Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantioselective annulation of enals with 2-naphthols by triazolium salts derived from L-phenylalanine†
Guo-Tai
Li
ab,
Qing
Gu
b and
Shu-Li
You
*abc
aSchool of Pharmacy, East China University of Science and Technology, 130 Mei-Long Road, Shanghai 200237, China. E-mail: slyou@sioc.ac.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, China
cCollaborative Innovation Center of Chemical Science and Engineering, Tianjin, China
First published on 30th April 2015
Abstract
A series of chiral triazolium salts have been synthesized from methyl L-phenylalaninate hydrochloride. The NHCs derived from this class of novel triazolium salts were found to be highly efficient catalysts in the annulation reaction of enals and 2-naphthols. These reactions proceeded with high chemoselectivity and wide substrate scope affording enantioenriched β-arylsplitomicins in good yields with up to 96% ee.
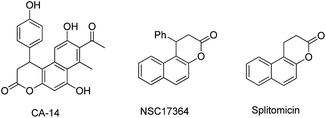
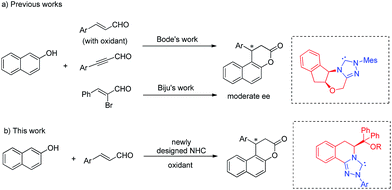
The naphthopyran-3-one core is a characteristic structural motif existing in a variety of biologically active natural products and pharmaceuticals (Fig. 1).1 Specifically, β-arylsplitomicin and its analogues have recently been extensively investigated and they were found to exhibit highly interesting biological activities such as recombinant SIRT2 inhibition, antiproliferative properties and tubulin hyperacetylation in MCF7 breast cancer cells.1c–e,g Moreover, they are also important synthetic intermediates in both organic synthesis and medicinal chemistry. Consequently, various approaches to access these β-arylsplitomicin scaffolds have been established. Although the synthesis of racemic β-arylsplitomicin derivatives has been extensively carried out,2 limited progress on the synthesis of enantioenriched β-arylsplitomicins has been reported.1g,3 In 2008, Jung and coworkers reported a cyclization of β-naphthol with propiolic acid, followed by a chiral rhodium-catalyzed conjugated addition of arylboronic acids to benzo[f]chromen-3-one, delivering the β-arylsplitomicin in excellent enantioselectivity but with poor yield (18%).1g Lately, Zhang and Feng employed a chiral bifunctional thiourea–tertiary amine-catalyzed annulation between β-naphthols and akylidene Meldrum's acids to afford β-arylsplitomicins in good yields but with moderate enantioselectivity.3a
Recently, N-heterocyclic carbene (NHC)4-catalyzed reactions involving α,β-unsaturated acyl azolium intermediates generated from enals,3b,5 ynals,6 α-bromoenals,7 α,β-unsaturated acyl fluorides,8 α,β-unsaturated esters,8a,9 and α,β-unsaturated carboxylic acids10 have received great attention and enjoyed rapid development. In 2009, Bode and coworkers reported an elegant work on the NHC-catalyzed enantioselective Claisen rearrangement of ynals and 2-naphthols, affording β-phenylsplitomicin in 79% yield with 68% ee.3b,c Later, the asymmetric annulation of 2-bromoenals and β-naphthols was also realized by Biju and coworkers to deliver β-phenylsplitomicin in moderate yield and enantioselectivity.3d Despite these pioneering studies, highly enantioselective synthesis of β-arylsplitomicins by NHC catalysis remains to be an unsolved project (Fig. 2). In particular, the chemoselectivity of annulation over ester byproduct formation is responsible for the low yield of the desired splitomicin product. As part of our continuous interest in NHC catalysis,5d,11 we envisioned that the introduction of novel chiral triazolium salts might provide a solution for this highly challenging but interesting reaction. In this paper, we report the synthesis of novel NHCs derived from L-phenylalanine and their excellent performance in the annulation reaction of enals with 2-naphthols.5
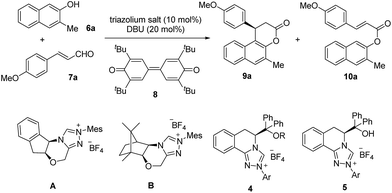
Our study began with the preparation of a series of triazolium salts from the commercially available methyl L-phenylalaninate hydrochloride (Scheme 1). The synthesis commenced with the transformation of L-phenylalaninate hydrochloride to the lactam.12 The addition of a Grignard reagent to the ester group in lactam 1 and protection of the hydroxyl group by TMSOTf or TBSOTf afforded the corresponding silyl ethers.13 Several homologous triazolium salts 4a–4g were then prepared by following the procedures developed by Rovis and coworkers.14 Desilylation of 4a–4b under acidic reflux conditions gave triazolium salts 5a–5b bearing a free hydroxyl group.15 Furthermore, the structure of 4a was confirmed by an X-ray crystallographic analysis as shown in Scheme 1.
