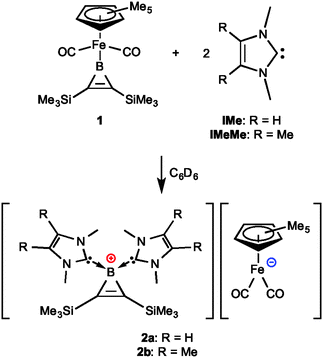
Carbene-induced synthesis of the first borironium cations using the [(η5-C5Me5)Fe(CO)2]− anion as an unlikely leaving group†
Holger
Braunschweig
*,
Rian D.
Dewhurst
and
Katharina
Ferkinghoff
Institut für Anorganische Chemie, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany. E-mail: h.braunschweig@uni-wuerzburg.de
First published on 29th October 2015
Abstract
Reaction of N-heterocyclic carbenes with ferroborirene complex [{(η5-C5Me5)Fe(CO)2}{BC2(SiMe3)2}] results in heterolytic Fe–B bond cleavage, yielding borironium ions, a new class of boron-containing heterocycles. The reaction rests on the surprising ability of the reactive [(η5-C5Me5)Fe(CO)2]− anion to act as a leaving group. The properties of these species were investigated by multinuclear NMR spectroscopy, as well as single-crystal X-ray diffraction.
Organocatalysis using N-heterocyclic carbenes (NHCs) is currently a thriving field. This concept is based on the remarkable ability of these carbenes to mediate organic reactions, usually resulting from initial deprotonations, conjugate additions and substitutions by the NHC.1 In contrast, the use of free, uncoordinated N-heterocyclic carbenes to mediate organometallic reactions is much less well understood. One notable exception is a report by Grubbs et al. of Fe–Fe bond formation from the Fe0 complex bis(cyclooctatetraene)iron catalysed by NHCs.2 The use of uncoordinated carbenes to break metal–element bonds is rarer still. Our foray into this chemistry began with the surprising realisation that NHCs are also able to cleave Fe–B bonds of ferroborirenes, results that are reported in this communication.
Borirenes are unsaturated, three-membered rings of the form BC2R3. Their two π electrons mean that borirenes satisfy Hückel's rule for aromaticity, which is consistent with their generally observed stability. Early routes to borirenes involved rearrangements and photogenerated borylene species, greatly limiting the possible structural diversity of the products.3 In 2005 we presented the synthesis of borirenes by transfer of borylene ligands from group 6 borylene complexes of the form [(OC)5M{BN(SiMe3)2}] (M = Cr, Mo) to alkynes.4 This synthetic route has enabled us to expand the palette of known bis(borirenes) to include singly and doubly boryl-substituted examples, as well as directly-connected and (hetero)arylene-spaced bis(borirenes). The borylene transfer strategy has also opened up the synthesis of both boron-5 and carbon-metallated6 borirenes, systems where two borirenes are connected by σ bonds through a metal center,7 and even a mononuclear complex with a η3-coordinated borirene ligand.8
Recently, we have established that the boron atoms of certain borirenes4c (as well as their saturated analogues boriranes9) can accept strong Lewis bases such as N-heterocyclic carbenes and 4-dimethylaminopyridine, forming base-stabilised borirenes. However, it should be noted that the latter only binds to relatively electron-poor arylborirenes. Given their assumed electron deficiency, we were interested in determining whether the ferroborirene complex [(η5-C5Me5)(OC)2Fe{BC2(SiMe3)2}] (1, Fig. 1) was also susceptible to quaternisation by Lewis bases. However, we were surprised to find that strongly donating NHCs in fact led to cleavage of the Fe–B bond in this complex, providing to the first examples of borironium cations, and the surprising ejection of [(η5-C5Me5)(OC)2Fe]− (which also acts as counteranion) as a leaving group.
