Lithiation-driven structural transition of VO2F into disordered rock-salt LixVO2F
Ruiyong Chen*a,
Emad Maawadb,
Michael Knappc,
Shuhua Rend,
Přemysl Berane,
Raiker Witterf and
Rolf Hempelmanna
aJoint Electrochemistry Lab, KIST Europe/Saarland University, 66123 Saarbrücken, Germany. E-mail: r.chen@kist-europe.de; ruiyong.chen@uni-saarland.de
bInstitute of Materials Research, Helmholtz-Zentrum Geesthacht, 22607 Hamburg, Germany
cInstitute for Applied Materials, Karlsruhe Institute of Technology, 76021 Karlsruhe, Germany
dInstitute of Nanotechnology, Karlsruhe Institute of Technology, 76021 Karlsruhe, Germany
eNuclear Physics Institute, Academy of Sciences of the Czech Republic, 25068 Řež near Prague, Czech Republic
fTechnomedicum, Tallinn University of Technology, 19086 Tallinn, Estonia
First published on 4th July 2016
Abstract
We synthesize a new vanadium oxyfluoride VO2F (rhombohedral, R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c) through a simple one-step ball-milling route and demonstrate its promising lithium storage properties with a high theoretical capacity of 526 mA h g−1. Similar to V2O5, VO2F transfers into an active disordered rock-salt (Fm
c) through a simple one-step ball-milling route and demonstrate its promising lithium storage properties with a high theoretical capacity of 526 mA h g−1. Similar to V2O5, VO2F transfers into an active disordered rock-salt (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) phase after initial cycling against the lithium anode, as confirmed by diffraction and spectroscopic experiments. The newly formed nanosized LixVO2F remains its crystal structure over further cycling between 4.1 and 1.3 V. A high capacity of 350 mA h g−1 at 2.5 V was observed at 25 °C and 50 mA g−1. Furthermore, superior performance was observed for VO2F in comparison with a commercial crystalline V2O5, in terms of discharge voltage, voltage hysteresis and reversible capacity.
m) phase after initial cycling against the lithium anode, as confirmed by diffraction and spectroscopic experiments. The newly formed nanosized LixVO2F remains its crystal structure over further cycling between 4.1 and 1.3 V. A high capacity of 350 mA h g−1 at 2.5 V was observed at 25 °C and 50 mA g−1. Furthermore, superior performance was observed for VO2F in comparison with a commercial crystalline V2O5, in terms of discharge voltage, voltage hysteresis and reversible capacity.
1. Introduction
High-performance intercalation cathode materials for rechargeable Li-ion batteries are of technical importance.1–5 Great efforts are devoted to the search of new electrode materials with extended lithium storage capacity. Vanadium is abundant in the crust of the earth. Owing to the multiple oxidation states of vanadium and the varied crystal structure of vanadium oxides and vanadates, vanadium-based electrodes have been widely investigated as alternatives with increased energy densities.6 Among them, orthorhombic V2O5 attracts tremendous attention as cathode materials for accommodating guest cations such as Li+ ions as well as Mg2+ and Al3+.6–9 It is well recognized that V2O5 transforms into several LixV2O5 phases depending on the content of Li+ intercalated.10,11 The theoretical capacities of V2O5 for two and three lithium intercalation are 294 and 442 mA h g−1, respectively, which are larger than that for classic cathode materials such as LiFePO4 (170 mA h g−1) and LiCoO2 (140 mA h g−1). Intercalation of the third lithium into V2O5 occurs at a voltage below 1.9 V, which leads to an irreversible phase transition into disordered rock-salt (ω-LixV2O5, Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m).11 Lithiation induced structural transition to disordered rock-salt has been observed for other materials, such as Li2MoO4,12 LiVO3 (ref. 13) and Li1.211Mo0.467Cr0.3O2.14 Disordered rock-salt was also found as intermediate phase during the lithiation reactions of FeOF,15 Li- and Mn-rich layered materials16 and spinels.17 Recent progress in surveying new cathode materials has demonstrated that disordered rock-salt structure is suitable to access high capacity with superior Li+ chemical diffusion and rigid host framework.14,18–20
m).11 Lithiation induced structural transition to disordered rock-salt has been observed for other materials, such as Li2MoO4,12 LiVO3 (ref. 13) and Li1.211Mo0.467Cr0.3O2.14 Disordered rock-salt was also found as intermediate phase during the lithiation reactions of FeOF,15 Li- and Mn-rich layered materials16 and spinels.17 Recent progress in surveying new cathode materials has demonstrated that disordered rock-salt structure is suitable to access high capacity with superior Li+ chemical diffusion and rigid host framework.14,18–20
Variation and modification of V2O5 have led to new opportunities to optimize the electrochemical properties. Interestingly, the characteristic lithiation plateau for crystalline orthorhombic V2O5 was not observed for amorphous V2O5,21,22 graphene sheets and carbon nanotubes modified V2O5 (ref. 23 and 24) and hydrated V2O5.25,26 Meanwhile, enhanced cycling stability and reversible capacity have been achieved. A direct modification of the anion sublattice through fluorine incorporation/substitution in electrode materials has been proven to be an effective strategy to modify and optimize electrochemical performance.20,27–31 Fluorinated materials are generally synthesized through solution routes using highly toxic and corrosive fluorine-containing acids,32–34 or by a direct fluorination under reactive conditions of F2 gas at high temperature.35 So far, various coordination polymers containing vanadium oxyfluoride matrix have been synthesized and structurally determined.36,37 However, the existence of inorganic vanadium oxyfluorides is rather rare. VOF3 is the only commercially available material, which is extremely sensitive to air and moisture and easy to decompose through hydrolysis. In an earlier work,38 an orange-yellow powder VO2F was identified by reaction of VOF3 with Me3SiOSiMe3 in acetonitrile under an inert atmosphere. However, the structure and applications of VO2F were unexplored over decades. Recently, VO2F solid powders have been synthesized under extreme reaction conditions of high pressure and high temperature (4 GPa and 800 °C).39 A topotactic intercalation reaction with 0.42 Li+ has been observed.39 The structural transition mechanisms remained unclear, regarding to the change in voltage profiles when cycling at a broad voltage range.
