Berberine as a novel light-up i-motif fluorescence ligand and its application in designing molecular logic systems†
Lijun
Xu‡
ab,
Shanni
Hong‡
ab,
Na
Sun
ab,
Kewei
Wang
a,
Lu
Zhou
a,
Liya
Ji
a and
Renjun
Pei
*a
aKey Laboratory of Nano-Bio Interface, Division of Nanobiomedicine, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou, 215123, China. E-mail: rjpei2011@sinano.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 21st October 2015
Abstract
Berberine, a new light-up fluorescence ligand, for i-motif structures has been reported. This interaction enabled the development of label-free DNA-based logic gates in response to input signals.
In recent years, the research on nucleic acid beyond its genetic role has aroused more and more interest and attention in designing biosensors, logic gates and nanodevices.1 Based on hydrogen bonds and stacking interactions between its base pairs, DNA could assemble several kinds of structures, such as the well-known duplex, triplex, A-motif, G-quadruplex, and i-motif structures.2 Among them, i-motif, a second type of quadruple besides G-quadruplex, is composed of cytosine-rich sequences and stabilized under moderately acidic conditions through the formation of hemi-protonated C·C+ base pair. I-motif sequences, generally known as the complementary strand of the G-quadruplex, are widely present in gene promoter regions and play biologically important roles, for example, gene transcriptional regulation.3 Several proteins and ligands have been shown to specifically bind to C-rich DNA, but much less is known about the biological function of i-motif DNA.4 By virtue of pH-dependent folding of C-rich DNA, nanomachines, switches and sensors were widely developed.5 To develop i-motif based sensors and to provide further evidence towards their potential biological roles, fluorophores or other labelling agents were commonly covalently attached to the C-rich sequences.6 However, additional labelling is expensive and complex, and may potentially interfere with the folding–unfolding transitions. Using specific fluorescence ligands, a label-free strategy was alternated to monitor the target induced DNA conformation conversion based on the non-covalent interaction (e.g., end-stacking).7 In recent years, several fluorescent dyes have been reported, such as [Ru(phen)2(dppz)]2+,8 crystal violet,9 DMSB,10 and thioflavin T,11 which are capable of binding i-motif structures, showing an increase in the fluorescence. Compared to G-quadruplexes,12 there are still limited i-motif binding ligands which could be used as probes.4a
Berberine (BBR), an isoquinoline alkaloid from plants, has diverse biological activities, such as antitumor, anti-inflammatory, antibacterial, and antileishmania. One important mechanism of anticancer originates from its ability to form complexes with DNA and RNA, which generates additional effects such as telomerase and topoisomerase inhibition. Many studies have been reported on its biological activity against nucleic acid related diseases, and on the chemical–physical aspects of its nucleic acid binding behaviour.13 When interacting with duplex,14 poly(A),15 triplex,16 or G-quadruplex structures,17 the weakly fluorescent berberine molecules undergo a dramatic increase in fluorescence. These characteristics make berberine an ideal precursor for designing probes and drugs that target nucleic acid structures. A previous study has suggested that berberine possibly interacted with i-motif structures, but lacked enough evidence.18 To the best of our knowledge, berberine as an i-motif fluorescence ligand is not reported, impairing a full understanding of the nucleic acid binding properties.
Herein we investigated the interaction between berberine and i-motif structures by fluorescence spectroscopy and circular dichroism (CD) spectroscopy. The result shows that berberine as a fluorescence probe is capable of generating a turn-on response to an i-motif structure involving C·C+ base pairs. Although i-motif structures have been widely employed to construct logic gates, the label-free strategy has rarely been reported, mainly because of the lack of fluorescence i-motif ligands.9,19 Here based on the recognition of berberine to the conformation transition of i-motif DNA, we conceived a concept for fluorescent label-free DNA logic gates in response to various chemical inputs. As a proof of concept, first we designed an INHIBIT logic gate that responds to H+ and Ag+.
