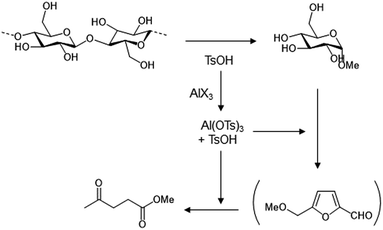
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A practical and efficient synthesis of methyl levulinate from cellulosic biomass catalyzed by an aluminum-based mixed acid catalyst system†
K.
Tominaga
*ab,
K.
Nemoto
a,
Y.
Kamimura
a,
A.
Yamada
c,
Y.
Yamamoto
c and
K.
Sato
ab
aNational Institute of Advanced Industrial Science and Technology (AIST), Central 5, 1-1-1 Higashi, Tsukuba, Ibaraki 305-8569, Japan. E-mail: k-tominaga@aist.go.jp
bInstitute for Catalysis, Hokkaido University, Kita21, Nishi10, Kita-ku, Sapporo, Hokkaido 001-0021, Japan
cOrganic Chemistry Research Lab., Ube Industries, Ltd., 1978-5, Kogushi, Ube, Yamaguchi 755-8633, Japan
First published on 4th July 2016
Abstract
Methyl levulinate is a promising building block which can be derived from cellulosic biomass. In this paper, a combination of aluminum compounds and organic sulfonic acids was found to be an efficient catalyst system for direct methyl levulinate synthesis from both microcrystalline cellulose and wood powder. Electrospray ionization mass analysis revealed the formation of aluminum sulfonate complexes in the reaction solution. The reaction properties of this catalyst system suggested that cooperative catalysis of aluminum sulfonates and organic sulfonic acids in methanol was responsible for the efficient formation of methyl levulinate.
Introduction
Recently, levulinic acid (LA) has attracted much attention because it has been recognized as a key building block that can be derived from cellulosic biomass.1,2 Specialty chemicals, such as agricultural chemicals,3 a methyl methacrylate substitute,4 and a bisphenol A substitute,5 as well as commodity chemicals, such as butene,6 succinic acid,7 and adipinic acid,8 can be synthesized from LA.Many synthesis methods of LA from cellulose have been reported. Conventionally, sulfuric acid is used for this reaction, but a much larger stoichiometric amount of sulfuric acid compared to cellulose is required and the yield of LA is between 60 and 70% based on the glucose units in cellulose.9
To replace sulfuric acid with more efficient acids that can act even at lower amounts, many kinds of acid catalysts such as metal salts,10 heteropolyacids,11 and solid acids12 have been examined to date. Among the metal salts, CrCl3 has reported to be the most effective in forming LA from cellulose (yields of 67% after the reaction at 200 °C for 3 h), but it was difficult to reuse it because of the formation of a less reactive chromium oxide.10b As for the heteropolyacids, ionic-liquid-substituted heteropolyacids were found to be effective in forming LA in 63% yield via the reaction at 140 °C for 12 h.11b Although the catalyst was shown to be reusable for the hydrolysis of cellulose, its reusability for LA formation remains unclear. Typical solid acids such as Amberlyst and Nafion have also shown catalytic activities for the synthesis of LA from cellulose.12c The LA yield reached over 60% when the reaction was carried out in aqueous γ-valerolactone solvent at 160 °C; however, large amounts of the catalyst, almost the same weight as cellulose, and a long reaction time of 16 h were required. Therefore, a more efficient and recyclable catalyst system for LA synthesis from cellulose is eagerly anticipated.
Cellulose has very unique properties; its crystal structure is strongly bonded with both hydrogen bonds and van der Waals forces.13 In order to decompose cellulose to glucose, both types of bonds have to be dissociated efficiently. The following two hypotheses can therefore be postulated: (1) the optimum acid for the decomposition of cellulose to glucose is different to that for the transformation of glucose to LA, and (2) because cellulose has a strong affinity to water, the reaction should be carried out in protonic organic solvents.
