Ag3O4 grafted NiO nanosheets for high performance supercapacitors†
Inbamani Manohara
Babu
ab,
Kamatchi Kamaraj
Purushothaman
*b and
Gopalan
Muralidharan
a
aDepartment of Physics, Gandhigram Rural University, Gandhigram-624302, Tamilnadu, India
bDepartment of Physics, TRP Engineering College (SRM Group), Irungalur, Tamilnadu, India. E-mail: purushoth_gri@yahoo.co.in; Tel: +91-431-2908050
First published on 31st October 2014
Abstract
Ag3O4 grafted NiO nanosheets were successfully synthesized via hydrothermal route. The morphological and structural analysis was performed using scanning transmission electron microscopy (STEM), scanning electron microscopy (SEM) and X-ray diffraction (XRD) studies. The grafting of Ag3O4 on NiO nanosheets enhanced the conductivity of the nanosheets and resulted in a maximum specific capacitance of 1504 F g−1 for 5 wt% of Ag on NiO (AGN2) at a scan rate of 3 mV s−1. The porous nature of AGN2 provides larger paths for ions, which significantly increases the intercalation of ions and utilization rate of electrode materials. Galvanostatic charge discharge (GCD) analysis reveals a specific capacitance of 1524 F g−1 for AGN2 at a current density of 1 A g−1 with excellent cyclic stability (12% loss after 1500 cycles). An asymmetric supercapacitor device (AGN2//RGO) achieved a specific capacitance of 274 F g−1 with an energy density of 37 Wh kg−1 and the power density of 499 W kg−1 at a discharge current of 0.2 A g−1.
1. Introduction
Over the past decades, the energy demand has tremendously increased due to increase in population, vehicles and energy based appliances, which results in a huge utilization of fossil fuels, CO2 emissions and environmental issues. To overcome such issues, an advanced energy conversion, as well as storage device is needed. Recent interest has focused towards electrochemical capacitors (ECs, also called supercapacitors (SC)), as a type of efficient energy storage device, which fills the gap between batteries and conventional capacitors. Supercapacitors exhibit a long cycle life (up to 104 cycles),1–3 excellent charge–discharge capability and environment friendly nature. These qualities make them an ideal choice for a wide range of potential applications like electric vehicles, short-term power sources for mobile electronic devices and other renewable energy storage systems.4–9Supercapacitors are classified as: pseudocapacitors and EDLC's (Electric Double Layer Capacitors – carbon based capacitors). A pseudocapacitive electrode material undergoes Faradaic redox reactions, which delivers higher energy density compared to carbon based electrode materials (non-faradaic reactions). Among the transition metal oxides (RuO2, NiO, Co3O4 and MnO2),10 nickel oxide (NiO) is one of the most important electrode material owing to its low cost, abundance, and good electrochemical capacitance (2584 F g−1 within 0.5 V).11 However, a low electrical conductivity and crystal shrinkage/expansion during the cycling process retards their motion towards practical applications. Both the problems should be addressed to produce commercially useful supercapacitors. To overcome this barrier two strategies may be adopted (i) form mixed transition metal oxides (NiO–TiO2, NiCo2O4, NiCo2O4@MnO2, Nix Co1−xO, Ni–Cu hydroxides, NiO–CdS),12–17 and carbon based (CNT, graphene)18,19 composites and (ii) a selective conductive coating on the electrode material. Cheng et al.20 reported that the incorporation of Ag on Co3O4 enhances the conductivity and results in a higher specific capacitance. MnO2–Au nanoporous electrode delivers an excellent rate performance.21 Au decorated NiO was reported to exhibit an excellent electrochemical performance.22 Silver oxide is an interesting material due to its multiple oxidation states (Ag+, Ag2+, Ag3+)23 with good electrical conductivity. Among the oxides of silver, Ag3O4 is an interesting candidate due to its mixed valance states, i.e., silver oxide (Ag(III) and Ag(II)) with a composition of Ag2O3 and AgO, and electrochemical redox reaction leads to the formation of metallic silver (Ag3O4 → AgO → Ag2O → Ag).24 This property inspired us to think about grafting nickel oxide with silver oxide to increase conductivity, for the first time using a hydrothermal method. The loading level of silver on NiO was varied and its effects on the structural, morphological and electrochemical properties of nickel oxide are reported and discussed in this paper.
