Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
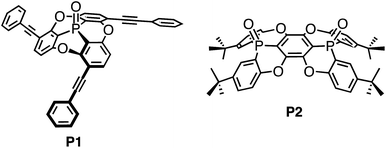
Twofold fused concave hosts containing two phosphorus atoms: modules for the sandwich-type encapsulation of fullerenes in variable cavities†
Masaki
Yamamura
 *,
Daigo
Hongo
and
Tatsuya
Nabeshima
*
*,
Daigo
Hongo
and
Tatsuya
Nabeshima
*
Graduate School of Pure and Applied Sciences, Tsukuba Research Center for Interdisciplinary Materials Science, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki, 305-8571 Japan. E-mail: myama@chem.tsukuba.ac.jp; nabesima@chem.tsukuba.ac.jp
First published on 24th July 2015
Abstract
The design and synthesis of extended concave host P2 by fusion of two concave phosphorus-containing units is reported. Co-crystallization of P2 and the fullerene guests C60 and C70 afforded the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes (P2)2 ⊃ C60 and (P2)2 ⊃ C70, in which the two concave surfaces of P2 encapsulate the convex surface of the fullerenes in a sandwich fashion. Interestingly, the orientation of the two P2 molecules with respect to each other was observed to be flexible, resulting in the formation of a variety of cavity shapes. MALDI-TOF mass, NMR, and UV-vis absorption spectra supported the formation of host–guest complexes between P2 and the fullerenes in solution. The affinity of P2, containing two phosphorus atoms, towards fullerenes was significantly enhanced relative to P1 with one phosphorus atom.
1 host–guest complexes (P2)2 ⊃ C60 and (P2)2 ⊃ C70, in which the two concave surfaces of P2 encapsulate the convex surface of the fullerenes in a sandwich fashion. Interestingly, the orientation of the two P2 molecules with respect to each other was observed to be flexible, resulting in the formation of a variety of cavity shapes. MALDI-TOF mass, NMR, and UV-vis absorption spectra supported the formation of host–guest complexes between P2 and the fullerenes in solution. The affinity of P2, containing two phosphorus atoms, towards fullerenes was significantly enhanced relative to P1 with one phosphorus atom.
Introduction
The recognition of fullerenes is one of the most intensively pursued research subjects in contemporary supramolecular and host–guest chemistry.1 The applications of fullerene recognition are manifold and reach from fullerene purification2 and nanoscale organisation3 to the formation of photovoltaic cells.4 A central paradigm for the design of molecular hosts is the generation of well-defined three-dimensional architectures so that suitable arrangements of the binding sites are obtained.5 For the recognition and capture of fullerenes, which usually do not contain any functional groups, only weak interactions such as π–π and CH–π interactions are available, and therefore aromatic compounds have often been used as binding sites. Even though benzene rings represent a relatively small binding site, recent studies have demonstrated a very high affinity for fullerene derivatives arising from the macrocyclic arrangement of benzene rings (carbon-nanorings),6 and can therefore be considered as an analogy of crown ether chemistry. Coordinative self-assembly of small molecules is also effective to capture fullerenes.7 Concave π-conjugated molecules8,9 are also promising binding sites for fullerenes, as their concave surfaces resemble and match the convex π-surface of fullerenes.10,11 Although individual concave molecules exhibit merely a weak affinity towards fullerenes, the fusion of multiple molecular units can substantially increase this affinity.12,13 Recently, we reported phosphorus-containing concave molecule P1 (Chart 1),14,15 and although four host molecules perfectly wrapped around the convex surface of C60 in the crystalline state, the host–guest interaction between the host and the C60 guest was found to be negligible in organic solvents. Concave P1 is considered to be a good molecular host fragment, but a carefully crafted arrangement of multiple such fragments is necessary for an efficient recognition of C60.Therefore, we have designed and synthesised the extended concave molecule P2 by fusing two phosphorus-containing concave units similar to P1. The expanded concave surface of P2 is expected to enhance the affinity of P2 towards fullerene guests through increased concave–convex interactions.