Based on these hypotheses, we developed an effective catalyst system for the synthesis of methyl levulinate (MeLev) in methanol.14 This catalyst system consists of two different kinds of acids, a Brønsted and Lewis acid; it has been demonstrated that the former mainly catalyzes the solvolysis of cellulose and the latter the transformation of sugar to MeLev. As a result, the yield of MeLev reached 75% when the reaction was carried out at 180 °C for 5 h and when In(OTf)3 and 2-naphthalenesulfonic acid (2-NSA) were used as the catalyst system. This catalyst system can also be used for the direct synthesis of MeLev from wood biomass; the yield of MeLev was 97% based on the glucose units of cellulose in cedar powder.
However, both indium and trifluoromethanesulfonic acid are expensive materials and the practical application of this catalyst system has therefore been limited. In this paper, we report an improved catalyst system for the synthesis of MeLev from both cellulosic and wood biomass using inexpensive and easily available aluminum compounds in place of rare metals or trifluoromethanesulfonic acid.
Experimental
All reagents were of research grade and used without further purification. Al(acac)3, AlBr3, and AlCl3 were purchased from Wako Pure Chemical Industry. Al(OH)3, Al2(SO4)3, AlI3, In(OTf)3, 1-pyrenesulfonic acid (1-PSA), and microcrystalline cellulose (<20 μm) were purchased from Sigma-Aldrich. Al(OEt)3, benzenesulfonic acid (BSA), and 2-naphthalenesulfonic acid (2-NSA) were purchased from Tokyo Chemical Industry. Al(OAc)3, p-toluenesulfonic acid (PTSA), glucose, and methanol were purchased from Kishida Chemical. 5-Methoxymethylfurfural (MMF) prepared according to a literature procedure.15 Wood powders were supplied by Nippon Paper Industries. The content of cellulose, hemicellulose, and lignin in the wood powders were determined using a conventional procedure.16 These wood powders were cutter-milled and sieved to decrease the particle size in the range of 100 to 425 μm.In a typical experiment for MeLev synthesis from cellulose, to a 50 mL stainless steel autoclave equipped with a magnetic stirring bar, microcrystalline cellulose (2.5 mmol as glucose units), Al precursor (0.02 mmol), p-toluenesulfonic acid (0.20 mmol), and methanol (20.0 mL) were added and the apparatus was purged with N2 (0.5 MPa). Then, the apparatus was heated to 180 °C and maintained at this temperature for 5 h with stirring. After the apparatus was cooled to room temperature and depressurized, the reaction solution was recovered and analyzed by high-performance liquid chromatography (HPLC).
HPLC analyses were carried out on a JASCO LC-2000Plus system. The quantitative analysis of sugars was performed with an Aminex HPX-87H column (250 × 4.0 mm I.D., Bio-Rad Laboratories, Inc.) using a 7.5 mM aqueous solution of sulfuric acid as the mobile phase and 2-methyl tetrahydrofuran as the internal standard. The quantitative analysis of MeLev was performed on a Scherzo SS-C18 column (250 × 4.0 mm I.D., Imtakt Corp.) using a 20 vol% aqueous solution of methanol containing 10 mM formic acid as the mobile phase and 2-methyl tetrahydrofuran as the internal standard.
Electrospray ionization mass spectrometry (ESI-MS) analysis was carried out on a Waters ZQ-2000 spectrometer. The needle and cone voltage were +4.0 kV and 50 V, respectively. The sample solution was filtered and directly introduced into the apparatus at an infusion rate of 20 μL min−1.