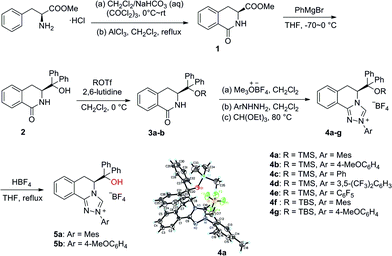 | ||
| Scheme 1 Synthesis of chiral triazolium salts derived from L-phenylalanine and the X-ray crystal structure of 4a. | ||
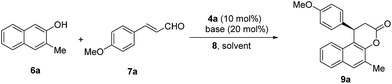
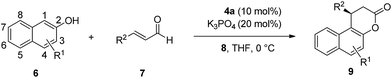
With these triazolium salts in hand, we began to test their catalytic activity in the annulation reaction between 3-methylnaphthalen-2-ol (6a) and (E)-3-(4-methoxyphenyl)acrylaldehyde (7a). To our delight, in the presence of 10 mol% N-Mes substituted triazolium salt 4a, 20 mol% DBU and 1 equivalent of quinone 8 as oxidant in THF, the reaction proceeded smoothly to afford the corresponding β-arylsplitomicin in 68% yield and 78% ee, along with 24% yield of byproduct ester 10a (Table 1, entry 3). Interestingly, the enantioselectivity obtained by 4a is much higher than those obtained by previously reported NHC precursors A and B (Table 1, entries 1–2). The ee value of the product could be further increased to 84% ee when 1 equivalent of 7a was used (Table 1, entry 5). However when 7a was used in excess, it was difficult to isolate 9a from the reaction mixture due to the similar Rf value between 7a and 9a. Other NHC precursors with different substituents were then investigated. As summarized in Table 1, NHC precursors 4b and 4c, bearing 4-MeOC6H4 and C6H5 groups on the N atom, respectively, gave moderate enantioselectivity and low conversions (Table 1, entries 7–8). In the presence of NHC precursors either bearing an electron-withdrawing group such as 3,5-(CF3)2C6H3 (4d) and C6F5 (4e) or a TBS-protected hydroxyl group (4f, 4g), this annulation reaction became sluggish and only gave trace amounts of the desired product (Table 1, entries 9–12). Notably, NHC precursor 5a bearing a free OH group gave comparable results (Table 1, entry 5 vs. entry 13).15
| Entry | Triazolium salt | 6a/7a | Time (h) | Yieldb (%) | eec (%) | 9a/10ad |
|---|---|---|---|---|---|---|
| a Reaction conditions: 7a (0.2 mmol), 8 (0.2 mmol), triazolium salt (0.02 mmol), DBU (0.04 mmol) in THF (2.0 mL) at rt. b Isolated yield for 9a. c Determined by HPLC. d Determined by 1H NMR of the crude reaction mixture. e Isolated yield for 10a. f Determined after isolation. | ||||||
| 1 | A | 3 | 4 | 74 | −24 | — |
| 2 | B | 3 | 15 | 58 | −24 | — |
| 3 | 4a | 3 | 2 | 68 | 78 | — |
| 4 | 4a | 2 | 2 | 68 | 81 | — |
| 5 | 4a | 1 | 2 | 61 | 84 | 1/0.40 |
| 6 | 4a | 0.5 | 2 | — | 85 | — |
| 7 | 4b | 1 | 24 | 34 | 53 | — |
| 8 | 4c | 1 | 48 | 8 | 51 | — |
| 9 | 4d | 1 | 30 | <5 | — | — |
| 10 | 4e | 1 | 30 | <5 | — | — |
| 11 | 4f | 1 | 30 | 24 (30)e | 75 | 1/1.27f |
| 12 | 4g | 1 | 48 | 7 | 42 | — |
| 13 | 5a | 1 | 2.5 | 62 | 84 | 1/0.42 |
| 14 | 5b | 1 | 24 | 30 | 54 | — |