Herein, we synthesize rhombohedral VO2F (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c) by a more facile ball-milling process and study the electrochemically driven structural change as cathode material for lithium batteries. The theoretical capacity of VO2F is 526 mA h g−1 based on the assumed intercalation reaction:
c) by a more facile ball-milling process and study the electrochemically driven structural change as cathode material for lithium batteries. The theoretical capacity of VO2F is 526 mA h g−1 based on the assumed intercalation reaction:
| VO2F + xLi+ + xe− ↔ LixVO2F | (1) |
2. Experimental
VO2F powders were synthesized by a simple ball-milling process (WC milling jar with volume of 80 mL, and balls with diameter of 10 mm, 450 rpm for 24 h) using commercial VOF3 (99%, Sigma-Aldrich) and V2O5 (≥99.6%, Sigma-Aldrich) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, molar ratio) as precursors. Precursors were added into the WC jar in an argon-filled glovebox. After ball-milling, the powders were collected and stored in a glovebox. The as-milled VO2F was used as electrode materials without further treatment.
1, molar ratio) as precursors. Precursors were added into the WC jar in an argon-filled glovebox. After ball-milling, the powders were collected and stored in a glovebox. The as-milled VO2F was used as electrode materials without further treatment.
Synchrotron X-ray diffraction (SXRD) patterns were collected at Petra-III beamline P02.1 (λ = 0.2076 Å) at DESY in Hamburg, Germany. Powders were sealed into 0.5 mm glass capillaries in an argon-filled glovebox. Rietveld structure refinements were performed using FullProf program.40
Powder neutron diffraction (ND) experiments were carried out at the CANAM (Center of Accelerators and Nuclear Analytical Methods, LM2011019) infrastructure, Czech Republic. The powder sample of about 1 g was sealed in cylindrical vanadium container with a diameter of 6 mm. The primary neutron beam was monochromatized by a copper mosaic monochromator (reflection 220) providing a neutron beam wavelength of λ = 1.46 Å. ND patterns were collected in the 2θ range from 4 to 144° with step of 0.08° and with total time for one diffraction pattern of about 18 h.
Solid-state 19F and 51V magic angle spinning (MAS) nuclear magnetic resonance (NMR) experiments were carried out at resonance frequencies of 338.4 and 94.5 MHz, respectively, on a Bruker Avance spectrometer with a 1.8 mm MAS probe (40 kHz). Experiments were carried out at room temperature with 90°/180°-pulses of 0.8/1.6 μs at 190 W for 19F, and pulse durations of 2.25 μs at 260 W for 51V. 19F and 51V spectra were referenced to CFCl3 (0 ppm) and V2O5 powder (0 ppm), respectively.
A slurry consisting of as-milled VO2F (72 wt%), carbon black (18 wt%) and poly(vinylidene fluoride) (10 wt%) in dimethylformamide was coated onto a stainless steel current collector. The electrodes were dried at 100 °C under vacuum. VO2F/Li Swagelok cells were assembled using Li foil anode, 1 M LiPF6 in ethylene carbonate/dimethyl carbonate (EC/DMC, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as electrolyte and two discs of glass fiber as separator in an Ar-filled glovebox. Galvanostatic charge/discharge was carried out between 4.1 and 1.3 V at 25 °C and 50 mA g−1.
1 v/v) as electrolyte and two discs of glass fiber as separator in an Ar-filled glovebox. Galvanostatic charge/discharge was carried out between 4.1 and 1.3 V at 25 °C and 50 mA g−1.
For ex situ structural analysis of the cycled samples, VO2F and carbon black composite was used as working electrode without binders. Electrodes were collected and washed using DMC, and then dried at room temperature in an Ar-filled glovebox.