Results and discussion
Representative results of MeLev syntheses from microcrystalline cellulose are summarized in Table 1. When only PTSA was used as the acid catalyst, MeLev was obtained in 47% yield, together with MMF in 2% yield, and sugars in 13% yield after the reaction at 180 °C for 5 h (entry 1). The reaction solution was brown in color and contained a small amount of colloidal particles which appeared to be humin. As an aluminum compound, Al(acac)3 is air and moisture stable, and thus easily handled. When only Al(acac)3 was used as the catalyst, the reaction barely proceeded and white cellulose powder remained in the reaction solution because its acidity was too weak to solvolyze cellulose (entry 2). However, when Al(acac)3 was used in combination with PTSA, cellulose was efficiently converted to MeLev in 72% yield (entry 3). As observed in entry 1, the reaction solution contained a small amount of colloidal humin. The yield of MeLev was almost the same as that obtained with our previous catalyst system of In(OTf)3 and PTSA (entry 14).| Entry | Lewis acid | Brønsted acid | Yieldb/% | ||
|---|---|---|---|---|---|
| MeLev | MMF | Sugarsc | |||
| a Conditions: Lewis acid (0.02 mmol), Brønsted acid (0.20 mmol), microcrystalline cellulose (405 mg, 2.5 mmol as glucose units), MeOH (20.0 mL), N2 (0.5 MPa), 180 °C, 5 h. b HPLC yields based on the glucose units in cellulose. c Total amount of fructose, glucose, and α-methylglycoside. | |||||
| 1 | — | PTSA | 47 | 2 | 13 |
| 2 | Al(acac)3 | — | — | — | 1 |
| 3 | Al(acac)3 | PTSA | 72 | 1 | 1 |
| 4 | Al(OH)3 | PTSA | 64 | — | — |
| 5 | Al(OAc)3 | PTSA | 66 | — | 4 |
| 6 | Al(OEt)3 | PTSA | 69 | 1 | 1 |
| 7 | Al2(SO4)3 | PTSA | 63 | — | — |
| 8 | AlI3 | PTSA | 67 | 1 | 1 |
| 9 | AlBr3 | PTSA | 64 | 1 | 1 |
| 10 | AlCl3 | PTSA | — | — | 8 |
| 11 | Al(OH)3 | BSA | 61 | 1 | — |
| 12 | Al(OH)3 | 2-NSA | 74 | — | — |
| 13 | Al(OH)3 | 1-PSA | 49 | 4 | 16 |
| 14 | In(OTf)3 | PTSA | 73 | — | 1 |
As for the effects of Al precursors, other common Al salts such as Al(OH)3, Al(OAc)3, Al(OEt)3, Al2(SO4)3, AlI3, and AlBr3 gave slightly lower MeLev yields than Al(acac)3 (entries 3–9), while AlCl3 significantly inhibited the solvolysis of cellulose and white cellulose powder remained unreacted (entry 10). This inhibition was specifically caused by HCl formed from AlCl3 and PTSA; when the reaction shown in entry 1 was carried out in the presence of 0.06 mmol of HCl (corresponding to 0.02 mmol of AlCl3) and 0.2 mmol of PTSA, the solvolysis of cellulose was also inhibited and white cellulose ponder was remained. No such inhibition was observed in the cases of other halide salts and their catalytic activity increased in the order AlCl3 ≪ AlBr3 < AlI3.
Among the organic sulfonic acids tested, 2-NSA was the most effective; the yield of MeLev reached 74% when 2-NSA was used in combination with Al(OH)3 (entries 11–13). The catalytic activity decreased in the order 2-NSA > PTSA > BSA > 1-PSA. Because all these sulfonic acids have almost the same acidity, there must be an optimal aromatic ring size for catalysis in this reaction.
The effect of Al/PTSA ratio is shown in Fig. 1. In the absence of Al salt, MeLev was formed in only moderate yield, while its yield drastically increased with the addition of just a 1/200 molar amount of Al(acac)3 to PTSA. The maximum yield of MeLev was obtained at Al/PTSA = 1/40. Above this ratio, the yield of MeLev decreased gradually, reaching just 10% when equimolar amounts of Al(acac)3 and PTSA were used. At this ratio, a significant amount of cellulose powder was observed to be unreacted, probably because almost amount of PTSA bonded to Al(acac)3 to form an Al sulfonate compound.