With 4a as the NHC precursor, further optimization of the reaction conditions was carried out. The results are summarized in Table 2. Various solvents such as THF, toluene, CH2Cl2, ether and dioxane were tolerated well, providing the desired product in moderate to good yields and enantioselectivity. The reaction in THF gave the highest ee (Table 2, entry 1, 61% yield, 84% ee), although toluene gave a higher yield (Table 2, entry 2, 83% yield, 74% ee). Lowering the reaction temperature to 0 °C led to an increased yield and ee (Table 2, entry 8, 72% yield, 85% ee).
| Entry | Solvent | Base | T (°C) | Time (h) | Yieldb (%) | eec (%) | 9a/10ad |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 6a (0.2 mmol), 7a (0.2 mmol), 8 (0.2 mmol), 4a (0.02 mmol), base (0.04 mmol) in solvent (2.0 mL). b Isolated yield for 9a. c Determined by HPLC. d Determined by 1H NMR of the crude reaction mixture. e Determined after isolation. | |||||||
| 1 | THF | DBU | rt | 3 | 61 | 84 | 1/0.40 |
| 2 | Toluene | DBU | rt | 18 | 83 | 74 | 1/0.16 |
| 3 | Et2O | DBU | rt | 3 | 64 | 78 | 1/0.42 |
| 4 | DCM | DBU | rt | 48 | 76 | 65 | 1/0.19 |
| 5 | Dioxane | DBU | rt | 16 | 57 | 80 | 1/0.62 |
| 6 | PhOMe | DBU | rt | 12 | 75 | 73 | 1/0.13e |
| 7 | CH3CN | DBU | rt | 48 | 43 | 53 | 1/0.30e |
| 8 | THF | DBU | 0 | 23 | 72 | 85 | 1/0.32 |
| 9 | Cyclohexane | DBU | rt | 24 | 39 | 81 | 1/0.27e |
| 10 | THF | KOAc | 0 | 72 | 72 | 84 | 1/0.27 |
| 11 | THF | Cs2CO3 | 0 | 2 | 67 | 86 | 1/0.31 |
| 12 | THF | Cs2CO3 | −10 | 8 | 73 | 86 | 1/0.20 |
| 13 | THF | K2CO3 | 0 | 8 | 72 | 85 | 1/0.28 |
| 14 | THF | K3PO4 | 0 | 8 | 72 | 87 | 1/0.23 |
| 15 | THF | KOtBu | 0 | 1.5 | 72 | 80 | 1/0.38 |
| 16 | THF | KHCO3 | 0 | 72 | 61 | 83 | 1/0.28 |
| 17 | THF | KHMDS | 0 | 1.5 | 73 | 81 | 1/0.36 |
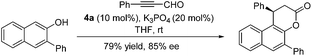
Several bases including DBU, KOAc, Cs2CO3, K3PO4, KHCO3 and KHMDS were further evaluated, and most of them gave the desired product in good yields and enantioselectivity (Table 2, entries 10–17, 61–73% yields, 80–87% ee). Among them, K3PO4 gave the best result (Table 2, entry 14, 72% yield, 87% ee). Other NHC precursors synthesized by utilizing different Grignard reagents were evaluated in this annulation reaction; unfortunately no better results were obtained compared with 4a (see the ESI† for details). Under the optimized conditions (10 mol% of 4a and 20 mol% of K3PO4 in THF at 0 °C), the reactions of various 2-naphthols with α,β-unsaturated aldehydes were tested to investigate the generality of the reaction. The results are summarized in Table 3. Firstly, the effect of the substituents on the naphthol ring was investigated. Various 3-substituted 2-naphthols (OMe, OBn, Bn, 4-CF3C6H4 and OCH2CHCH2) were all tolerated well, and their corresponding annulation products were obtained in 46–90% yields and 84–90% ee (Table 3, entries 1–7). When 3-Br, 3-Ph and 3-PhCONH substituted 2-naphthols were used, relatively longer time was required to obtain the satisfactory results (Table 3, entries 8–10; 73–84% yields, 76–95% ee). When the 6-COOMe 2-naphthol was used, the annulation product was obtained in 86% yield and 83% ee (Table 3, entry 11), and the 7-OMe 2-naphthol gave excellent enantioselectivity with moderate yield (Table 3, entry 12; 62% yield, 91% ee). Next a wide range of substituted cinnamaldehydes bearing either an electron-donating or electron-withdrawing group were further tested. In all cases, the annulation proceeded smoothly to afford their corresponding products in good yields and enantioselectivity (Table 3, entries 15–23; 82–96% yields, 71–96% ee). Notably, (E)-3-(furan-2-yl)acrylaldehyde and (E)-but-2-enal were also suitable substrates, affording annulation products in 91% yield, 85% ee and 65% yield, 75% ee respectively (Table 3, entries 24–25).
| Entry | R1 | R2 | 9 | Time (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 6 (0.2 mmol), 7 (0.2 mmol), 8 (0.2 mmol), 4a (0.02 mmol), K3PO4 (0.04 mmol) in THF (2.0 mL) at 0 °C unless noted otherwise. b Isolated yield for 9. c Determined by HPLC. d At rt. | ||||||
| 1 | 3-Me | 4-MeOC6H4 | 9a | 12 | 73 | 87 |
| 2 | H | 4-MeOC6H4 | 9b | 11 | 46 | 88 |
| 3 | 3-OMe | 4-MeOC6H4 | 9c | 18 | 75 | 88 |
| 4 | 3-OBn | 4-MeOC6H4 | 9d | 4 | 79 | 90 |
| 5 | 3-OCH2CHCH2 | 4-MeOC6H4 | 9e | 13 | 76 | 88 |
| 6 | 3-Bn | 4-MeOC6H4 | 9f | 5 | 90 | 84 |
| 7 | 3-(4-CF3C6H4) | 4-MeOC6H4 | 9g | 5.5 | 87 | 88 |
| 8 | 3-Ph | 4-MeOC6H4 | 9h | 24 | 84 | 95 |
| 9 | 3-Br | 4-MeOC6H4 | 9i | 24 | 73 | 82 |
| 10 | 3-PhCONH | 4-MeOC6H4 | 9j | 5 days | 73 | 76 |
| 11 | 6-COOMe | 4-MeOC6H4 | 9k | 12 | 86 | 83 |
| 12 | 7-OMe | 4-MeOC6H4 | 9l | 12 | 62 | 91 |
| 13 | H | Ph | 9m | 36 | 60 | 85 |
| 14 | 3-Me | Ph | 9n | 24 | 65 | 79 |
| 15 | 3-Ph | Ph | 9o | 8.5 | 82 | 91 |
| 16 | 3-Ph | 2-MeC6H4 | 9p | 36 | 89 | 96 |
| 17 | 3-Ph | 2-MeOC6H4 | 9q | 30 | 85 | 92 |
| 18 | 3-Ph | 4-MeC6H4 | 9r | 6 | 92 | 92 |
| 19 | 3-Ph | 4-Me2NC6H4 | 9s | 36 | 91 | 92 |
| 20 | 3-Ph | 4-FC6H4 | 9t | 1.5 | 94 | 86 |
| 21 | 3-Ph | 4-ClC6H4 | 9u | 2 | 95 | 85 |
| 22 | 3-Ph | 4-BrC6H4 | 9v | 6 | 96 | 85 |
| 23 | 3-Me | 4-MeCO2C6H4 | 9w | 5.5 | 82 | 71 |
| 24 | 3-Ph | 2-Furyl | 9x | 3 | 91 | 85 |
| 25d | 3-Ph | Me | 9y | 5 days | 65 | 75 |
In order to determine the absolute configuration of the products, a crystal of enantiopure 9h was obtained and X-ray crystallographic analysis determined its configuration as R.