2. Experimental section
2.1 Synthesis of Ag3O4 grafted NiO
Analytical grade nickel nitrate, silver nitrate, sodium lauryl sulfate (SDS) and urea were purchased from Sigma Aldrich and used without further purification. In a typical synthesis, Ni(NO3)2·6H2O and Ag(NO3)2·6H2O were taken as the precursors. Initially, 0.1081 g of SDS was dissolved in 40 mL of double distilled water and stirred for 30 min at room temperature. Subsequently, 0.4523 g of Ni(NO3)2·6H2O was dissolved in 10 mL of double distilled water and 0.0104 g (corresponds to 2.5 wt% of Ag) of Ag(NO3)2·6H2O was separately dissolved in 10 mL of double distilled water. The precursor solutions were slowly added to the SDS solution under constant stirring. Finally, 0.1081 g of urea was dissolved in 15 mL of double distilled water and the same was added to the solution containing SDS and the precursors. The resultant solution was allowed to stir for 3 h at room temperature. The as-obtained solution was transferred into 100 mL Teflon lined stainless steel autoclave and it was maintained at 160 °C for 24 h. After cooling to room temperature, a solid green color precipitate was obtained. It was repeatedly washed with ethanol and double distilled water. Ag3O4 grafted NiO was obtained by annealing the green powder at 300 °C for 2 h in an atmosphere of air. The silver level was varied from 2.5 to 10 wt% in steps of 2.5 wt% to prepare the Ag3O4 grafted NiO and the samples were named AGN1, AGN2, AGN3 and AGN4, respectively.2.2 Material characterization
The morphology of the samples was analyzed by scanning electron microscopy with an energy dispersive X-ray spectrometer (SEM, JSM-6390-JEOL) and a scanning transmission electron microscope (STEM, FE-QUANTA). The crystalline nature of the sample was identified using X-ray diffraction analysis using a PANAlytical X'PERT-PRO X-ray diffractometer with CuKα radiation. An automated adsorption apparatus (Micromeritics ASAP 2020 V3.00 H) was used to analyze the textural characteristics of the sample using physisorption at 77 K. Conductivity measurements were made by using a two probe HIOKI 3532-50LCR Hi Tester (pellet method).2.3 Fabrication of the electrodes and electrochemical measurements
Initially, the current collector (Ni foam) was repeatedly washed with acetone/double distilled water to ensure that the surface was clean. The electrodes used for evaluating the electrochemical properties of the synthesized Ag3O4 grafted NiO were prepared by mixing the electroactive material (85%), activated carbon (10%) and poly(tetrafluoroethylene) (PTFE 5%) with a few drops of ethanol to form a homogenous slurry. The slurry was coated onto the current collector (1 cm2 of nickel foam) and was dried at 80 °C for 8 h. The electrochemical properties of the samples were investigated under a three-electrode cell configuration with Ag3O4 grafted NiO as the working electrode, platinum wire as the counter electrode, and Ag/AgCl as the reference electrode with a 2 M KOH solution as the electrolyte. The Cyclic Voltammetry (CV), galvanostatic charge-discharge (GCD) and Electrochemical Impedance Spectroscopy (EIS) measurements (including equivalent circuit fitting) were carried out using an Electrochemical Workstation (CHI 660D, USA). Cyclic voltammograms were recorded in the potential range of 0 to 0.55 V and the specific capacitance was calculated from the average current developed in the CV plot. GCD measurements were performed at different current densities within the potential range of −0.15 to 0.4 V. Impedance studies on the electrode materials were conducted between 0.01 Hz to 100 kHz at an amplitude of 5 mV.2.4 Fabrication of the asymmetric supercapaitor
A two electrode system was fabricated by coating AGN2 (1 mg) and RGO (reduced graphene oxide, 1 mg) on Ni foams of area 1 sq cm, to serve as the anode and cathode, respectively. A polypropylene separator (Celgard-2400) was used as the separator to make a sandwich type device with 6 M KOH solution as the electrolyte. RGO was prepared by a modified Hummers method.253. Results and discussions
3.1 Structural and morphological investigations