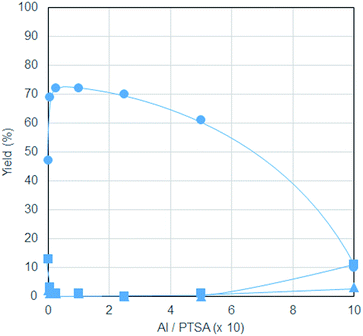 | ||
| Fig. 1 Effect of Al/PTSA ratio ● = MeLev, ▲ = MMF, ■ = sugars. Conditions: Al(acac)3 and PTSA (total amount: 0.22 mmol), microcrystalline cellulose (405 mg, 2.5 mmol as glucose units), MeOH (20.0 mL), N2 (0.5 MPa), 180 °C, 5 h. All the yields were determined by the same method described in Table 1. | ||
The effect of reaction temperature is shown in Fig. 2. MeLev formation proceeded even at 160 °C, while 16% sugars remained unreacted. The optimum reaction temperature for MeLev formation lies at around 180 °C, above which its yield decreases slightly.
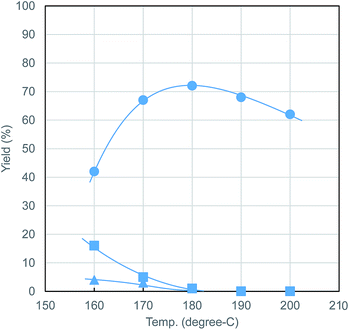 | ||
| Fig. 2 Effect of reaction temperature ● = MeLev, ▲ = MMF, ■ = sugars. Conditions: Al(acac)3 (0.02 mmol), PTSA (0.20 mmol), microcrystalline cellulose (405 mg, 2.5 mmol as glucose units), MeOH (20.0 mL), N2 (0.5 MPa), 5 h. All the yields were determined by the same method described in Table 1. | ||
The time course of the reaction at 180 °C (Fig. 3) shows that only small amounts of sugars and MMF were formed in the initial stage, which means that solvolyzed sugars were readily transformed to MMF and then MeLev. The formation of MeLev is almost complete within 3 h.
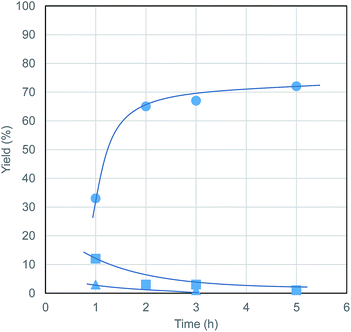 | ||
| Fig. 3 Time course of MeLev formation from cellulose ● = MeLev, ▲ = MMF, ■ = sugars. Conditions: Al(acac)3 (0.02 mmol), PTSA (0.20 mmol), microcrystalline cellulose (405 mg, 2.5 mmol as glucose units), MeOH (20.0 mL), N2 (0.5 MPa), 180 °C. All the yields were determined by the same method described in Table 1. | ||
The catalyst system is recyclable. After distillation of the solvent and products, the residue was recovered and used as the catalyst for the next run, in which the same amount of microcrystalline cellulose as the first run was newly added. As shown in Fig. 4, although the yield of MeLev decreased slightly, it remained above 60%.