Highly sensitive to acidic conditions, the intramolecular i-motif structure could rapidly form due to the N3 protonation in cytosine.20 Ag+ has been recently reported to stabilize cytosine–cytosine (C–C) mismatches through the formation of C–Ag+–C base pairs, whereas other metal ions have no or slight effects on the C–C mismatch.21 These specific interactions of cytosine enable the development of a novel logic gate (Scheme 1). In the absence of H+ and Ag+, berberine has very weak interaction with hTeloC in a random coil state, and therefore exhibits weak fluorescence. In the presence of H+, hTeloC folds into an i-motif structure through the formation of hemi-protonated C·C+ base pairs.22 Berberine shows fluorescence enhancement upon binding to the formed i-motif. In the presence of Ag+, hTeloC would be folded based on the formation of C–Ag+–C base pairs.21b The weak interaction of berberine with the Ag+-folded structure would result in a low fluorescence response. When H+ and Ag+ coexist, the fluorescence intensity is low as in the case of only Ag+ existing in the sensing system.
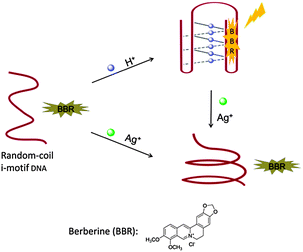 | ||
| Scheme 1 Working principle of the INHIBIT logic gate based on the conformation transition of hTeloC in the presence of H+ or/and Ag+. | ||
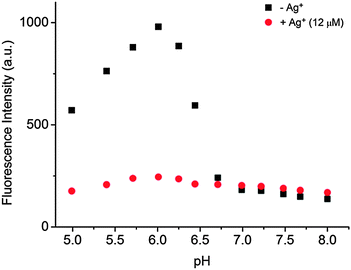
Fig. 1 indicates the fluorescence intensity in response to H+ and Ag+ that proves the feasibility of the present strategy. In the absence of Ag+, the fluorescence intensity at 530 nm of the system was obviously increased upon lowering the pH from 8.00 to 6.01 for i-motif formation. Previously, we showed that the fluorescence of both berberine itself and the berberine–DNA complex is pH-insensitive between pH 5.0 and 8.0,16 therefore the fluorescence enhancement is derived from the interaction between berberine and i-motif structures formed in acidic solution. While continuing to lower pH from 6.01 to 4.99, the fluorescence slightly decreased, which is basically consistent with the situation using thioflavin T as an i-motif probe.11 When the pH value was high, the weak fluorescence emission demonstrated that the hTeloC strand in a random coil state had slight interactions with berberine and could not enhance its fluorescence. As pH was reduced from 8.00 to 6.01, the emission enhancement was almost 7.2-fold, deriving from the strong interaction between berberine and i-motif structures. This sensor responds sensitively in the pH range of 6.01–8.00, which makes it a suitable probe for physiological H+ concentration detection. The fluorescence spectra of the BBR/hTeloC system at different pH values are shown in Fig. S1 (ESI†). In the presence of Ag+, the fluorescence intensities at different pH values in the range from 8.0 to 5.0 are obviously lower than that in the absence of Ag+. Due to little effects of Ag+ on the fluorescence of berberine in PB buffer,16 the weak fluorescence suggests that Ag+ favors the formation of another DNA structure which could not enhance the fluorescence of berberine.