The purity, phase and crystal structure of the samples were examined by XRD analysis. Fig. 1 shows the XRD patterns of the Ag3O4 grafted NiO. The diffraction peaks located at 43.2° and 62.9° correspond to the (200) and (220) planes of cubic structured NiO (JCPDS file no. 78-0643), which is in very good agreement with the reported results.26–30 The other peaks at 34.37°, 37.82°, 38.62°, 65.02°, 75.82° and 78.17° could be indexed to the (012) (112) (022) (113) (222) (143) crystal planes of monoclinic structured Ag3O4 (JCPDS file no. 84-1261). The NiO diffraction peaks become sharper and intense with the increase in the Ag level, which confirms the enhancement in the crystalline nature of NiO and results in an increase in the conductivity of the material (Fig. S1†).Fig. 2 shows the SEM and STEM images of the Ag3O4 grafted NiO. The concentration of Ag alters the morphology of the NiO. Initially NiO nanosheets are formed due to the anionic surfactant.31 AGN2 shows a flower like structure with good cross linked nanosheets. The increase in Ag content leads to the formation of an ordered structure up to 5 wt% and further increase in Ag content results in the collapse of the flowerlike structure and the formation of individual nanosheets. The grafting of Ag3O4 on the NiO system is clearly shown in Fig. 2e. The presence of Ag in the NiO system is confirmed by the energy dispersive X-ray analysis (Fig. S2†).
3.2 Electrochemical behavior
Cyclic voltammetry (CV), galvanostatic charge-discharge (GCD) and electrochemical impedance analysis were performed to evaluate the electrochemical performance of the Ag3O4 grafted NiO electrodes. The cyclic voltammograms were recorded in a voltage range from 0 to 0.55 V at a wide range of scan rates ranging from 3 to 20 mV s−1. Typical CV curves of the Ag3O4 grafted NiO are shown in Fig. 3a–d. The shape of the CV curves clearly reveals the pseudocapacitive behavior of the electrodes, which is totally different from EDLCs. | ||
| Fig. 3 (a–d) Cyclic voltammograms of the Ag3O4 grafted electrodes at different scan rates. (e) Effect of the scan rate on specific capacitance. | ||
The current response is found to be proportionally increased with the increasing scan rates, which reveals that the rate of the electronic and ionic transport are not limited by higher scan rates.32 The specific capacitance (Cs) was estimated using the relation,
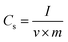 | (1) |
The effect of scan rate on the specific capacitance is shown in Fig. 3e. At lower scan rates, ions have enough time to reach the inner electrode sites and this results in a higher specific capacitance while at higher scan rates the ions are not able to access the inner regions.38
To quantify the specific capacitance and rate capability of the Ag3O4 grafted NiO electrodes, GCD measurements were carried out. The nonlinearity in the discharge curves (Fig. 4) shows the pseudocapacitive behavior of the electrodes. The specific capacitance (F g−1) was obtained using the following equation.
 | (2) |
Energy and power density are the two crucial factors to evaluate the commercial application of electrochemical supercapacitors. A good electrochemical supercapacitor is expected to provide a high energy density or high specific capacitance at high charge–discharge rates (current densities). The Ragone plot (power density vs. energy density) of the Ag3O4 grafted NiO electrodes are shown in Fig. 5a. The energy and power densities of the samples were calculated from the charge–discharge curves at different current densities using following equations:
 | (3) |
 | (4) |
The cycle life is another important factor to evaluate the performance of supercapacitors. The percentage retention of the capacitance of AGN2 is shown in Fig. 5b. The specific capacitance does not show any appreciable change during the first 200 cycles, whereas it gradually increases up to 400 cycles, indicating the activation of the electrode material. Beyond 400 cycles, the specific capacitance remains unaffected up to 1400 cycles and finally it shows 88% capacity retention at 1500 cycles. It is worth mentioning that the cyclic stability of the Ag3O4 grafted NiO (5 wt% of Ag) is better compared to other reported results.45–47 The flower like structure may provide the easiest intercalation/deintercalation paths for ions without much change in the structure for prolonged cycles, and thus results in a higher cyclic stability.
To elucidate the electrical conductivity and ion transfer ability of the electrodes, electrochemical impedance analysis was performed. Fig. 5c shows the Nyquist plot of the Ag3O4 grafted NiO electrode materials (AGN1, AGN2, AGN3 and AGN4) recorded in a frequency range of 0.01 Hz to 100 kHz at the potential of 0.4 V. The equivalent fitting circuit is shown in Fig. 5d, which contains the solution resistance Rs, charge transfer resistance Rct, double layer capacitance (Cdl), pseudocapacitive capacitance (CL) and Warburg impedance (W). The faradaic pseudocapacitance CL is charged via a faradaic resistance Rct, and CL is leaked by a desorption faradaic resistance RL. The impedance spectrum is composed of a semicircle at the high frequency region followed by one linear component (Warburg) at the low frequency region, which shows the frequency dependence of ion diffusion in the electrolyte to the electrode interface. The charge transfer resistances (Rct) of the electrode materials AGN1, AGN2, AGN3 and AGN4 are 1.39, 1.31, 0.96, and 0.37 Ω, respectively. Liu et al.48 reported three dimensional tubular arrays of MnO2–NiO nanoflakes with an Rct of 18.7 Ω. CoxNi1−xO nanorods synthesized via a bio inspired method shows an Rct of 2.29 Ω.49 Lee et al.50 reported a charge transfer resistance of 5 Ω for NiO/CNT nanocomposites prepared via the precipitation method. Compared to other reports, the electrodes prepared in the present work exhibit lower Rct values and this probably increased the conductivity of the electrodes due to excellent electrolyte accessibility.51 The increase in the Ag level decreases the Rct value and this is in good agreement with the DC conductivity studies, but the formation of compact crystallites and change in morphology may play a vital role in deciding the electrochemical performance of the electrodes.
In the view of potential applications, asymmetric supercapacitors were fabricated to evaluate the capacitive features of the Ag3O4 grafted NiO. The working electrodes were fabricated as discussed earlier in the Experimental section. Fig. 6a shows the schematic illustration of the fabrication of the asymmetric supercapacitor, which consists of an anode (AGN2), polypropylene separator (Celgard-2400), cathode (reduced graphene oxide-RGO) and electrolyte (6 M KOH). The electrochemical performance of RGO is shown in Fig. S7.† The CV and GCD analysis were carried out to explore the electrochemical performance of the two electrode system. The potential window of 1 V (0–1 V) was fixed for both the analysis. The CV curves (Fig. 6b) show excellent redox peaks i.e., capacitance arises mainly due to the peudocapacitive behavior of the material prepared in the present work. The specific capacitance estimated from the CV are 186, 140, 84, 55, 44 and 37 F g−1 at a scan rate of 2, 3, 5, 10, 15 and 20 mV s−1, respectively. The GCD analysis exhibits the specific capacitance of 274, 135, 80 and 30 F g−1 (Fig. 6c) at 0.2, 0.5, 1 and 2 A g−1, respectively, with a maximum energy density of 38 Wh kg−1 at the power density of 499 W kg−1. Fig. S8† shows the Ragone plot of the asymmetric supercapacitor.
4. Conclusion
A simple and facile hydrothermal approach was used to fabricate Ag3O4 grafted NiO nanosheets. Electrochemical measurements reveal the pseudocapacitive nature of the Ag3O4 grafted NiO with the maximum specific capacitance of 1524 F g−1 (AGN2) at a current density of 1 A g−1. The enhanced electrochemical performance is attributed to the Ag3O4 grafting on NiO and the formation of flower like cross linked nanosheets with a mesoporous structure. 88% capacity retention was observed even after 1500 continuous charge–discharge cycles at a current density of 5 A g−1. An asymmetric supercapacitor (AGN2//RGO) was constructed and it exhibits the maximum specific capacitance of 274 F g−1 at a discharge current of 0.2 A g−1 with the power density of 499 W kg−1 and these results make the Ag3O4 grafted NiO (AGN2) an optimistic potential nominee for electrochemical energy storage.Acknowledgements
One of the authors (K.K.P.) thanks The Department of Science and Technology-SERC, New Delhi, India, for providing the financial support under Young Scientist scheme (No. SR/FTP/PS-030/2011) and Nanotechnology Research Centre, SRM University, Kattankulathur, India, for providing XRD and STEM facility.References
- C. Yuan, J. Li, L. Hou, X. Zhang, L. Shen and X. W. D. Lou, Ultrathin Mesoporous NiCo2O4 Nanosheets Supported on Ni Foam as Advanced Electrodes for Supercapacitors, Adv. Funct. Mater., 2012, 22, 4592–4597 CrossRef CAS.
- H. Wang, Q. Gao and L. Jiang, Facile Approach to Prepare Nickel Cobaltite Nanowire Materials for Supercapacitors, Small, 2011, 7, 2454–2459 CAS.
- S. Vijayakumar, S. Nagamuthu and G. Muralidharan, Supercapacitor Studies on NiO Nanoflakes Synthesized through a Microwave Route, ACS Appl. Mater. Interfaces, 2013, 5, 2188–2196 CAS.
- Y. G. Wang, H. Q. Li and Y. Y. Xia, Ordered Whiskerlike Polyaniline Grown on the Surface of Mesoporous Carbon and its Electrochemical Capacitance Performance, Adv. Mater., 2006, 18, 2619–2623 CrossRef CAS.
- R. Liu and S. B. Lee, MnO2/poly (3,4-ethylenedioxythiophene) coaxial nanowires by one-step co-electrodepositon for electrochemical energy storage, J. Am. Chem. Soc., 2008, 130, 2942–2943 CrossRef CAS PubMed.
- X. Du, C. Wang, M. Chen, Y. Jiao and J. Wang, Electrochemical Performances of Nanoparticle Fe3O4/Activated Carbon Supercapacitor Using KOH Electrolyte Solution, J. Phys. Chem. C, 2009, 113, 2643–2646 CAS.
- O. Barbieri, M. Hahn, A. Herzog and R. K. Otz, Capacitance Limits of High Surface Area Activated Carbons for Double Layer Capacitors, Carbon, 2005, 43, 1303–1310 CrossRef CAS PubMed.
- L. L. Zhang and X. S. Zhao, Carbon Based Materials as Supercapacitor Electrodes, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- C. Xu, J. Sun and L. Gao, Synthesis of Novel Hierarchical Graphene/Polypyrrole Nanosheet Composites and their Superior Electrochemical Performance, J. Mater. Chem., 2011, 21, 11253–11258 RSC.
- J. Ji, L. L. Zhang, H. Ji, Y. Li, X. Zhao, X. Bai, F. Fan, F. Zhang and R. S. Rouff, Nanoporous Ni(OH)2 Thin film on 3D Ultrathin-Graphite Foam for Asymmetric Supercapacitor, ACS Nano, 2013, 7, 6237–6243 CrossRef CAS PubMed.
- D. H. Shin, J. S. Lee, J. Jun and J. Jang, Fabrication of Amorphous Carbon-Coated NiO Nanofibers for Electrochemical Capacitor Applications, J. Mater. Chem. A, 2014, 2, 3364–3371 CAS.
- J. B. Wu, R. Q. Guo, X. H. Huang and Y. Lin, Construction of Self-Supported TiO2/NiO Core–Shell Nanorod Arrays for Electrochemical Capacitor Application, J. Power Sources, 2013, 243, 317–322 CrossRef CAS PubMed.
- W. Zhou, D. Kong, X. Jia, C. Ding, C. Cheng and G. Wen, NiCo2O4 Nanosheet Supported Hierarchical Core–Shell Arrays for High-Performance Supercapacitors, J. Mater. Chem. A, 2014, 2, 6310–6315 CAS.
- K. Xu, W. Li, Q. Liu, B. Li, X. Liu, L. An, Z. Chen, R. Zou and J. Hu, Hierarchical Mesoporous NiCo2O4@MnO2 Core–Shell Nanowire Arrays on Nickel Foam for Aqueous Asymmetric Supercapacitors, J. Mater. Chem. A, 2014, 2, 4795–4802 CAS.
- Y. M. Wang, X. Zhang, G. Y. Gao, Y. Q. Zhao, C. L. Xu and H. L. Li, Controllable Synthesis of 3D NixCo1−xO with Different Morphologies for High-Capacity Supercapacitors, J. Mater. Chem. A, 2013, 1, 13290–13300 CAS.
- L. Zhang, C. Tang, X. Yin and H. Gong, Substrate-Assisted Self Organization of Ni–Cu Spherical Double Hydroxide (SDH) and its Excellent Pseudo-capacitive Performance, J. Mater. Chem. A, 2014, 2, 4660–4666 CAS.
- S. H. Kang, K. Zhu, N. R. neale and A. J. Frank, Hole Transport in Sensitized CdS–NiO Nanoparticle Photocathods, Chem. Commun., 2011, 47, 10419–10421 RSC.
- M. Hakamada, A. Moriguchi and M. Mabuchi, Fabrication Carbon Nanotube/NiOx(OH)y Nanocomposite by Pulsed Electrodeposition for Supercapacitor Applications, J. Power Sources, 2014, 245, 324–330 CrossRef CAS PubMed.
- M. S. Wu, Y. P. Lin, C. H. lin and J. T. Lee, Formation of Nano-Scaled Crevices and Spacers in NiO Attached Graphene Oxide Nanosheets for Supercapacitor Applications, J. Mater. Chem., 2012, 22, 2442–2448 RSC.
- H. Cheng, Z. G. Lu, J. Q. Deng, C. Y. Chung, K. L. Zhang and Y. Y. Li, A Facile Method to Improve the High Rate Capability of Co3O4 Nanowire Array Electrodes, Nano Res., 2010, 3, 895–901 CrossRef CAS.
- A. L. M. Reddy, M. M. Shaijumon, S. R. Gowda and P. M. Ajayan, Multisegmented Au–MnO2/Carbon Nanotube Hybrid Co-Axial Arrays fro High Power Supercapacitor Applications, J. Phys. Chem. C, 2010, 114, 658–663 CAS.
- B. Qu, L. Hu, Y. Chen, C. Li, Q. Li, Y. Wang, W. Wei, L. Chen and T. Wang, Rational Design of Au–NiO Hierarchical Structures with Enhanced Rate Performance for Supercapacitors, J. Mater. Chem. A, 2013, 1, 7023–7026 CAS.
- N. L. Yong, A. Ahamad and A. W. Mohammad, Synthesis and Characterization of Silver Oxide Nanoparticles by a Novel Method, Int. J. Sci. Eng. Res., 2013, 4, 155–158 Search PubMed.
- Y. Cheng, M. Yan and Z. Jiang, Electrochemical Behavior and Reduction Mechanism of High Valence Silver Oxide in Alkaline Solution, Electrochem. Solid-State Lett., 2005, 10, F5–F8 CrossRef PubMed.
- K. K. Purushothaman, B. Saravanakumar, I. M. Babu, B. Sethuraman and G. Muralidharan, Nanostructured CuO/Reduced Graphene Oxide Composites for Hybrid Supercapacitors, RSC Adv., 2014, 4, 23485–23491 RSC.
- N. Chopra, L. Claypoole and L. G. Bachas, Morphological Control of Ni/NiO Core/Shell Nanoparticles and Production of Hollow NiO Nanostructures, J. Nanopart. Res., 2010, 12, 2883–2893 CrossRef CAS.
- D. B. Kuang, B. X. Lei, Y. P. Pan, X. Y. Yu and C. Y. Su, Fabrication of Novel Herarchical β-Ni(OH)2 and NiO Microspheres via an Easy Hydrothermal Process, J. Phys. Chem. C, 2009, 113, 5508–5513 CAS.
- M. G. Ma, J. F. Zhu, J. X. Jang and R. C. Sun, Hydrothermal-Polyol Route to Synthesis of β-Ni(OH)2 and NiO in Mixed Solvents of 1,4-Butanediol and Water, Mater. Lett., 2009, 63, 1791–1793 CrossRef CAS PubMed.
- G. Zhang, L. Yu, H. E. Hostera and X. L. Wen, Synthesis of One-dimensional Hierarchical NiO Hollow Nanostructures with Enhanced Supercapacitive Performance, Nanoscale, 2013, 5, 877–881 RSC.
- Q. X. Xia, K. S. Hui, K. N. hui, D. H. Hwang, S. K. Lee, W. Zhau, Y. R. Cho, S. H. Kwon, Q. M. Wang and Y. G. Son, A Facile Synthesis Method of Hierarchically Porous NiO Nanosheets, Mater. Lett., 2012, 69, 69–71 CrossRef CAS PubMed.
- K. K. Purushothaman, I. Manohara Babu, B. Sethuraman and G. Muralidharan, Nanosheet-Assembled NiO Microstructures for High-Performance Supercapacitors, ACS Appl. Mater. Interfaces, 2013, 5, 10767–10773 CAS.
- J. H. Kim, S. H. Kang, K. Zhu, J. Y. Kim, N. R. Neale and A. J. Frank, Ni-NiO Core–Shell Inverse Opal Electrodes for Supercapaacitors, Chem. Commun., 2011, 47, 5214–5216 RSC.
- B. Zhang, W. Li, J. Sun, G. He, R. Zou, J. Hu and Z. Chen, NiO/MnO2 Core/Shell Nanocomposites for High Performance Pseudocapacitors, Mater. Lett., 2014, 114, 40–43 CrossRef CAS PubMed.
- H. Chen, J. Jiang, L. Zhang, T. Qi, D. Xia and H. Wan, Facilely Synthesized Porous NiCo2O4 Flower Like Nanostructures for High-Rate-Supercapacitors, J. Power Sources, 2014, 248, 28–36 CrossRef CAS PubMed.
- J. B. Condon, Surface Area and Porosity Determinations by Physisorption: Measurements and Theory, Elsevier, Amsterdam, 1st edn, 2006 Search PubMed.
- S. M. Zhang and H. C. Zeng, Self-Assembled Hollow Spheres of Ni(OH)2 and their Derived Nanomaterials, Chem. Mater., 2009, 21, 871–883 CrossRef CAS.
- Y. Q. Zou and Y. Wang, NiO Nanosheets Grown on Graphene Nanosheets as Superior Anode Materials for Li-ion Batteries, Nanoscale, 2011, 3, 2615–2620 RSC.
- X. H. Xia, J. P. Tu, Y. Q. Zhang, X. L. Wang, C. D. Gu, X. B. Zhao and H. J. Fan, Metal Oxide Core/Shell Nanowire Arrays on Conductive Substrates for Electrochemical Energy Storage, ACS Nano, 2012, 6, 5531–5538 CrossRef CAS PubMed.
- M. Huang, F. Li, Y. J. Jun, X. Z. Yu, L. Z. Xiao and G. Xing, Facile Synthesis of single-Crystalline NiO Nanosheet Arrays on Ni Foam for High-Performance Supercapacitors, CrystEngComm, 2014, 16, 2878–2884 RSC.
- Y. Lei, J. Li, Y. Wang, L. Gu, Y. Chang, H. Yuan and D. Xiao, Rapid Microwave-Assisted Green Synthesis of 3D Hierarchical Flower-Shaped NiCo2O4 Microspheres for High-Performance Supercapacitor, ACS Appl. Mater. Interfaces, 2014, 6, 1773–1780 CAS.
- M. Huang, F. Li, Y. X. Zhang, B. Li and X. Gao, Hierarchical NiO nanoflake coated CuO flower core–shell nanostructure for supercapacitor, Ceram. Int., 2014, 40, 5333–5338 CrossRef PubMed.
- X. Tian, C. Cheng, L. Qian, B. Zheng, H. Yuan, S. Xie, D. Xiao and M. M. F. Choi, Microwave-Assisted Non-Aqueous Homogenous Precipitation of Nanoball-like Mesoporous α-Ni(OH)2 as a Precursor for NiOx and its Application as a Pseudocapacitor, J. Mater. Chem., 2012, 22, 8029–8035 RSC.
- S. Xiong, C. Yuan, X. Zhangb and Y. Qiana, Mesoporous NiO with Hierarchical Nanostructures by Quasi-nanotubes/Nanowires/Nanorods Self-Assembly: Controllable Preparation and Application in Supercapacitors, CrystEngComm, 2011, 13, 626–632 RSC.
- X. Zhang, W. Shi, J. Zhu, W. Zhao, J. Ma, S. Mhaisalkar, T. L. Maria, Y. Yang, H. Zhang, H. H. Hng and Q. Yan, Synthesis of Porous Nanocrystals with Controllable Surface Area and Their Application as Supercapacitor Electrodes, Nano Res., 2010, 3, 643–652 CrossRef CAS PubMed.
- Z. Xing, Q. Chu, X. Ren, C. Ge, A. H. Qusti, A. M. Asiri, A. O. Al-Youbi and X. Sun, Ni3S2 Coated ZnO Array for High-Performance Supercapacitors, J. Power Sources, 2014, 245, 463–467 CrossRef CAS PubMed.
- C. H. Tang, X. Yin and H. Gong, Superior Performance of Asymmetric Supercapcitors Based on a Directly Grown Commercial Mass 3D Co3O4@Ni(OH)2 Core–Shell Electrodes, ACS Appl. Mater. Interfaces, 2013, 5, 10574–10582 CAS.
- L. Huang, D. Chen, Y. Ding, Z. L. Wang, Z. Zeng and M. Liu, Hybrid Composite Ni(OH)2@NiCo2O4 Grown on Carbon Fiber Paper for High-Performance Supercapacitors, ACS Appl. Mater. Interfaces, 2013, 5, 11159–11162 CAS.
- J. Liu, J. Jiang, M. Bosman and H. J. Fan, Three-Dimensional Tubular arrays of MnO2–NiO Nanoflakes with High Areal Pseudoapacitance, J. Mater. Chem., 2012, 22, 2419–2426 RSC.
- J. Xiao and S. Yang, Bio-Inspired Synthesis of NaCl Type CoxNi1−x O (0< x <1) Nanorods on Reduced Graphene Oxide Sheets for Screening Asymmetric Electric Capacitors, J. Mater. Chem., 2012, 22, 12253–12262 RSC.
- J. Y. Lee, K. Liang, K. H. An and Y. H. Lee, NickelOxide/Carbon Nanotubes Nanocomposites for Electrochemical Capacitance, Synth. Met., 2005, 150, 153–157 CrossRef CAS PubMed.
- J. F. Zang, S. J. Bao, C. M. Li, H. J. Bian, X. Q. Cui, Q. L. Bao, C. Q. Sun, J. Guo and K. R. Lian, Well-Aligned Cone-Shaped Nanostructure of Polypyrrole/RuO2 and its Electrochemical Supercapacitor, J. Phys. Chem. C, 2008, 112, 14843–14847 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ta04586f |
| This journal is © The Royal Society of Chemistry 2015 |