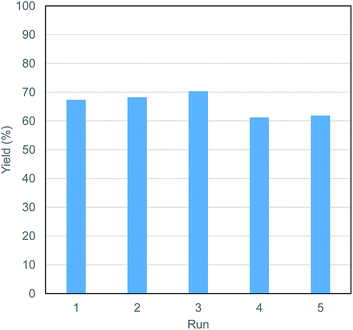 | ||
| Fig. 4 Catalyst recyclability. Conditions: Al(acac)3 (0.02 mmol), PTSA (0.20 mmol), microcrystalline cellulose (405 mg in each run, 2.5 mmol as glucose units), MeOH (20.0 mL), N2 (0.5 MPa), 180 °C, 5 h. The distilled residue was used as the catalyst for the next run without any treatment. All the yields were determined by the same method described in Table 1. | ||
In order to investigate the catalyst species in the reaction, the MeOH solution of Al(acac)3 and PTSA heat-treated at 180 °C under 0.5 MPa N2 for 2 h was analyzed by ESI-MS (Fig. 5). The detected species were as follows: TsOH2+ (a, m/z = 173), Al(OTs)H+ (b, m/z = 199), Al(OTs)(OMe)+ (c, m/z = 229), Al(OTs)(OMe)(H2O)+ (c(H2O), m/z = 247), Al(OTs)(OMe)(MeOH)+ (c(MeOH), m/z = 261), Al(OTs)(OMe)(MeOH)(H2O)+ (c(H2O)(MeOH), m/z = 279), Al(OTs)2+ (d, m/z = 369), Al(OTs)2(H2O)+ (d(H2O), m/z = 387), Al(OTs)2(MeOH)+ (d(MeOH), m/z = 401), Al(OTs)3H+ (e, m/z = 541), Al(OTs)3H(H2O)+ (e(H2O), m/z = 559), Al(OTs)3H(MeOH)+ (e(MeOH), m/z = 573), Al2(OTs)4OH+ (f, m/z = 755), and Al2(OTs)5+ (g, m/z = 909). All of these species are in good agreement with their calculated isotope distribution spectra (see ESI Fig. S2–S8†).
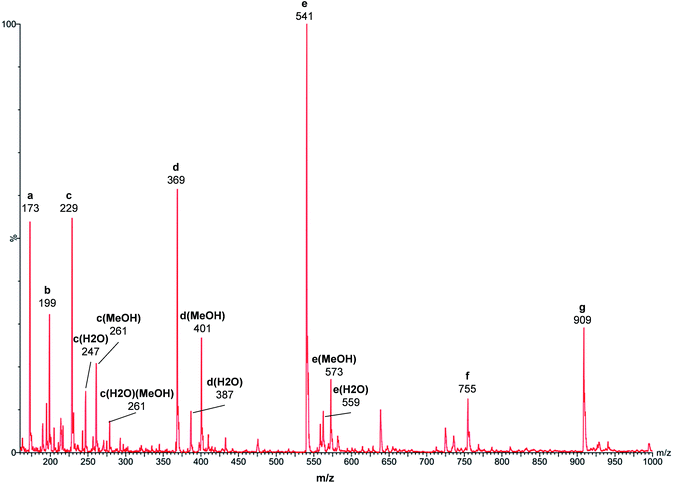 | ||
| Fig. 5 ESI-MS spectrum of the catalyst species in MeOH. Sample: Al(acac)3 (0.02 mmol), PTSA (0.20 mmol), MeOH (20.0 mL), N2 (0.5 MPa), 180 °C, 2 h. | ||
The ESI-MS results shown in Fig. 5 suggest that Al(acac)3 reacts preferably with PTSA in MeOH to form Al(OTs)3, a Lewis acid. Yang et al. reported that Al(OTs)3 could be formed from Al2O3 and PTSA in water.17 Likewise, in the cooperative catalysis of Al compounds and PTSA, Al(OTs)3 would be in situ formed and act as a Lewis acid.
The effect of the Al/PTSA ratio (Fig. 1) shows that the formation of MeLev from cellulose is drastically enhanced by a very small amount of Al species in comparison with PTSA and that the yield of MeLev decreased as the Al/PTSA ratio increased. These results suggest that the methanolysis of cellulose to sugars is mainly catalyzed by PTSA and that the main role of Al(OTs)3 lies in enhancing the formation of MeLev from sugars. To confirm this, we compared the reaction of glucose in MeOH using a catalyst system consisting of Al(acac)3 and PTSA (Al/PTSA = 1/3) with that using only PTSA (Table 2). The results show that the combination of Al(acac)3 and PTSA showed much higher catalytic activity for MeLev formation from glucose than PTSA alone.