Results and discussion
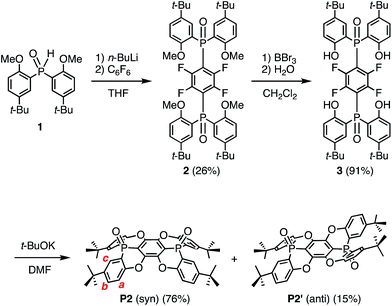
Synthesis of the fused concave host
Concave host P2 was synthesised in three steps (Scheme 1), starting from the reaction between hexafluorobenzene and two equivalents of an anion, which was generated from the deprotonation of 1 with n-BuLi. Thus, 1,4-bis(phosphoryl)tetrafluorobenzene 2 was obtained in 26% yield.16 Subsequent treatment of 2 with BBr3 resulted in the removal of four methyl groups to afford 3 in 91% yield. Deprotonation of 3 with t-BuOK furnished concave P2 (76%) and its anti-isomer P2′ (15%) in excellent stereo-selectivity, whereby the intramolecular SNAr reaction is the key step. The molecular structures of P2 and P2′ were determined unequivocally by single crystal X-ray diffraction analysis (Fig. S27†).Single crystals of P2, suitable for X-ray crystallographic analysis, were obtained by recrystallization from CHCl3/hexane, and the crystal structure clearly showed the concave shape of P2 (Fig. 1). The two phosphorus atoms, P1 and P2, are positioned slightly above (0.167/0.269 Å) the plane of the central benzene ring A.17 The P–O axes are aligned almost vertically with respect to A, comprising dihedral O–P–C–C angles of 82.0° (P1) and 88.2° (P2). Accordingly, no mirror planes pass through the two phosphorus atoms, resulting in a molecular structure of P2 with low symmetry. One P–O bond is twisted in clockwise direction, while the other is twisted in the opposite direction. Torsion angles of 24.2° and 17.4° were observed between the central ring A and the terminal B and C rings, respectively. Much larger values were observed between A and D (32.2°) or E (38.4°). This distorted structure is probably caused by steric repulsion between the B/D or C/E benzene rings. In contrast to the molecular structure in the crystal, the 1H NMR spectrum displayed four magnetically equivalent terminal benzene rings (B–E), most likely due to a fast interconversion of the twisting on the NMR timescale.
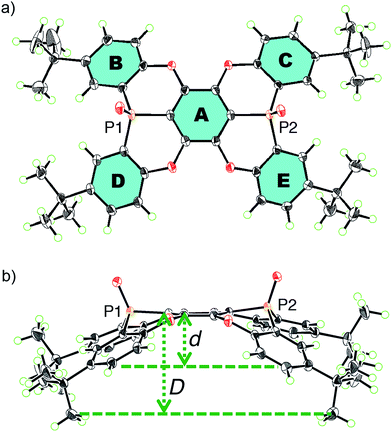 | ||
| Fig. 1 (a) Top and (b) side views of an ORTEP drawing of P2 with thermal ellipsoids set at 50% probability. | ||
For extended concave hosts such as P2, two depth parameters can be defined in order to characterise the concave surface (Fig. S26†). The first (d) is defined as the distance between the centroids of A and the plane of the t-Bu-substituted carbon atoms in B–E (Fig. 1). The second (D) is defined as the distance between the centroids of A and the plane of the terminal methyl groups. For P2, depth values of 1.8 Å (d) and 3.6 Å (D) were observed, i.e.P2 may be considered a deep concave host, if the methyl groups are included as host fragments.