To our great delight, when an ynal or 2-bromoenal was used, this annulation reaction occurred without external oxidant to give comparable results by running the reaction at room temperature (eqn (1) and (2)).
 | (1) |
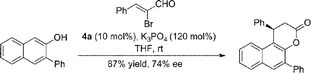 | (2) |
In order to shed light on the reaction mechanism,16 compound 10o was synthesized and subjected to the identical reaction conditions. Product 9o was isolated in 22% yield with 48% ee after 7 days at room temperature, along with 6h in 44% yield (eqn (3)). In addition, real time monitoring of the reaction of 6a with 7a by 1H NMR showed that there was little change in the ratio of product 9a to byproduct 10a during the progress of the reaction, which suggested that an α,β-unsaturated acyl azolium intermediate is directly generated from the enal with the NHC and oxidant rather than from byproduct 10a (see the ESI† for details).
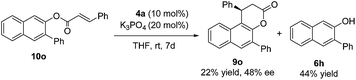 | (3) |
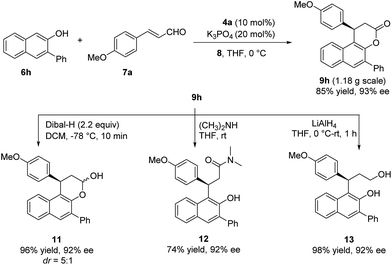
A gram-scale reaction between 6h and 7a was carried out to give the desired product in 85% yield and 93% ee without any loss of either yield or enantioselectivity, which further demonstrated the practicality of this methodology. Highly enantioenriched products obtained here can undergo diverse transformations. For example, the reduction of 9h by DIBAL–H gave hemiacetal 11 in 96% yield and 92% ee. Ring opening of 9h by dimethylamine or LiAlH4 led to amide 12 or diol 13 respectively in high yield without loss of enantiomeric purity (Scheme 2).
Conclusions
We have synthesized a series of novel chiral triazolium salts derived from L-phenylalanine. The NHC derived from 4a is found to be a highly efficient catalyst for annulation of enals with 2-naphthols under oxidative conditions. Structurally diverse β-arylsplitomicins were formed in good yields with up to 96% ee. Understanding the excellent performance of these novel triazolium salts and further exploration of their application in asymmetric reactions are currently underway in our lab.Acknowledgements
We thank the National Basic Research Program of China (973 Program 2015CB856600) and National Natural Science Foundation of China (21272253, 21332009, 21361140373, 21421091) for generous financial support.Notes and references
- (a) G. Speranza, A. D. Meo, P. Manitto, D. Monti and G. Fontana, J. Agric. Food Chem., 1996, 44, 274 CrossRef CAS; (b) P. Pahari, M. K. Kharel, M. D. Shepherd, S. G. van Lanen and J. Rohr, Angew. Chem., Int. Ed., 2012, 51, 1216 CrossRef CAS PubMed; (c) A. Bedalov, T. Gatbonton, W. P. Irvine, D. E. Gottschling and J. A. Simon, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 15113 CrossRef CAS PubMed; (d) J. Posakony, M. Hirao, S. Stevens, J. A. Simon and A. Bedalov, J. Med. Chem., 2004, 47, 2635 CrossRef CAS PubMed; (e) M. Freitag, J. Schemies, T. Larsen, K. E. Gaghlab, F. Schulz, T. Rumpf, M. Jung and A. Link, Bioorg. Med. Chem., 2011, 19, 3669 CrossRef CAS PubMed; (f) R. Jangir and N. P. Argade, RSC Adv., 2014, 4, 5531 RSC; (g) R. C. Neugebauer, U. Uchiechowska, R. Meier, H. Hruby, V. Valkov, E. Verdin, W. Sippl and M. Jung, J. Med. Chem., 2008, 51, 1203 CrossRef CAS PubMed.