| Entry | Catalyst | Yieldb/% | ||
|---|---|---|---|---|
| MeLev | MMF | Sugarsc | ||
| a Conditions: PTSA (0.06 mmol), Al(acac)3 (0.02 mmol), glucose (2.5 mmol), MeOH (20.0 mL), N2 (0.5 MPa), 180 °C, 5 h. b HPLC yields based on the amount of glucose. c Total amount of fructose, glucose, and α-methylglycoside. | ||||
| 1 | PTSA | 20 | — | 51 |
| 2 | Al(acac)3 + PTSA (Al/PTSA = 1/3) | 62 | — | 11 |
A reaction mechanism can be proposed, as shown in Scheme 1. In the first step, cellulose is solvolyzed with methanol to produce sugars, catalyzed by the Brønsted acid PTSA. PTSA also reacts with Al precursors to form a Lewis acid, Al(OTs)3, which in combination with PTSA catalyze the conversion of sugars to MeLev via MMF. The cooperation of the Brønsted and Lewis acids is responsible for the high yield of MeLev obtained from cellulose, as shown in our previous reports.14
It is well known that water molecules are closely associated with the cellulose matrix.18 When water is used as the solvent, such close interactions may inhibit the hydrolysis of cellulose with acid. In contrast, although little is known about the interaction of methanol in cellulose matrix, the proton affinity of methanol is much lower than that of water, making solvolysis of cellulose possible with a small amount of Brønsted acid in methanol. Li and Hu reported another effect of methanol; the formation of humin is suppressed due to protection of the aldehyde groups of glucose and hydroxymethylfurfural (HMF) by methanol.19 The same effect appeared to occur here, which would be responsible for the efficient transformation of cellulose to MeLev.
Finally, we applied the catalyst system to MeLev synthesis from actual wood biomass. The content of cellulose, hemicellulose, and lignin in the wood powders are shown in Table 3 and the results of MeLev synthesis from them are summarized in Table 4. Both cedar and eucalyptus powder were effectively converted to MeLev with a maximum yield of 80% based on the amount of cellulose in the wood powder with this catalyst system. These results suggest that the lignin component does not so inhibit catalysis. This catalyst system also works under a higher concentration of raw materials; in the presence of 10 wt% cedar powder, MeLev was formed in a maximum yield of 74%. We also examined a scale-up experiment; under a ten times scaled-up condition using 10 wt% cedar powder, MeLev was confirmed to be formed in 72% yield (entry 5, see ESI†).
| Entry | Raw material | Conc. (g L−1) | Yieldc/% | ||
|---|---|---|---|---|---|
| MeLev | MMF | Sugarsd | |||
| a Conditions: Al(acac)3 (0.02 mmol), PTSA (0.20 mmol), wood powder (0.50 g), MeOH (20.0 mL), N2 (0.5 MPa), 180 °C, 5 h. b Conditions: Al(acac)3 (0.08 mmol), PTSA (0.80 mmol), wood powder (2.0 g), MeOH (20.0 mL), N2 (0.5 MPa), 180 °C, 5 h. c Based on the amount of glucose units in cellulose. d Total amount of fructose, glucose, and α-methylglycoside. e Ten times scaled-up condition. | |||||
| 1a | Cedar | 25 | 80 | — | 5 |
| 2b | Cedar | 100 | 74 | — | 1 |
| 3a | Eucalyptus | 25 | 70 | — | 8 |
| 4b | Eucalyptus | 100 | 63 | — | 2 |
| 5e | Cedar | 100 | 72 | — | 3 |
Conclusions
Based on the two hypotheses described in the Introduction, we developed a practical catalyst system for MeLev synthesis from wood biomass using Al compounds and PTSA; although it is inexpensive and simple, it affords a high yield of MeLev and is recyclable. According to hypothesis (1), the combination of different kinds of acids is effective for this reaction; the solvolysis of cellulose to sugars is catalyzed by Brønsted acidic PTSA and the conversion of sugars to MeLev is significantly enhanced by Lewis acidic Al(OTs)3 formed in situ from Al compounds and PTSA. Besides, according to hypothesis (2), MeOH is an efficient solvent for the MeLev synthesis from cellulose and cellulosic biomass. This is because of its lower affinity to cellulose and its function of protecting the highly reactive aldehyde groups of sugars and MMF to prevent undesirable humin formation.Acknowledgements
This study was financially supported by the New Energy and Industrial Technology Development Organization (NEDO), Japan.Notes and references
- (a) T. Werpy and G. Petersen, Top Value Added Chemicals from Biomass. Volume I – Results of Screening for Potential Candidates from Sugars and Synthesis Gas, U. S. D. o. Energy, 2004 Search PubMed; (b) J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539 RSC.