The reaction of one equivalent of the NHC N,N′-dimethylimidazol-2-ylidene (IMe) with one equivalent of the ferroborirene complex 1 in toluene at room temperature provided an 11B NMR spectrum with a new signal at δ ∼ 33 along with a sizeable starting material peak (δ 63.5). This suggested that an incomplete reaction had taken place and led us to double the amount of carbene added to the mixture. Thus, in separate experiments, two equivalents of the NHCs IMe and N,N′-dimethyl(3,4-dimethyl)imidazol-2-ylidene (IMeMe) were combined with 1 at room temperature (Fig. 1), leading in both cases to full consumption of 1 as judged by 11B NMR spectroscopy. The orange salt 2a precipitated directly from the solution, while the red salt 2b was obtained after removal of solvent, washing with pentane and recrystallisation from THF. Their 11B NMR spectra show singlets at low frequency indicative of tetracoordination of the boron atom (2a: δ −32.6; 2b: δ −33.3). The 1H NMR signals corresponding to the trimethylsilyl protons of the salts 2a,b (2a: δ 0.11; 2b: δ 0.14) do not differ appreciably, nor do the signals for the C5Me5 protons (2a: δ 1.85; 2b: δ 1.84). 13C NMR signals at δ ∼ 232 can be assigned to the carbonyl carbon atoms of the counteranions. 13C NMR signals at δ ∼ 163 (two signals for 2a, one signal for 2b) can be assigned to either the carbene carbon or borironium carbon nuclei, in comparison to borirenes reported previously, but could not be distinguished due to their similarity in chemical shift.4
Single crystals of the borironium ions 2a and 2b suitable for X-ray diffraction were obtained by slow evaporation from THF at −30 °C (see Fig. 2 and Table 1). The NHC units bind the boron atoms at slightly wider angles (2a: 114.0(1)°; 2b: 112.6(1)°) than expected for a perfect tetrahedron, while the three-membered rings create extremely acute endocyclic C–B–C angles (2a: 48.5(1)°; 2b: 48.69(8)°). As would be expected, the structural parameters of 2a and 2b are, almost without exception, very similar. The structures also show strong similarities to those of previously-reported NHC-stabilised neutral borirenes (IMe)BR1CR2CR3 (A: R1 = N(SiMe3)2, R2 = Ph, R3 = BNMe2; B: R1 = N(SiMe3)2, R2 = R3 = BNMe2; C: R1 = N(SiMe3)2, R2 = SiMe3, R3 = BNMe2; D: R1 = R2 = Mes, R3 = Ph).4 The B–Ccarbene distances of 2a (1.588(3), 1.594(3) Å) and 2b (1.599(2), 1.603(2) Å) are only slightly smaller than those of A–D (1.612–1.629 Å). The endocyclic B–C bonds of 2a (1.608(2), 1.607(3) Å) and 2b (1.604(2), 1.602(2) Å) fall well inside the range of those of A–D (1.561–1.628 Å), but are slightly longer than the only endocyclic bond between boron and a silicon-bound carbon atom (in compound C, 1.581(3) Å). Similarly, the endocyclic C–B–C angles of 2a (48.5(1)°) and 2b (48.69(8)°) are barely smaller than those of A–D (48.4–49.7°). The endocyclic B–C bonds of 2a,b are also effectively identical to those found in anionic boratirenes of the form [R2BC2R′2]− prepared by Schuster (1.578(8), 1.597(8) Å)10 and Yamaguchi (1.590, 1.596 Å).11
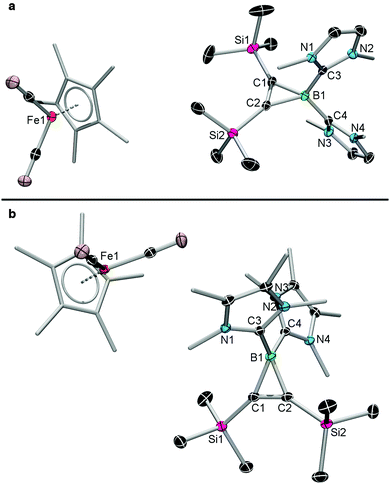 | ||
| Fig. 2 Crystallographically-derived molecular structures of 2a,b. Thermal ellipsoids are shown at the 50% probability level. Hydrogen atoms are omitted for clarity. Selected bond lengths for 2a,b are listed in Table 1. | ||
| 2a | 2b | |
|---|---|---|
| Distances | ||
| B–C1 | 1.608(2) | 1.604(2) |
| B–C2 | 1.607(3) | 1.602(2) |
| B–C3 | 1.588(3) | 1.599(2) |
| B–C4 | 1.594(3) | 1.603(2) |
| C1–Si1 | 1.847(2) | 1.849(1) |
| C2–Si2 | 1.851(2) | 1.848(1) |
| Angles | ||
| C1–B–C2 | 48.5(1) | 48.69(8) |
| C1–C2–B | 65.8(1) | 65.7(1) |