The in situ V K-edge X-ray absorption near-edge structure (XANES) spectra were collected at beamline KMC-2 at Bessy II, Berlin, in transmission mode. A home-made cell consisting of two Al plates with rectangular apertures in the center and two sheets of Kapton windows glued on both sides was used.41 The slurry containing 70 wt% active material, 20 wt% carbon black, and 10 wt% poly(vinylidene fluoride) in dimethylformamide was cast onto a carbon-coated Al foil and dried overnight. The loading of active material on the Al foil was about 5.5 mg cm−2. Li foil was used as counter electrode. 1 M LiPF6 in EC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was used as electrolyte. A porous Celgard film was soaked in the electrolyte and used as separator. The cell was placed between two ionization chambers and cycled between 4.1 and 1.3 V versus lithium at a current rate of 52 mA g−1, and then at 113 mA g−1 at room temperature. The spectra were recorded every 10 to 15 min during one discharge/charge cycle over about 12 h. All spectra were background corrected and normalized with the Athena software package.42
1, v/v) was used as electrolyte. A porous Celgard film was soaked in the electrolyte and used as separator. The cell was placed between two ionization chambers and cycled between 4.1 and 1.3 V versus lithium at a current rate of 52 mA g−1, and then at 113 mA g−1 at room temperature. The spectra were recorded every 10 to 15 min during one discharge/charge cycle over about 12 h. All spectra were background corrected and normalized with the Athena software package.42
3. Results and discussion
3.1. Synthesis and structure of pristine material
Stoichiometric amounts of commercial V2O5 and VOF3 were used as a starting mixture to obtain the target compound VO2F through the following one-step mechanochemical reaction in a closed milling jar:| V2O5 + VOF3 → 3VO2F | (2) |
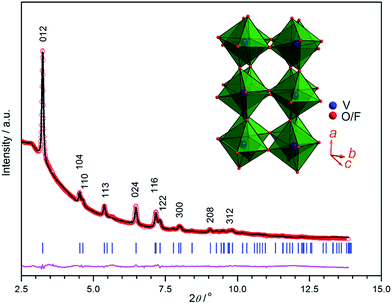
Formation of VO2F product was identified by recording its SXRD pattern (Fig. 1). After the mechanochemical ball-milling reaction, all V2O5 and VOF3 precursors have converted into new phase without any further thermal processing. Rietveld refinement of the diffraction pattern was performed using a starting crystal structure model reported by Pérez-Flores et al.39 Refinement with the space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c (no. 167) gave a good fit to the experimental data. Such phase assumption is also supported by the characteristic splitting of 104 and 110 peaks and the presence of 113 reflection.43 No impurities can be detected for the as-milled powders. Pristine VO2F crystallizes with a rhombohedral symmetry (VF3-type crystal structure), consisting of tilted corner-shared V(O/F)6 octahedra, as depicted in the inset of Fig. 1. It has been observed that such structure with flexible corner-connected octahedral network has large structural tunability through tilting and octahedral rotation.44 The refined cell parameters and average crystallite size of pristine VO2F are summarized in Table 1. The c/a value of the ball-milled nanosized VO2F (2.5383) is slightly smaller than that (2.5512) for a high temperature and high pressure (800 °C and 4 GPa) synthesized microsized VO2F material (about 1 to 60 μm).39 O and F share the Wyckoff 18e sites with nominal site occupancy of 2/3 and 1/3, respectively. Nevertheless, it cannot be refined accurately based on the X-ray diffraction data due to the near identical scattering properties of O and F. The O/F atomic coordinates are refined to be (0.4212(6), 0, 0.25). The isotropic thermal displacement parameters (Uiso) for V and O/F sites are 0.037(5) and 0.029(7), respectively. The calculated material density is 3.408 g cm−3, which is close to that for an isostructural VF3 (3.363 g cm−3).45
c (no. 167) gave a good fit to the experimental data. Such phase assumption is also supported by the characteristic splitting of 104 and 110 peaks and the presence of 113 reflection.43 No impurities can be detected for the as-milled powders. Pristine VO2F crystallizes with a rhombohedral symmetry (VF3-type crystal structure), consisting of tilted corner-shared V(O/F)6 octahedra, as depicted in the inset of Fig. 1. It has been observed that such structure with flexible corner-connected octahedral network has large structural tunability through tilting and octahedral rotation.44 The refined cell parameters and average crystallite size of pristine VO2F are summarized in Table 1. The c/a value of the ball-milled nanosized VO2F (2.5383) is slightly smaller than that (2.5512) for a high temperature and high pressure (800 °C and 4 GPa) synthesized microsized VO2F material (about 1 to 60 μm).39 O and F share the Wyckoff 18e sites with nominal site occupancy of 2/3 and 1/3, respectively. Nevertheless, it cannot be refined accurately based on the X-ray diffraction data due to the near identical scattering properties of O and F. The O/F atomic coordinates are refined to be (0.4212(6), 0, 0.25). The isotropic thermal displacement parameters (Uiso) for V and O/F sites are 0.037(5) and 0.029(7), respectively. The calculated material density is 3.408 g cm−3, which is close to that for an isostructural VF3 (3.363 g cm−3).45