- Recent reviews: (a) H. Kobayashi and A. Fukuoka, Green Chem., 2013, 15, 1740 RSC; (b) S. Van de Vyver, J. Geboers, P. A. Jacobs and B. F. Sels, ChemCatChem, 2011, 3, 82 CrossRef CAS; (c) W. Deng, Q. Zhang and Y. Wang, Catal. Today, 2014, 234, 31 CrossRef CAS; (d) S. Dutta and S. Pal, Biomass Bioenergy, 2014, 62, 182 CrossRef CAS; (e) P. J. Deuss, K. Barta and J. G. de Vries, Catal. Sci. Technol., 2014, 4, 1174 RSC; (f) M. J. Climent, A. Corma and S. Iborra, Green Chem., 2014, 16, 516 RSC; (g) F. H. Isikgor and C. Remzi Becer, Polym. Chem., 2015, 6, 4497 RSC; (h) A. Mukherjee, M. J. Dumont and V. Raghauan, Biomass Bioenergy, 2015, 72, 143 CrossRef CAS.
- J. J. Bozell, L. Moens, D. C. Elliott, Y. Wang, G. G. Neuenscwander, S. W. Fitzpatrick, R. J. Bilski and J. L. Jarnefeld, Resour., Conserv. Recycl., 2000, 28, 227 CrossRef.
- L. E. Manzer, Appl. Catal., A, 2004, 272, 249 CrossRef CAS.
- (a) Y. Guo, K. Li and J. H. Clark, Green Chem., 2007, 9, 839 RSC; (b) Y. Guo, K. Li, X. Yu and J. H. Clark, Appl. Catal., B, 2008, 81, 182 CrossRef CAS; (c) H.-F. Liu, F.-X. Zeng, L. Deng, B. Liao, H. Pang and Q.-X. Guo, Green Chem., 2013, 15, 81 RSC.
- (a) J. Q. Bond, D. M. Alonso, D. Wang, R. M. West and J. A. Dumesic, Science, 2010, 327, 1110 CrossRef CAS PubMed; (b) J. Q. Bond, D. Wang, D. M. Alonso and J. A. Dumesic, J. Catal., 2011, 281, 290 CrossRef CAS.
- (a) I. Podolean, V. Kuncser, N. Gheorghe, D. Macovei, V. I. Parvulescu and S. M. Coman, Green Chem., 2013, 15, 3077 RSC; (b) S. Dutta, L. Wu and M. Mascal, Green Chem., 2015, 17, 2335 RSC.
- J.-P. Lange, J. Z. Vestering and J. Haan, Chem. Commun., 2007, 3488 RSC.
- (a) J. Dahlmann, Chem. Ber., 1968, 101, 4251 CrossRef CAS; (b) F. Camacho, P. González-Tello, E. Jurado and A. Robles, J. Chem. Technol. Biotechnol., 1996, 67, 350 CrossRef CAS; (c) R. W. Torget, J. S. Kim and Y. Y. Lee, Ind. Eng. Chem. Res., 2000, 39, 2817 CrossRef CAS; (d) J. S. Kim, Y. Y. Lee and R. W. Torget, Appl. Biochem. Biotechnol., 2001, 91, 331 CrossRef PubMed; (e) N. S. Mosier, A. Sarikaya, C. M. Ladisch and M. R. Ladisch, Biotechnol. Prog., 2001, 17, 474 CrossRef CAS PubMed; (f) B. Girisuta, L. P. B. M. Janssen and H. J. Heeres, Ind. Eng. Chem. Res., 2007, 46, 1696 CrossRef CAS.
- (a) K. Seri, T. Sakaki, M. Shibata, Y. Inoue and H. Ishida, Bioresour. Technol., 2002, 81, 257 CrossRef CAS PubMed; (b) L. Peng, L. Lin, J. Zhang, J. Zhuang, B. Zhang and Y. Gong, Molecules, 2010, 15, 5258 CrossRef CAS PubMed; (c) F. H. Lv, R. Bi, Y. H. Liu, W. S. Li and X. P. Zhou, Catal. Commun., 2014, 49, 78 CrossRef CAS; (d) L. Zhou, H. Zou, J. Nan, L. Wu, X. Yang, Y. Su, T. Lu and J. Xu, Catal. Commun., 2014, 50, 13 CrossRef CAS.
- (a) Y. Ogasawara, S. Itagaki, K. Yamaguchi and N. Mizuno, ChemSusChem, 2011, 4, 519 CrossRef CAS PubMed; (b) Z. Sun, M. Cheng, H. Li, T. Shi, M. Yuan, X. Wang and Z. Jiang, RSC Adv., 2012, 2, 9058 RSC; (c) F. Rataboul and N. Essayem, Ind. Eng. Chem. Res., 2011, 50, 799 CrossRef CAS.
- (a) J. Potvin, E. Sorlien, J. Hegner, B. DeBoef and B. L. Lucht, Tetrahedron Lett., 2011, 52, 5891 CrossRef CAS; (b) S. Van de Vyver, J. Thomas, J. Geboers, S. Keyzer, M. Smet, W. Dehaen, P. A. Jacobs and B. F. Sels, Energy Environ. Sci., 2011, 4, 3601 RSC; (c) D. M. Alonso, J. M. R. Gallo, M. A. Mellmer, S. G. Wettstein and J. A. Dumesic, Catal. Sci. Technol., 2013, 3, 927 RSC; (d) D. Ding, J. Wang, J. Xi, X. Liu, G. Lu and Y. Wang, Green Chem., 2014, 16, 3846 RSC; (e) S. S. Joshi, A. D. Zodge, K. V. Pandare and B. D. Kulkarni, Ind. Eng. Chem. Res., 2014, 53, 18796 CrossRef CAS; (f) Y. Zuo, Y. Zhang and Y. Fu, ChemCatChem, 2014, 6, 753 CrossRef CAS; (g) D. Ding, J. Xi, J. Wang, X. Liu, G. Lu and Y. Wang, Green Chem., 2015, 17, 4037 RSC.
- (a) Y. Nishiyama, P. Langan and H. Chanzy, J. Am. Chem. Soc., 2002, 124, 9074 CrossRef CAS PubMed; (b) Y. Nishiyama, J. Sugiyama, H. Chanzy and P. Langan, J. Am. Chem. Soc., 2003, 125, 14300 CrossRef CAS PubMed.
- (a) K. Tominaga, A. Mori, Y. Fukushima, S. Shimada and K. Sato, Green Chem., 2011, 13, 810 RSC; (b) K. Nemoto, K. Tominaga and K. Sato, Chem. Lett., 2014, 43, 1327 CrossRef CAS.
- H. Zhu, Q. Cao, C. Li and X. Mu, Carbohydr. Res., 2011, 346, 2016 CrossRef CAS PubMed.
- Y. Teramoto, S. Lee and T. Endo, Bioresour. Technol., 2008, 99, 8856 CrossRef CAS PubMed.
- J. Yang and Y. Yuan, Catal. Lett., 2009, 131, 643 CrossRef CAS.
- H. Hatakeyama and T. Hatakeyama, Thermochim. Acta, 1998, 308, 3 CrossRef CAS.
- X. Hu and C.-Z. Li, Green Chem., 2011, 13, 1676 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra15638j |
| This journal is © The Royal Society of Chemistry 2016 |