment of Material and Life Science, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
bRigaku Corporation, Matsubara-cho 3-9-12, Akishima, Tokyo 196-8666, Japan
First published on 7th July 2016
Abstract
We demonstrate, for the first time, that a hydrogen-bonded low-density organic framework can be applied as a platform to achieve periodic alignment of paired molecules of C60, which is the smallest example of a finite-numbered cluster of C60. The framework is a layered assembly of a hydrogen-bonded 2D hexagonal network (LA-H-HexNet) composed of dodecadehydrotribenzo[18]annulene derivatives.
Dimensionally-controlled assemblies of functional π-conjugated molecules are of substantial interest from optical and electronic viewpoints. In particular, those of fullerene (C60) derivatives have been intensively studied because of their specific optoelectronic properties such as a significant electron accepting ability originating in the spherical π-system.1,2
Crystalline lattices are a potential platform to achieve controlled arrangements of C60 molecules. To date, molecularly-isolated arrangements (Fig. 1a)3 and one-dimensionally (1-D) (Fig. 1b)2d,4 or two-dimensionally (2-D) (Fig. 1c)5 aligned infinite structures of C60 have been reported in various crystalline frameworks. On the other hand, isolated arrangements of finite-numbered clusters of C60 molecules are still challenging to construct due to the lack of a general methodology, although seven examples have been reported for an isolated-dimer structure of C60 (Fig. 1d), which is the smallest example of a finite-numbered cluster.6,7
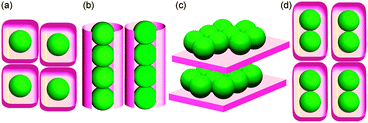 | ||
| Fig. 1 Schematic representation of dimensionally-controlled alignments of C60. (a) Molecularly-isolated, (b) one-dimensional, (c) two-dimensional, and (d) finite-number-isolated arrangements. | ||
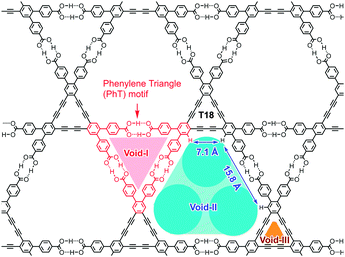
In this study, we propose that a hydrogen-bonded low density framework can be applied as a platform to construct an isolated arrangement of finite-numbered clusters of C60 molecules. Previously, we have reported that C3-symmetric cyclic planar π-conjugated molecules with three 4,4′-dicarboxy-o-terphenyl moieties can form hydrogen-bonded 2D hexagonal network (H-HexNet) structures with multiple voids and that the H-HexNet sheets are stacked without interpenetration to give low-density layered assemblies of H-HexNets (LA-H-HexNets).8 In particular, a H-HexNet of dodecadehydrotribenzo[18]annulene derivative T18 (Fig. 2)8a,c can provide a space suitable for aligning C60 molecules.
T18 was synthesised using our reported method8a and was initially crystallized via slow evaporation of a mixed solution of o-dichlorobenzene (oDCB) and N,N-dimethylformamide (DMF) at 50 °C to yield P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) crystal T18-oDCB as shown in Fig. 3a–c.‡
crystal T18-oDCB as shown in Fig. 3a–c.‡
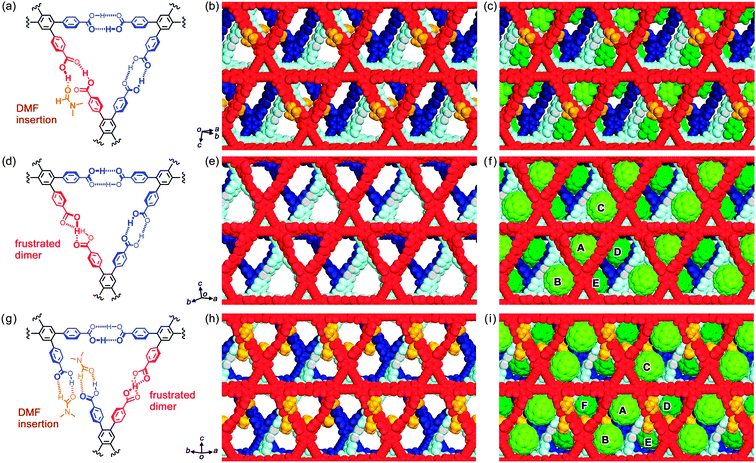
T18-oDCB assembles into a H-HexNet framework by forming a so-called phenylene triangle (PhT) motif, in which one pair of carboxy dimers is truncated by DMF. The HexNet sheets are stacked in a XX′ manner without interpenetration, where the adjacent layers X and X′ are related by an inversion center, affording a LA-H-HexNet structure. In the center of triangular void-I, oDCB molecules are located, indicating that oDCB may act as a template to stabilize the PhT motif.
Subsequently, crystallization of T18 was performed under the above mentioned conditions in the presence of C60, yielding two types of LA-H-HexNet crystals including C60 molecules (T18-C60-1 and T18-C60-2), as shown in Fig. 3d–i.‡ These are formed concomitantly and a condition for the selective preparation of either of the two forms has not been established yet in spite of our great efforts. The final convergence factors of these crystals, such as relatively high R values, are mostly predetermined by the nature of the crystals showing large triclinic cells and extensive disorder involving chlorine atoms.
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) crystal T18-C60-1 consists of T18, C60, and oDCB in a ratio of 1
crystal T18-C60-1 consists of T18, C60, and oDCB in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. T18 molecules assemble into a H-HexNet framework by forming a PhT motif with three hydrogen-bonded dimers of carboxyphenyl groups, one of which exhibits geometrical frustration8 (Fig. 3d), and the H-HexNet sheet is stacked to give a LA-H-HexNet structure (Fig. 3e). Within the H-HexNet layer, C60 molecules are located in two positions: one molecule labelled A (C60A) is in contact with the rim of a PhT (void-I); the others (C60B or C60C) are at the corners of non-regular hexagonal spaces (void-II) (Fig. 3f). In both cases, interactions between the H-HexNet framework and C60 molecules are van der Waals forces such as π⋯π and CH⋯π contacts. In Table 1, intermolecular distances between the centres of adjacent C60 molecules (L) are listed. In crystal T18-C60-1, C60 molecules are molecularly isolated. For example, the distances L for C60A–C60B and C60A–C60C are 13.2 Å and 14.9 Å, respectively.
4. T18 molecules assemble into a H-HexNet framework by forming a PhT motif with three hydrogen-bonded dimers of carboxyphenyl groups, one of which exhibits geometrical frustration8 (Fig. 3d), and the H-HexNet sheet is stacked to give a LA-H-HexNet structure (Fig. 3e). Within the H-HexNet layer, C60 molecules are located in two positions: one molecule labelled A (C60A) is in contact with the rim of a PhT (void-I); the others (C60B or C60C) are at the corners of non-regular hexagonal spaces (void-II) (Fig. 3f). In both cases, interactions between the H-HexNet framework and C60 molecules are van der Waals forces such as π⋯π and CH⋯π contacts. In Table 1, intermolecular distances between the centres of adjacent C60 molecules (L) are listed. In crystal T18-C60-1, C60 molecules are molecularly isolated. For example, the distances L for C60A–C60B and C60A–C60C are 13.2 Å and 14.9 Å, respectively.
| Crystal | C60 pair | L/Å | Type | Symmetry code |
|---|---|---|---|---|
| a L denotes the distance between the centroids of two C60 molecules. | ||||
| T18-C60-1 | A–B | 13.17 | Intra-layer | B: (−x, 1 − y, − z) |
| A–C | 14.92 | Intra-layer | C: (1 − x, − y, 1 − z) | |
| A–D | 13.82 | Inter-layer | D: (1 + x, y, z) | |
| A–E | 13.16 | Inter-layer | E: (1 − x, 1 − y, − z) | |
| T18-C60-2 | A–B | 11.15 | Intra-layer | B: (−x, 1 − y, 2 − z) |
| A–C | 16.36 | Intra-layer | C: (1 − x, 1 − y, 3 − z) | |
| A–D | 16.28 | Intra-layer | D: (1 + x, y, z) | |
| A–E | 15.12 | Intra-layer | E: (1 − x, 1 − y, 2 − z) | |
| A–F | 16.30 | Intra-layer | F: (x, 1 + y, z) | |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) crystal T18-C60-2 consists of T18, DMF, C60, and oDCB in a ratio of 1
crystal T18-C60-2 consists of T18, DMF, C60, and oDCB in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The crystal density of T18-C60-2 is slightly higher (1.518 vs. 1.481 g−1 cm−3) and C60 is rotationally less disordered than in T18-C60-1. One of the hydrogen-bonded dimers in the PhT motif of T18-C60-2 is completely dissociated, and each of the carboxy groups forms a heterodimer with a DMF molecule (Fig. 3g), although the H-HexNet framework itself is retained owing to the other two homodimers. The H-HexNet sheets are again stacked in a XX′ manner, affording a LA-H-HexNet framework as in the case of the former two (Fig. 3h). Interestingly, void-II in T18-C60-2 accommodates two C60 molecules (C60A and C60B) at its corners and the resulting dimeric array of C60 is isolated from the adjacent dimers by the H-HexNet framework (Fig. 3i). The distance (L1) between the nearest two molecules (C60A and C60B) is 11.2 Å and that (L2) for the second nearest two molecules (C60A and C60E) is 15.12 Å. This is regarded as the smallest system of an infinite-number-isolated array of C60.
4. The crystal density of T18-C60-2 is slightly higher (1.518 vs. 1.481 g−1 cm−3) and C60 is rotationally less disordered than in T18-C60-1. One of the hydrogen-bonded dimers in the PhT motif of T18-C60-2 is completely dissociated, and each of the carboxy groups forms a heterodimer with a DMF molecule (Fig. 3g), although the H-HexNet framework itself is retained owing to the other two homodimers. The H-HexNet sheets are again stacked in a XX′ manner, affording a LA-H-HexNet framework as in the case of the former two (Fig. 3h). Interestingly, void-II in T18-C60-2 accommodates two C60 molecules (C60A and C60B) at its corners and the resulting dimeric array of C60 is isolated from the adjacent dimers by the H-HexNet framework (Fig. 3i). The distance (L1) between the nearest two molecules (C60A and C60B) is 11.2 Å and that (L2) for the second nearest two molecules (C60A and C60E) is 15.12 Å. This is regarded as the smallest system of an infinite-number-isolated array of C60.
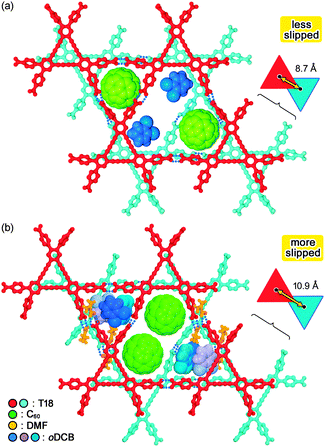
To conduct a more detailed comparison of these two structures, we next focused on the bi-layer of the rhombic framework, which is a motif of the H-HexNet sheet (Fig. 4). The two adjacent rhombic frameworks are stacked in an inverted manner, giving three partitioned voids: one central space and two side spaces. In T18-C60-1, each of the side spaces is occupied by one C60 molecule and the central one accommodates oDCB molecules (Fig. 4a), although they are disordered within it. In T18-C60-2, on the other hand, each of the side spaces is filled with three oDCB molecules and the central one is filled with two C60 molecules (Fig. 4b). It is not trivial that the width of the central space of T18-C60-2 is expanded by slipping the rhombic frameworks by 2.2 ŧ compared with those of T18-C60-1 to accommodate bulky C60 molecules (inset in Fig. 4). In addition, the existence of hydrogen-bonded DMF molecules contributes to subtle structural tuning to achieve the present molecular arrangements.