Circular dichroism (CD) spectroscopy was performed to characterize the conformation-switching response of hTeloC to H+ and Ag+ (Fig. S2, ESI†). The CD spectrum of hTeloC in pH 7.5 solution exhibited a positive band at 275 nm and a negative band at ca. 250 nm, suggesting the presence of unfold DNA in a random-coil state. In the presence of H+, the strong positive band at 287 nm and the negative one at 253 nm indicated the formation of an i-motif structure at pH 6.0.23 When 12 μM Ag+ was present at pH 6.0 and 7.5, the CD spectra of hTeloC were consistent with the reported results, suggesting a folded structure.21b
To provide a further insight into the interaction between berberine and the i-motif structure, the absorption spectra of berberine in the absence and presence of i-motif structures were obtained. Progressive addition of hTeloC into pH 6.01 PB buffer solution caused obvious hypochromic and bathochromic effects with three isosbestic points (Fig. S3, ESI†), demonstrating the interaction of berberine with i-motif structures and the equilibrium between the bound and free berberine molecules. The spectral changes are similar to that of berberine interacting with duplex, poly (A), triplex, and G-quadruplex structures through partial intercalation or other binding modes.14,24
A fluorescence titration experiment of berberine with hTeloC was carried out in PB buffer (10 mM, pH 6.01). As shown in Fig. S4 (ESI†), the fluorescence intensities at 530 nm enhanced with the increase in hTeloC concentration and gave an enhancement of ca. 118-fold upon binding with 40 μM hTeloC. Based on 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding, the dissociation constant of BBR was estimated to be 19.6 μM for i-motif structures, much weaker than that with the telomeric G-quadruplex (ca. 1.79 μM).17
1 binding, the dissociation constant of BBR was estimated to be 19.6 μM for i-motif structures, much weaker than that with the telomeric G-quadruplex (ca. 1.79 μM).17
The fluorescent intercalator displacement (FID) assay is widely employed to establish DNA binding behaviors of small molecules. It relies on the reduction of fluorescence of a DNA-bound dye upon displacement by a DNA binder.25 Crystal violet (CV) has been concluded from molecular docking that CV binds to i-motif structures by end-stacking.9 Here, a competitive displacement assay was conceived to investigate the binding mode of berberine with i-motif structures based on the loss of fluorescence of CV (Fig. S5, ESI†). Due to no or slight interaction of CV with the i-motif structure formed by hTeloC (data not shown), an i-motif sequence named C29 was used. Upon addition of berberine into the CV/C29 system at pH 6.0, the fluorescence of CV at 625 nm (excited at 580 nm) slightly decreased relative to that without berberine, suggesting that CV in the bound state was not effectively replaced by berberine. Meanwhile, when excited at 350 nm, the fluorescence of berberine at 530 nm enhanced nearly as well as that in the absence of CV (data not shown). The result demonstrates that berberine and CV could simultaneously bind with i-motif structures in distinct binding modes, respectively. It is speculated therefore that berberine possibly binds with the groove of i-motif structures.
The reversibility is very important for real-time monitoring and the operation of logic gates, therefore the reversible response to pH was investigated next (Fig. S6, ESI†). When the pH was cycled between 6 and 8 by alternated addition of HNO3 and NaOH, the fluorescence intensity oscillated consistently and rapidly with the transition of DNA conformation and the binding mode of BBR. The gradual reduction of fluorescence over the circles is mainly due to the dilution effect and the accumulation of salt upon acid or base addition.16
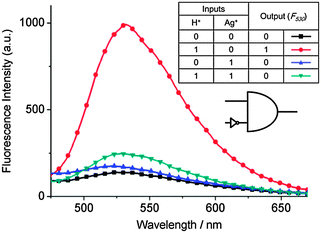
Based on H+/Ag+ modulating the fluorescence change in the BBR/hTeloC sensing system, a label-free INHIBIT logic gate was established. For the operation of this logic gate, the presence of H+ (pH 6.0, i1) or Ag+ (12 μM, i2) corresponded to an input of “1”, and their absence to “0”. The higher fluorescence intensities at 530 nm gave an output signal of “1”, otherwise an output signal of “0”. Fig. 2 shows the fluorescence spectra and the truth table of the logic gate system corresponding to four input combinations of H+ and Ag+: (i1 = 0, i2 = 0), (i1 = 1, i2 = 0), (i1 = 0, i2 = 1), and (i1 = 1, i2 = 1). The fluorescence intensity at the (i1 = 1, i2 = 0) state was respectively 7.2, 5.8 and 4.0-fold higher than that of (i1 = 0, i2 = 0), (i1 = 0, i2 = 1), and (i1 = 1, i2 = 1) states. Therefore the INHIBIT logic gate was confirmed.