| Samples | As-prepared | 1st charge | 2nd discharge |
|---|---|---|---|
| Space group | R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c c |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
| Lattice constant/Å | a = 5.1374(4) | a = 4.0932(6) | a = 4.1110(8) |
| c = 13.0405(2) | |||
| Lattice volume/Å3 | 298.07 | 68.58 | 69.48 |
| Crystallite size/nm | 34 | 3 | 4 |
Fig. 2 shows solid state 51V and 19F MAS NMR spectra for the as-milled VO2F and the starting precursors. The NMR chemical shifts are sensitive to components of chemical shielding and quadrupole tensor, as a consequence of a change in crystallographic structure. A single sharp 51V resonance line at 0 ppm was observed for V2O5 and a single resonance line at −168 ppm was observed for VOF3. In contrast, VO2F shows a 51V resonance line at 5 ppm (fwhm 83 ppm) with a broad shoulder at −68 ppm (fwhm 230 ppm) (Fig. 2a), indicating the change in the local bonding environments surrounding vanadium. Such observations are in agree with the formation of a new phase, as detected by diffraction experiments. Meanwhile, large difference in the 19F NMR was observed for VO2F and VOF3 (Fig. 2b). VOF3 has one single sharp 19F resonance line at −138 ppm, whereas VO2F has multiple 19F resonance lines with the strongest one at −112 ppm. The 51V resonance line at 5 ppm and the 19F line at −138 ppm for VO2F sample may suggest that certain unreacted residuals exist in the final product after ball-milling. These residuals may exist as amorphous state, which are not detectable by diffraction methods. The presence of several weak 19F resonance lines at −98, −119 and −145 ppm may arise from local structure distortion (such as short-range O/F ordering)46 or unidentified species.
 | ||
| Fig. 2 (a) 51V and (b) 19F MAS NMR for the as-milled VO2F and starting precursors V2O5, VOF3. The asterisks denote the spinning sidebands. | ||
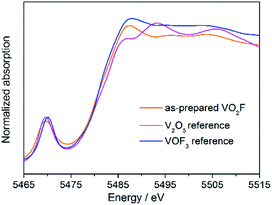
Fig. 3 shows the V K-edge XANES spectra for the as-milled VO2F and the starting materials V2O5 and VOF3. The similarity in the absorption edges with strong pre-edge peaks at about 5469 eV indicates that V exist mainly as V5+ in the as-milled VO2F. Owing to the high oxidation state of V and an open framework crystal structure, the nanosized VO2F is considered to be able to store more lithium ions without metal–O/F bond cleavage, as other vanadium-based oxides and vanadates.47–49
3.2. Electrochemical properties
Deep lithiation of VO2F with the attempt to approach the theoretical capacity, as expected in eqn (1), has never been studied and understood before. The electrochemical properties were evaluated using a VO2F/Li Swagelok cell. Fig. 4a shows the first three discharge/charge voltage profiles measured at 25 °C and 50 mA g−1. VO2F was first discharged from its open-circuit voltage (OCV, 3.7 V) to 1.3 V, which shows a lithiation capacity of about 460 mA h g−1, corresponding to 1.75 Li+ intercalation per formula unit. Several plateau-like voltage steps located at 3.5, 2.8 and 1.7 V were observed, suggesting a continuous structural change upon lithiation, as observed typically for a crystalline orthorhombic V2O5 (Fig. 4b). These voltage steps were further highlighted in the dQ/dV curve in Fig. 4c.Afterwards, the cell was recharged to 4.1 V, which exhibits a sloping charge profile and a charge capacity of about 370 mA h g−1. This indicates that certain amounts of lithium ions were trapped after first discharge. Li trapping inside crystalline V2O5 has been also observed due to phase transition.21 It is estimated that the cycled LixVO2F has a composition of about x = 0.34 after first discharge/recharge cycle. Further cycling of the Li0.34VO2F between 1.3 and 4.1 V leads to steady sloping voltage profiles (Fig. 4a) and high coulombic efficiencies (99.3%, Fig. 4d). The sloping cycling curves indicate a single phase solid solution reaction. The second discharge capacity is about 350 mA h g−1, suggesting a solid solution range between Li0.34VO2F and Li1.67VO2F, assuming that no side reactions occur. Thus, the ball-milling synthesized nanosized VO2F shows significant gains in the deliverable capacity over cycling between 4.1 and 1.3 V, compared to the microsized particles.39 After 50 cycles, the capacity decays to about 215 mA h g−1 (Fig. 4d). The capacity decay of VO2F over 50 cycles is 0.7% per cycle. This is superior to that for V2O5 nanoparticles (3.2% decay per cycle over 20 cycles),50 and close to that for V2O5 nanowires supported on graphene (0.6% decay per cycle over 50 cycles)51 cycled between 4 and 1.5 V.