The isolated C60 pair observed in the present system is a unique type of C60 array. Indeed, a survey of the Cambridge Structural Database (CSD) revealed that, among the structure-available 200 registries of C60 containing crystals, seven crystal structures contained an isolated dimer of a neutral parent C60 molecule as summarised in Table 2. Crystals referred to by the Ref Codes BIBVUE,6b LIZSIX,6c and VAJYIP6a have crystalline lattices composed of porphyrin arrays connected through van der Waals interactions. Notably, in the case of BIBVUE, a C60 dimer with intermolecular distance L1 = 9.95 Å is well isolated from the adjacent dimers (L2 = 14.23 Å, L12 = 4.29 Å). In DAYXAE,6e FUMBIZ,6d NIFXUV,6f and NIFYAC,6f C60 molecules form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes with a bowl-shaped or tripodal receptor, and the complexes are arranged through van der Waals contacts. Although the present C60 pairs have relatively long intermolecular distances (i.e., L1 = 11.2 Å), the pairs are well separated from adjacent pairs (L2 = 15.1 Å and L12 = 4.0 Å). It should be noted that it is almost impossible to predict and design an arrangement of C60 molecules in a crystalline lattice constructed by non-directional van der Waals interactions, although a sophisticated C60-isolated pair has been achieved. The present system is the first example of an isolated C60 dimeric pair within a well-defined, hydrogen-bonded, low density framework.
1 host–guest complexes with a bowl-shaped or tripodal receptor, and the complexes are arranged through van der Waals contacts. Although the present C60 pairs have relatively long intermolecular distances (i.e., L1 = 11.2 Å), the pairs are well separated from adjacent pairs (L2 = 15.1 Å and L12 = 4.0 Å). It should be noted that it is almost impossible to predict and design an arrangement of C60 molecules in a crystalline lattice constructed by non-directional van der Waals interactions, although a sophisticated C60-isolated pair has been achieved. The present system is the first example of an isolated C60 dimeric pair within a well-defined, hydrogen-bonded, low density framework.
| Crystal/RefCodea | Host compound | L 1 /Å | L 2 /Å | dL12d/Å | Type of framework | Ref. |
|---|---|---|---|---|---|---|
a Crystals denoted by RefCodes were extracted by a CSD search.
b
L
1 denotes the intermolecular distance between the centroids of the nearest two C60 molecules.
c
L
2 denotes the intermolecular distance between the centroids of the second nearest two C60 molecules.
d
L
12 denotes differences between L1 and L2.
e HB 2D lattice denotes a 2D lattice connected by a hydrogen bonded network.
f vdW lattice denotes a lattice constructed through van der Waals contacts.
g H/G complex denotes that C60 and a host compound form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex, which subsequently constructs crystals through van der Waals contacts. 1 complex, which subsequently constructs crystals through van der Waals contacts.
|
||||||
| T18-C60-2 | T18 | 11.15 | 15.12 | 3.97 | HB 2D latticee | This work |
| BIBVUE | Porphyrin array | 9.94 | 14.23 | 4.29 | vdW latticef | 6b |
| LIZSIX | Porphyrin array | 10.21 | 12.51 | 2.30 | vdW latticef | 6c |
| VAJYIP | Zn porphyrin dimer | 9.93 | 13.37 | 3.43 | vdW latticef | 6a |
| DAYXAE | Bowl shaped π-system | 9.94 | 13.07 | 3.13 | H/G complexg | 6e |
| FUMBIZ | Tripodal anthracene system | 9.76 | 11.70 | 1.94 | H/G complexg | 6d |
| NIFXUV | Calix[5]arene derivative | 10.18 | 11.08 | 0.90 | H/G complexg | 6f |
| NIFYAC | Calix[5]arene derivative | 10.17 | 11.20 | 1.03 | H/G complexg | 6f |
In conclusion, we adopted a low-density layered assembly of a hydrogen-bonded hexagonal network (LA-H-HexNets) of dodecadehydrotribenzo[18]annulene derivatives T18 to align C60, yielding two types of C60 included crystals: T18-C60-1 and T18-C60-2. In the latter, an alignment of an isolated molecular pair of C60 partitioned by 2D hexagonal frameworks was achieved. This arrangement is the smallest example of a finite-numbered cluster of C60. These results imply that the present LA-H-HexNet can be applied as a platform to align functional molecules, aimed at developing for example, artificial photosynthetic systems.9 Selective preparation of T18-C60-1 and T18-C60-2 and the subsequent investigation of their optical and electronic properties are currently underway.
This work was supported by a Grant-in-Aid for Scientific Research (C) (JPT15K04591) and for Scientific Research on Innovative Areas: π-System Configuration (JP15H00998) from MEXT Japan. We would like to thank Prof. Hidehiro Sakurai, Osaka University, for NMR measurements and Prof. Mikiji Miyata, Osaka University, for fruitful discussions. Finally, we would like to thank the referrers for their important and constructive comments, which led to the improvement of the manuscript. Crystallographic data were partly collected using synchrotron radiation at the BL38B1 and BL02B2 in the SPring-8 with the approval of JASRI (Proposal No. 2014B1976, 2015B1397, and 2015B1685).
Notes and references
- (a) D. M. Guldi, F. Zerbetto, V. Georgakilas and M. Prato, Acc. Chem. Res., 2005, 38, 38–43 CrossRef CAS PubMed; (b) S. S. Babu, H. Möhwald and T. Nakanishi, Chem. Soc. Rev., 2010, 39, 4021–4035 RSC; (c) F. Giacalone and N. Martín, Adv. Mater., 2010, 22, 4220–4248 CrossRef CAS PubMed.
- (a) S. Zhou, C. Burger, B. Chu, M. Sawamura, N. Nagahama, M. Toganoh, U. E. Hackler, H. Isobe and E. Nakamura, Science, 2001, 291, 1944–1947 CrossRef CAS PubMed; (b) M. Sawamura, K. Kawai, Y. Matsuo, K. Kanie, T. Kato and E. Nakamura, Nature, 2002, 419, 702–705 CrossRef CAS PubMed; (c) Y. Yamamoto, G. Zhang, W. Jin, T. Fukushima, N. Ishii, A. Saeki, S. Seki, S. Tagawa, T. Minari, K. Tsukagoshi and T. Aida, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 21051–21056 CrossRef CAS PubMed; (d) H. Nobukuni, Y. Shimazaki, H. Uno, Y. Naruta, K. Ohkubo, T. Kojima, S. Fukuzumi, S. Seki, H. Sakai, T. Hasobe and F. Tani, Chem. – Eur. J., 2010, 16, 11611–11623 CrossRef CAS PubMed.
- D. Canevet, E. M. Pérez and N. Martín, Angew. Chem., Int. Ed., 2011, 50, 9248–9259 CrossRef CAS PubMed.
- (a) L. J. Barbour, G. W. Orr and J. L. Atwood, Chem. Commun., 1998, 1901–1902 RSC; (b) A. L. Litvinov, D. V. Konarev, A. Y. Kovalevsky, I. S. Neretin, Y. L. Slovokhotov, P. Coppens and R. N. Lyubovskaya, CrystEngComm, 2002, 4, 618–622 RSC; (c) A. Sygula, F. R. Fronczek, R. Sygula, P. W. Rabideau and M. M. Olmstead, J. Am. Chem. Soc., 2007, 129, 3842–3843 CrossRef CAS PubMed; (d) C. Pariya, C. R. Sparrow, C.-K. Back, G. Sandi, F. R. Fronczek and A. W. Maverick, Angew. Chem., Int. Ed., 2007, 46, 6305–6308 CrossRef CAS PubMed; (e) J. Kobayashi, Y. Domoto and T. Kawashima, Chem. Commun., 2009, 6186–6188 RSC.
- (a) E. M. Veen, P. M. Postma, H. T. Jonkman, A. L. Spek and B. L. Feringa, Chem. Commun., 1999, 1709–1710 RSC; (b) D. V. Konarev, A. Y. Kovalevsky, D. V. Lopatin, A. V. Umrikhin, E. I. Yudanova, P. Coppens, R. N. Lyubovskaya and G. Saito, Dalton Trans., 2005, 1821–1825 RSC; (c) D. V. Konarev, S. S. Khasanov, A. Y. Kovalevsky, D. V. Lopatin, V. V. Rodaev, G. Saito, B. Nafradi, L. Forro and R. N. Lyubovskaya, Cryst. Growth Des., 2008, 8, 1161–1172 CrossRef CAS; (d) S.-X. Fa, L.-X. Wang, D.-X. Wang, L. Zhao and M.-X. Wang, J. Org. Chem., 2014, 79, 3559–3571 CrossRef CAS PubMed.
- (a) A. L. Litvinov, D. V. Konarev, A. Y. Kovalevsky, P. Coppens and R. N. Lyubovskaya, CrystEngComm, 2003, 5, 137–139 RSC; (b) D. V. Konarev, A. L. Litvinov, I. S. Neretin, N. V. Drichko, Y. L. Slovokhotov, R. N. Lyubovskaya, J. A. K. Howard and D. S. Yufit, Cryst. Growth Des., 2004, 4, 643–646 CrossRef CAS; (c) L. H. Tong, J.-L. Wietor, W. Clegg, P. R. Raithby, S. I. Pascu and J. K. M. Sanders, Chem. – Eur. J., 2008, 14, 3035–3044 CrossRef CAS PubMed; (d) J. Kobayashi, Y. Domoto and T. Kawashima, Chem. Lett., 2010, 39, 134–135 CrossRef CAS; (e) B. T. King, M. M. Olmstead, K. K. Baldridge, B. Kumar, A. L. Balch and J. A. Gharamaleki, Chem. Commun., 2012, 48, 9882–9884 RSC; (f) T. Haino, M. Yanase and Y. Fukazawa, Tetrahedron Lett., 1997, 38, 3739–3742 CrossRef CAS.
- Covalently connected arrays of C60 are also known, see; J. L. Segura and N. Martín, Chem. Soc. Rev., 2000, 29, 13–25 RSC.
- (a) I. Hisaki, S. Nakagawa, N. Tohnai and M. Miyata, Angew. Chem., Int. Ed., 2015, 54, 3008 CrossRef CAS PubMed; (b) I. Hisaki, N. Ikenaka, N. Tohnai and M. Miyata, Chem. Commun., 2016, 52, 300–303 RSC; (c) I. Hisaki, S. Nakagawa, N. Ikenaka, Y. Imamura, M. Katouda, M. Tashiro, H. Tsuchida, T. Ogoshi, H. Sato, N. Tohnai and M. Miyata, J. Am. Chem. Soc., 2016, 138, 6617–6628 CrossRef CAS PubMed.
- T. Hasegawa, K. Ohkubo, I. Hisaki, M. Miyata, N. Tohnai and S. Fukuzumi, Chem. Commun., 2016, 52, 7928–7931 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, CSD searches. CCDC 1481305, 1481306 and 1487277. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc04310k |
‡ Crystal data for T18-oDCB: (C72H36O12)·3(C6H4Cl2)·(C3H7N), Fw = 1607.08, a = 12.0893(3) Å, b = 17.1939(4) Å, c = 24.6711(6) Å, α = 104.880(2)°, β = 90.0510(19)°, γ = 98.3470(19)°, V = 4899.8(2) Å3, T = 213 K, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 2, 30849 collected, 15945 unique (Rint = 0.052) reflections, the final R1 and wR2 values 0.1254 (I > 2.0σ(I)) and 0.4149 (all data), respectively. Crystal data for T18-C60-1: (C72H36O12)·(C60)·4(C6H4Cl2), Fw = 2400.74, a = 13.8208(4) Å, b = 17.4812(6) Å, c = 24.2884(9) Å, α = 76.082(3)°, β = 87.204(3)°, γ = 70.991(3)°, V = 5382.5(3) Å3, T = 100 K, triclinic, space group P , Z = 2, 30849 collected, 15945 unique (Rint = 0.052) reflections, the final R1 and wR2 values 0.1254 (I > 2.0σ(I)) and 0.4149 (all data), respectively. Crystal data for T18-C60-1: (C72H36O12)·(C60)·4(C6H4Cl2), Fw = 2400.74, a = 13.8208(4) Å, b = 17.4812(6) Å, c = 24.2884(9) Å, α = 76.082(3)°, β = 87.204(3)°, γ = 70.991(3)°, V = 5382.5(3) Å3, T = 100 K, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 2, 90205 collected, 28131 unique (Rint = 0.102) reflections, the final R1 and wR2 values 0.1497 (I > 2.0σ(I)) and 0.4352 (all data), respectively. Crystal data for T18-C60-2: (C72H36O12)·(C60)·2(C3H7NO)·4(C6H4Cl2), Fw = 2547.93, a = 16.2768(7) Å, b = 16.3003(7) Å, c = 23.6077(8) Å, α = 94.242(3)°, β = 5.588(3)°, γ = 115.579(4)°, V = 5575.6(4) Å3, T = 100 K, triclinic, space group P , Z = 2, 90205 collected, 28131 unique (Rint = 0.102) reflections, the final R1 and wR2 values 0.1497 (I > 2.0σ(I)) and 0.4352 (all data), respectively. Crystal data for T18-C60-2: (C72H36O12)·(C60)·2(C3H7NO)·4(C6H4Cl2), Fw = 2547.93, a = 16.2768(7) Å, b = 16.3003(7) Å, c = 23.6077(8) Å, α = 94.242(3)°, β = 5.588(3)°, γ = 115.579(4)°, V = 5575.6(4) Å3, T = 100 K, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 2, 77 , Z = 2, 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 collected, 20 670 collected, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 249 unique (Rint = 0.0984) reflections, thes final R1 and wR2 values 0.1414 (I > 2.0σ(I)) and 0.4453 (all data), respectively. CCDC numbers: T18-oDCB (CCDC 1487277), T18-C60-1 (CCDC 1481306) and T18-C60-2 (CCDC 1481305). 249 unique (Rint = 0.0984) reflections, thes final R1 and wR2 values 0.1414 (I > 2.0σ(I)) and 0.4453 (all data), respectively. CCDC numbers: T18-oDCB (CCDC 1487277), T18-C60-1 (CCDC 1481306) and T18-C60-2 (CCDC 1481305). |
| § Distances between the centroids and the molecular planes of the two overlapped T18 cores are 7.9 Å and 3.7 Å, respectively, for T18-C60-1 and 10.1 Å and 4.0 Å, respectively, for T18-C60-2. Based on these distances, the slipping distances of the two overlapped T18 cores A and B can be estimated as 8.7 Å for T18-C60-1 and 10.9 Å for T18-C60-2. |
| This journal is © The Royal Society of Chemistry 2016 |