Furthermore, AND and OR logic gates were constructed (Fig. S7 and S8, ESI†). For the outputs of both gates, the higher fluorescence at 530 nm was defined as “1”, otherwise as “0”. In the AND system, the presence of H+ (pH 6.01) or cysteine (Cys) was defined as an input of “1”, and their absence as “0”. Cysteine is well known to destroy the C–Ag+–C complex because Ag+ is apt to interact with thiols.26 Without H+ and/or Cys, the fluorescence was lower because effective i-motif structures were absent at pH 8.00 and/or in the presence of Ag+. With both inputs, berberine interacted with the formed H+-stabilized i-motif structures, exhibiting strong fluorescence. This result was consistent with the AND logic behavior. In the OR system, H+ and Telo 21 (a G-quadruplex-forming sequence) were used as two inputs. In the presence of K+, Telo 21 sequences form G-quadruplex structures which significantly increased the fluorescence of berberine.17 With H+ and/or Telo 21, the fluorescence was strong due to the presence of i-motif and/or G-quadruplex structures, otherwise the fluorescence was weak. Therefore, the OR logic gate is achieved. The results show that berberine is a useful fluorescence probe for designing label-free logic gates based on i-motif structures. While such logic operations are still preliminary, complex logic circuits would be expected to achieve in the future.27
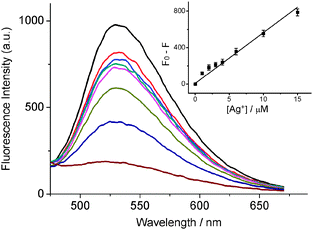
Next, we tested the performance of the INHIBIT logic gate in Ag+ detection. The detection of highly toxic Ag+ is of great importance, due to its potential release from plants to the environment.28 As shown in Fig. 1, the fluorescence responds sensitively to Ag+ at pH 6.01, therefore PB buffer of pH 6.01 is employed for probing Ag+. First the sensor's sensitivity towards Ag+ was investigated. Fig. 3 shows the fluorescence spectra upon adding different concentrations of Ag+ into the sensing system. With successive addition of Ag+ from 0 to 15 μM, the fluorescence intensity gradually decreased due to the dissociation of the BBR/i-motif complex, while the emission peak was almost not shifted. In comparison, the fluorescence was quenched at 8.0 and not sensitive to different concentrations of Ag+ (Fig. S9, ESI†). The loss of fluorescence was sensitive to Ag+ in a concentration-dependent manner, and a linear relationship was achieved in the wide concentration range from 0 to 15 μM. A detection limit of 410 nM for Ag+ was obtained based on a signal-to-noise ratio (S/N) of 3.
Furthermore the specificity of the sensor was evaluated (Fig. S10, ESI†). It was found that Ag+ resulted in an obvious change in the fluorescence intensity, while there was a nearly negligible fluorescence change in the presence of other metal ions. Besides, the fluorescence response to Ag+ was almost not influenced when Ag+ coexisted with other metal ions. The excellent selectivity is mainly attributed to the high specificity and affinity of the C–Ag+–C base pair and the masking agent. To test the practical performance of this Ag+ assay, Ag+ in artificially contaminated tap water was detected (Table S1, ESI†). The recovery of Ag+ was in the range of 98.9–115.1%.
In conclusion, the interaction of berberine with i-motif structures was confirmed, which was an important supplement to its nucleic acid binding behaviour. Berberine was also demonstrated as an excellent light-up fluorescence ligand to monitor pH-driven conformation conversion of i-motif structures. Label-free fluorescence logic gates, such as INHIBIT, AND and OR, were designed based on i-motif recognition by berberine. The results demonstrate that berberine is a useful i-motif fluorescence ligand, which could find many applications in the fields of medicine, sensors, logic gates, and nanodevices.
This work was financially supported by the National Natural Science Foundation of China (21275156) and the CAS Hundred Talents program.
Notes and references
- (a) J. Bath and A. J. Turberfield, Nat. Nanotechnol., 2007, 2, 275–284 CrossRef CAS PubMed; (b) J. Liu, Z. Cao and Y. Lu, Chem. Rev., 2009, 109, 1948–1998 CrossRef CAS PubMed; (c) W. Zhou, P.-J. J. Huang, J. Ding and J. Liu, Analyst, 2014, 139, 2627–2640 RSC; (d) I. Willner, B. Shlyahovsky, M. Zayats and B. Willner, Chem. Soc. Rev., 2008, 37, 1153–1165 RSC.