The first discharge curve is different from the subsequent charge/discharge curves, indicating that lithium storage proceeds through a different mechanism after the first discharge. Similar voltage profile changes upon lithiation have been previously observed for other materials such as cubic TiOF2,52 rhombohedral Li2MoO4,12 orthorhombic V2O5 (ref. 10) and monoclinic LiVO3 (ref. 53) with discharge voltages centered at 1.2 V (Ti4+/Ti2+), 2.1 V (Mo6+/Mo5+), 2.5 V (V5+/V3.5+) and 2.3 V (V5+/V4+), respectively. Most of these materials evolve into a disordered rock-salt phase after one step lithiation. The same behavior is thus expected for the lithiation of VO2F.
3.3. Structural transition into disordered rock-salt phase
After cycling, clear structural changes were observed from the SXRD patterns. Several broad diffraction peaks located at 2θ of 5.76°, 8.16° and 9.98° for the cycled VO2F were observed (Fig. 5), revealing that VO2F transfers to a new phase. Moreover, the diffraction peaks are located at lower d-values for the first charge sample, compared to that for the second discharge sample, which is reasonable due to intercalation of lithium. This suggests the formation of a stable intercalation phase. All diffraction peaks can be well indexed to a cubic cell Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, as labeled by Miller indices in Fig. 5. The refined lattice constants are summarized in Table 1. The cubic lattice volume expands slightly from the first charge (a = 4.0932 Å) to the second discharge (a = 4.1110 Å). For the first charged sample, the diffraction peaks are rather broad and weak. In addition, the refined crystallite size for the cycled samples decreases to about 3 nm (Table 1).
m, as labeled by Miller indices in Fig. 5. The refined lattice constants are summarized in Table 1. The cubic lattice volume expands slightly from the first charge (a = 4.0932 Å) to the second discharge (a = 4.1110 Å). For the first charged sample, the diffraction peaks are rather broad and weak. In addition, the refined crystallite size for the cycled samples decreases to about 3 nm (Table 1).
 | ||
Fig. 5 SXRD patterns and Rietveld refinement using space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m for the 1st charge and 2nd discharge VO2F samples. Difference plots are shown in blue below the patterns. m for the 1st charge and 2nd discharge VO2F samples. Difference plots are shown in blue below the patterns. | ||
Fig. 6a shows the d-spacing patterns for the cycled samples from combined SXRD and ND diffraction. For the second discharged sample, several diffraction peaks with d-spacings of 2.38 Å (111 diffraction peak) and 1.24 Å (311 diffraction peak) were exclusively observed from the ND diffraction. Other diffraction peaks (200, 220 and 222) can be well resolved from both ND and SXRD.20,27 Complementary diffraction data from XRD and ND confirm that the cycled VO2F (LixVO2F) crystallizes in a disorder rock-salt structure (Fig. 6b). Such phase transition occurs during the first lithiation step. Several intermediate phases are likely to form with progressive lithiation and need to be further identified in the future.
 | ||
| Fig. 6 (a) d-Spacing values from SXRD and ND diffraction of the cycled VO2F. (b) Disordered rock-salt unit cell. | ||
It is well-known that deep lithiation of V2O5 (below about 1.9 V) leads to an irreversible structural transition into disordered rock-salt ω-Li3V2O5 phase.10 For comparison, Fig. 7 shows the voltage load curves from the second cycles for VO2F, a commercial orthorhombic V2O5 and a previously reported Li2VO2F.20 All three materials show sloping voltage profiles from the second cycles, due to the formation of a similar disordered rock-salt crystal structure. VO2F with mixed O/F anions show high onset discharge voltage (4.1 V) compared to V2O5 (3.7 V). It is reasonable that mixed O/F anionic sublattice favors high intercalation voltage. The redox reactions of V2O5 occur in a narrower voltage range between 3.7 and 1.6 V, compared to that for VO2F and Li2VO2F.20 In addition, V2O5 shows the largest voltage hysteresis and the lowest coulombic efficiency (89.5%).
 | ||
| Fig. 7 Voltage load curves at 25 °C from the second cycles for the rhombohedral VO2F (50 mA g−1), in comparison with that for a previously reported Li2VO2F (23 mA g−1)20 and a commercial orthorhombic V2O5 (20 mA g−1). Inset shows the corresponding dQ/dV plots. | ||
The load curves for VO2F and Li2VO2F are well overlapped except for the low voltage regions. VO2F exhibits gentle charge/discharge slope below about 2.2 V, compared to Li2VO2F. Accordingly, VO2F shows a slightly higher capacity of about 30 mA h g−1 at the low voltage regions (as also observed in the dQ/dV plots in the inset of Fig. 7), although cycling at slightly higher current density (50 mA g−1), compared to that for Li2VO2F (23 mA g−1). Such difference may arise from the different crystallite sizes of both materials, which is about 10 nm for Li2VO2F and about 3 nm for newly formed disordered rock-salt VO2F nanophase. The ultrafine nanoparticles have an enlarged accessible surface area for Li+ ions. It is considered that the increased capacity at low voltage may arise from an enhanced intercalation process.20,54 Moreover, based on the calculated composition of Li1.67VO2F at the end of the second discharge, it is considered that further lithium intercalation (with x > 1.67) into disordered rock-salt lattice (with about 16% cation sites unoccupied) may occur at lower discharge cutoff voltages (below 1.3 V).