| C2–C1–B | 65.7(1) | 65.6(1) |
| C3–B1–C4 | 114.0(1) | 112.6(1) |
It should be noted that while derivatives of the [(η5-C5R5)Fe(CO)2]− (R = H, Ph) anion have been structurally characterised,12 the structures of 2a,b comprise the first structural authentication of the pentamethyl analogue of this anion. The metallate anions of the salts 2a,b show slightly shorter Fe–C(O) distances (2a: 1.717(2), 1.728(2) Å; 2b: 1.724(1), 1.726(1) Å), and slightly longer C–O distances (2a: 1.186(2), 1.191(2) Å; 2b: 1.1784(18), 1.1797(17) Å), than neutral FeII complexes of the form [(η5-C5R5)Fe(CO)2R], in line with the greater electron density at the metal of the formally Fe0 anions. The metallate anions of the salts 2a,b can be compared to the two reported structures of fully ion-separated anions of [(η5-C5R5)Fe(CO)2]− anions, namely the parent cyclopentadienyl analogue [K2(18-crown-6)2(C5H5)][(η5-C5H5)Fe(CO)2] (Fe–C(O) 1.727(6), 1.735(7) Å; C–O 1.187(8), 1.188(7) Å; (O)C–Fe–C(O) 89.5(3)°) and the pentaphenylcyclopentadienyl derivative [PPN][(η5-C5Ph5)Fe(CO)2] (PPN = bis(triphenylphosphine)iminium chloride) (Fe–C(O) 1.714(6), 1.715(6) Å; C–O 1.182(7), 1.186(7) Å; (O)C–Fe–C(O) 88.0(3)°). However, these show no major differences from 2a,b apart from slightly more acute (O)C–Fe–C(O) angles (2a: 93.66(8)°; 2b: 91.99(6)°).
Our description of the cations of 2a,b is based on their effective [BR2L2]+ structure, making them borirene (i.e. unsaturated BC2 ring) derivatives of boronium cations (four-coordinate boron cations13), thus we have used the term “borironium” cations. However, 11B NMR data of published acyclic boronium cations show a remarkably narrow range of chemical shifts (δ 0 to +12),13 of which the 11B NMR signals of 2a,b (2a: δ −32.6; 2b: δ −33.3) fall significantly outside. These signals are much closer to that of the reported boratirene salt K[Ph2BC2Ph2] (δ −16).10 These facts, combined with the aforementioned structural similarity of the cations to boratirenes, suggest that the cations have considerable “bis(imidazolium) boratirene” character.
Recently a number of neutral compounds with boron in the +1 oxidation state have been prepared with two donor units, these being cyclic (alkyl)(amino)carbenes (CAACs), oxazol-2-ylidenes, cyclopropenylidenes, isonitriles, carbon monoxide, and one case where the boron is ligated by one CAAC and one N-heterocyclic carbene (NHC).14 However, it should be noted that the cations of 2a,b are the first compounds – cationic or otherwise – in which a boron atom is ligated by two NHCs, as well as the first boronium salts where the boron atom is ligated by two carbenes of any type. Presumably the small size of the borironium rings in 2a,b is the key factor allowing two bulky NHCs to bind to the boron atom.
The results herein comprise a highly unusual carbene-induced Fe–B bond cleavage reaction, leading to the first examples of three-membered heterocyclic borironium cations. The reactions also involve the ejection of a reactive [(η5-C5Me5)Fe(CO)2]− anion as a leaving group under remarkably mild conditions, allowing the first structural characterisation of this anion as an isolated unit. Investigations into the reactivity of these novel borironium cations are currently underway.
Financial support for this work from the European Research Council is gratefully acknowledged.
Notes and references
- (a) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606 CrossRef CAS PubMed; (b) S. J. Ryan, L. Candish and D. W. Lupton, Chem. Soc. Rev., 2013, 42, 4906 RSC.
- V. Lavallo and R. H. Grubbs, Science, 2009, 326, 559 CrossRef CAS PubMed.
- (a) C. Habben and A. Meller, Chem. Ber., 1984, 117, 2531 CrossRef; (b) C. Pues and A. Berndt, Angew. Chem., Int. Ed., 1984, 23, 313 CrossRef; (c) B. Pachaly and R. West, Angew. Chem., Int. Ed., 1984, 23, 454 CrossRef; (d) J. J. Eisch, B. Shafii, J. D. Odom and A. L. Rheingold, J. Am. Chem. Soc., 1990, 112, 1847 CrossRef CAS; (e) J. J. Eisch, B. Shafii and A. L. Rheingold, J. Am. Chem. Soc., 1987, 109, 2526 CrossRef CAS.
- (a) H. Braunschweig, T. Herbst, D. Rais and F. Seeler, Angew. Chem., Int. Ed., 2005, 44, 7461 CrossRef CAS PubMed; (b) H. Braunschweig, T. Herbst, D. Rais, S. Ghosh, T. Kupfer, K. Radacki, A. G. Crawford, R. M. Ward, T. B. Marder, I. Fernández and G. Frenking, J. Am. Chem. Soc., 2009, 131, 8989 CrossRef CAS PubMed; (c) H. Braunschweig, A. Damme, R. D. Dewhurst, S. Ghosh, T. Kramer, B. Pfaffinger, K. Radacki and A. Vargas, J. Am. Chem. Soc., 2013, 135, 1903 CrossRef CAS PubMed.
- H. Braunschweig, I. Fernández, G. Frenking, K. Radacki and F. Seeler, Angew. Chem., Int. Ed., 2007, 46, 5215 CrossRef CAS PubMed.
- (a) H. Braunschweig, Q. Ye, K. Radacki, P. Brenner, G. Frenking and S. De, Inorg. Chem., 2011, 50, 62 CrossRef CAS PubMed; (b) H. Braunschweig, Q. Ye, K. Radacki and T. Kupfer, Dalton Trans., 2011, 40, 3666 RSC.
- H. Braunschweig, A. Damme, R. D. Dewhurst, H. Kelch, B. B. Macha, K. Radacki, A. Vargas and Q. Ye, Chem. – Eur. J., 2015, 21, 2377 CrossRef CAS PubMed.
- H. Braunschweig, R. D. Dewhurst, K. Radacki, C. W. Tate and A. Vargas, Angew. Chem., Int. Ed., 2014, 53, 6263 CrossRef CAS PubMed.
- H. Braunschweig, C. Claes, A. Damme, A. Deißenberger, R. D. Dewhurst, C. Hörl and T. Kramer, Chem. Commun., 2015, 51, 1627 RSC.
- M. A. Kropp and G. B. Schuster, J. Am. Chem. Soc., 1989, 111, 2316 CrossRef CAS.
- A. Iida, S. Saito, T. Sasamori and S. Yamaguchi, Angew. Chem., Int. Ed., 2013, 52, 3760 CrossRef CAS PubMed.
- (a) E. Hey-Hawkins and H. G. von Schnering, Z. Naturforsch., 1991, 46b, 621 Search PubMed; (b) I. Sänger, T. I. Kückmann, F. Dornhaus, M. Bolte, M. Wagner and H.-W. Lerner, Dalton Trans., 2012, 41, 6671 RSC; (c) B. T. Carter, M. P. Castellani, A. L. Rheingold, S. Hwang, S. E. Longacre and M. G. Richmond, Organometallics, 2002, 21, 373 CrossRef CAS.
- W. E. Piers, S. C. Bourke and K. D. Conroy, Angew. Chem., Int. Ed., 2005, 44, 5016 CrossRef CAS PubMed.
- (a) R. Kinjo, B. Donnadieu, M. A. Celik, G. Frenking and G. Bertrand, Science, 2011, 333, 610 CrossRef CAS PubMed; (b) L. Kong, Y. Li, R. Ganguly, D. Vidovic and R. Kinjo, Angew. Chem., Int. Ed., 2014, 53, 9280 CrossRef CAS PubMed; (c) D. A. Ruiz, M. Melaimi and G. Bertrand, Chem. Commun., 2014, 50, 7837 RSC; (d) L. Kong, R. Ganguly, Y. Li and R. Kinjo, Chem. Sci., 2015, 6, 2893 RSC; (e) H. Braunschweig, R. D. Dewhurst, F. Hupp, M. Nutz, K. Radacki, C. W. Tate, A. Vargas and Q. Ye, Nature, 2015, 522, 327 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic and crystallographic details. CCDC 1422335 and 1422336. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc07503c |
| This journal is © The Royal Society of Chemistry 2016 |