- (a) J. Choi and T. Majima, Chem. Soc. Rev., 2011, 40, 5893–5909 RSC; (b) Y. Du and X. Zhou, Chem. Rec., 2013, 13, 371–384 CrossRef CAS PubMed.
- (a) K. Gehring, J.-L. Leroy and M. Guéron, Nature, 1993, 363, 561–565 CrossRef CAS PubMed; (b) J. A. Brazier, A. Shah and G. D. Brown, Chem. Commun., 2012, 48, 10739–10741 RSC.
- (a) H. A. Day, P. Pavlou and Z. A. E. Waller, Bioorg. Med. Chem., 2014, 22, 4407–4418 CrossRef CAS PubMed; (b) S. Kendrick, H.-J. Kang, M. P. Alam, M. M. Madathil, P. Agrawal, V. Gokhale, D. Yang, S. M. Hecht and L. H. Hurley, J. Am. Chem. Soc., 2014, 136, 4161–4171 CrossRef CAS PubMed; (c) X. Li, Y. Peng, J. Ren and X. Qu, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 19658–19663 CrossRef CAS PubMed.
- (a) Y. Dong, Z. Yang and D. Liu, Acc. Chem. Res., 2014, 47, 1853–1860 CrossRef CAS PubMed; (b) J. Choi and T. Majima, Photochem. Photobiol., 2013, 89, 513–522 CrossRef CAS PubMed.
- (a) J.-L. Mergny, Biochemistry, 1999, 38, 1573–1581 CrossRef CAS PubMed; (b) I. J. Lee, J. W. Yi and B. H. Kim, Chem. Commun., 2009, 5383–5385 RSC; (c) J. Choi, S. Kim, T. Tachikawa, M. Fujitsuka and T. Majima, J. Am. Chem. Soc., 2011, 133, 16146–16153 CrossRef CAS PubMed; (d) I. J. Lee, M. Park, T. Joo and B. H. Kim, Mol. BioSyst., 2012, 8, 486–490 RSC; (e) A. Dembska, P. Rzepecka and B. Juskowiak, J. Fluoresc., 2013, 23, 807–812 CrossRef CAS PubMed; (f) B. Xu, X. Wu, E. Yeow and F. Shao, Chem. Commun., 2014, 50, 6402–6405 RSC.
- D.-L. Ma, H.-Z. He, K.-H. Leung, H.-J. Zhong, D. S.-H. Chan and C.-H. Leung, Chem. Soc. Rev., 2013, 42, 3427–3440 RSC.
- S. Shi, J. Zhao, X. Geng, T. Yao, H. Huang, T. Liu, L. Zheng, Z. Li, D. Yang and L. Ji, Dalton Trans., 2010, 39, 2490–2493 RSC.
- D. L. Ma, M. H. Kwan, D. S. Chan, P. Lee, H. Yang, V. P. Ma, L. P. Bai, Z. H. Jiang and C. H. Leung, Analyst, 2011, 136, 2692–2696 RSC.
- (a) Y. Shi, H. Sun, J. Xiang, H. Chen, Q. Yang, A. Guan, Q. Li, L. Yu and Y. Tang, Chem. Commun., 2014, 50, 15385–15388 RSC; (b) Y. Shi, H. Sun, J. Xiang, L. Yu, Q. Yang, Q. Li, A. Guan and Y. Tang, Anal. Chim. Acta, 2015, 857, 79–84 CrossRef CAS PubMed.
- I. J. Lee, S. P. Patil, K. Fhayli, S. Alsaiari and N. M. Khashab, Chem. Commun., 2015, 51, 3747–3749 RSC.
- L. Lv, Z. Guo, J. Wang and E. Wang, Curr. Pharm. Des., 2012, 18, 2076–2095 CrossRef CAS PubMed.
- K. Bhadra and G. S. Kumar, Med. Res. Rev., 2011, 31, 821–862 CrossRef CAS PubMed.
- D. Debnath, G. S. Kumar, R. Nandi and M. Maiti, Indian J. Biochem. Biophys., 1989, 26, 201–208 CAS.
