High performance shape memory polyimides based on π–π interactions†
Qihua
Wang
a,
Yongkang
Bai
*ab,
Yu
Chen
ab,
Junping
Ju
ab,
Fei
Zheng
ab and
Tingmei
Wang
a
aState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, 730000, P. R. China
bUniversity of Chinese Academy of Sciences, Beijing, 100039, P. R. China. E-mail: yongkangbai@foxmail.com
First published on 31st October 2014
Abstract
A series of polyimides (PIs) with different chain structures were synthesized by a two-step method. The influences of chain conformations on their thermal and mechanical properties were investigated by dynamic mechanical analysis, thermogravimetric analysis, as well as by a universal testing machine. All PIs exhibited high glass transition temperature (>240 °C) and thermal decomposition temperature (>480 °C). Moreover, all these PIs could show shape memory properties more or less, especially ODA–BPDA with a shape recovery ratio (Rr) greater than 93%. The high value of Rr was mainly due to the existence of π–π interaction, a type of non-covalent interaction. The dependence of Rr on π–π interaction was investigated in detail by UV-vis spectroscopy. Finally, atomic oxygen (AO) exposure experiments showed that the shape memory properties of ODA–BPDA were affected little by the erosion due to AO.
1. Introduction
Over the past few decades, shape memory polymers (SMPs) have been widely studied for the development of novel smart materials.1–5 SMP have an interesting ability of recovering their original shape from a temporary deformed shape upon exposure to external stimulus. The prerequisite for endowing polymers with this ability (shape memory effect, SME) involves two aspects: molecular switches (soft segments) and netpoints (hard segments).6,7 The molecular switches are reversibly sensitive to certain stimuli and responsible for shape fixation, whereas the netpoints could be chemical cross-linked points or physical cross-linked points, which are responsible for shape recovery.Various types of polymers have been developed as thermally sensitive SMPs, such as polyurethanes, cross-linked polyolefins, polysiloxanes, and poly(methyl-acrylates).8–12 Although these materials possess excellent shape memory properties, their low transition temperature (<150 °C), mechanical strength and stability restrict their application in engineering field, especially in the aerospace industry. Materials used for these special fields require higher switching temperatures and mechanical strengths. These high temperature applications range from expandable structures such as solar arrays and antennas to shape morphing surfaces such as airplane wings and inflatable protection systems; therefore, high temperature SMPs have emerged in recent years. For example, Weiss13,14 reported a high temperature SMP (>250 °C) prepared from the metal salts of a sulfonated poly (ether ether ketone) ionomer. The ionic and dipolar interactions between metal sulfonate groups provide physical cross-linked points, endowing the ionomer with high shape recovery ratio. However, the introduction of sulfonate groups reduced the heat resistance of the materials. Polyimide (PI) materials are also good candidates for high temperature shape memory application because of their outstanding thermal and chemical stability, high glass transition temperatures (Tg), and good mechanical strength.15–18 In addition, PI also possesses radiation resistant properties, and it is used in the aerospace industry.18,19 Previous research have focused on the shape memory properties of PI composites with the emphasis on the effect of doping of nanoparticles. For instance, Yoonessi et al.20 prepared PI nanocomposites reinforced by graphene, which showed shape memory behavior with a switching temperature of 230 °C. Moreover, they found that the shape memory properties of PI could be improved with the addition of graphene. In addition, Vaia et al.21 prepared lightly cross-linked PI composites reinforced by single wall carbon nanotubes, which also exhibited good shape memory properties at 220 °C. Although the introduction of carbon fillers could endow PIs with good shape memory properties, the complexity of the synthesis process and the reduction of thermal stability restrict their application. Moreover, the feasibility of their application in the space environment after doping still needs to be further investigated.
Herein, a series of PIs with different molecular structures were synthesized to check whether neat thermoplastic PIs could show high shape memory properties. The thermal, mechanical and shape memory properties of these PIs were investigated. Interestingly, we found that all the PIs could show shape memory properties. The influences of chain structure and cycle time on shape memory properties were also carefully investigated to confirm the crucial factor of shape memory properties for PIs. We also found that the addition of nanosilica to the PI networks could further improve the shape memory properties due to the introduction of hydrogen bonds. Moreover, the shape memory properties of PIs were only slightly affected after atomic oxygen (AO) exposure, which indicated a great potential for the application of PIs in space environment as smart materials.
2. Experimental section
2.1 Materials
4,4′-Oxydianiline (ODA) was supplied by Sinopharm Group Chemical Reagent Co., Ltd. 4,4′-Oxydiphthalic dianhydride (ODPA) was purchased from Shanghai Research Institute of Synthetic Resin. 3,4′-Oxydianiline (34ODA) and 3,3′,4,4′-biphenyltetracarboxylic dianhydride (BPDA) were received from Changzhou Sunlight Pharmaceutical Co., Ltd. Nanosilica was obtained from Sigma, and its average grain diameter was about 14 nm. N-Methyl-2-pyrrolidone (NMP) was obtained from Shanghai Kefeng Industry & Commerce Co., Ltd.2.2 Preparation of neat polyimide and silica hybrid films
Polyimides (PIs) with different molecular structures were prepared by a two-step method:22 synthesis of polyamic acid (PAA) and imidization. Briefly, dianiline (0.01 mol) was first dissolved in NMP (50 mL) under protection of Ar. Then, dianhydride (0.01 mol) was slowly added to the solution and the polymerization reaction was allowed to stand for 24 h at room temperature to afford a PAA solution. The resulting solution was cast onto a glass support and thermal imidization was carried out at 70 °C for 4 h, 100 °C for 1 h, 200 °C for 1 h and 300 °C for 1 h to obtain a PI film.The main process of preparing silica hybrid films was similar to that of pure PI, with the exception of the addition of nanosilica after the synthesis of PAA. The solution mixture was continuously stirred for another 12 h before imidization.
2.3 Materials characterization
The glass transition temperatures (Tg) of PI and hybrid films were determined by dynamic mechanical analysis (Netzsch, DMA 242C). The samples were cut into dimensions of ∼20 × 3 × 0.05 mm for DMA tests.3 For the thermo-mechanical properties, the samples were heated from room temperature (25 °C) to 400 °C at a rate of 5 °C min−1. Thermogravimetric analysis (TGA) was performed under a nitrogen atmosphere on a Netzsch-STA449F3 series thermal analysis system using a heating rate of 10 °C min−1.The mechanical properties were measured with a universal testing machine (Shimadzu AG-X) with a strain speed of 5 mm min−1 at room temperature. The tested samples were cut to dog-bone type dimensions according to ISO527-2/1BB. The UV-vis spectra were obtained on a Hitachi U-3010 spectrophotometer.
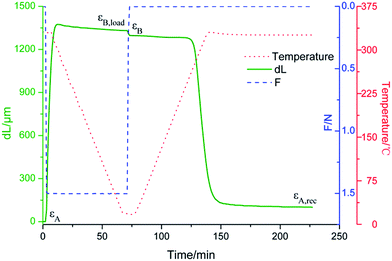
The shape memory properties were measured by DMA under the tension mode by the following process: (1) the sample with a constant force was stretched at an elevated temperature (Thigh = Tg + 50 °C), (2) the sample was then cooled to a low temperature (Tlow = 20 °C) at a rate of 5 °C min−1, and held at that temperature for 5 min, (3) the external load was then removed and the sample was held for another 5 min, and finally (4) the sample was reheated to Thigh at 5 °C min−1 and held for more than 1 h to observe the shape recovery process. The shape memory properties were then evaluated by shape fixation (Rf) and shape recovery ratio (Rr) according to eqn (1) and (2). Rf represents the capability of fixing the temporary shape, whereas Rr represents the capability of memorizing the original shape. In the equations, εx represents the strain of each shape obtained from the strain curve obtained from DMA. The effects of AO on the shape memory properties were analyzed on a ground-based simulation facility at the Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences.
| Rf = (εB − εA)/(εB,load − εA) | (1) |
| Rr = (εB − εA,rec)/(εB − εA) | (2) |
3. Results and discussion
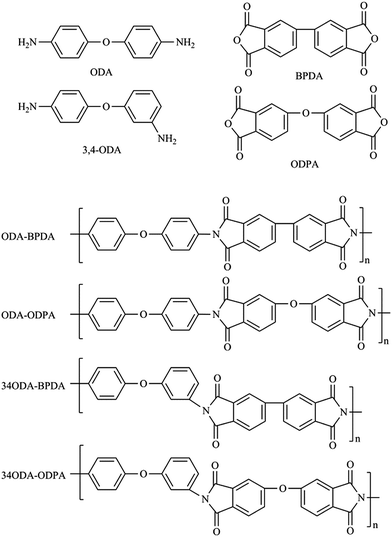
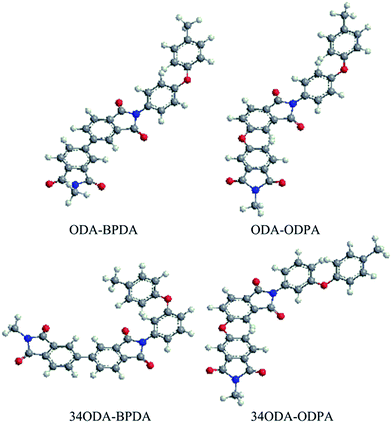
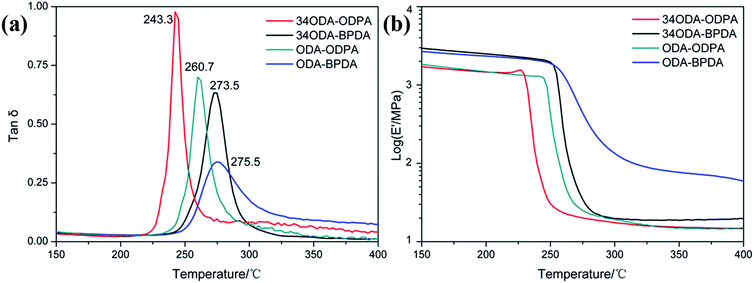
In this study, a series of PIs with different molecular structures were synthesized to investigate whether PIs could exhibit shape memory properties. They were named as ODA–BPDA, ODA–ODPA, 34ODA–BPDA and 34ODA–ODPA, according to the dianhydride and dianiline used. As is well known, the chemical structure of polymers will exert a great influence on their transition temperatures and mechanical properties.15,23 Thus, the structures of monomers and PI polymers are presented in Fig. 1 and the 3D model structures of PIs are shown in Fig. 2. According to the images, it is apparent that the chain rigidity of the PIs prepared from BPDA is higher than those prepared from ODPA. The chain linearity of the PIs prepared from ODA is higher than those prepared from 34ODA. In general, the ODA–BPDA possessed the highest chain rigidity and linearity among all the PIs in this study. Then, the effect of the structure of PIs on their thermal, mechanical and shape memory properties were carefully investigated and discussed in the subsequent parts.Being thermally induced SMPs, the glass transition temperatures (Tg) of the PIs were firstly investigated by DMA. In Fig. 3(a), the values of Tg determined from the maxima of the loss factor (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) curves are shown. It is apparent that the Tg increased in the order 34ODA–ODPA < ODA–ODPA < 34ODA–BPDA < ODA–BPDA as a result of the increase in chain rigidity. The increase in chain rigidity can be determined from the decrease of tan
δ) curves are shown. It is apparent that the Tg increased in the order 34ODA–ODPA < ODA–ODPA < 34ODA–BPDA < ODA–BPDA as a result of the increase in chain rigidity. The increase in chain rigidity can be determined from the decrease of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ. Moreover, the higher chain linearity was also a reason for the higher Tg of the PIs synthesized from ODA. The storage modulus (E′) curves in Fig. 3(b) also show the same trend of Tg. Thermo-stability was studied by TGA and the results are given in Fig. S1.† The onset of thermal decomposition, Td95, of the PIs also increased in the same order as Tg. Therefore, the ODA–BPDA with the highest chain rigidity and linearity possessed the maximum Tg and Td95 among these PIs.
δ. Moreover, the higher chain linearity was also a reason for the higher Tg of the PIs synthesized from ODA. The storage modulus (E′) curves in Fig. 3(b) also show the same trend of Tg. Thermo-stability was studied by TGA and the results are given in Fig. S1.† The onset of thermal decomposition, Td95, of the PIs also increased in the same order as Tg. Therefore, the ODA–BPDA with the highest chain rigidity and linearity possessed the maximum Tg and Td95 among these PIs.
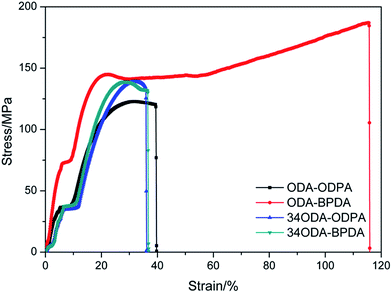
The influence of chain conformation on mechanical properties was investigated by a universal testing machine. As presented in Fig. 4 and Table S1,† ODA–BPDA along with high chain rigidity and linearity also showed excellent mechanical strength. Its tensile strength and tensile modulus exceeded 180 MPa and 2000 MPa, respectively, whereas the elongation at break was more than 110%. Compared with ODA–BPDA, the mechanical strength of the other PIs greatly decreased due to the decrease in chain rigidity and linearity; however, their tensile strength still exceeded 120 MPa.
From the above studies, it can be concluded that all the PIs showed good thermal and mechanical properties, especially ODA–BPDA. Shape memory properties were investigated in the subsequent parts. The shape recovery process of the PIs can be observed in Fig. 5. All the polymer samples were first folded at 300 °C and subsequently cooled to room temperature to fix the temporary shapes. Then, the samples were again reheated to 300 °C to observe the shape recovery process. It is interesting to note that all the samples showed considerable SME. ODA–BPDA recovered almost its original shape within seconds, whereas the ODA–ODPA showed the lowest shape recovery ratio.
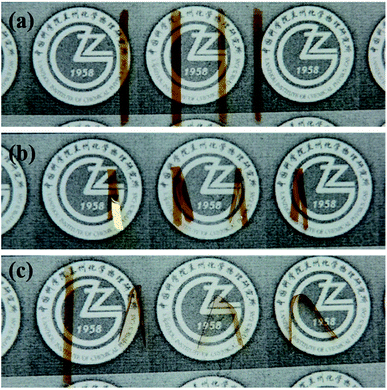 | ||
| Fig. 5 Shape recovery process of the PIs: samples from left to right are ODA–BPDA, ODA–ODPA, 34ODA–BPDA and 34ODA–ODPA. (a) Original samples, (b) folded samples, (c) recovered samples. | ||
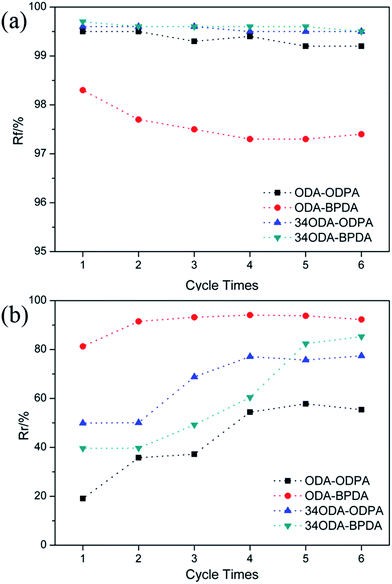
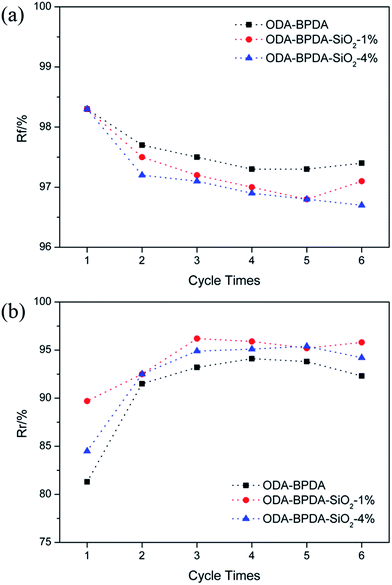
To more accurately investigate the shape memory properties, cyclic thermo-mechanical tests were performed using DMA in the tensile mode. A representative shape memory cycle of ODA–BPDA is presented in Fig. 6. As observed from the strain curve, ODA–BPDA exhibited excellent shape memory properties with the shape fixation (Rf) and shape recovery ratio (Rr) reaching 97.5% and 93.2%, respectively. Fig. 7 shows the Rf and Rr of the PIs during different cycle times. As can be seen in Fig. 7(a), all the four PIs exhibited high Rf, which was only slightly affected with the increase of cycle time. According to the previous studies, the high values of Rf are obtained mainly due to the dramatic decrease of E′ after glass transition, as can be seen in Fig. 3(b). Moreover, this was also the reason for the Rf of ODA–BPDA to be slightly smaller than the others. Unlike Rf, the Rr increased with increasing cycle time, especially for 34ODA–BPDA and ODA–ODPA. The former increased from 39.6% to 85.4%, after six circulations, while the latter increased from 19.1% to 57.4%. This phenomenon was different compared to the other SMPs because the Rr of a material without SME does not usually increase, according to the previous studies.24,25 Surely, according to previous studies,26,27 the shape recovery of SMPs usually need a training process due to the irreversible segment-chain orientation and relaxation effects in the polymer networks; however, this training process usually needs only one or two cycles like ODA–BPDA. Therefore, we attempted to determine the reason for Rr to increase with increase in cycle times for PIs. In addition, the reason for thermoplastic PIs to show high Rr was also investigated.
It is well known that the prerequisite to achieve a SME involves two aspects: molecular switches and netpoints. Molecular switches are reversibly sensitive to certain external stimuli and are responsible for shape fixing, similar to PI in this study. Netpoints could be physically or chemically cross-linked points and are responsible for shape recovery. For thermoplastic PIs, the non-covalent interactions might be playing the main role of netpoints. In Yoonessi's study,20 they attributed the netpoints to the intermolecular entanglement; however, this was not sufficient to endow PIs with high Rr. It is well known that π–π interactions, also called π–π stacking, play an important role in PIs because PIs have large amounts of aromatic groups.16,28,29 Therefore, we assumed that π–π interaction performed the role of physical cross-linked points, which endowed PIs with good shape memory properties.
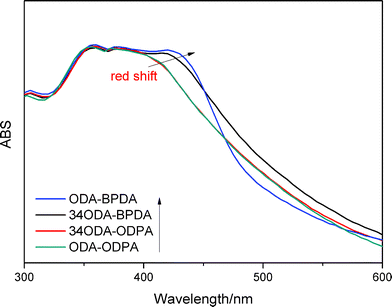
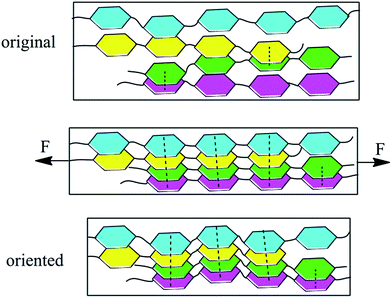
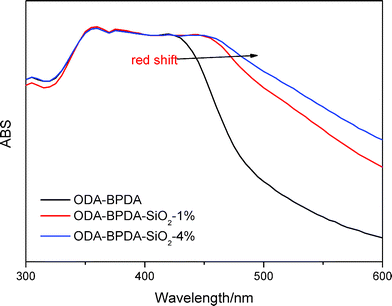
π–π interactions are a type of non-covalent interaction, which have significant potential application in healable materials, supramolecular chemistry and biochemistry.30–33 π–π interactions are also a type of conjugative effect between aromatic groups, which affect the chemical environment of benzene, leading to the red shift of UV absorption wavelength.33,34 Therefore, UV-vis spectra were used to monitor the effect of π–π interactions in this study. Fig. 8 shows the UV-vis spectra of PIs. The broad absorption peaks were ascribed to the electronic transitions of benzene. The absorption wavelength could have increased due to the increase of π–π interactions. As shown in the spectra, the maximum absorption wavelength (λmax) increased in the order 34ODA–ODPA, ODA–ODPA < 34ODA–BPDA < ODA–BPDA, indicating the enhancement of the π–π interactions. The higher value of λmax for ODA–BPDA suggested the presence of stronger π–π interactions, leading to more netpoints in the polymer networks. Therefore, this may be a reason why ODA–BPDA had a higher Rr than the other PIs. To further confirm the effect of π–π interactions on shape recovery, the UV-vis spectra of the PIs after different cycle times were also investigated. As presented in Fig. 9, λmax increased with increasing cycle times, indicating the enhancement of π–π interactions. Moreover, it was also observed that the λmax of ODA–BPDA increased in the first two cycles but remained about the same in the following cycles, whereas that of ODA–ODPA increased until the fourth cycle. Interestingly, the changing trend of λmax (in both ODA–BPDA and ODA–ODPA) was in accordance with the variation of Rr, as shown in Fig. 7. This indicated that cyclic thermo-mechanical process could increase the π–π interactions in the PIs. It is well known that π–π interactions occur when aromatic groups approach each other face to face.30 During the stretching process, the PI chains oriented along the stretching direction and came close to each other, as vividly presented in Fig. 10. Thus, the π–π interactions (dotted line in Fig. 10) easily occurred, and most of them could be reserved, leading to the increase of the netpoints. Therefore, the red shift occurred after the cyclic tests and Rr increased with each cycle. In addition, the formation of π–π interactions also preserved the orientation of the polymer chains, which is another reason for the increase in Rr. The orientation of the PIs was characterized by polarizing microscopy (POM) because anisotropic polymers show light field under POM, while isotropic polymers exhibit dark field. Fig. S2† shows the POM images of ODA–BPDA after different cycle times. Being an isotropic polymer, the original sample showed dark field under POM. Then, it became bright due to the orientation of the polymer chains after the cyclic tests.
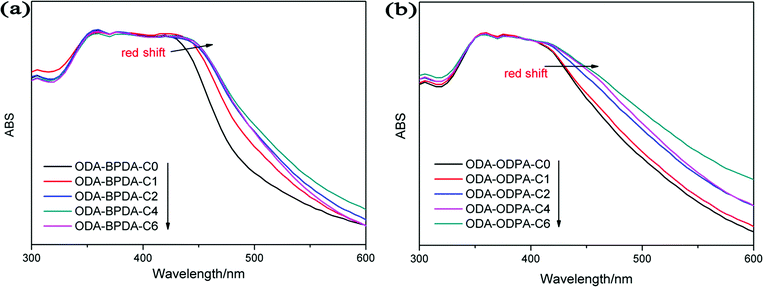 | ||
| Fig. 9 Red shift of UV-vis absorption wavelength induced by cyclic tests for (a) ODA–BPDA and (b) ODA–ODPA. | ||
Through the above investigation, it can be concluded that π–π interactions indeed played an important role in shape memory properties by acting as the physical cross-linked points, and then by fixing the orientation of the molecular chains. The former effect increased the netpoints, which was closely related to the shape recovery. The latter effect decreased the irreversible orientation in the subsequent cycle, which led to a higher Rr in the following cycles. In addition, we found that ODA–BPDA was again better than other PIs in terms of shape recovery ratio. The reason for this phenomenon may also be ascribed to the higher chain rigidity and linearity of ODA–BPDA because the π–π interactions easily occurred between the molecular chains. It was interesting to note that ODA–BPDA exhibited the best thermal, mechanical and shape memory properties due to its chain conformation. However, it would be more interesting to investigate whether its shape memory properties could be further improved. In this study, hydrogen bonds were introduced by the addition of nanosilica to increase the physical cross-linked netpoints. In the composite networks, nanosilica with abundant hydroxyl groups on the surface acted as the H-bond donors, while the PI chains with many polar groups acted as the H-bond acceptors. Therefore, nanosilica acted as the netpoints in the polymer networks. As presented in Fig. 11, the introduction of only 1% wt nanosilica indeed increased the Rr of ODA–BPDA. However, the Rr was not further improved with the further addition of nanosilica (4% wt) due to the decrease of the dispersibility of SiO2 in the PI. The influence of hydrogen bonds on the π–π interactions was also investigated, as shown in Fig. 12. We found that the λmax obviously increased after the addition of nanosilica. This indicated that the π–π interactions were increased due to the introduction of hydrogen bonds. Therefore, the effect of nanosilica on Rr could be divided into two aspects: the increase of π–π interactions and the introduction of hydrogen bonds. Both these factors increased the netpoints, leading to the promotion of shape memory properties.
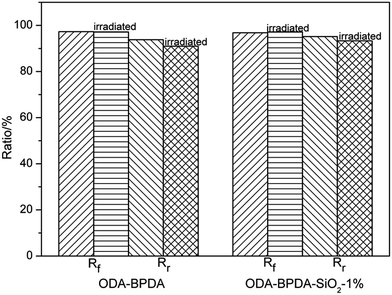
To confirm the influence of space environment on the shape memory properties, an atomic oxygen (AO) exposure experiment, which is the main cause of erosion effects in low Earth orbit, was performed in a ground-based simulation facility. The impinging kinetic energy was about 5 eV and every sample was exposed to AO for 3 h, which corresponds to the specimens being mounted on an international space station flying in a 345 km track for 233 days. As shown in Fig. 13, Rf for ODA–BPDA and its composites was almost the same after the exposure, while Rr slightly decreased due to the erosion effects of AO. However, the irradiated PIs still featured excellent shape memory properties with Rr greater than 91%. This experiment indicated that the ODA–BPDA and its composites could exhibit excellent shape memory properties after AO erosion.
4. Conclusions
A series of PIs with different chain conformations were prepared by a two-step method. With a higher chain rigidity and linearity, the ODA–BPDA showed better performance than the other PIs in this study. Its glass transition temperature and onset thermal decomposition temperature were over 275 °C and 480 °C, respectively. Tensile tests revealed its high mechanical properties with the tensile modulus and tensile strength of over 2000 MPa and 180 MPa, respectively. Its elongation at break was above 110%, indicating its high toughness. Moreover, it exhibited outstanding shape memory properties with Rf and Rr reaching 97.5% and 93.2%, respectively. Its shape memory properties were further improved by the addition of nanosilica because of the introduction of hydrogen bonds. Finally, an AO exposure experiment was performed in a ground-based simulation facility. This revealed that the ODA–BPDA could still show excellent shape memory properties even after the exposure. In general, the ODA–BPDA with these outstanding properties possesses great application potential in the aerospace industry as a smart material. In addition, ODA–BPDA is also a commercialized material, which has been widely used in industry. The discovery of its shape memory properties could also play a guiding role on the industrial application of PIs in the future.Acknowledgements
The financial supports from the National Science Foundation for Distinguished Young Scholars of China (grant no. 51025517), the National Nature Science Foundation of China (NSFC, grant no. 51305431) and the National Defense Basic Scientific Research Project (A1320110011) are duly acknowledged.References
- Y. Liu, H. Du, L. Liu and J. Leng, Smart Mater. Struct., 2014, 23, 023001–023022 CrossRef.
- Q. Zhao, M. Behl and A. Lendlein, Soft Matter, 2013, 9, 1744–1755 RSC.
- A. Lendlein and S. Kelch, Angew. Chem., Int. Ed., 2002, 41, 2034–2057 CrossRef CAS.
- M. Behl and A. Lendlein, Mater. Today, 2007, 10, 20–28 CrossRef CAS.
- W. M. Huang, C. L. Song, Y. Q. Fu, C. C. Wang, Y. Zhao, H. Purnawali, H. B. Lu, C. Tang, Z. Ding and J. L. Zhang, Adv. Drug Delivery Rev., 2013, 65, 515–535 CrossRef CAS PubMed.
- J. Leng, X. Lan, Y. Liu and S. Du, Prog. Mater. Sci., 2011, 56, 1077–1135 CrossRef CAS PubMed.
- J. Hu, Y. Zhu, H. Huang and J. Lu, Prog. Polym. Sci., 2012, 37, 1720–1763 CrossRef CAS PubMed.
- S. Zhou, L. Wang, X. Yang, H. M. Chen, G. Yang, T. Gong and W. B. Li, Polym. Chem., 2013, 4, 4461–4468 RSC.
- L. Zhang, Y. Jiang, Z. Xiong, X. Liu, H. Na, R. Zhang and J. Zhu, J. Mater. Chem. A, 2013, 1, 3263–3267 CAS.
- J. Zhao, M. Chen, X. Wang, X. Zhao, Z. Wang, Z.-M. Dang, L. Ma, G.-H. Hu and F. Chen, ACS Appl. Mater. Interfaces, 2013, 5, 5550–5556 CAS.
- J. M. Cuevas, R. Rubio, L. German, J. M. Laza, J. L. Vilas, M. Rodriguez and L. M. Leon, Soft Matter, 2012, 8, 4928–4935 RSC.
- W. Voit, T. Ware, R. R. Dasari, P. Smith, L. Danz, D. Simon, S. Barlow, S. R. Marder and K. Gall, Adv. Funct. Mater., 2010, 20, 162–171 CrossRef CAS.
- Y. Shi, M. Yoonessi and R. A. Weiss, Macromolecules, 2013, 46, 4160–4167 CrossRef CAS.
- Y. Shi and R. A. Weiss, Macromolecules, 2014, 47, 1732–1740 CrossRef CAS.
- G. Ragosta, M. Abbate, P. Musto and G. Scarinzi, J. Mater. Sci., 2012, 47, 2637–2647 CrossRef CAS.
- L. Luo, J. Yao, X. Wang, K. Li, J. Huang, B. Li, H. Wang, Y. feng and X. Liu, Polymer, 2014, 55, 4258–4269 CrossRef CAS PubMed.
- B. Tang, D. Cai, J. Sun, J. Wang and L. Dai, Polym. Compos., 2013, 34, 2076–2081 CrossRef CAS.
- D. Cai, J. Su, M. Huang, Y. Liu, J. Wang and L. Dai, Polym. Degrad. Stab., 2011, 96, 2174–2180 CAS.
- Y. Wang, T. Wang and Q. Wang, Tribol. Int., 2014, 78, 47–59 CrossRef CAS PubMed.
- M. Yoonessi, Y. Shi, D. A. Scheiman, M. Lebron-Colon, D. M. Tigelaar, R. A. Weiss and M. A. Meador, ACS Nano, 2012, 6, 7644–7655 CrossRef CAS PubMed.
- H. Koerner, R. J. Strong, M. L. Smith, D. H. Wang, L.-S. Tan, K. M. Lee, T. J. White and R. A. Vaia, Polymer, 2013, 54, 391–402 CrossRef CAS PubMed.
- J. Liu, Y. Gao, F. Wang, D. Li and J. Xu, J. Mater. Sci., 2002, 37, 3085–3088 CrossRef CAS.
- I. Ronova and M. Bruma, Struct. Chem., 2010, 21, 1013–1020 CrossRef CAS.
- Y. Bai, Y. Chen, Q. Wang and T. Wang, J. Mater. Chem. A, 2014, 2, 9169–9177 CAS.
- M. Heuchel, J. Cui, K. Kratz, H. Kosmella and A. Lendlein, Polymer, 2010, 51, 6212–6218 CrossRef CAS PubMed.
- C. Véchambre, A. Buléon, L. Chaunier, C. Gauthier and D. Lourdin, Macromolecules, 2011, 44, 9384–9389 CrossRef.
- H. J. Qi, T. D. Nguyen, F. Castro, C. M. Yakacki and R. Shandas, J. Mech. Phys. Solids, 2008, 56, 1730–1751 CrossRef CAS PubMed.
- S. Burattini, H. M. Colquhoun, J. D. Fox, D. Friedmann, B. W. Greenland, P. J. F. Harris, W. Hayes, M. E. Mackay and S. J. Rowan, Chem. Commun., 2009, 6717–6719, 10.1039/b910648k.
- S. Burattini, B. W. Greenland, W. Hayes, M. E. Mackay, S. J. Rowan and H. M. Colquhoun, Chem. Mater., 2010, 23, 6–8 CrossRef.
- S. Grimme, Angew. Chem., Int. Ed., 2008, 47, 3430–3434 CrossRef CAS PubMed.
- F. Schlosser, M. Moos, C. Lambert and F. Würthner, Adv. Mater., 2013, 25, 410–414 CrossRef CAS PubMed.
- L. Zhang, J. Wu, N. Sun, X. Zhang and L. Jiang, J. Mater. Chem. A, 2014, 2, 7666–7668 CAS.
- K. Kano, H. Minamizono, T. Kitae and S. Negi, J. Phys. Chem. A, 1997, 101, 6118–6124 CrossRef CAS.
- A. M. Smith, R. J. Williams, C. Tang, P. Coppo, R. F. Collins, M. L. Turner, A. Saiani and R. V. Ulijn, Adv. Mater., 2008, 20, 37–41 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ta05058d |
| This journal is © The Royal Society of Chemistry 2015 |