3.4. In situ V K-edge XANES
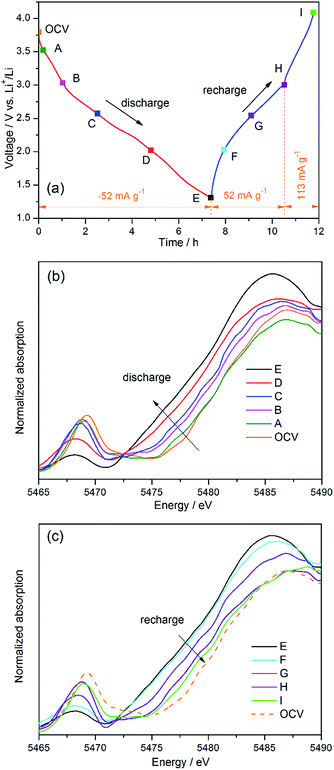
Changes in electronic structures for VO2F over galvanostatic cycling were examined by recording the V K-edge XANES spectra during an in situ discharge/charge experiment for a VO2F/Li cell (Fig. 8a). On discharge (from stage OCV to stage E), the main edge shifts to lower energy region by about 3 eV (Fig. 8b). Meanwhile, the intensity of the pre-edge peak decreases and the peak also shifts slightly to lower energy region. This confirms the reduction of vanadium upon lithiation. Based on the observed capacity (1.32 Li+ capacity), it is considered that the pristine V5+ ions are reduced to an average oxidation state of V3.68+. The spectral evolution from stage E to stage I is largely reversible upon recharge to 4.1 V. After recharge to 4.1 V, the main edge shifts back to higher energy region, together with increase in the intensity of the pre-edge peak (Fig. 8c). This suggests that recovery of the V oxidation state near V5+. However, the spectrum after recharge is not well overlapped with that of the pristine sample, implying a change in electronic structure due to phase transition.4. Conclusions
In summary, VO2F powders have been synthesized through ball-milling V2O5 and VOF3 and have been explored as new high capacity/energy density (theoretically 526 mA h g−1 and 1315 W h kg−1) cathode material for rechargeable Li-batteries. First lithiation down to 1.3 V leads to an irreversible phase transition from rhombohedral into a new active disordered rock-salt nanophase, as confirmed by SXRD and ND. Such new phase contributes a high reversible Li+ intercalation capacity of about 350 mA h g−1 at 2.5 V (i.e., 875 W h kg−1). The voltage/structure changes of VO2F were compared with that for a commercial V2O5 and a previously reported Li2VO2F. From the second charge/discharge cycles, similar sloping voltage profiles were observed for these materials with disordered rock-salt crystal structure. Compared to V2O5, VO2F shows higher discharge voltage, lower voltage hysteresis and higher coulombic efficiency. In comparison with Li2VO2F, a slight extension of the deliverable capacity was observed for VO2F owing to its ultrafine particle size. Such insights into structural mechanisms may guide the design of other related materials for battery applications. Further investigations need to be carried out to improve the cycling stability and rate performance through controlling the nanoarchitectures, as often strategically used for V2O5 and other metal oxides.55,56Acknowledgements
We acknowledge the allocated beamtime at Petra-III (P02.1 beamline, DESY, Hamburg, Germany), Bessy II (KMC-2 beamline, Berlin, Germany), and at the CANAM infrastructure (Ministry of Education, Youth and Sports of the Czech Republic, project: LM2011019, NMI3-II, grant no. 283883).References
- M. S. Whittingham, Chem. Rev., 2014, 114, 11414 CrossRef CAS PubMed.
- K. Mizushima, P. C. Jones, P. J. Wiesman and J. B. Goodenough, Mater. Res. Bull., 1980, 15, 783 CrossRef CAS.
- A. K. Padhi, K. S. Nanjundaswamy and J. B. Goodenough, J. Electrochem. Soc., 1997, 144, 1188 CrossRef CAS.
- K. Kang, Y. S. Meng, J. Bréger, C. P. Grey and G. Ceder, Science, 2006, 311, 977 CrossRef CAS PubMed.
- A. R. Armstrong and P. G. Bruce, Nature, 1996, 381, 499 CrossRef CAS.