Encapsulation of fullerenes
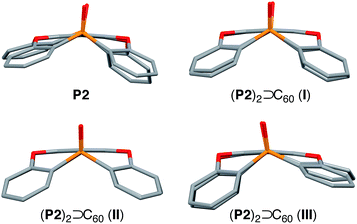
Three different types of black single co-crystals of P2 and C60 (I–III), suitable for X-ray crystallographic analysis, were obtained by diffusing hexane vapour into solutions of P2 and C60 in toluene/CHCl3, anisole, or CHCl3, respectively. These crystals consist of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes (P2)2 ⊃ C60 with centrosymmetric space groups P21/c (I), C2/m (II), and C2/c (III). In I–III, the two concave surfaces of P2 surround the convex surface of C60 in a sandwich fashion, and the differences between I–III is ascribed to different solvates.18 The orientation of the encapsulated C60 guest molecule could not be determined accurately, due to the presence of disorder. This is contrary to the previously reported tetrahedral host–guest complex between C60 and P1, for which no disorder of C60 within the tetrahedral cavity was observed.14
1 host–guest complexes (P2)2 ⊃ C60 with centrosymmetric space groups P21/c (I), C2/m (II), and C2/c (III). In I–III, the two concave surfaces of P2 surround the convex surface of C60 in a sandwich fashion, and the differences between I–III is ascribed to different solvates.18 The orientation of the encapsulated C60 guest molecule could not be determined accurately, due to the presence of disorder. This is contrary to the previously reported tetrahedral host–guest complex between C60 and P1, for which no disorder of C60 within the tetrahedral cavity was observed.14
In I, two host molecules encapsulated C60 in a sandwich fashion, i.e. the host molecules occupy opposing apex positions of the guest (Fig. 2a). The distance between the A centroids in the two hosts is 13.2 Å, which corresponds to the sum of a benzene ring and the diameter of C60. In contrast, distances of 6.8–7.0 Å were observed between the centroids of B–E and C60, which is longer than the sum of the van der Waals radii of C60 and a carbon atom (6.5 Å). The observed lengths thus suggested that the fullerene should be in direct contact with the two concave surfaces of both host molecules. The P1–P2 axes of the two host molecule are offset by 67.6° with respect to each other (Fig. 2b), reflecting the twisted arrangement of the two P2 molecules in I. In II, two comparable, yet crystallographically independent (P2)2 ⊃ C60 complexes are contained within the asymmetric unit (Fig. S29 and S30†). The host molecules are arranged in a similar manner to I, and the distance between the A centroids of the two hosts is 13.2 Å (Fig. 2c). In contrast to I, the P1–P2 axes of the two hosts are aligned in II (Fig. 2d), reflecting a horizontal arrangement of the two P2 molecules in II. In III, the two hosts do not occupy opposing apex positions of the C60 guest, as one host molecule is positioned at a latitudinal angle of 54.3° relative to the apex position (Fig. 2e), reflecting the misalignment of the two P2 molecules in III. Based on these observations, it can be concluded that the surface of C60 is too large to be covered entirely by two P2 host molecules, but simultaneously too small to be covered by three P2 host molecules. This mismatch in size should be at least partially responsible for the formation of different crystal forms in these host–guest complexes.
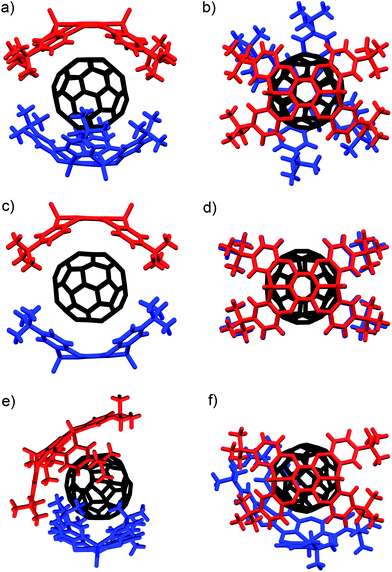 | ||
| Fig. 2 Molecular structures of the (P2)2 ⊃ C60 forms I–III. (a) Side and (b) top view of I (twisted); (c) side and (d) top view of II (horizontal); (e) side and (f) top view of III (misaligned). | ||
The internal structure of the P2 hosts was observed to vary in the different crystals (Fig. 3). In I, a significantly smaller deviation of the torsion angles (29.5–37.4°) between the central (A) and terminal benzene rings (B–E) in P2 was observed (Table 1) relative to that in uncomplexed P2 (17.4–38.4°). While the corresponding deviation in II was observed to be even smaller (33.9–35.7°), that in III is larger (17.3–41.5°), and hence, P2 should be able to adapt its structure according to the specific architecture of the host–guest complex.