- R. Nandi, D. Debnath and M. Maiti, Biochim. Biophys. Acta, 1990, 1049, 339–342 CrossRef CAS.
- L. Xu, Y. Guo, J. Wang, L. Zhou, Y. Zhang, S. Hong, Z. Wang, J. Zhang and R. Pei, Chem. – Asian J., 2015, 10, 1126–1129 CrossRef CAS PubMed.
- W.-J. Zhang, T.-M. Ou, Y.-J. Lu, Y.-Y. Huang, W.-B. Wu, Z.-S. Huang, J.-L. Zhou, K.-Y. Wong and L.-Q. Gu, Bioorg. Med. Chem., 2007, 15, 5493–5501 CrossRef CAS PubMed.
- N. K. Sharma, P. Lunawat and M. Dixit, Am. J. Biochem. Biotechnol., 2011, 7, 130–134 CrossRef CAS.
- (a) D. Miyoshi, M. Inoue and N. Sugimoto, Angew. Chem., Int. Ed., 2006, 45, 7716–7719 CrossRef CAS PubMed; (b) J. Zhou, S. Amrane, D. N. Korkut, A. Bourdoncle, H. Z. He, D. L. Ma and J. L. Mergny, Angew. Chem., Int. Ed., 2013, 52, 7742–7746 CrossRef CAS PubMed; (c) T. Li, L. Zhang, J. Ai, S. Dong and E. Wang, ACS Nano, 2011, 5, 6334–6338 CrossRef CAS PubMed; (d) J. Elbaz, Z.-G. Wang, R. Orbach and I. Willner, Nano Lett., 2009, 9, 4510–4514 CrossRef CAS PubMed.
- C. Chen, M. Li, Y. Xing, Y. Li, C.-C. Joedecke, J. Jin, Z. Yang and D. Liu, Langmuir, 2012, 28, 17743–17748 CrossRef CAS PubMed.
- (a) A. Ono, S. Cao, H. Togashi, M. Tashiro, T. Fujimoto, T. Machinami, S. Oda, Y. Miyake, I. Okamoto and Y. Tanaka, Chem. Commun., 2008, 4825–4827 RSC; (b) H. A. Day, C. Huguin and Z. A. Waller, Chem. Commun., 2013, 49, 7696–7698 RSC.
- S. Ahmed, A. Kintanar and E. Henderson, Nat. Struct. Biol., 1994, 1, 83–88 CrossRef CAS PubMed.
- J. Kypr, I. Kejnovská, D. Renčiuk and M. Vorlíčková, Nucleic Acids Res., 2009, 37, 1713–1725 CrossRef CAS PubMed.
- (a) R. C. Yadav, G. S. Kumar, K. Bhadra, P. Giri, R. Sinha, S. Pal and M. Maiti, Bioorg. Med. Chem., 2005, 13, 165–174 CrossRef CAS PubMed; (b) D. Bhowmik, S. Das, M. Hossain, L. Haq and G. Suresh Kumar, PLoS One, 2012, 7, e37939 CAS; (c) A. Arora, C. Balasubramanian, N. Kumar, S. Agrawal, R. P. Ojha and S. Maiti, FEBS J., 2008, 275, 3971–3983 CrossRef CAS PubMed.
- W. C. Tse and D. L. Boger, Acc. Chem. Res., 2004, 37, 61–69 CrossRef CAS PubMed.
- L. C. Gruen, Biochim. Biophys. Acta, 1975, 386, 270–274 CrossRef CAS.
- (a) R. H. Katz, Contemporary logic design, Benjamin Cummings, CA, 1994 Search PubMed; (b) V. Balzani, A. Credi and M. Venturi, ChemPhysChem, 2003, 3, 49–59 CrossRef PubMed; (c) A. P. De Silva and S. Uchiyama, Nat. Nanotechnol., 2007, 2, 399–410 CrossRef CAS PubMed; (d) B. M. Frezza, S. L. Cockroft and M. R. Ghadiri, J. Am. Chem. Soc., 2007, 129, 14875–14879 CrossRef CAS PubMed.
- H. T. Ratte, Environ. Toxicol. Chem., 1999, 18, 89–108 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc08242k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |