One-pot synthesis of porous nickel cobalt sulphides: tuning the composition for superior pseudocapacitance†
Haichao
Chen
ab,
Jianjun
Jiang
*a,
Yuandong
Zhao
a,
Li
Zhang
a,
Danqing
Guo
a and
Dandan
Xia
a
aSchool of Optical and Electronic Information, Huazhong University of Science and Technology (HUST), Wuhan 430074, China. E-mail: jiangjj@mail.hust.edu.cn
bCollege of Materials Science & Engineering, China Jiliang University, Hangzhou 310018, China
First published on 7th October 2014
Abstract
Nickel cobalt sulphides with different stoichiometric nickel and cobalt contents have been synthesized and used as pseudocapacitive materials for supercapacitors. The as-employed polyol method is robust enough to use a one-pot synthesis of nickel cobalt sulphides with similar porous morphology and crystal structure at different nickel and cobalt ratios. The electrochemical performance of the nickel cobalt sulphides can be easily tuned by the varying the Ni and Co content. Owing to the combined contributions from both Ni and Co ions, the bimetallic Ni–Co sulphides show a superior pseudocapacitance compared to monometallic Co and Ni sulphides in terms of high specific capacitance, excellent rate capability and long cycle stability. In particular, the Ni1.5Co1.5S4 sample shows the highest specific capacitance of 1093 F g−1 at 1 A g−1, superior rate capability of 69% capacitance retention after a 50-fold increase in current densities, and longer cycle stability with increased specific capacitance of 108% of capacitance retention after 2000 cycles. In addition, the Ni1.5Co1.5S4 was also used to assemble an asymmetric supercapacitor with reduced graphene oxide and attains excellent capacitive performance with high specific capacitance (113 F g−1 at 1 A g−1), high energy density (37.6 W h kg−1 at 775 W kg−1) and high power density (23.25 kW kg−1 at 17.7 W h kg−1).
Introduction
The ever-increasing energy demand caused by the rapid development of economy and the fast-growing use of electronics devices, has stimulated researchers to pursue high performance energy-storage devices and technologies.1 Among these charge storage systems, supercapacitors have been explored as the most promising power sources compared to other energy-storage devices (such as lithium ion batteries and Ni–MH batteries) in terms of higher power density, shorter charge time and longer cycle life. These are the optimal qualities for applications requiring quick release of energy, such as electric vehicles, hybrid electric vehicles, high power portable electronics and backup energy systems.2–4 Generally, the performance of supercapacitors is mainly determined by the properties of the electroactive materials. Therefore, numerous efforts have been made to design new electroactive materials to optimize the performance of supercapacitors.5,6Recently, transition metal sulphides have been demonstrated to be the materials of choice for high performance supercapacitors. Metal sulphides have been intensively researched in applications for fuel cells,7,8 solar cells,9–11 and Li-ion batteries,12–14 in view of their excellent performance. When used as the electroactive material for supercapacitors, the electrochemical properties of various binary sulphides, such as CoSx,15–18 NiSx,17–20 WS2,21 CuS,22 have been thoroughly explored in alkaline electrolytes. In particular, Co sulphides and Ni sulphides have been widely investigated because of their high theoretical capacitance, high redox activity, low cost and low toxicity. However, binary Co sulphide and Ni sulphide usually suffer from a disadvantage of low specific capacitance and poor cycle life, which is not beneficial for their use in high performance supercapacitors.
Compared to binary systems, ternary Ni–Co sulphide has recently been proven to possess superior electrochemical performance. For example, our group synthesized an NiCo2S4 urchin-like nanostructure, which exhibits a considerably higher specific capacitance of 1149 F g−1 over binary Co sulphide and showed an improved cycle stability and rate property compared to NiS.23 Furthermore, when we introduced Ni foam in the preparation process as a substrate to support the electroactive materials, NiCo2S4 nanotube arrays on Ni foam were synthesized. A capacitive performance with ultra-high specific capacitance of 14.39 F cm−2 at 5 mA cm−2, long cycle stability, and improved rate capability were achieved even at a high mass loading of 6 mg cm−2.24 The outstanding capacitive performance of Ni–Co sulphides can be attributed to the higher electrochemical activity and the high conductivity of NiCo2S4.23,25 Moreover, the combined contributions from both Ni and Co ions in Ni–Co sulphides provide better redox reactions for electrochemical processes. Despite the extensive research on the supercapacitive performance of Ni–Co sulphides, the preparation of nickel cobalt sulphides with different Ni and Co ratios is still rare. To the best of our knowledge, no work has been performed to explore and then compare the supercapacitive performance of nickel cobalt sulphides with different Ni and Co ratios, despite the fact that nickel and cobalt ratio has a great effect on the capability of nickel cobalt compounds.26,27
Herein, based on a one-pot polyol method, a series of Ni–Co sulphides with different Ni and Co ratios have been prepared. 3 mmol of the stoichiometric Ni(CH3COO)2·4H2O and Co(CH3COO)2·4H2O was used as the precursor to synthesize the Ni–Co sulphides. To facilitate the description, we term the Ni–Co sulphides as Ni–Co–S-n, where n denotes the molar weight of Ni(CH3COO)2·4H2O and n = 0, 1, 1.5, 2 and 3, which signify that the molar ratios of Ni(CH3COO)2·4H2O and Co(CH3COO)2·4H2O are 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, respectively. Due to the peculiar synthesis method, the Ni–Co sulphides are all porous and loosely assembled. The Ni–Co sulphides, especially Ni–Co–S-1.5, exhibit high specific capacitance, excellent rate capability and superior cycle stability, making them promising electroactive materials for supercapacitors.
0, respectively. Due to the peculiar synthesis method, the Ni–Co sulphides are all porous and loosely assembled. The Ni–Co sulphides, especially Ni–Co–S-1.5, exhibit high specific capacitance, excellent rate capability and superior cycle stability, making them promising electroactive materials for supercapacitors.
Experimental section
Synthesis of Ni–Co sulphides
All of the chemicals were of analytical grade and used as received. In a typical synthesis procedure, Ni(CH3COO)2·4H2O and Co(CH3COO)2·4H2O, altogether 3 mmol were first dissolved in 60 ml of ethylene glycol. Then, 6 mmol of thiourea was added to the solution; after stirring for about 20 min, the solution was transferred into an 80 ml Teflon-lined stainless steel autoclave and heated at 200 °C for 6 h. After cooling naturally to ambient temperature, the Ni–Co sulphides were collected and washed several times with deionized water and ethanol. After that, the samples were dried at 60 °C for 24 h.Synthesis of the reduced graphene oxide nanosheets
The reduced graphene (RGO) nanosheets were prepared by the hydrothermal reduction of the graphene oxide (GO) nanosheets; the detailed method is discussed in a previously reported study.24Materials characterization
The Ni–Co sulphides were characterized by XRD (Philips X'Pert Pro; Cu Kα, λ = 0.1542 nm), XPS (Kratos, AXIS-ULTRA DLD-600W), SEM (FEI Quanta 200 and Sirion 200, JEOL JSM–7100F), TEM (JEOL JEM–2100F) equipped with SAED, a N2 adsorption–desorption isotherms (Micromeritics ASAP 2020 analyzer, USA).Electrochemical measurements
For preparation of the working electrode, the as-synthesized Ni–Co sulphides were mixed with acetylene black and poly(tetrafluoroethylene) in a mass ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by adding a small amount of ethanol. The mixture was drop dried into a circular nickel foam (d = 1.2 cm) and then pressed at 10 MPa. The diameter of the circular nickel foam was 1.2 cm, thus its area was 1.13 cm2. For electrochemical evaluations, 6 M KOH was used as the electrolyte, Platinum foil (1 × 1 cm2) was used as the counter electrode and Hg/HgO electrodes were used as the reference electrode. All of the electrochemical properties of the Ni–Co sulphides were measured using a three-electrode glass configuration by a CHI 660D electrochemical workstation. All of the measurements were performed at room temperature without removal of oxygen from the electrolyte. The mass loading of the electroactive materials in each electrode is 2–3 mg. For electrochemical measurements, the electrode was first activated by a few cycles of CV test, and then CV tests at different scan rates and GCD measurements at different current densities were performed. Finally, repeated GCD tests at 10 A g−1 were measured for 2000 cycles to determine the cycle stability. All the electrochemical performance of each sample was measured several times; the electrochemical performance of each sample was documented by taking an average. After 2000 charge and discharge cycles, the Ni1.5Co1.5S4 was separated from the Ni foam by sonication in ethanol and collected via volatilizing the ethanol at 80 °C.
1 by adding a small amount of ethanol. The mixture was drop dried into a circular nickel foam (d = 1.2 cm) and then pressed at 10 MPa. The diameter of the circular nickel foam was 1.2 cm, thus its area was 1.13 cm2. For electrochemical evaluations, 6 M KOH was used as the electrolyte, Platinum foil (1 × 1 cm2) was used as the counter electrode and Hg/HgO electrodes were used as the reference electrode. All of the electrochemical properties of the Ni–Co sulphides were measured using a three-electrode glass configuration by a CHI 660D electrochemical workstation. All of the measurements were performed at room temperature without removal of oxygen from the electrolyte. The mass loading of the electroactive materials in each electrode is 2–3 mg. For electrochemical measurements, the electrode was first activated by a few cycles of CV test, and then CV tests at different scan rates and GCD measurements at different current densities were performed. Finally, repeated GCD tests at 10 A g−1 were measured for 2000 cycles to determine the cycle stability. All the electrochemical performance of each sample was measured several times; the electrochemical performance of each sample was documented by taking an average. After 2000 charge and discharge cycles, the Ni1.5Co1.5S4 was separated from the Ni foam by sonication in ethanol and collected via volatilizing the ethanol at 80 °C.
The electrochemical performance of the asymmetric supercapacitor was measured using a stainless steel two-electrode cell (Hefei Kejing Mater. Technol. CO., Ltd). The mass loading of RGO used in the negative electrode of the asymmetric supercapacitor was determined by balancing the charge storage in the positive and negative electrodes following the equation Q+ = Q−. The charge stored in positive electrode or negative electrode can be calculated by equation: Q = C × ΔV × m, where C represents the specific capacitance of each electrode, ΔV is the potential window of each electrode, and m is the mass loading of each electrode. To balance the charge storage in the positive and negative electrode, the mass ratio of positive electrode and negative electrode is m+/m− = (C− × ΔV−)/(C+ × ΔV+). Therefore, the mass of RGO can be determined by the mass of Ni–Co sulphide and the electrochemical performance of both Ni–Co sulphide and RGO.
Results and discussion
Fig. 1 shows the powder XRD patterns of the nickel cobalt sulphides with different Ni and Co molar ratios. For the sample prepared with the individual precursor Ni(CH3COO)2·4H2O or Co(CH3COO)2·4H2O, all diffraction peaks in Fig. 1 are assigned to cubic Co3S4 (JCPDS 42-1448) or NiS (JCPDS 75-0613), respectively. When Ni(CH3COO)2·4H2O and Co(CH3COO)2·4H2O were simultaneously used as the precursor, the Co seemed to be more prominent in defining the structure of mixed sulfides; all the diffraction peaks in the XRD patterns of Ni–Co–S-1, Ni–Co–S-1.5 and Ni–Co–S-2 samples behaved similar to those in the Co3S4 sample. For Ni–Co–S-1, the overall diffraction peaks can be indexed to the NiCo2S4 phase (JCPDS 43-1477). When the Ni and Co molar ratio is increased to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the sample also possessed nearly the same XRD pattern, except that all the diffraction peaks moved towards the low angle direction. This does not necessarily indicate that the NiS phase is absent from the Ni–Co sulphide samples; we consider that the formation of Co3S4 phase is predominant. When the Ni and Co molar ratio was increased to 2
3, the sample also possessed nearly the same XRD pattern, except that all the diffraction peaks moved towards the low angle direction. This does not necessarily indicate that the NiS phase is absent from the Ni–Co sulphide samples; we consider that the formation of Co3S4 phase is predominant. When the Ni and Co molar ratio was increased to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, except for those peaks that belong to the Co3S4 phase, we observed weak diffraction peaks from the (101) plane and (102) plane diffraction of NiS phase located at 34.6° and 45.8°, respectively, as shown by the arrows in Fig. 1. However, even at a Ni and Co ratio up to 2
1, except for those peaks that belong to the Co3S4 phase, we observed weak diffraction peaks from the (101) plane and (102) plane diffraction of NiS phase located at 34.6° and 45.8°, respectively, as shown by the arrows in Fig. 1. However, even at a Ni and Co ratio up to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the XRD diffraction peaks from NiS phase are still negligible compared to Co3S4 phase, indicating that the sample was almost entirely composed of the Co3S4 phase.
1, the XRD diffraction peaks from NiS phase are still negligible compared to Co3S4 phase, indicating that the sample was almost entirely composed of the Co3S4 phase.
 | ||
| Fig. 1 XRD pattern of the nickel cobalt sulphides; the arrows represent the peaks from the NiS phase in Ni–Co–S-2. | ||
From the XRD pattern of the NiS sample (Fig. 1), it can be found that the NiS shows a different diffraction pattern compared to the Co3S4 phase, and the diffraction peaks of the NiS phase are all sharp and strong. In bimetallic Ni–Co sulphides, no clear diffraction peaks from NiS phase are found, demonstrating that the Ni and Co ions undergo chemical interaction and form the Co3S4 phase. Based on the various reports concerning the preparation of Ni-based or Co-based compounds, it can be found that Ni2+ is considerably stable than the Co2+. Even after the hydrothermal or annealing treatment, Ni ions can maintain the valence of +2 in Ni-based compounds.19,20,28–31 However, in the synthesis process of the Co-based compound, part of the Co2+ ions tend to be oxidized into Co3+, and the coexistence of Co2+ and Co3+ will force the Co-based compounds to demonstrate a Co3O4 or Co3S4 phase.8,15,32–35 Therefore, due to the coexistence of Co2+, Co3+ and S2− in our experiment, Co3S4 is synthesized instead of CoS. In the periodic table, because of the location of Ni (no. 28), adjacent to Co (no. 27), the radii of the Ni and the Co atom are close; hence, Ni ions can partially replace of the Co ions while maintaining the Co3S4 phase. As a result, all the bimetallic Ni–Co sulphide samples share XRD patterns similar to the Co3S4 samples. The mean grain sizes of the Ni–Co sulphides are calculated based on the Scherrer equation. From the (311) diffraction peak of the XRD patterns in Fig. 1, the mean grain sizes of the Co3S4, Ni–Co–S-1, Ni–Co–S-1.5 and Ni–Co–S-2 samples can be calculated to be 3.8 nm, 4.6 nm, 5.1 nm, and 5.4 nm, respectively. From the (102) diffraction peak of the XRD pattern, the mean grain size of the NiS sample can be calculated to be 16.6 nm. Apparently, with increase in Co content, the mean grain sizes of the samples gradually increase, indicating increase in inner structural order of the samples.
To determine the elemental composition and the chemical state of the Ni–Co sulphides in the near-surface range, the Ni–Co–S-1.5 sample was measured by X-ray photoelectron spectroscopy (XPS). Fig. 2a is the survey spectrum of the sample, wherein the attributions of all the peaks have been marked. Evidently, all the peaks can be ascribed to the elements Ni, Co, S, O, C, and N. The C (as reference), O, N elements can be attributed to the exposure of air. Therefore, the chemical composition in the near-surface range of the product is Ni, Co, and S, which is in good agreement with our experiment. No other element is found from the XPS spectrum, indicating the high purity of the sample. The high-resolution Co 2p, Ni 2p and S 2p spectra were fitted by the Gaussian fitting method, and both of them can be best fitted with two spin–orbit doublets and two shake-up satellites (marked “Sat.”). For the Co 2p XPS spectrum, the first doublet is located at 778.7 and 793.7 eV, whereas the second is situated at 781.3 and 797.2 eV, corresponding to a spin–orbit splitting value of Co 2p1/2 and Co 2p3/2 of 15.0 eV and 15.9 eV, can be ascribed to Co3+ and Co2+, respectively.23 As regards the Ni 2p XPS spectrum, the binding energy at 853.3 eV in Ni 2p3/2 and 870.6 eV in Ni 2p1/2 corresponds to the spin–orbit characteristic of Ni2+, and the binding energy at 856.2 eV in Ni 2p3/2 and 874.0 eV in Ni 2p1/2 are characteristic of Ni3+.36 Clearly, except for the divalent state, a portion of Ni atoms in Ni–Co–S-1.5 show a trivalent state, suggesting that the coordination mode of Ni ions with S2− has altered compared to the NiS sample, which is consistent with the XRD results. The location of Ni at the Co3+ site in the Co3S4 phase may compel Ni2+ to lose one electron and show a valence of +3. In the S 2p spectrum, the peaks at 161.5 eV and 162.6 eV correspond to the S 2p3/2 and S 2p1/2, respectively, which is typical of the coordination of sulphur ions with metal ions. According to the XPS analysis, the near-surface composition of the Ni–Co–S-1.5 sample is composed of Co2+, Co3+, Ni2+, Ni3+ and S2−. Therefore, the formula of Ni–Co–S-1.5 can be expressed as Ni1.5Co1.5S4. Similarly, the Ni–Co–S-1 and Ni–Co–S-2 samples can be expressed as NiCo2S4 and Ni2CoS4, respectively.
Based on the above analysis, it is clear that Ni–Co sulphides have been successfully prepared by a simple polyol method, in which Ni(CH3COO)2·4H2O and Co(CH3COO)2·4H2O are used to provide the Ni2+ and Co2+, respectively, ethylene glycol serves as the solvent and thiourea is used as the sulfur source for the generation of S2−. The relevant chemical reactions involved in the preparation of Ni–Co sulphides can be expressed as follows. For Co3S4, Ni–Co–S-1, Ni–Co–S-1.5 and Ni–Co–S-2 samples, the equation is as follows:
 | (1) |
| Ni2+ + S2− = NiS | (2) |
The morphology and structure of the as-synthesized Ni–Co sulphides were characterized by scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HRTEM). Fig. 3 shows the SEM images of the Ni–Co sulphides with different Ni and Co molar ratios. The Ni–Co sulphides have high similarity in morphology and structure in spite of the different Ni and Co molar ratios in their components. All the samples are composed of numerous interconnected particles. The diameters of these particles range from hundreds of nanometres to several micrometres. As the Ni and Co molar ratio varies, the Ni–Co sulphide samples only slightly change in nanoparticles size without a clear change in morphology and structure. The detailed morphology and structural features of the samples were further demonstrated by TEM measurement; the corresponding results are shown in Fig. 4. It is identical to the SEM measurements; the Ni–Co sulphides under different Ni and Co content show similar morphology and structure. Interestingly, the particles of the Ni–Co sulphides are porous and loosely assembled. The as-synthesized samples are so loose that even for particle diameter up to 200 nm, electrons can still penetrate through the samples while TEM measurements are being taken. At higher magnification of the TEM images (the inset of Fig. 4a–e), it is can be found that all the Ni–Co sulphide samples are composed of numerous interconnected nanoparticles. To determine the elemental distribution of the sample, the elemental mapping of the Ni, Co and S elements of the Ni1.5Co1.5S4 sample was measured using a SEM equipped with elemental mapping, and the result is shown in Fig. 4f. Evidently, the Ni, Co and S elements are uniformly distributed in the sample, demonstrating the coexistence of the Ni and Co ions in the Ni–Co sulphides.
 | ||
| Fig. 3 SEM images of the Ni–Co sulphides: (a and b) Co3S4, (c and d) Ni–Co–S-1, (e and f) Ni–Co–S-1.5, (g and h) Ni–Co–S-2, (i and j) NiS. | ||
Selected area electron diffraction (SAED) measurement was also employed to further determine the crystalline phase and the purity of the Ni–Co sulphides. All the SAED patterns exhibit well-defined diffraction rings, indicating the polycrystalline nature of all the samples. Apparently, the Ni–Co–S-1, Ni–Co–S-1.5 and Ni–Co–S-2 samples show the same SAED pattern as the Co3S4 sample, demonstrating their high similarity in crystal structure, which in good agreement with the XRD results. The diffraction rings in Fig. 5a–d can be entirely assigned to the (220), (311), (400), (511), (440) planes of the Co3S4 phase. From the SAED pattern in Fig. 5a–d, no observable diffraction ring contributing to the NiS phase is found, indicating that the samples is almost entirely composed of the Co3S4 phase. The NiS sample exhibits a different SAED pattern; as shown in Fig. 5e, and the diffraction rings can be indexed to the (100), (101), (102), (110) planes of NiS, matching well with the XRD results.
 | ||
| Fig. 5 SAED patterns of the Ni–Co sulphides: (a) Co3S4, (b) Ni–Co–S-1, (c) Ni–Co–S-1.5, (d) Ni–Co–S-2, (e) NiS. | ||
To investigate the porous characteristics of the Ni–Co sulphides, the samples were characterized using a nitrogen adsorption–desorption isotherm; the corresponding results are shown in Fig. 6. The hysteresis loops at a relative pressure of 0.6–1.0 demonstrate that all the isotherms can be categorized as type IV, indicating the presence of mesoporous structures in the Ni–Co sulphide samples. According to the Barrett–Joyner–Halenda (BJH) method, the pore size distribution curves can be calculated from the desorption branch of the isotherms (the inset in Fig. 6), which demonstrates that the pores in the sample were mesopores and macropores with a broad pore-size distribution. The average pore sizes of Ni–Co–S-0, Ni–Co–S-1, Ni–Co–S-1.5, Ni–Co–S-2 and Ni–Co–S-3 samples are 17.8, 7.5, 17.4, 6.4 and 8.3 nm, respectively, indicating that the Co3S4 and Ni1.5Co1.5S4 samples show considerably higher average pore size than the NiCo2S4, Ni2CoS4 and NiS samples. The Brunauer–Emmett–Teller (BET) areas of the Ni–Co–S-0, Ni–Co–S-1, Ni–Co–S-1.5, Ni–Co–S-2 and Ni–Co–S-3 samples are 21.3, 15.8, 19.5, 9.6 and 4.2 m2 g−1, respectively. Therefore, except for the Co3S4 sample, the Ni1.5Co1.5S4 shows considerably higher BET area than the Ni–Co sulphides for other Ni and Co content. The porous structure is beneficial for the Ni–Co sulphides to provide more electroactive sites and facilitate complete contact with the electrolyte. The relatively high size of the pores is beneficial for the fast diffusion of the electrolyte ions into electroactive materials. Therefore, superior electrochemical performance can be expected for the as-synthesized Ni–Co sulphides when used as electrode materials for supercapacitors.
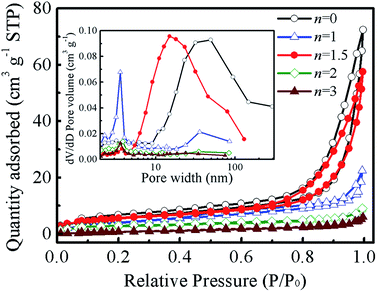 | ||
| Fig. 6 Nitrogen adsorption–desorption isotherm and pore-size distribution curves (inset) of the Ni–Co–S-n. | ||
The electrochemical properties of the nickel cobalt sulphides were evaluated by CV and GCD measurements in a three-electrode configuration with a Hg/HgO reference electrode in 6 M KOH aqueous electrolyte. Fig. 7a shows the representative CV curves of the nickel cobalt sulphides in a potential window ranging from 0 to 0.6 V at a scan rate of 10 mV s−1. Apparently, a pair of redox peaks can be positively observed in the CV curves of Ni–Co–S-1, Ni–Co–S-1.5, Ni–Co–S-2, NiS, and two pairs is found for the Co3S4 sample, indicating the faradaic pseudocapacitance nature of the nickel cobalt sulphides in the electrochemical process. The redox peaks can be attributed to redox reactions as follows:24,37
| NixCo3−xS4 + 2OH− ↔ NixS4−yOH + (3 − x)CoSyOH + 2e− | (3) |
| CoSyOH + OH− ↔ CoSyO + H2O + e− | (4) |
| NiS + OH− ↔ NiSOH + e− | (5) |
The theoretical value of a pseudo-capacitive electrode material can be calculated according to the formula.
| C = (n × F)/(M × V) | (6) |
From CV curves of the Ni–Co sulphides in Fig. 7a, it is also found that the location of the redox peaks shift to a more cathodic potential with the increase of Ni content, which results from the relatively higher potential of redox reaction between NiS and NiSOH, as described in eqn (5). The integrated area of the Ni–Co sulphides changed as the Ni and Co content varied. In general, the integrated area of an electrode material is proportional to its capacitance performance. Therefore, our work provides an effective method to optimize the supercapacitive performance of the sulphides by tuning the transition-metal composition. In particular, Ni–Co–S-1.5 shows the highest integrated area, signifying the highest specific capacitance. The maximum specific capacitance of the Ni–Co–S-1.5 can further be proven by the GCD curves at a current density of 5 A g−1 (Fig. 7b). The Ni–Co–S-1.5 shows the highest charge and discharge times compared to those of monometallic Co and Ni sulphides and bimetallic NixCo3−x sulphides (x = 1, 2), demonstrating its highest specific capacitance.
Based on the GCD measurements at different current densities, specific capacitance can be calculated. Fig. 7c shows the specific capacitance versus current density curves of the Ni–Co sulphides. The Ni–Co–S-1.5 exhibits the highest specific capacitance at all measured current densities compared to NiS, Ni–Co–S-1, Ni–Co–S-2 and Co3S4, which is in good agreement with the analysis of CV and GCD curves in Fig. 7a and b. Based on the above analysis, the NixCo3−xS4 (x = 0, 1, 1.5, 2) possess similar theoretical capacitances even at different Ni and Co ratios. The Ni1.5Co1.5S4 shows considerably higher specific capacitance than the Ni2CoS4 in Fig. 7c, which can be attributed to the increased specific surface and improved electrochemical activity due to the increase of Co content. In general, a certain amount of Co is beneficial to improve the supercapacitive performance in Ni–Co based compounds. However, except for the influence of specific surface, Co ions seem to be more electrochemically inactive than Ni ions; therefore, too much Co in Ni–Co sulphides also harms their specific capacitance. The NixCo3−xS4 (x = 0, 1, 1.5) samples deliver gradually decreased specific capacitance as the Co content increases, as shown in Fig. 7c. Since NiCo2S4 was first reported as the electroactive materials for supercapacitors by our research group,23 we and a few other research groups have focused on Ni–Co sulphides as electroactive materials for supercapacitors. Except for NiCo2S4, the supercapacitive properties of Ni2CoS4 have also been explored.37,38 However, to the best of our knowledge, the supercapacitive performance of Ni1.5Co1.5 sulphide has not been explored, despite the fact revealed by this work that the Ni1.5Co1.5 sulphide delivers a considerably higher supercapacitive performance than the NixCo3−x sulphide (x = 1, 2). A specific capacitance up to 1093 F g−1 is achieved for Ni–Co–S-1.5 at 1 A g−1, which is higher than the monometallic sulphide Co3S4, NiS and the bimetallic sulphides Ni–Co–S-1, Ni–Co–S-2, and also considerably higher than the various reports about Ni–Co sulphides, including CoNi2S4 nanoparticles,38 NiCo2S4 nanoplates,39 NiCo2S4 nanotube arrays on nickel foam,40 and NixCo3−xS4 hollow nanoprisms.41 Moreover, the specific capacitance is much higher than various Ni–Co based compounds, such as NiCo2O4,26,42–44 Ni–Co hydroxides,45,46 and also considerably higher than some reported ternary compounds such as NiWO46 and CoMoO4.47,48 The high specific capacitance demonstrates the superior capacitive performance of the as-synthesized Ni–Co sulphides. When the current density increases 50 times to 50 A g−1, the specific capacitance of Ni–Co–S-1.5 can still retain 750 F g−1, corresponding to a capacitance retention of 69%, demonstrating its high rate capability.
The rate capability of the as-synthesized Ni–Co sulphides is further compared by capacitance retention versus current density curves in Fig. 7d. After a 50-fold increase in current density, the capacitance retention of the Co3S4, Ni–Co–S-1, Ni–Co–S-1.5, Ni–Co–S-2, NiS are 40%, 69%, 69%, 58%, 42%, respectively. Apparently, the bimetallic nickel cobalt sulphides Ni–Co–S-1, Ni–Co–S-1.5, Ni–Co–S-2 possess improved rate capability than the monometallic nickel and cobalt sulphide, demonstrating that the coexistence of Co ions and Ni ions contributes to the improvement of the rate capability. Tuning the Ni and Co content can tune the electrochemical performance; this phenomenon commonly exists in various Ni–Co compounds such as Ni–Co oxides26,27 and Ni–Co hydroxides.49,50 However, this is the first effort to tune the capability of the Ni–Co sulphides. The improvement of the supercapacitive performance can be ascribed to the increased charging efficiency due to improved electrical conductivity and increased electroactive sites due to possible valence interchange or charge hopping between Co and Ni cations.49,50
Electrochemical impedance spectroscopy (EIS) was used to evaluate the electrical resistance responses of the Ni–Co sulphides. The EIS measurements were performed at open-circuit potential in the frequency region of 100 kHz to 0.01 Hz with an ac perturbation of 5 mV. Fig. 7e shows the Nyquist plots of the Ni–Co sulphides. Clearly, all the curves consist of a semicircle in the high-frequency region and a straight line at the low-frequency region. The bimetallic NixCo3−x sulphides (x = 1, 1.5, 2) show a smaller semicircle in the high-frequent region, demonstrating their lower charge-transfer resistance, which can best explain the higher rate capability of the bimetallic Ni–Co sulphides. The EIS spectra are fitted based on the equivalent circuit model in the inset of Fig. 7e, wherein Rs is the bulk solution resistance, Rct is the charge-transfer resistance, Cdl is the double-layer capacitance, and W is the Warburg resistance. The Rct of the Ni–Co–S-0, Ni–Co–S-1, Ni–Co–S-1.5, Ni–Co–S-2 and Ni–Co–S-3 are 0.24 Ω, 0.07 Ω, 0.06 Ω, 0.09 Ω and 0.42 Ω, respectively. Apparently, the bimetallic Ni–Co sulphides show considerably lower charge-transfer resistance than the monometallic sulphides Co3S4 and NiS, demonstrating that the coexistence of Ni and Co ions in the sulphides contribute to the reduction of the charge-transfer resistance. The low charge-transfer resistance can be attributed to the high conductivity of the bimetallic sulphides.23,25 High charge transfer conductivity facilitates the fast transfer of electrons in the charge–discharge process, giving rise to the high rate capability of the bimetallic sulphides. In particular, the Ni–Co–S-1.5 sample shows the lowest charge transfer conductivity, indicating its superior supercapacitive performance. Moreover, the bimetallic NixCo3−x sulphides (x = 1, 1.5, 2) exhibit a closer to vertical asymptote compared with NiS and Co3S4 samples, signifying their better electrochemical capacitance. In particular, Ni1.5Co1.5S4 exhibits the highest slope, demonstrating that Ni1.5Co1.5S4 possesses the highest capacitive performance, which is in good agreement with the specific capacitance in Fig. 7c.
As long cycle life is of vital importance for supercapacitor applications, the cycle stability was measured by repeating GCD test at a current density of 10 A g−1, as shown in Fig. 7f. Instead of the performance decay in various sulphides, the specific capacitance of Ni–Co–S-1.5 and Ni–Co–S-2 increase in the first few hundred cycles and then tend towards stability in the subsequent cycles. After 2000 cycles, the specific capacitances of Ni–Co–S-1.5, Ni–Co–S-2 still retain 108% and 104% of the initial specific capacitance, respectively. Fig. 7g shows the first and last 5 cycles of the Ni1.5Co1.5S4 during a 2000 cycle long-term cycling test. The increase in the charge and discharge time can further demonstrate the increase in the capacitive performance. For Ni–Co–S-1, after 2000 cycles, the specific capacitance can still retain 97%, which is still remarkable. The cycling stability of the as-synthesized Ni–Co sulphides is even higher than various reported ternary compounds such as NiCo2O4 (ref. 51) and Ni3V2O8.5 The specific capacitance of the NiS sample can only maintain 89% after 2000 cycles. Clearly, bimetallic Ni–Co sulphides show superior cycle stability compared to NiS. In general, the sulphides, especially NiS, deliver a poor cycling performance during cycling. However, the sulphides in our work show remarkable electrochemical cycle stability, which is favourable for their practical applications.
After 2000 charge and discharge cycles, the Ni1.5Co1.5S4 sample was also characterized to demonstrate the evolution of the Ni–Co sulphides during the long-term cycling. Fig. 8a shows the SEM image of the Ni1.5Co1.5S4 after 2000 cycles. The sample is composed of numerous particles, which is considerably larger than the sample before cycling. Clearly, the Ni1.5Co1.5S4 sample has been agglomerated during the long-term cycling, which can be further confirmed by the TEM measurement, as shown in Fig. 8b. Interestingly, at high magnification, it is found that the surface of these particles is covered by various nanosheets. Electroactive materials can be gradually activated during the repeated charge–discharge process. According to some previous reports, the activity of the electroactive material usually involves the erosion of the electroactive materials' surface and the formation of new structure on the surface. For example, Yu et al.52 synthesized a Co9S8 nanotube and found the surface covered with a layer of nanosheets after long-term cycling. Li et al.53 prepared a Co3O4@NiCo2O4 hybrid structure and found the hybrid electrode to be covered by a uniform wrapping after 2000 charge–discharge cycles. The newly formed nanosheets on the surface of the Ni1.5Co1.5S4 may provide more electroactive sites, resulting in increased specific capacitance during the long-term cycling (Fig. 7f). The electrochemical properties of Ni1.5Co1.5S4 were also measured by CV and EIS tests before and after 2000 charge–discharge cycles to demonstrate the evolution in electrochemical performance of Ni–Co sulphides during long-term cycling. The electrochemical performance of Ni and Co ions in Ni–Co sulphides usually varies during a long-term cycling process. For example, in the urchin-like NiCo2S4 sample prepared via a precursor transformation method,23 the redox peaks from both of Ni and Co ions significant changed after 5000 cycles. However, the CV curves (Fig. 9a) of the as-synthesized Ni1.5Co1.5S4 are highly similar in shape before and after 2000 charge–discharge cycles. Moreover, the location of the redox peaks did not obviously vary. These results can all demonstrate the high stability of the Ni1.5Co1.5S4 during long-term cycling. After 2000 charge–discharge cycles, the integral area of the CV curves slightly increased, indicating an increase of specific capacitance, which is in good agreement with the cycling performances of the Ni1.5Co1.5S4 as shown in Fig. 7f. Fig. 9b shows the Nyquist plots of the Ni1.5Co1.5S4 before and after 2000 charge–discharge cycles. It is can be found that the curve becomes more vertical in the low-frequency range after 2000 cycles, indicating that the electroactive material becomes more capacitive after long-term cycling, which further confirms the increase in the specific capacitance of the Ni1.5Co1.5S4 during cycling.
 | ||
| Fig. 8 (a) SEM and (b–d) TEM images of the Ni1.5Co1.5S4 measured after 2000 charge and discharge cycles. | ||
 | ||
| Fig. 9 (a) CV curves and (b) EIS curves of the Ni1.5Co1.5S4 measured before and after 2000 charge and discharge cycles. | ||
To evaluate the capacitive performance of the Ni–Co sulphides in practical applications, the Ni1.5Co1.5S4 sample was used as the positive electrode to assemble asymmetric supercapacitors, wherein the RGO was used as the negative electrode and 6 M KOH was used as electrolyte. Fig. 10a shows the CV curves of the Ni1.5Co1.5S4 and RGO at a scan rate of 5 mV s−1. The potential window of RGO is 10 V, and the potential window of Ni1.5Co1.5S4 electrode is 0.55 V. When the potential is higher than 0.55 V, the Ni1.5Co1.5S4 electrode starts to release oxygen, as demonstrated by the rapid increase in current density as shown in Fig. 10a. Based on the specific capacitances and the potential windows of the CV curves determined from Fig. 10a, the mass ratio of Ni1.5Co1.5S4 and RGO is determined to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.64. Owing to the different working potential of the Ni1.5Co1.5S4 and RGO (Fig 10a), the voltage of the Ni1.5Co1.5S4//RGO asymmetric supercapacitor can be extended to 1.55 V, which can be further demonstrated by the CV and GCD curves in Fig. 10b and c, respectively. The specific capacitances of the asymmetric supercapacitors can be calculated from the GCD curves. The current densities and specific capacitances are calculated relative to the total mass of the active materials in two electrodes. Even so, a specific capacitance up to 113 F g−1 can still be attained at 1 A g−1. After 20-fold increase in current density, the specific capacitance retains 64 F g−1, corresponding to 56.6% of the initial value. The energy density and power density of the asymmetric supercapacitor can be calculated according to the GCD measurement, and the result is shown in Fig. 10e. An energy density up to 37.6 W h kg−1 is obtained at a power density of 775 W kg−1. After the power density increased to 23.25 kW kg−1, the energy density can still retain 17.7 W h kg−1, indicating high power performance of the asymmetric supercapacitors. The Ni1.5Co1.5S4//RGO asymmetric supercapacitor delivers considerably higher energy density than the NiCo2S4 nanotube arrays on Ni foam24 and porous NiCo2O4 flower-like nanostructures42 assembled asymmetric supercapacitors, which all use the same method and prepared RGO as negative electrode, indicating superior capacitive performance of the Ni1.5Co1.5S4. In addition, the long-term cycling stability is also measured by repeated charge–discharge test. After 5000 cycles, the asymmetric supercapacitor only shows a slight decrease in discharge time (the inset of Fig. 10f), and 90.5% of the initial capability can still be retained, indicating excellent cycling stability of the Ni1.5Co1.5S4//RGO asymmetric supercapacitor.