| P2 | (P2)2 ⊃ C60 (I) | (P2)2 ⊃ C60 (II) | (P2)2 ⊃ C60 (III) | |
|---|---|---|---|---|
| Bowl depth | ||||
| d (Å) | 1.81 | 2.40 | 2.32 | 2.04 |
| D (Å) | 3.56 | 4.60 | 4.22 | 4.07 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Torsion angles | ||||
| A–B (°) | 24.2 | 29.5 | 33.9 | 23.2 |
| A–C (°) | 17.4 | 33.8 | 35.7 | 17.3 |
| A–E (°) | 32.2 | 37.4 | 33.9 | 41.5 |
| A–F (°) | 38.4 | 34.4 | 35.7 | 41.0 |
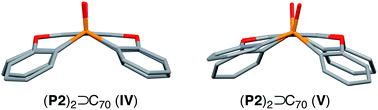
Using C70 as a guest molecule, two different 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes (P2)2 ⊃ C70 (IV, V) were obtained by crystallization from different solvents (Fig. 4). Similar to (P2)2 ⊃ C60, the two host molecules encapsulated C70 in a sandwich fashion. The P21212 space group of IV, prepared from CHCl3/toluene, is non-centrosymmetric with a Flack χ value of −0.03(3). In IV, the P1–P2 axes of the two host molecules are almost perfectly aligned vertically with respect to the long axis of C70 (Fig. 4a), but the centroids of the A rings of the two P2 molecules are slightly misaligned, resulting in a faulting-like chiral architecture of IV. Conversely, crystal V, obtained from CHCl3/CS2, crystallises in the centrosymmetric space group C2/c. In V, the two host molecules encapsulated C70 with their P1–P2 axes offset by 37.1° with respect to the long axis of C70 (Fig. 4b), and the two P2 molecules are also misaligned. In IV, the torsion angle range between the central (A) and peripheral benzene rings (B–E) of P2 (29.1–33.8°) was significantly narrower than that in V (13.2–37.3°) (Table 2). Similarly to the (P2)2 ⊃ C60 host–guest complexes, the torsion angles are flexible and are thus able to facilitate different host–guest architectures (Fig. 5). To the best of our knowledge, no reports exist on concave hosts encapsulating fullerene guests in such a variable fashion.
1 host–guest complexes (P2)2 ⊃ C70 (IV, V) were obtained by crystallization from different solvents (Fig. 4). Similar to (P2)2 ⊃ C60, the two host molecules encapsulated C70 in a sandwich fashion. The P21212 space group of IV, prepared from CHCl3/toluene, is non-centrosymmetric with a Flack χ value of −0.03(3). In IV, the P1–P2 axes of the two host molecules are almost perfectly aligned vertically with respect to the long axis of C70 (Fig. 4a), but the centroids of the A rings of the two P2 molecules are slightly misaligned, resulting in a faulting-like chiral architecture of IV. Conversely, crystal V, obtained from CHCl3/CS2, crystallises in the centrosymmetric space group C2/c. In V, the two host molecules encapsulated C70 with their P1–P2 axes offset by 37.1° with respect to the long axis of C70 (Fig. 4b), and the two P2 molecules are also misaligned. In IV, the torsion angle range between the central (A) and peripheral benzene rings (B–E) of P2 (29.1–33.8°) was significantly narrower than that in V (13.2–37.3°) (Table 2). Similarly to the (P2)2 ⊃ C60 host–guest complexes, the torsion angles are flexible and are thus able to facilitate different host–guest architectures (Fig. 5). To the best of our knowledge, no reports exist on concave hosts encapsulating fullerene guests in such a variable fashion.
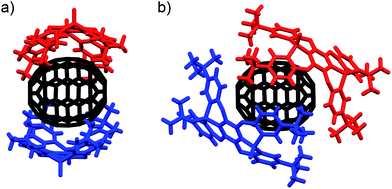 | ||
| Fig. 4 Side view of the molecular structures of the (P2)2 ⊃ C70 forms (a) IV (faulting-like) and (b) V (misaligned). | ||
| (P2)2 ⊃ C70 (IV) | (P2)2 ⊃ C70 (V) | |
|---|---|---|
| Bowl depth | ||
| d (Å) | 2.05 | 1.83 |
| D (Å) | 4.16 | 3.47 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Torsion angles | ||
| A–B (°) | 31.9 | 13.2 |
| A–C (°) | 29.1 | 30.3 |
| A–E (°) | 29.6 | 37.3 |
| A–F (°) | 33.8 | 30.5 |
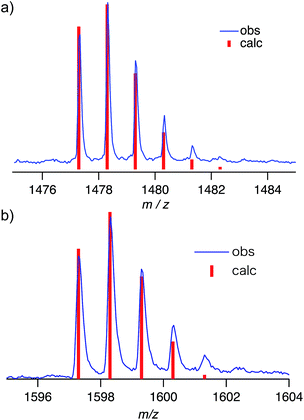
Negative-mode MALDI-TOF mass spectra of (P2)2 ⊃ C60 and (P2)2 ⊃ C70 revealed prominent peaks at m/z = 1478.3 and 1598.3, which were assigned to the corresponding 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes (Fig. 6). This result suggested that the concave–convex interactions between hosts and guests should be strong enough to preserve the host–guest complex at least partially even during ionization. However, no peaks corresponding to the 2
1 complexes (Fig. 6). This result suggested that the concave–convex interactions between hosts and guests should be strong enough to preserve the host–guest complex at least partially even during ionization. However, no peaks corresponding to the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes (P2)2 ⊃ C60 and (P2)2 ⊃ C70 were observed, even though this stoichiometry was found in the crystal structure. This result indicated that the interactions with the second host molecules in the 2
1 complexes (P2)2 ⊃ C60 and (P2)2 ⊃ C70 were observed, even though this stoichiometry was found in the crystal structure. This result indicated that the interactions with the second host molecules in the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes might be too weak in order to be observed. Therefore, NMR titration experiments were carried out in order to evaluate the concave–convex interactions in the binary organic solvent mixture CDCl3/CS2 (1
1 complexes might be too weak in order to be observed. Therefore, NMR titration experiments were carried out in order to evaluate the concave–convex interactions in the binary organic solvent mixture CDCl3/CS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v). In the 31P NMR spectra, the signal for P2 experienced a downfield shift (ΔδP: 0.20 ppm) upon addition of three equivalents of C60 (Fig. 7a).
3 v/v). In the 31P NMR spectra, the signal for P2 experienced a downfield shift (ΔδP: 0.20 ppm) upon addition of three equivalents of C60 (Fig. 7a).
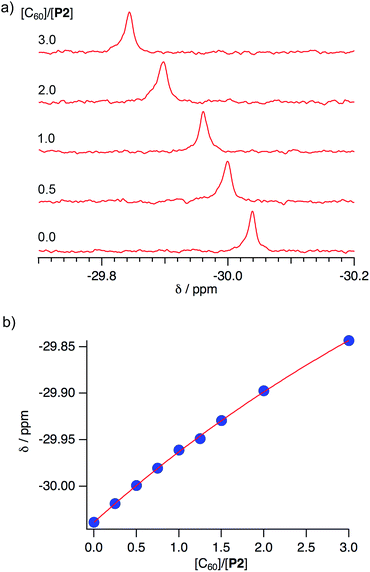 | ||
Fig. 7 (a) Spectral changes in the 31P NMR spectra (243 MHz) of P2 in CDCl3/CS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) upon addition of C60, and (b) 1 3 v/v) upon addition of C60, and (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm. 1 binding isotherm. | ||
In the 1H NMR spectra, signals for the protons attached to the peripheral benzene rings were shifted slightly upfield (ΔδH for Ha: −0.0049; Hb: −0.0038; Hc: 0.0028 ppm; see Scheme 1 and Fig. S21†). This result clearly demonstrated the interactions of P2 with C60 in this solvent mixture, while the interaction between the anti-isomer P2′ or previously reported P1 and C60 was observed to be negligible. A Job plot analysis confirmed the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes between P2 and C60 (Fig. S19†), and the absence of any indications for the formation of a 2
1 complexes between P2 and C60 (Fig. S19†), and the absence of any indications for the formation of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex is consistent with the results obtained from mass spectrometry measurements. Non-linear least-squares analysis for the change of the chemical shift afforded association constants (Ka) between P2 and C60 of 210 ± 20 M−1 (Fig. 7b),19 while between P2 and C70 a Ka value of 200 ± 30 M−1 was estimated (Fig. S22†). Accordingly, P2 exhibited an enhanced affinity for fullerenes relative to P1, containing one phosphorus atom, even though no selectivity for either C60 or C70 was observed. This result is consistent with the observation of multiple host–guest architectures in host–guest complexes between P2 and fullerenes, which may reflect that P2 is unable to selectively recognize the convex surface of specific fullerenes, due to its ability to accommodate various structures in accordance with the guest shape. A comparison of the observed Ka values with those of previously reported concave hosts, such as calixarenes and their analogues confirmed that P2 exhibits a moderate affinity towards C60.20
1 complex is consistent with the results obtained from mass spectrometry measurements. Non-linear least-squares analysis for the change of the chemical shift afforded association constants (Ka) between P2 and C60 of 210 ± 20 M−1 (Fig. 7b),19 while between P2 and C70 a Ka value of 200 ± 30 M−1 was estimated (Fig. S22†). Accordingly, P2 exhibited an enhanced affinity for fullerenes relative to P1, containing one phosphorus atom, even though no selectivity for either C60 or C70 was observed. This result is consistent with the observation of multiple host–guest architectures in host–guest complexes between P2 and fullerenes, which may reflect that P2 is unable to selectively recognize the convex surface of specific fullerenes, due to its ability to accommodate various structures in accordance with the guest shape. A comparison of the observed Ka values with those of previously reported concave hosts, such as calixarenes and their analogues confirmed that P2 exhibits a moderate affinity towards C60.20
Spectral titration experiments based on the UV-vis absorption also confirmed the formation of host–guest complexes in solution. Upon addition of P2 to a CHCl3/toluene solution of C60, the weak absorption at 450–600 nm, which is associated with the forbidden excitation of C60, gradually increased (Fig. 8), while P2 is transparent in this region (Fig. S18†). In contrast, addition of P2′ to a CHCl3/toluene solution of C60 did not change the UV-vis absorption spectrum (Fig. S25†). This result suggested that the stronger host–guest interaction between P2 and C60 relative to P2′ and C60 is responsible for the spectral change.
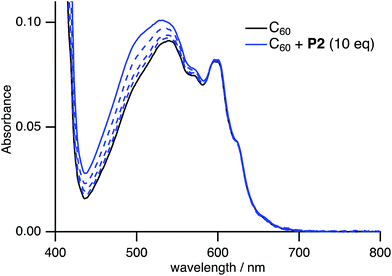 | ||
Fig. 8 Change of the UV-vis absorption spectrum of C60 in CHCl3/toluene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v) upon addition of P2 ([C60] = 0.10 mM, 0 ≤ [P2]/[C60] ≤ 10). 4 v/v) upon addition of P2 ([C60] = 0.10 mM, 0 ≤ [P2]/[C60] ≤ 10). | ||
Conclusions
In this study, we have disclosed the synthesis of the extended concave host P2, containing two phosphorus atoms. Moreover, we have demonstrated that P2 may serve as a suitable host for fullerenes. In co-crystals with C60 and C70, two molecules of P2 bind to the convex surface of the fullerenes in a sandwich fashion. Interestingly, the orientation of the two P2 molecules with respect to each other is flexible, resulting in the formation of a variety of cavity shapes. MALDI-TOF mass, NMR, and UV-vis absorption spectra supported the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes between P2 and the fullerenes in solution. Relative to P1 with one phosphorus atom, the affinity of P2, containing two phosphorus atoms, towards fullerenes was significantly enhanced. Accordingly, the expansion of the concave surface by fusing two phosphorus-containing concave units should result in an effective recognition of fullerenes. Considering that two molecules of P2 are necessary for the encapsulation of fullerene, fusion of two P2 molecules should enhance the affinity even further. Control over the cavity shape should be possible via a judicious choice of the connecting moiety between these two P2 units. Studies in this direction are currently in progress in our laboratory.
1 host–guest complexes between P2 and the fullerenes in solution. Relative to P1 with one phosphorus atom, the affinity of P2, containing two phosphorus atoms, towards fullerenes was significantly enhanced. Accordingly, the expansion of the concave surface by fusing two phosphorus-containing concave units should result in an effective recognition of fullerenes. Considering that two molecules of P2 are necessary for the encapsulation of fullerene, fusion of two P2 molecules should enhance the affinity even further. Control over the cavity shape should be possible via a judicious choice of the connecting moiety between these two P2 units. Studies in this direction are currently in progress in our laboratory.
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (26810015) from MEXT, Japan. This work was partly supported by Grants-in-Aid for Scientific Research on Innovative Areas “π-System Figuration: Control of Electron and Structural Dynamism for Innovative Functions” (15H00985) and “Photosynergetics” (15H01079) from MEXT, Japan.Notes and references
- (a) N. Martin and J.-F. Nierengarten, Supramolecular Chemistry of Fullerenes and Carbon Nanotubes, Wiley-VCH, 2012 Search PubMed; (b) F. D'Souza and K. M. Kadish, Handbook of Carbon Nano Materials Volume 1. Syntheses and Supramolecular Systems, World Scientific Publishers, 2011 Search PubMed.
- (a) J. L. Atwood, G. A. Koutsantonis and C. L. Raston, Nature, 1994, 368, 229 CrossRef CAS PubMed; (b) T. Suzuki, K. Nakashima and S. Shinkai, Chem. Lett., 1994, 699 CrossRef CAS; (c) Y. Shoji, K. Tashiro and T. Aida, J. Am. Chem. Soc., 2004, 126, 6570 CrossRef CAS PubMed; (d) T. Haino, C. Fukunaga and Y. Fukazawa, Org. Lett., 2006, 8, 3545 CrossRef CAS PubMed.
- (a) T. Haino, Y. Matsumoto and Y. Fukazawa, J. Am. Chem. Soc., 2005, 127, 8936 CrossRef CAS PubMed; (b) G. Fernández, E. M. Pérez, L. Sánchez and N. Martín, J. Am. Chem. Soc., 2008, 130, 2410 CrossRef PubMed.
- (a) T. Hasobe, H. Imahori, P. V. Kamat, T. K. Ahn, S. K. Kim, D. Kim, A. Fujimoto, T. Hirakawa and S. Fukuzumi, J. Am. Chem. Soc., 2005, 127, 1216 CrossRef CAS PubMed; (b) A. Takai, M. Chkounda, A. Eggenspiller, C. P. Gros, M. Lachkar, J.-M. Barbe and S. Fukuzumi, J. Am. Chem. Soc., 2010, 132, 4477 CrossRef CAS PubMed.
- J.-M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, VCH, Weinheim, 1995, p. 14 Search PubMed.
- For reports describing binding constants of carbon nanorings and their fullerene analogues, see: (a) T. Kawase, K. Tanaka, N. Fujiwara, H. R. Darabi and M. Oda, Angew. Chem., Int. Ed., 2003, 42, 1624 CrossRef CAS PubMed; (b) T. Kawase, K. Tanaka, Y. Seirai, N. Shiono and M. Oda, Angew. Chem., Int. Ed., 2003, 42, 5597 CrossRef CAS PubMed; (c) T. Iwamoto, Y. Watanabe, T. Sadahiro, T. Haino and S. Yamago, Angew. Chem., Int. Ed., 2011, 50, 8342 CrossRef CAS PubMed; (d) T. Iwamoto, Y. Watanabe, H. Takaya, T. Haino, N. Yasuda and S. Yamago, Chem.–Eur. J., 2013, 19, 14061 CrossRef CAS PubMed; (e) H. Isobe, S. Hitosugi, T. Yamasaki and R. Iizuka, Chem. Sci., 2013, 4, 1293 RSC; (f) T. Matsuno, S. Sato, R. Iizuka and H. Isobe, Chem. Sci., 2015, 6, 909 RSC.
- (a) W. Meng, B. Breiner, K. Rissanen, J. D. Thoburn, J. K. Clegg and J. R. Nitschke, Angew. Chem., Int. Ed., 2011, 50, 3479 CrossRef CAS PubMed; (b) K. Suzuki, K. Takao, S. Sato and M. Fujita, J. Am. Chem. Soc., 2010, 132, 2544 CrossRef CAS PubMed; (c) N. Kishi, Z. Li, K. Yoza, M. Akita and M. Yoshizawa, J. Am. Chem. Soc., 2011, 133, 11438 CrossRef CAS PubMed; (d) T. Nakamura, H. Ube, R. Miyake and M. Shionoya, J. Am. Chem. Soc., 2013, 135, 18790 CrossRef CAS PubMed.
- (a) W. E. Barth and R. G. Lawton, J. Am. Chem. Soc., 1966, 88, 380 CrossRef CAS; (b) L. T. Scott, M. M. Hashemi and M. S. Bratcher, J. Am. Chem. Soc., 1992, 114, 1920 CrossRef CAS.
- H. Sakurai, T. Daiko and T. Hirao, Science, 2003, 301, 1878 CrossRef CAS PubMed.
- (a) T. Kawase and H. Kurata, Chem. Rev., 2006, 106, 5250 CrossRef CAS PubMed; (b) E. M. Pérez and N. Martín, Chem. Soc. Rev., 2008, 37, 1512 RSC.
- (a) S. Mizyed, P. E. Georghiou, M. Bancu, B. Cuadra, A. K. Rai, P. Cheng and L. T. Scott, J. Am. Chem. Soc., 2001, 123, 12770 CrossRef CAS PubMed; (b) P. E. Georghiou, A. H. Tran, S. Mizyed, M. Bancu and L. T. Scott, J. Org. Chem., 2005, 70, 6158 CrossRef CAS PubMed; (c) B. Bredenkötter, S. Henne and D. Volkmer, Chem.–Eur. J., 2007, 13, 9931 CrossRef PubMed; (d) C. G. Claessens and T. Torres, Chem. Commun., 2004, 1298 RSC; (e) S. Shimizu, S. Nakano, T. Hosoya and N. Kobayashi, Chem. Commun., 2011, 47, 316 RSC.
- Even though corannulene, which is a representative fullerene fragment, was not able to capture C60 in solution, the fusion of two corannulene moieties resulted in a drastic increase of its affinity towards C60; see: (a) A. Sygula, F. R. Fronczek, R. Sygula, P. W. Rabideau and M. M. Olmstead, J. Am. Chem. Soc., 2007, 129, 3842 CrossRef CAS PubMed; (b) L. N. Dawe, T. A. AlHujran, H.-A. Tran, J. I. Mercer, E. A. Jackson, L. T. Scott and P. E. Georghiou, Chem. Commun., 2012, 48, 5563 RSC.
- For the self-assembly of concave molecules, resulting in the encapsulation of fullerene, see: C. G. Claessens and T. Torres, Chem. Commun., 2004, 1298 RSC.
- (a) M. Yamamura, T. Saito and T. Nabeshima, J. Am. Chem. Soc., 2014, 136, 14299 CrossRef CAS PubMed; (b) M. Yamamura, K. Sukegawa and T. Nabeshima, Chem. Commun., 2015, 51, 12080 RSC.
- For phosphorus-containing concave molecules, see: (a) F. C. Krebs, P. S. Larsen, J. Larsen, C. S. Jacobsen, C. Boutton and N. Thorup, J. Am. Chem. Soc., 1997, 119, 1208 CrossRef CAS; (b) G. K. H. Madsen, F. C. Krebs, B. Lebech and F. K. Larsen, Chem.–Eur. J., 2000, 6, 1797 CrossRef CAS.
- For the regioselectivity of aromatic nucleophilic substitutions between phosphide anions and perfluorobenzene, see: S. Sasaki, Y. Tanabe and M. Yoshifuji, Bull. Chem. Soc. Jpn., 1999, 72, 563 CrossRef CAS.
- The central benzene ring A is slightly distorted, exhibiting a root-mean square deviation of 0.041 Å.
- The orientation of the solvent molecules incorporated in the lattice could not be determined due to high levels of disorder.
- S. Akine, TitrationFit, program for analyses of host-guest complexation, Kanazawa University, Kanazawa, Japan, 2013 Search PubMed.
- (a) K. Tsubaki, K. Tanaka, T. Kinoshita and K. Fuji, Chem. Commun., 1998, 895 RSC; (b) T. Haino, M. Yanase, C. Fukunaga and Y. Fukazawa, Tetrahedron, 2006, 62, 2025 CrossRef CAS PubMed; (c) R. Inoue, M. Hasegawa, T. Nishinaga, K. Yoza and Y. Mazaki, Angew. Chem., Int. Ed., 2015, 54, 2734 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1401373–1401379. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc02224j |
| This journal is © The Royal Society of Chemistry 2015 |