- Synthesis of racemic β-arylsplitomicins: (a) J. D. Simpson and H. Stephen, J. Chem. Soc., 1956, 1382 RSC; (b) S. Aoki, C. Amamoto, J. Oyamada and T. Kitamura, Tetrahedron, 2005, 61, 9291 CrossRef CAS PubMed; (c) Z. Zhang, Y. Ma and Y. Zhao, Synlett, 2008, 1091 CAS; (d) S. Gao, C. H. Tsai and C.-F. Yao, Synlett, 2009, 949 CAS; (e) D. P. Kamat, S. G. Tilve and V. P. Kamat, Tetrahedron Lett., 2012, 53, 4469 CrossRef CAS PubMed; (f) M. M. Naik, D. P. Kamat, S. G. Tilve and V. P. Kamat, Tetrahedron, 2014, 70, 5221 CrossRef CAS PubMed.
- Synthesis of enantioenriched β-arylsplitomicins: (a) J.-Y. Wang, H. Zhang, Y.-H. Liao, W.-C. Yuan, Y.-J. Feng and X.-M. Zhang, Synlett, 2012, 23, 796 CrossRef CAS PubMed; (b) J. Kaeobamrung, J. Mahatthananchai, P. Zheng and J. W. Bode, J. Am. Chem. Soc., 2010, 132, 8810 CrossRef CAS PubMed; (c) J. Mahatthananchai, J. Kaeobamrung and J. W. Bode, ACS Catal., 2012, 2, 494 CrossRef CAS PubMed; (d) S. R. Yetra, A. Bhunia, A. Patra, M. V. Mane, K. Vanka and A. T. Biju, Adv. Synth. Catal., 2013, 355, 1089 CrossRef CAS PubMed.
- Selected reviews on NHC catalysis: (a) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606 CrossRef CAS PubMed; (b) V. Nair, S. Vellalath and B. P. Babu, Chem. Soc. Rev., 2008, 37, 2691 RSC; (c) A. T. Biju, N. Kuhl and F. Glorius, Acc. Chem. Res., 2011, 44, 1182 CrossRef CAS PubMed; (d) V. Nair, R. S. Menon, A. T. Biju, C. R. Sinu, R. R. Paul, A. Jose and V. Sreekumar, Chem. Soc. Rev., 2011, 40, 5336 RSC; (e) X. Bugaut and F. Glorius, Chem. Soc. Rev., 2012, 41, 3511 RSC; (f) J. Douglas, G. Churchill and A. D. Smith, Synthesis, 2012, 44, 2295 CrossRef CAS PubMed; (g) A. Grossmann and D. Enders, Angew. Chem., Int. Ed., 2012, 51, 314 CrossRef CAS PubMed; (h) J. Izquierdo, G. E. Hutson, D. T. Cohen and K. A. Scheidt, Angew. Chem., Int. Ed., 2012, 51, 11686 CrossRef CAS PubMed; (i) C. E. I. Knappke, A. Imami and A. J. von Wangelin, ChemCatChem, 2012, 4, 937 CrossRef CAS PubMed; (j) H. U. Vora, P. Wheeler and T. Rovis, Adv. Synth. Catal., 2012, 354, 1617 CrossRef CAS PubMed; (k) S. De Sarkar, A. Biswas, R. C. Samanta and A. Studer, Chem.–Eur. J., 2013, 19, 4664 CrossRef CAS PubMed; (l) S. J. Ryan, L. Candish and D. W. Lupton, Chem. Soc. Rev., 2013, 42, 4906 RSC; (m) D. C. M. Albanese and N. Gaggero, Eur. J. Org. Chem., 2014, 5631 CrossRef CAS PubMed; (n) J. Mahatthananchai and J. W. Bode, Acc. Chem. Res., 2014, 47, 696 CrossRef CAS PubMed; (o) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485 CrossRef CAS PubMed.
- (a) S. De Sarkar and A. Studer, Angew. Chem., Int. Ed., 2010, 49, 9266 CrossRef CAS PubMed; (b) B. Wanner, J. Mahatthananchai and J. W. Bode, Org. Lett., 2011, 13, 5378 CrossRef CAS PubMed; (c) A. Biswas, S. De Sarkar, R. Fröhlich and A. Studer, Org. Lett., 2011, 13, 4966 CrossRef CAS PubMed; (d) Z.-Q. Rong, M.-Q. Jia and S.-L. You, Org. Lett., 2011, 13, 4080 CrossRef CAS PubMed; (e) A. G. Kravina, J. Mahatthananchai and J. W. Bode, Angew. Chem., Int. Ed., 2012, 51, 9433 CrossRef CAS PubMed; (f) S. Bera, R. C. Samanta, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2014, 53, 9622 CrossRef CAS PubMed.
- (a) Z.-Q. Zhu and J.-C. Xiao, Adv. Synth. Catal., 2010, 352, 2455 CrossRef CAS PubMed; (b) E. Lyngvi, J. W. Bode and F. Schoenebeck, Chem. Sci., 2012, 3, 2346 RSC; (c) F. Romanov-Michailidis, C. Besnard and A. Alexakis, Org. Lett., 2012, 14, 4906 CrossRef CAS PubMed; (d) Y. Lu, W.-F. Tang, Y. Zhang, D. Du and T. Lu, Adv. Synth. Catal., 2013, 355, 321 CrossRef CAS PubMed; (e) Z.-Q. Zhu, X.-L. Zheng, N.-F. Jiang, X. Wan and J.-C. Xiao, Chem. Commun., 2011, 47, 8670 RSC.
- (a) F.-G. Sun, L.-H. Sun and S. Ye, Adv. Synth. Catal., 2011, 353, 3134 CrossRef CAS PubMed; (b) C. Yao, D. Wang, J. Lu, T. Li, W. Jiao and C. Yu, Chem.–Eur. J., 2012, 18, 1914 CrossRef CAS PubMed; (c) B. Zhang, P. Feng, Y. Cui and N. Jiao, Chem. Commun., 2012, 48, 7280 RSC; (d) C.-S. Yao, W.-H. Jiao, Z.-X. Xiao, R. Liu, T.-J. Li and C.-X. Yu, Tetrahedron, 2013, 69, 1133 CrossRef CAS PubMed; (e) S. R. Yetra, T. Kaicharla, S. S. Kunte, R. G. Gonnade and A. T. Biju, Org. Lett., 2013, 15, 5202 CrossRef CAS PubMed; (f) S. Mondal, S. R. Yetra, A. Patra, S. S. Kunte, R. G. Gonnade and A. T. Biju, Chem. Commun., 2014, 50, 14539 RSC; (g) Q. Ni, X.-X. Song, G. Raabe and D. Enders, Chem.–Asian J., 2014, 9, 1535 CrossRef CAS PubMed; (h) S. R. Yetra, T. Roy, A. Bhunia, D. Porwal and A. T. Biju, J. Org. Chem., 2014, 79, 4245 CrossRef CAS PubMed.
- (a) S. J. Ryan, L. Candish and D. W. Lupton, J. Am. Chem. Soc., 2009, 131, 14176 CrossRef CAS PubMed; (b) S. J. Ryan, L. Candish and D. W. Lupton, J. Am. Chem. Soc., 2011, 133, 4694 CrossRef CAS PubMed; (c) L. Candish and D. W. Lupton, J. Am. Chem. Soc., 2013, 135, 58 CrossRef CAS PubMed; (d) L. Candish, C. M. Forsyth and D. W. Lupton, Angew. Chem., Int. Ed., 2013, 52, 9149 CrossRef CAS PubMed.
- (a) J. Cheng, Z. Huang and Y. R. Chi, Angew. Chem., Int. Ed., 2013, 52, 8592 CrossRef CAS PubMed; (b) L. Candish, A. Levens and D. W. Lupton, J. Am. Chem. Soc., 2014, 136, 14397 CrossRef CAS PubMed.
- X.-Y. Chen, Z.-H. Gao, C.-Y. Song, C.-L. Zhang, Z.-X. Wang and S. Ye, Angew. Chem., Int. Ed., 2014, 53, 11611 CrossRef CAS PubMed.
- (a) Y. Li, Z. Feng and S.-L. You, Chem. Commun., 2008, 2263 RSC; (b) G.-Q. Li, Y. Li, L.-X. Dai and S.-L. You, Adv. Synth. Catal., 2008, 350, 1258 CrossRef CAS PubMed; (c) M.-Q. Jia, Y. Li, Z.-Q. Rong and S.-L. You, Org. Biomol. Chem., 2011, 9, 2072 RSC; (d) K.-J. Wu, G.-Q. Li, Y. Li, L.-X. Dai and S.-L. You, Chem. Commun., 2011, 47, 493 RSC; (e) M.-Q. Jia and S.-L. You, Chem. Commun., 2012, 48, 6363 RSC; (f) M.-Q. Jia and S.-L. You, ACS Catal., 2013, 3, 622 CrossRef CAS.
- (a) J. H. Tsai, L. R. Takaoka, N. A. Powell and J. S. Nowick, Org. Synth., 2002, 78, 220 CrossRef CAS; (b) D. Dou, P. Viwanathan, Y. Li, G. He, K. R. Alliston, G. H. Lushington, J. D. Brown-Clay, R. Padmanabhan and W. C. Groutas, J. Comb. Chem., 2010, 12, 836 CrossRef CAS PubMed.
- (a) Y.-R. Zhang, L. He, X. Wu, P.-L. Shao and S. Ye, Org. Lett., 2007, 10, 277 CrossRef PubMed; (b) D. Enders and J. Han, Tetrahedron: Asymmetry, 2008, 19, 1367 CrossRef CAS PubMed.
- M. S. Kerr, J. R. de Alaniz and T. Rovis, J. Org. Chem., 2005, 70, 5725 CrossRef CAS PubMed.
- For leading references on the application of NHCs bearing a free hydroxyl group: (a) L. He, Y.-R. Zhang, X.-L. Huang and S. Ye, Synthesis, 2008, 2825 CAS; (b) L. Baragwanath, C. A. Rose, K. Zeitler and S. J. Connon, J. Org. Chem., 2009, 74, 9214 CrossRef CAS PubMed; (c) L.-H. Sun, L.-T. Shen and S. Ye, Chem. Commun., 2011, 47, 10136 RSC; (d) H. Lv, W.-Q. Jia, L.-H. Sun and S. Ye, Angew. Chem., Int. Ed., 2013, 52, 8607 CrossRef CAS PubMed; (e) L.-H. Sun, Z.-Q. Liang, W.-Q. Jia and S. Ye, Angew. Chem., Int. Ed., 2013, 52, 5803 CrossRef CAS PubMed; (f) H.-M. Zhang, Z.-H. Gao and S. Ye, Org. Lett., 2014, 16, 3079 CrossRef CAS PubMed; (g) Z.-Q. Liang, Z.-H. Gao, W.-Q. Jia and S. Ye, Chem.–Eur. J., 2015, 21, 1868 CrossRef CAS PubMed.
- (a) J. Mahatthananchai, P. Zheng and J. W. Bode, Angew. Chem., Int. Ed., 2011, 50, 1673 CrossRef CAS PubMed; (b) R. C. Samanta, B. Maji, S. De Sarkar, K. Bergander, R. Fröehlich, C. Müeck-Lichtenfeld, H. Mayr and A. Studer, Angew. Chem., Int. Ed., 2012, 51, 5234 CrossRef CAS PubMed; (c) E. Lyngvi, J. W. Bode and F. Schoenebeck, Chem. Sci., 2012, 3, 2346 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: CCDC 1051429 and 1051473. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc00731c |
| This journal is © The Royal Society of Chemistry 2015 |