2.64. Owing to the different working potential of the Ni1.5Co1.5S4 and RGO (Fig 10a), the voltage of the Ni1.5Co1.5S4//RGO asymmetric supercapacitor can be extended to 1.55 V, which can be further demonstrated by the CV and GCD curves in Fig. 10b and c, respectively. The specific capacitances of the asymmetric supercapacitors can be calculated from the GCD curves. The current densities and specific capacitances are calculated relative to the total mass of the active materials in two electrodes. Even so, a specific capacitance up to 113 F g−1 can still be attained at 1 A g−1. After 20-fold increase in current density, the specific capacitance retains 64 F g−1, corresponding to 56.6% of the initial value. The energy density and power density of the asymmetric supercapacitor can be calculated according to the GCD measurement, and the result is shown in Fig. 10e. An energy density up to 37.6 W h kg−1 is obtained at a power density of 775 W kg−1. After the power density increased to 23.25 kW kg−1, the energy density can still retain 17.7 W h kg−1, indicating high power performance of the asymmetric supercapacitors. The Ni1.5Co1.5S4//RGO asymmetric supercapacitor delivers considerably higher energy density than the NiCo2S4 nanotube arrays on Ni foam24 and porous NiCo2O4 flower-like nanostructures42 assembled asymmetric supercapacitors, which all use the same method and prepared RGO as negative electrode, indicating superior capacitive performance of the Ni1.5Co1.5S4. In addition, the long-term cycling stability is also measured by repeated charge–discharge test. After 5000 cycles, the asymmetric supercapacitor only shows a slight decrease in discharge time (the inset of Fig. 10f), and 90.5% of the initial capability can still be retained, indicating excellent cycling stability of the Ni1.5Co1.5S4//RGO asymmetric supercapacitor.
Conclusions
In summary, a series of Ni–Co sulphides with different Ni and Co content have been prepared by a simple polyol method. All the Ni–Co sulphides are composed of numerous interconnected particles, which are porous and loosely assembled. The bimetallic Ni–Co sulphides, especially for Ni1.5Co1.5S4, show superior pseudocapacitance than monometallic Co and Ni sulphides in terms of high specific capacitance, excellent rate capability and long cycle stability. This work demonstrates that the coexistence of nickel and cobalt ions and their appropriate content are vital for superior pseudocapacitive performance. Therefore, it is feasible to explore high performance supercapacitors by engineering electroactive materials with multicomponent and optimize their electrochemical performance by tuning the content of every component.Acknowledgements
This work is financial supported by the National Natural Science Foundation of China (no. 51302097) and Wuhan Planning Project of Science and Technology (no. 2013011801010594). The authors also thank the Analysis and Testing Center of HUST for samples testing support.Notes and references
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845 CrossRef CAS PubMed.
- B. E. Conway, in Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications, Kluwer Acaemic/Plenum Press, New York, 1999 Search PubMed.
- G. Wang, L. Zhang and J. Zhan, Chem. Soc. Rev., 2012, 41, 797 RSC.
- K. Wang, H. Wu, Y. Meng and Z. Wei, Small, 2014, 10, 14 CrossRef CAS PubMed.
- M. C. Liu, L. B. Kong, L. Kang, X. Li, F. C. Walsh, M. Xing, C. Lu, X. J. Ma and Y. C. Luo, J. Mater. Chem. A, 2014, 2, 4919 CAS.
- L. Y. Niu, Z. Li, Y. Xu, J. Sun, W. Hong, X. Liu, J. Wang and S. Yang, ACS Appl. Mater. Interfaces, 2013, 5, 8044 CAS.
- H. Wang, Y. Liang, Y. Li and H. Dai, Angew. Chem. Int., Ed., 2011, 50, 10969 CrossRef CAS PubMed.
- N. Mahmood, C. Zhang, J. Jiang, F. Liu and Y. Hou, Chem.–Eur. J., 2013, 19, 5183 CrossRef CAS PubMed.
- M. K. Wang, A. M. Anghel, B. Marsan, N. L. C. Ha, N. Pootrakulchote, S. M. Zakeeruddin and M. Gratzel, J. Am. Chem. Soc., 2009, 131, 15967 Search PubMed.
- J. Tang, Z. Huo, S. Brittman, H. Gao and P. Yang, Nat. Nanotechnol., 2011, 6, 568 CrossRef CAS PubMed.
- T. Dufaux, J. Boettcher, M. Burghard and K. Kern, Small, 2010, 6, 1868 CrossRef CAS PubMed.
- K. Chang and W. Chen, ACS Nano, 2011, 5, 4720 CrossRef CAS PubMed.
- D. D. Vaughn, O. D. Hentz, S. Chen, D. H. Wang and R. E. Schaak, Chem. Commun., 2012, 48, 5608 RSC.
- Z. Wang, L. Pan, H. Hu and S. Zhao, CrystEngComm, 2010, 12, 1899 RSC.
- Q. Wang, L. Jiao, H. Du, Y. Si, Y. Wang and H. Yuan, J. Mater. Chem., 2012, 22, 21387 RSC.
- F. Tao, Y. Q. Zhao, G. Q. Zhang and H. L. Li, Electrochem. Commun., 2007, 9, 1282 CrossRef CAS PubMed.
- S. Peng, L. Li, H. Tan, R. Cai, W. Shi, C. Li, S. G. Mhaisalkar, M. Srinivasan, S. Ramakrishna and Q. Yan, Adv. Funct. Mater., 2014, 24, 2155 CrossRef CAS.
- X. Xia, C. Zhu, J. Luo, Z. Zeng, C. Guan, C. F. Ng, H. Zhang and H. J. Fan, Small, 2014, 10, 766 CrossRef CAS PubMed.
- T. Zhu, Z. Wang, S. Ding, J. S. Chen and X. W. Lou, RSC Adv., 2011, 1, 397 RSC.
- J. Yang, X. Duan, Q. Qin and W. Zheng, J. Mater. Chem. A, 2013, 1, 7880 CAS.
- S. Ratha and C. S. Rout, ACS Appl. Mater. Interfaces, 2013, 5, 11427 CAS.
- T. Zhu, B. Xia, L. Zhou and X. W. Lou, J. Mater. Chem., 2012, 22, 7851 RSC.
- H. C. Chen, J. J. Jiang, L. Zhang, H. Z. Wan, T. Qi and D. D. Xia, Nanoscale, 2013, 5, 8879 RSC.
- H. C. Chen, J. J. Jiang, L. Zhang, D. D. Xia, Y. D. Zhao, D. Q. Guo, T. Qi and H. Z. Wan, J. Power Sources, 2014, 254, 249 CrossRef CAS PubMed.
- J. Xiao, L. Wan, S. Yang, F. Xiao and S. Wang, Nano Lett., 2014, 14, 831 CrossRef CAS PubMed.
- H. Wang, Q. Gao and L. Jiang, Small, 2011, 7, 2454 CAS.
- J. Chang, J. Sun, C. Xu, H. Xu and L. Gao, Nanoscale, 2012, 4, 6786 RSC.
- J. W. Lang, L. B. Kong, W. J. Wu, Y. C. Luo and L. Kang, Chem. Commun., 2008, 35, 4213 RSC.
- S. Vijayakumar, S. Nagamuthu and G. Muralidharan, ACS Appl. Mater. Interfaces, 2013, 5, 2188 CAS.
- B. Wang, J. S. Chen, Z. Wang, S. Madhavi and X. W. Lou, Adv. Energy Mater., 2012, 2, 1188 CrossRef CAS.
- J. Yang, X. Duan, W. Guo, D. Li, H. Zhang and W. Zheng, Nano Energy, 2014, 5, 74 CrossRef CAS PubMed.
- X. Wang, X. L. Wu, Y. G. Guo, Y. Zhong, X. Cao, Y. Ma and J. Yao, Adv. Funct. Mater., 2010, 20, 1680 CrossRef CAS.
- W. Du, R. Liu, Y. Jiang, Q. Lu, Y. Fan and F. Gao, J. Power Sources, 2013, 227, 101 CrossRef CAS PubMed.
- Q. Chen, H. Li, C. Cai, S. Yang, K. Huang, X. Wei and J. Zhong, RSC Adv., 2013, 3, 22922 RSC.
- A. N. Grace, R. Ramachandran, M. Vinoba, S. Y. Choi, D. H. Chu, Y. Yoon, S. C. Nam and S. K. Jeong, Electroanalysis, 2014, 26, 199 CrossRef CAS.
- Q. Liu, J. Jin and J. Zhang, ACS Appl. Mater. Interfaces, 2013, 5, 5002 CAS.
- L. Mei, T. Yang, C. Xu, M. Zhang, L. Chen, Q. Li and T. Wang, Nano Energy, 2014, 3, 36 CrossRef CAS PubMed.
- W. Du, Z. Zhu, Y. Wang, J. Liu, W. Yang, X. Qian and H. Pang, RSC Adv., 2014, 4, 6998 RSC.
- J. Pu, F. Cui, S. Chu, T. Wang, E. Sheng and Z. Wang, ACS Sustainable Chem. Eng., 2014, 2, 809 CrossRef CAS.
- J. Pu, T. Wang, H. Wang, Y. Tong, C. Lu, W. Kong and Z. Wang, ChemPlusChem, 2014, 79, 577 CrossRef CAS.
- L. Yu, L. Zhang, H. B. Wu and X. W. Lou, Angew. Chem., 2014, 126, 3785 CrossRef.
- H. C. Chen, J. J. Jiang, L. Zhang, T. Qi, D. D. Xia and H. Z. Wan, J. Power Sources, 2014, 248, 28 CrossRef CAS PubMed.
- H. Jiang, J. Ma and C. Z. Li, Chem. Commun., 2012, 48, 4465 RSC.
- X. Y. Liu, Y. Q. Zhang, X. H. Xia, S. J. Shi, Y. Lu, X. L. Wang, C. D. Gu and J. P. Tu, J. Power Sources, 2013, 239, 157 CrossRef CAS PubMed.
- R. R. Salunkhe, K. Jang, S. W. Lee and H. Ahn, RSC Adv., 2012, 2, 3190 RSC.
- B. P. Bastakoti, Y. Kamachi, H. S. Huang, L. C. Chen, K. C. W. Wu and Y. Yamauchi, Eur. J. Inorg. Chem., 2013, 1, 39 CrossRef.
- M. C. Liu, L. B. Kong, X. J. Ma, C. Lu, X. M. Li, Y. C. Luo and L. Kang, New J. Chem., 2012, 36, 1713 RSC.
- M. C. Liu, L. B. Kong, C. Lu, X. M. Li, Y. C. Luo and L. Kang, Mater. Lett., 2013, 94, 197 CrossRef CAS PubMed.
- Y. Cheng, H. Zhang, C. V. Varanasi and J. Liu, Energy Environ. Sci., 2013, 6, 3314 CAS.
- X. Liu, R. Ma, Y. Bando and T. Sasaki, Adv. Mater., 2012, 24, 2148 CrossRef CAS PubMed.
- M. C. Liu, L. B. Kong, C. Lu, X. M. Li, Y. C. Luo, L. Kang, X. Li and F. C. Walsh, J. Electrochem. Soc., 2012, 159, A1262 CrossRef CAS PubMed.
- J. Yu, H. Wan, J. Jiang, Y. Ruan, L. Miao, L. Zhang, D. Xia and K. Xu, J. Electrochem. Soc., 2014, 161, A996 CrossRef CAS PubMed.
- Y. Li, Y. Zhang, Y. Li, Z. Wang, H. Fu, X. Zhang, Y. Chen, H. Zhang and X. Li, Electrochim. Acta, 2014, 145, 177 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The first 50 CV cycles of the Ni–Co–S-1.5 and Co3S4. See DOI: 10.1039/c4ta04420g |
| This journal is © The Royal Society of Chemistry 2015 |