- M. S. Whittingham, Chem. Rev., 2004, 104, 4271 CrossRef CAS PubMed.
- G. S. Gautam, P. Canepa, A. Abdellahi, A. Urban, R. Malik and G. Ceder, Chem. Mater., 2015, 27, 3733 CrossRef.
- P. Novák and J. Desilvestro, J. Electrochem. Soc., 1993, 140, 140 CrossRef.
- N. Jayaprakash, S. K. Das and L. A. Archer, Chem. Commun., 2011, 47, 12610 RSC.
- C. Delmas, H. Cognac-Auradou, J. M. Cocciantelli, M. Ménétrier and J. P. Doumerc, Solid State Ionics, 1994, 69, 257 CrossRef CAS.
- C. Delmas, S. Brèthes and M. Ménétrier, J. Power Sources, 1991, 34, 113 CrossRef CAS.
- D. Mikhailova, A. Voss, S. Oswald, A. A. Tsirlin, M. Schmidt, A. Senyshyn, J. Eckert and H. Ehrenberg, Chem. Mater., 2015, 27, 4485 CrossRef CAS.
- V. Pralong, V. Gopal, V. Caignaert, V. Duffort and B. Raveau, Chem. Mater., 2012, 24, 12 CrossRef CAS.
- J. Lee, A. Urban, X. Li, D. Su, G. Hautier and G. Ceder, Science, 2014, 343, 519 CrossRef CAS PubMed.
- K. M. Wiaderek, O. J. Borkiewicz, E. Castillo-Martínez, R. Robert, N. Pereira, G. G. Amatucci, C. P. Grey, P. J. Chupas and K. W. Chapman, J. Am. Chem. Soc., 2013, 135, 4070 CrossRef CAS PubMed.
- J. Zheng, P. Xu, M. Gu, J. Xiao, N. D. Browning, P. Yan, C. Wang and J.-G. Zhang, Chem. Mater., 2015, 27, 1381 CrossRef CAS.
- R. Chen, M. Knapp, M. Yavuz, R. Heinzmann, D. Wang, S. Ren, V. Trouillet, S. Lebedkin, S. Doyle, H. Hahn, H. Ehrenberg and S. Indris, J. Phys. Chem. C, 2014, 118, 12608 CAS.
- A. Urban, J. Lee and G. Ceder, Adv. Energy Mater., 2014, 4, 1400478 Search PubMed.
- N. Yabuuchi, M. Takeuchi, M. Nakayama, H. Shiiba, M. Ogawa, K. Nakayama, T. Ohta, D. Endo, T. Ozaki, T. Inamasu, K. Sato and S. Komaba, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 7650 CrossRef CAS PubMed.
- R. Chen, S. Ren, M. Knapp, D. Wang, R. Witter, M. Fichtner and H. Hahn, Adv. Energy Mater., 2015, 5, 1401814 Search PubMed.
- O. B. Chae, J. Kim, I. Park, H. Jeong, J. H. Ku, J. H. Ryu, K. Kang and S. M. Oh, Chem. Mater., 2014, 26, 5874 CrossRef CAS.
- S. Afyon, F. Krumeich, C. Mensing, A. Borgschulte and R. Nesper, Sci. Rep., 2014, 4, 7113 CrossRef CAS PubMed.
- Q. Liu, Z.-F. Li, Y. Liu, H. Zhang, Y. Ren, C.-J. Sun, W. Lu, Y. Zhou, L. Stanciu, E. A. Stach and J. Xie, Nat. Commun., 2015, 6, 6127 CrossRef PubMed.
- M. Sathiya, A. S. Prakash, K. Ramesha, J.-M. Tarascon and A. K. Shukla, J. Am. Chem. Soc., 2011, 133, 16291 CrossRef CAS PubMed.
- Y. Wang, K. Takahashi, K. Lee and G. Z. Cao, Adv. Funct. Mater., 2006, 16, 1133 CrossRef CAS.
- V. Petkov, P. N. Trikalitis, E. S. Bozin, S. J. L. Billinge, T. Vogt and M. G. Kanatzidis, J. Am. Chem. Soc., 2002, 124, 10157 CrossRef CAS PubMed.
- S. Ren, R. Chen, E. Maawad, O. Dolotko, A. A. Guda, V. Shapovalov, D. Wang, H. Hahn and M. Fichtner, Adv. Sci., 2015, 2, 1500128 Search PubMed.
- N. Recham, J.-N. Chotard, L. Dupont, C. Delacourt, W. Walker, M. Armand and J.-M. Tarascon, Nat. Mater., 2010, 9, 68 CrossRef CAS PubMed.
- N. Pereira, F. Badway, M. Wartelsky, S. Gunn and G. G. Amatucci, J. Electrochem. Soc., 2009, 156, A407 CrossRef CAS.
- M. Duttine, D. Dambournet, N. Penin, D. Carlier, L. Bourgeois, A. Wattiaux, K. W. Chapman, P. J. Chupas, H. Groult, E. Durand and A. Demourgues, Chem. Mater., 2014, 26, 4190 CrossRef CAS.
- J. Barker, M. Y. Saidi and J. L. Swoyer, J. Electrochem. Soc., 2004, 151, A1670 CrossRef CAS.
- A. P. Wilkinson, R. E. Josefsberg, L. C. Gallington, C. R. Morelock and C. M. Monaco, J. Solid State Chem., 2014, 213, 38 CrossRef CAS.
- A. Demourgues, N. Penin, E. Durand, F. Weill, D. Dambournet, N. Viadere and A. Tressaud, Chem. Mater., 2009, 21, 1275 CrossRef CAS.
- M. Leblanc, V. Maisonneuve and A. Tressaud, Chem. Rev., 2015, 115, 1191 CrossRef CAS PubMed.
- N. Louvain, Z. Karkar, M. El-Ghozzi, P. Bonnet, K. Guérin and P. Willmann, J. Mater. Chem. A, 2014, 2, 15308 CAS.
- S. L. Benjamin, W. Levason and G. Reid, Chem. Soc. Rev., 2013, 42, 1460 RSC.
- G. A. Senchyk, V. O. Bukhan'ko, A. B. Lysenko, H. Krautscheid, E. B. Rusanov, A. N. Chernega, M. Karbowiak and K. V. Domasevitch, Inorg. Chem., 2012, 51, 8025 CrossRef CAS PubMed.
- G. A. Kolta, D. W. A. Sharp and J. M. Winfield, J. Fluorine Chem., 1979, 14, 153 CrossRef CAS.
- J. C. Pérez-Flores, R. Villamor, D. Ávila-Brande, J. M. Gallardo Amores, E. Morán, A. Kuhn and F. García-Alvarado, J. Mater. Chem. A, 2015, 3, 20508 Search PubMed.
- J. Rodríguez-Carvajal, Phys. B, 1993, 192, 55 CrossRef.
- R. Chen, R. Heinzmann, S. Mangold, V. S. K. Chakravadhanula, H. Hahn and S. Indris, J. Phys. Chem. C, 2013, 117, 884 CAS.
- B. Ravel and M. Newville, J. Synchrotron Radiat., 2005, 12, 537 CrossRef CAS PubMed.
- B. Li, D. Wang, Y. Wang, B. Zhu, Z. Gao, Q. Hao, Y. Wang and K. Tang, Electrochim. Acta, 2015, 180, 894 CrossRef CAS.
- J. M. Rondinelli, S. J. May and J. W. Freeland, MRS Bull., 2012, 37, 261 CrossRef CAS.
- D. L. Perry, Handbook of inorganic compounds, Taylor & Francis, Boca Raton, FL, 2nd edn, 2011, p. 452 Search PubMed.
- L.-S. Du, A. Samoson, T. Tuherm and C. P. Grey, Chem. Mater., 2000, 12, 3611 CrossRef CAS.
- N. A. Chernova, M. Roppolo, A. C. Dillon and M. S. Whittingham, J. Mater. Chem., 2009, 19, 2526 RSC.
- R. Chen, S. Ren, M. Yavuz, A. A. Guda, V. Shapovalov, R. Witter, M. Fichtner and H. Hahn, Phys. Chem. Chem. Phys., 2015, 17, 17288 RSC.
- R. Chen, S. Ren, X. Mu, E. Maawad, S. Zander, R. Hempelmann and H. Hahn, ChemElectroChem, 2016, 3, 892 CrossRef CAS.
- S.-H. Ng, T. J. Patey, R. Büchel, F. Krumeich, J.-Z. Wang, H.-K. Liu, S. E. Pratsinis and P. Novák, Phys. Chem. Chem. Phys., 2009, 11, 3748 RSC.
- H. Liu and W. Yang, Energy Environ. Sci., 2011, 4, 4000 CAS.
- M. V. Reddy, S. Madhavi, G. V. Subba Rao and B. V. R. Chowdari, J. Power Sources, 2006, 162, 1312 CrossRef CAS.
- Z. Huang, L. Cao, L. Chen, Y. Kuang, H. Zhou, C. Fu and Z. Chen, J. Phys. Chem. C, 2016, 120, 3242 CAS.
- R. Chen, M. Knapp, M. Yavuz, S. Ren, R. Witte, R. Heinzmann, H. Hahn, H. Ehrenberg and S. Indris, Phys. Chem. Chem. Phys., 2015, 17, 1482 RSC.
- B. Yan, X. Li, Z. Bai, Y. Zhao, L. Dong, X. Song, D. Li, C. Langford and X. Sun, Nano Energy, 2016, 24, 32 CrossRef CAS.
- Y. Zhao, X. Li, B. Yan, D. Xiong, D. Li, S. Lawes and X. Sun, Adv. Energy Mater., 2016, 6, 1502175 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |