A facile palladium catalysed 3-component cascade route to functionalised isoquinolinones and isoquinolines†
Ronald
Grigg
*a,
Elghareeb E.
Elboray
ab,
Sunisa
Akkarasamiyo
ac,
Nutthawat
Chuanopparat
ac,
H. Ali
Dondas
d,
Hussien H.
Abbas-Temirek
b,
Colin W. G.
Fishwick
*a,
Moustafa F.
Aly
b,
Boonsong
Kongkathip
c and
Ngampong
Kongkathip
c
aSchool of Chemistry, University of Leeds, Leeds, LS6 9JT, UK. E-mail: r.grigg@leeds.ac.uk; c.w.g.fishwick@leeds.ac.uk; Fax: +44-0113 343 6565; Tel: +44-0113 343 6510
bDepartment of Chemistry, Faculty of Science at Qena, South Valley University, Qena, Egypt
cNatural Products and Organic Synthesis Research Unit (NPOS), Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Kasetsart University, Chatuchak, Bangkok 10900, Thailand
dDepartment of Chemistry, Faculty of Pharmacy, Mersin University, 33342, Mersin, Turkey
First published on 21st October 2015
Abstract
Palladium catalysed three component cascade process, involving coupling of 2-iodobenzoates, -benzaldehydes, or acetophenones with substituted allenes and ammonium tartrate as an ammonium surrogate, provides a novel and facile route to substituted functionalised isoquinolinones and isoquinolines in good yields.
Isoquinolinone and isoquinoline derivatives are important constituents of a diverse range of molecules of biological and pharmaceutical relevance as well as a common structural component within many alkaloids.1–3 However, little attention has been given to approaches to these systems which utilise metal-catalysed aryl–allene couplings, despite the obvious potential of this route to produce such systems with high degrees of chemo- and regioselectivity. Nonetheless, the potential of this approach has been underlined previously. Thus, Larock reported that annulation of a substituted allene with N-tosyl-2-iodobenzylamine under Pd(II) catalysis afforded isoquinolines as a mixture of three regio- and stereo-isomers.4 Additionally, 2-iodobenzaldehyde imines have also been used with Pd(0) catalysis to annulate substituted allenes giving isoquinoline derivatives.5 Ni(0)/chiral phosphine ligand mediated regio- and enantioselective synthesis of isoquinoline-1(2H)-one derivatives has been reported via denitrogenation or decarbonylation of N-aryl-1,2,3-benzotriazin-4(3H)-ones or N-substituted phthalimide, respectively, followed by intermolecular annulation with substituted allenes.6 Recently, Glorius et al., employed Rh(III) to catalyse C–H activation of N-(pivaloyloxy)benzamide involving intermolecular annulation with substituted allenes to furnish isoquinoline-1(2H)-ones.7
As part of our program of research into the development and application of palladium catalysed allene insertion cascades, we have previously reported a number of examples of three-component cascades for the synthesis of N-substituted 4-methylene-3,4-dihydro-1(2H)-isoquinolinones. A feature of these reactions is that, following an initial Pd-mediated intramolecular allene insertion, both intra- and intermolecular nucleophilic addition then occurs to give tetra-fused ring systems containing an isoquinolinone ring.8,9a These include, following the initial Pd catalysed intramolecular allene insertion, intermolecular nucleophilic addition involving N-allenyl-2-iodobenzamide,9b,c and nitrogen-tethered 1,6-enynes9d respectively. Additionally, we have also reported two types of cascade reactions that can furnish isoquinolines; (i) intermolecular allene insertion into the C–I bond of an aryl iodide linked N-nucleophile followed by intramolecular N-addition to the generated π-allyl,10 and (ii) intermolecular allene insertion to an aryl iodide carrying a dipolarophile/Michael acceptor followed by intermolecular N-addition of an azide/amine and finally intramolecular 1,3-dipolar cycloaddition/Michael addition.11
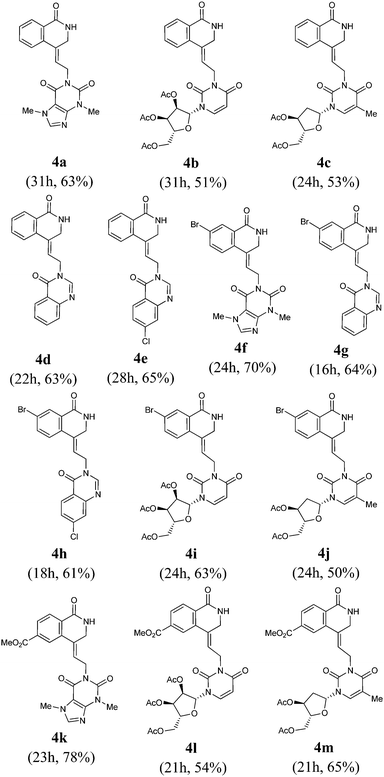
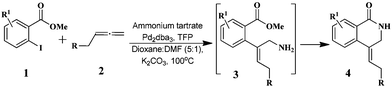
In the present study, we report a new approach utilising our “ammonium surrogate” technology12 as a novel ammonia source to furnish substituted functionalised isoquinolinone and isoquinoline derivatives. Thus, methyl 2-iodobenzoate derivatives 1 were reacted with a range of substituted allenes 2 in the presence of ammonium tartrate (6 equiv.), Pd2(dba)3 (2.5 mol%), TFP (10 mol%), and K2CO3 (3 equiv.), to afford isoquinolinones 4via intramolecular cyclisation of the intermediate 3 in 51–78% yield (Table 1). Z-Configuration of the exocyclic double bonds were established using NOE data (see the Experimental section) and in the absence of ammonium tartrate, no reaction occurred, and only starting materials were observed. This appears to be consistent with a mechanism involving the addition of ammonia to the π-allyl intermediate forming amine 3 which subsequently cyclises to give 4. Thus, the cyclisation step in 3 → 4 is faster than further allylation of the allyl-NH2 group. In the case of methyl 5-bromo-2-iodobenzoate (1, R1 = 5-Br), the reaction is chemoselective for oxidative addition at the C–I bond leaving the C–Br bond intact. It is also noteworthy that the ester moieties in 4k–m were unchanged under the reaction conditions.
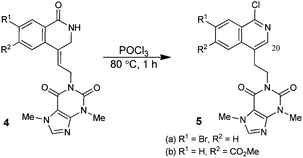
In order to briefly explore the potential of adducts 4 for further synthetic manipulation, compounds 4f and 4k, selected as representative examples, were converted into the corresponding 1-chloroisoquinolines 5a and 5b in the presence of POCl3, (Scheme 1). The assignment of the structures of the chlorination products to chloropyridines 5a and 5b followed from analysis of the 1H-NMR data for these compounds. This revealed the absence of allyl signals (typically a triplet at ∼6–6.5 ppm and doublet at ∼4.5–5 ppm respectively), and instead, comprised an AA′BB′ NMR pattern for the two methylene groups at 3–3.5 and 4–4.5 ppm respectively, consistent with the assigned structures (see ESI†).
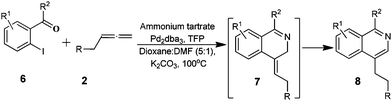
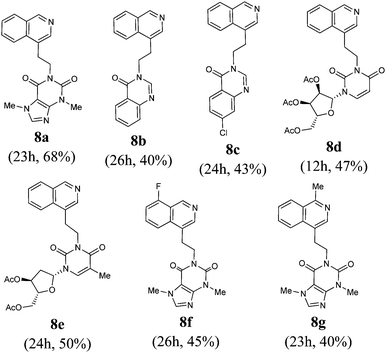
To further probe the scope of this process, the reaction of 2-iodobenzaldehydes/2′-iodoacetophenone 6 with substituted allenes 2 was explored. This reaction presumably goes via intermediate 7 which undergoes a 1,3-hydrogen rearrangement generating isoquinolines 8, Table 2. Analogously to isoquinoline 5a and 5b, 1H-NMR data (see ESI†) showed no indication of allyl signals but instead included an AA′BB′ pattern for the two methylene groups present in 8. The somewhat low yields of products from this reaction may reflect the thermal instability of the substrates or the products. This hypothesis appears to be supported by the isolation of theobromine (in the case of 8a, f and g), 2′,3′,5′-tri-O-acetyluridine (in case of 8d), 3′,5′-tri-O-acetylthymidine (in case of 8e), quinazolin-4-one (in case of 8b) and chloroquinazolin-4-one (in case of 8c) as by-products. It is noteworthy that thermal degradation of products was not observed in the preparation of isoquinolinones 4a–m.
In summary, a novel and powerful cascade approach has been applied to the synthesis of substituted functionalised isoquinolinone and isoquinoline derivatives via 3-component palladium catalysed cascade chemistry. The utility of ammonium tartrate as a novel ammonia source is underlined in this simple one-pot cascade protocol.
We acknowledge support from the University of Leeds, the Egyptian Government, South Valley University, Thailand Research Fund (TRF), Royal Golden Jubilee Program, the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Mersin University and The Scientific and Technological Research Council of Turkey (TUBİTAK), Commission on Higher Education, Ministry of Education and Kasetsart University Research and Development Institute.
Notes and references
- See for example: (a) J. D. Scott and R. M. Williams, Chem. Rev., 2002, 102, 1669–1730 CrossRef CAS PubMed; (b) S.-S. Lee, Y.-C. Lai, C.-K. Chen, L.-H. Tseng and C.-Y. Wang, J. Nat. Prod., 2007, 70, 637–642 CrossRef CAS PubMed; (c) A. Padwa and H. Zhang, J. Org. Chem., 2007, 72, 2570–2582 CrossRef CAS PubMed; (d) M. Matveenko, O. J. Kokas, M. G. Banwell and A. C. Willis, Org. Lett., 2007, 9, 3683–3685 CrossRef CAS PubMed; (e) C. T. Goralski, D. L. Hasha, D. R. Henton, R. C. Krauss, C. D. Pfeiffer and B. M. Williams, Org. Process Res. Dev., 1997, 1, 273–279 CrossRef CAS; (f) C.-J. Chou, L.-C. Lin, K.-T. Chen and C.-F. Chen, J. Nat. Prod., 1994, 57, 689–694 CrossRef CAS; (g) K. Iwasa, M. Moriyasu, Y. Tachibana, H. S. Kim, Y. Watanya, W. Wiegrebe, K. F. Bastow, M. Cosentino, M. Kozuka and K. H. Lee, Bioorg. Med. Chem., 2001, 9, 2781–2884 CrossRef.
- See for example: (a) Y. Asano, S. Kitamura, T. Ohra, F. Itoh, M. Kajino, T. Tamura, M. Kaneko, S. Ikeda, H. Igata, T. Kawamoto, S. Sogabe, S.-I. Matsumoto, T. Tanaka, M. Yamaguchi, H. Kimurab and S. Fukumoto, Bioorg. Med. Chem., 2008, 16, 4699–4714 CrossRef CAS PubMed; (b) R. Pellicciari, E. Camaioni, G. Costantino, L. Formentini, P. Sabbatini, F. Venturoni, G. Eren, D. Bellocchi, A. Chiarugi and F. Moroni, ChemMedChem, 2008, 3, 914–923 CrossRef CAS PubMed; (c) W.-H. Chueh and J.-Y. Lin, J. Agric. Food Chem., 2011, 59, 8021–8027 CrossRef CAS PubMed; (d) H.-P. Kuo, T.-C. Chuang, S.-C. Tsai, H.-H. Tseng, S.-C. Hsu, Y.-C. Chen, C.-L. Kuo, Y.-H. Kuo, J.-Y. Liu and M.-C. Kao, J. Agric. Food Chem., 2012, 60, 9649–9658 CrossRef CAS PubMed; (e) M. Jayaraman, B. M. Fox, M. Hollingshead, G. Kohlhagen, Y. Pommier and M. Cushman, J. Med. Chem., 2002, 45, 242–249 CrossRef CAS PubMed; (f) L. Ingrassia, F. Lefranc, J. Dewelle, L. Pottier, V. Mathieu, S. Spiegl-Kreinecker, S. Sauvage, M. El Yazidi, M. Dehoux, W. Berger, E. Van Quaquebeke and R. Kiss, J. Med. Chem., 2009, 52, 1100–1114 CrossRef CAS PubMed; (g) L. Chen, M. Conda-Sheridan, P. V. N. Reddy, A. Morrell, E.-J. Park, T. P. Kondratyuk, J. M. Pezzuto, R. d B. van Breemen and M. Cushman, J. Med. Chem., 2012, 55, 5965–5981 CrossRef CAS PubMed; (h) A. Chiarugi, E. Meli, M. Calvani, R. Picca, R. Baronti, E. Camaioni, G. Costantino, M. Marinozzi, D. E. Pellegrini-Giampietro, R. Pellicciari and F. Moroni, J. Pharmacol. Exp. Ther., 2003, 305, 943–949 CrossRef CAS PubMed; (i) X. Xiao and M. Cushman, J. Org. Chem., 2005, 70, 6496–6498 CrossRef CAS PubMed.
- See for example: (a) M. Álvarez and J. A. Joule, in Science of Synthesis, ed. D. S. Black, Thieme, Stuttgart, 2004, vol. 15, pp. 661–836 Search PubMed; (b) M. Álvarez and J. A. Joule, in Science of Synthesis, ed. D. S. Black, Thieme, Stuttgart, 2004, vol. 15, pp. 839–906 Search PubMed; (c) M. Chrzanowska and M. D. Rozwadowska, Chem. Rev., 2004, 104, 3341–3370 CrossRef CAS PubMed; (d) G. Zeni and R. C. Larock, Chem. Rev., 2006, 106, 4644–4680 CrossRef CAS PubMed; (e) J. Lu and H. Fu, J. Org. Chem., 2011, 76, 4600–4605 CrossRef CAS PubMed; (f) L. Ackermann and S. Fenner, Org. Lett., 2011, 13, 6548–6551 CrossRef CAS PubMed; (g) N. Guimond, C. Gouliaras and K. Fagnou, J. Am. Chem. Soc., 2010, 132, 6908–6909 CrossRef CAS PubMed; (h) K. Fujiwara, T. Kurahashi and S. Matsubara, Org. Lett., 2010, 12, 4548–4551 CrossRef CAS PubMed; (i) P. C. Too and S. Chiba, Chem. Commun., 2012, 48, 7634–7636 RSC.
- (a) T. Lechel, F. Pfrengle, H.-U. Reissig and R. Zimmer, ChemCatChem, 2013, 5, 2100–2130 CrossRef CAS; (b) R. C. Larock, N. G. Berrios-Peiia and C. A. Fried, J. Org. Chem., 1991, 56, 2615–2617 CrossRef CAS.
- J. J. H. Diederen, R. W. Sinkeldam, H.-W. Frühauf, H. Hiemstra and K. Vrieze, Tetrahedron Lett., 1999, 40, 4255–4258 CrossRef CAS.
- (a) M. Yamauchi, M. Morimoto, T. Miura and M. Murakami, J. Am. Chem. Soc., 2010, 132, 54–55 CrossRef CAS PubMed; (b) Y. Ochi, T. Kurahashi and S. Matsubara, Org. Lett., 2011, 13, 1374–1377 CrossRef CAS PubMed.
- H. Wang and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 7318–7322 CrossRef CAS PubMed.
- R. Grigg, T. Khamnaen, S. Rajviroongit and V. Sridharan, Tetrahedron Lett., 2002, 43, 2601–2603 CrossRef CAS.
- (a) R. Grigg, I. Köppen, M. Rasparini and V. Sridharan, Chem. Commun., 2001, 964–965 RSC; (b) M. Gardiner, R. Grigg, M. Kordes, V. Sridharan and N. Vicker, Tetrahedron, 2001, 57, 7729–7735 CrossRef CAS; (c) R. Grigg, V. Sridharan and L.-H. Xu, J. Chem. Soc., Chem. Commun., 1995, 1903–1904 RSC; (d) N. M. Groome, E. E. Elboray, M. W. Inman, H. A Dondas, R. M. Phillips, C. Kilner and R. Grigg, Chem. – Eur. J., 2013, 19, 2180–2184 CrossRef CAS PubMed.
- (a) R. Grigg, V. Sridharan and A. Thayaparan, Tetrahedron Lett., 2003, 44, 9017–9019 CrossRef CAS; (b) H. A. Dondas, C. W. G. Fishwick, X. Gai, R. Grigg, C. Kilner, N. Dumrongchai, B. Kongathip, N. Kongathip, C. Polysuk and V. Sridharan, Angew. Chem., Int. Ed., 2005, 44, 7570–7574 CrossRef CAS PubMed.
- (a) R. Grigg, M. Inman, C. Kliner, I. Köppen, J. Marchbank, P. Selby and V. Sridharan, Tetrahedron, 2007, 63, 6152–6169 CrossRef CAS; (b) X. Gai, R. Grigg, I. Köppen, J. Marchbank and V. Sridharan, Tetrahedron Lett., 2003, 44, 7445–7448 CrossRef CAS; (c) X. Gai, R. Grigg, S. Rajviroongit, S. Songarsa and V. Sridharan, Tetrahedron Lett., 2005, 46, 5899–5902 CrossRef CAS.
- R. Grigg, S. Akkarasamiyo, N. Chuanopparat, E. E. Elboray, M. F. Aly, H. H. Abbas-Temirek, B. Kongkathip and N. Kongkathip, Chem. Commun., 2013, 49, 2007–2009 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Full characterisation of all new compounds. See DOI: 10.1039/c5cc07526b |
| This journal is © The Royal Society of Chemistry 2016 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: