Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
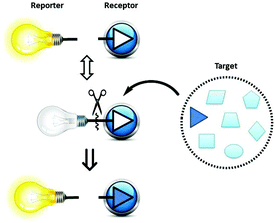
Reaction-based Indicator displacement Assay (RIA) for the selective colorimetric and fluorometric detection of peroxynitrite†
Xiaolong
Sun
a,
Karel
Lacina
ae,
Elena C.
Ramsamy
a,
Stephen E.
Flower
a,
John S.
Fossey
b,
Xuhong
Qian
c,
Eric V.
Anslyn
*d,
Steven D.
Bull
*a and
Tony D.
James
*a
aDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk; s.d.bull@bath.ac.uk
bSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham, West Midlands, B15 2TT, UK
cSchool of Pharmacy, East China University of Science and Technology, Meilong Road 130, Shanghai 200237, China
dDepartment of Chemistry and Biochemistry, The University of Texas at Austin, Austin, Texas 78712, USA. E-mail: anslyn@austin.utexas.edu
eCEITEC, Masaryk University, Kamenice 5, 62500, Brno, Czech Republic
First published on 6th March 2015
Abstract
Using the self-assembly of aromatic boronic acids with Alizarin Red S (ARS), we developed a new chemosensor for the selective detection of peroxynitrite. Phenylboronic acid (PBA), benzoboroxole (BBA) and 2-(N,N-dimethylaminomethyl)phenylboronic acid (NBA) were employed to bind with ARS to form the complex probes. In particular, the ARS–NBA system with a high binding affinity can preferably react with peroxynitrite over hydrogen peroxide and other ROS/RNS due to the protection of the boron via the solvent-insertion B–N interaction. Our simple system produces a visible colorimetric change and on–off fluorescence response towards peroxynitrite. By coupling a chemical reaction that leads to an indicator displacement, we have developed a new sensing strategy, referred to herein as RIA (Reaction-based Indicator displacement Assay).
Introduction
Peroxynitrite (ONOO−) – a combination of nitric oxide (NO˙) and the superoxide radical anion (O2−˙) – was first discovered as a biological endogenous oxidant in 1990.1 It is recognised as a strong oxidant with a very short lifetime (∼10 ms) in physiological and pathological processes. Peroxynitrite is a highly reactive molecule involved in cell signal transduction, and leads to apoptosis in HL-60 cells, and PC-12 cells. Many biomolecules are oxidised and/or nitrated by peroxynitrite-derived radicals, including DNA, tyrosine residues, thiols, and unsaturated fatty-acid-containing phospholipids. Endogenous peroxynitrite formation, and associated protein nitration, has been implicated in Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis, viral myocarditis, septic shock, cardiac allograft, transplant coronary artery disease, idiopathic dilated cardiomyopathy, atrial fibrillation, hypercholesterolemia, atherosclerosis, hypertension, diabetes, diabetic nephropathy, and traumatic brain injury.2,3 Thus, the importance of peroxynitrite has captured the attention of many groups who seek effective and selective approaches for its detection.Among the powerful tools available for ONOO− detection are small-molecule probes which are attractive owing to their high sensitivity, easy manipulation, coupled with widely available instrumentation. To the best of our knowledge, limited work has been carried out in the development of small-molecule probes for the selective colorimetric sensing of peroxynitrite.4,5 The successful application of indicator displacement arrays, by Anslyn,6–11 Severin,12 Singaram,13–18 and reaction-based small-molecular fluorescent probes for chemoselective ROS detection, developed by Chang,19,20 inspired us to combine these two approaches and develop RIA: “Reaction-based Indicator displacement Assay” for the selective detection of peroxynitrite (ONOO−) (Scheme 1).
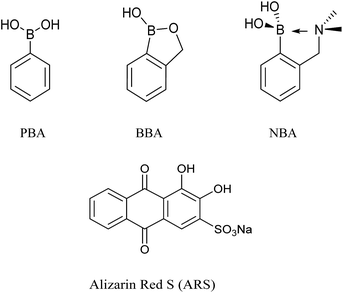
Phenylboronic acid (PBA, pKa = 8.83), benzoboroxole (BBA, pKa = 7.30), and 2-(N,N-dimethylaminomethyl)phenylboronic acid (NBA, pKa = 6.70) are three representative receptors among common boronic acid motifs (Fig. 1), which have been extensively applied in the area of monosaccharides sensing,21–23 while, Alizarin Red S (ARS), has been successfully employed as a general optical reporter for investigating the binding of boronic acids with carbohydrates (Fig. 1).23,24 Also, some of our previous research has used the binding and analyte-mediated release of Alizarin Red S from hydrogel-bound boronic acids.25
Bearing this previous work in mind, we decided to evaluate the sensing systems (ARS–BAs), which are formed by the conjugation of aromatic boronic acid receptors with Alizarin Red S in situ, for the selective detection of peroxynitrite.
Results and discussion
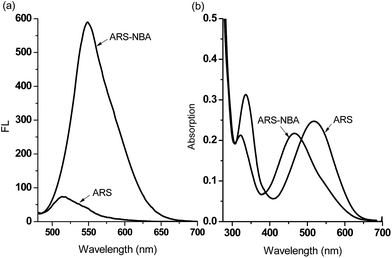
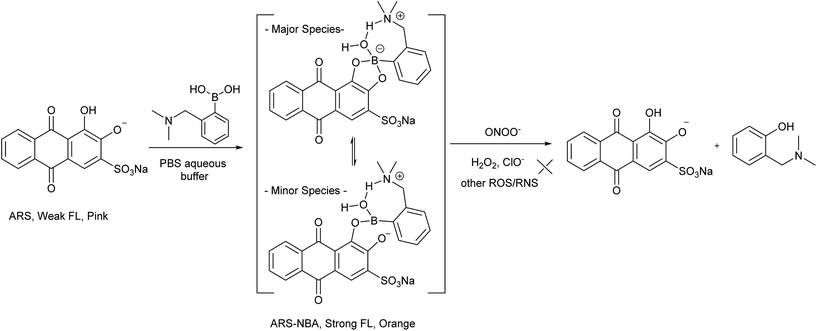
When buffered at neutral pH, we found only small changes occur i.e. only a slight blue-shift was observed in the UV-Vis absorption and a very small fluorescence response due to binding of PBA (200 μM), BBA (200 μM), NBA (200 μM) with ARS (50 μM), respectively (FS1, FS2). Also, the almost identical pink colours of the solutions clearly indicate weak binding between ARS and three boronic acid receptors at pH 7.30 (FS2). Therefore, we decided to use a higher pH working environment to enhance the binding of boronic acid with the catechol unit of ARS.As can be seen from Fig. 2 in 52.1% MeOH/H2O PBS buffer (pH 8.10), compounds NBA, PBA and BBA behaved differently with ARS and in particular for the fluorescence, there is ca. 15.0-fold fluorescence increase for NBA (F/F0) while only a 3.4-fold increase is observed for PBA and a 2.8-fold increase with BBA (FS3), respectively. In terms of absorption, NBA (200 μM) produced the largest shift in wavelength from λmax = 520 nm to λmax = 465 nm (55 nm blue-shift) on binding with ARS, in comparison with a 10 nm blue-shift for BBA and a 20 nm blue-shift for PBA was observed. A significant colour change from pink to orange in the presence of NBA (200 μM) with ARS (50 μM) was observed (FS4), while BBA (200 μM) and PBA (200 μM) did not change significantly even after one night of stirring. Based upon recent results, the binding between NBA and ARS (binding constant k = 7200 ± 92 M−1, FS5), is most likely enhanced by the N–B interaction via a solvent insertion (Scheme 2). From the boron NMR (FS6), the NBA boron is clearly sp3 in nature and remains sp3 with added ARS under neutral and alkaline conditions (16 ppm) due to solvent insertion. The binding between ARS and NBA results in two species (one major and one minor with approximately 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) as described by Benkovic.26 (Scheme 2)
1 ratio) as described by Benkovic.26 (Scheme 2)
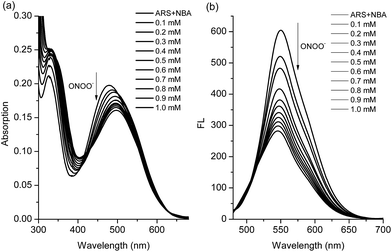
We have previously demonstrated the N–B interaction provides protection for boronic acids towards common oxidants resulting in selectivity towards stronger reactive oxygen species such as peroxynitrite (ONOO−). To a preformed sensing ensemble, peroxynitrite (ONOO−) mediated oxidation of the aryl boronate ARS adduct led to phenols and release of ARS, thereby giving a fluorescence decrease and an accompanying red-shifted absorbance wavelength (Fig. 3).
As can be seen from the dose-dependent absorption response in Fig. 3a, when various concentrations of ONOO− (0–1.0 mM) were added to a solution of ARS–NBA (ARS, 50 μM; NBA, 200 μM), a decrease in the absorption at λmax = 465 nm was observed with the appearance of a red-shifted band centred at 500 nm (A500nm/A465nm = 1.15). In the relationship between A500nm/A465nm and concentration of peroxynitrite, there was rapid absorption increase in the presence of peroxynitrite below 0.4 mM while the reaction between ARS–NBA and ONOO− (0.4–1.0 mM) was reduced due to saturation (FS7). In Fig. 3b, the fluorescence of the ARS–NBA complex was reduced to a ca. 0.45 of its original value (F (in the presence of ONOO−)/F0 (in the absence of ONOO−)) over a concentration range of ONOO− from 0 to 1 mM. A linear relationship (R2 = 0.994) was observed between the relative fluorescence ((F − F0)/F at λ550nm) and the peroxynitrite (ONOO−) concentration (0.4–1.0 mM, FS8).
Furthermore, due to the very short lifetime (∼10 ms) and low steady-state concentration (nM range) of peroxynitrite, we monitored the time dependant response towards peroxynitrite (using both UV-Vis and Fluorescence methods). From these time-drive experiments the chemical reaction between probe ARS–NBA and peroxynitrite proceeds rapidly (k′ = 4.39 s−1, t1/2 = 0.14 s, k2 = 8.78 × 104 s−1 M−1, FS9 and FS10). Using dose-dependent titrations, we obtained a LOD (limit of detection) of 5.4 μM for the sensing of peroxynitrite (FS8). Importantly, these results indicate that the ARS–NBA system with an N–B interaction produce significant colorimetric and fluorometric response towards peroxynitrite.
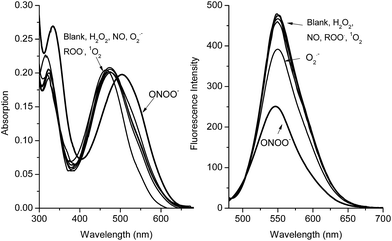
As reported previously, hydrogen peroxide (H2O2), hypochlorite (ClO−) and peroxynitrite (ONOO−) react with boronate-based compounds to produce the phenol analogues.27,28 We have previously demonstrated that the solvent-insertion interaction between the amine and boron provides a protection for the boronic acid towards oxidation and results in the selective detection of peroxynitrite (ONOO−) over hydrogen peroxide (H2O2) and hypochlorite (ClO−) and other ROS/RNS species.29,30 Noticeably, we also observed that H2O2 is much more reactive with increasing pH. However, when we use NBA (2-(N,N-dimethylaminomethyl)phenylboronic acid) with ARS this provides a complex with a strong N–B interaction resulting in the selective sensing of peroxynitrite (ONOO−) over hydrogen peroxide (H2O2) and other ROS/RNS species.
Both the absorption and emission spectra (Fig. 4) clearly demonstrate that the RIA system has excellent selectivity for peroxynitrite (ONOO−) over other common ROS. Additionally, high concentrations of H2O2 (1 mM) were not able to change the fluorescence and absorption intensity of the ARS–NBA complex (ARS, 50 μM; NBA, 200 μM) over 60 min (FS11), indicating, that the oxidation–reduction between boron and H2O2 has been prevented by the N–B interaction. The response of the ARS–NBA sensing system to hydroxyl HO˙ (500 μM), hypochlorite ClO− (500 μM), and peroxynitrite ONOO− (500 μM) (FS12) indicate that peroxynitrite reacts significantly more ((F − F0)/F = ca. 0.45) than with hydroxyl (ca. 0.29) and hypochlorite ClO− (ca. 0.35). Importantly, both, hydroxyl HO˙ (500 μM) and hypochlorite ClO− (500 μM) respond less than peroxynitrite ONOO− (500 μM) in the RIA system FS12. However, hypochlorite (100 μM) can quench the fluorescence and change the absorption of just the ARS dye (FS13). Since hydroxyl is produced from the Fenton reaction (Fe2+ + H2O2), and is inherently coloured. Therefore, while both hydroxyl HO˙, hypochlorite ClO− are not ideal ROS to evaluate the RIA colorimetric system, the system remains peroxynitrite selective (FS12). While, the other, ROS/RNS (NO, 1O2, ROO˙, O2−˙) (Fig. 4) do not change the absorption and emission spectra of the system significantly (FS14). Thus, the ARS–NBA sensing system can be used to selectively and sensitively detect peroxynitrite over other ROS/RNS in buffered media.
Experimental
Solvents and reagents
Solvents and reagents were reagent grade unless stated otherwise and were purchased from Fisher Scientific UK, Frontier Scientific Europe Ltd, TCI UK, Alfa Aesar and Sigma-Aldrich Company Ltd and were used without further purification.Fluorescence measurements
Fluorescence measurements were performed on a Perkin-Elmer Luminescence Spectrophotometer LS 50B and Gilden Photonics Ltd. FluoroSENS, utilising Starna Silica (quartz) cuvette with 10 mm path lengths, four faces polished. Data was collected via the Perkin-Elmer FL Winlab software package. All solvents used in fluorescence measurements were HPLC or fluorescence grade and the water was deionised.UV-Vis measurement
UV-Vis measurements were performed on a Perkin-Elmer Spectrophotometer. Absorption, utilising Starna Silica (quartz) cuvette with 10 mm path lengths, two faces polished. Data was collected via the Perkin-Elmer Lambda 20 software package. Further reprocess of the data was operated in OriginPro 8.0 graphing software.Preparation of ONOO−
Peroxynitrite stock solution was prepared by the reaction of hydrogen peroxide with sodium nitrite and stabilised in basic solution, which is frequently used for the in vitro experiments.31Owing to its short-lived oxidant species, especially in the physiological pH (half-life, 10 ms), the preparation of peroxynitrite was carried out in the sodium hydroxide solution by chemical synthesis with a quenched flow reactor: using equal flow rates of the following solutions at 0 °C: 0.6 M KNO2, a solution 0.6 M in HCl and 0.7 M in H2O2, and 3 M NaOH.31 The product solution was analysed spectrophotometrically. The stock solution of peroxynitrite was fresh-made, and the pH of the stock solution (pH = 13.5) was adjusted by 12 N hydrochloric acid to pH = 12 and re-analysed each time in the detection experiments. The concentration of peroxynitrite was estimated by using an extinction coefficient of 1670 ± 50 cm−1 M−1 at 302 nm in 0.1 N sodium hydroxide (aq.).
Preparation of −OCl, H2O2 and other ROS/RNS
The concentration of −OCl was determined from the absorption at 292 nm (ε = 350 M−1 cm−1);32 the concentration of H2O2 was determined from the absorption at 240 nm (ε = 43.6 M−1 cm−1); nitric oxide (NO) was prepared by treating a sodium nitrite solution (7.3 M) with sulfuric acid (3.6 M) and its stock solution (2.0 mM) was prepared by bubbling NO into deoxygenated deionised water for 30 min;331O2 was generated by the reaction of H2O2 with NaClO; ROO˙ was generated from 2,2′-azobis(2-amidinopropane) dihydrochloride; superoxide was generated from KO2; hydroxyl radical was generated by Fenton reaction.The UV-Vis spectra and fluorescent titrations with hydrogen peroxide were carried out at 25 °C in 1/15 M PBS buffer at pH 7.30 and 52.1% methanol/aqueous PBS buffer solution at pH 8.10.
Conclusion
Using an indicator displacement assay induced by a chemical oxidation, we investigated a new chemo/biosensor for the selective sensing of peroxynitrite, using the self-assembly of boronic acid NBA with ARS as the reporter. The ARS–NBA system displays minimal response towards hydrogen peroxide (H2O2) and other ROS/RNS due to the protection provided by a B–N solvent-insertion interaction, but a large absorption and fluorescence response with peroxynitrite (ONOO−). The boronic acid receptor NBA is a good candidate for the selective detection of peroxynitrite as part of dye-displacement arrays and for biological applications, such as drug design and cell labelling experiments. The large initial change of fluorescence intensity indicates that the system might also be used as a temporal fluorescent probe to map intracellular ONOO−. Systems that produce colour changes are particular interesting since they could be incorporated into a diagnostic test paper for ONOO−, similar to universal indicator papers for pH. More importantly, ratiometric probes increase the dynamic range and permit signal-rationing, thus they provide a built-in correction for monitoring environmental effects.34,35 Therefore, we believe that this simple but powerful RIA system can be extended into new applications for the sensing of reactive oxygen and nitrogen species (ROS and RNS) both in vitro and in vivo.Acknowledgements
TDJ and XS are grateful for financial support from China Scholarship Council (CSC) and University of Bath Full Fees Scholarship. The Catalysis And Sensing for our Environment (CASE) network is thanked for research exchange opportunities.36Notes and references
- J. S. Beckman, T. W. Beckman, J. Chen, P. A. Marshall and B. A. Freeman, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 1620–1624 CrossRef CAS
.
- P. Pacher, J. S. Beckman and L. Liaudet, Physiol. Rev., 2007, 87, 315–424 CrossRef CAS PubMed
.
- C. Szabo, H. Ischiropoulos and R. Radi, Nat. Rev. Drug Discovery, 2007, 6, 662–680 CrossRef CAS PubMed
.
- D. Yang, H.-L. Wang, Z.-N. Sun, N.-W. Chung and J.-G. Shen, J. Am. Chem. Soc., 2006, 128, 6004–6005 CrossRef CAS PubMed
.
- Q. Zhang, Z. Zhu, Y. Zheng, J. Cheng, N. Zhang, Y. T. Long, J. Zheng, X. Qian and Y. Yang, J. Am. Chem. Soc., 2012, 134, 18479–18482 CrossRef CAS PubMed
.
- S. L. Wiskur, H. Ait-Haddou, J. J. Lavigne and E. V. Anslyn, Acc. Chem. Res., 2001, 34, 963–972 CrossRef CAS PubMed
.
- S. C. McCleskey, P. N. Floriano, S. L. Wiskur, E. V. Anslyn and J. T. McDevitt, Tetrahedron, 2003, 59, 10089–10092 CrossRef CAS
.
- A. Goodey, J. J. Lavigne, S. M. Savoy, M. D. Rodriguez, T. Curey, A. Tsao, G. Simmons, J. Wright, S. J. Yoo, Y. Sohn, E. V. Anslyn, J. B. Shear, D. P. Neikirk and J. T. McDevitt, J. Am. Chem. Soc., 2001, 123, 2559–2570 CrossRef CAS PubMed
.
- Y. S. Sohn, A. Goodey, E. V. Anslyn, J. T. McDevitt, J. B. Shear and D. P. Neikirk, Biosens. Bioelectron., 2005, 21, 303–312 CrossRef CAS PubMed
.
- A. P. Umali and E. V. Anslyn, Curr. Opin. Chem. Biol., 2010, 14, 685–692 CrossRef CAS PubMed
.
- B. T. Nguyen and E. V. Anslyn, Coord. Chem. Rev., 2006, 250, 3118–3127 CrossRef CAS
.
- A. Buryak and K. Severin, Angew. Chem., Int. Ed., 2004, 43, 4771–4774 CrossRef CAS PubMed
.
-
A. Schiller, B. Vilozny, R. A. Wessling and B. Singaram, in Reviews in Fluorescence 2009, ed. C. D. Geddes, 2011, vol. 6, pp. 155–191 Search PubMed
.
- D. Cordes, A. Miller, S. Gamsey and B. Singaram, Anal. Bioanal. Chem., 2007, 387, 2767–2773 CrossRef CAS PubMed
.
- A. Schiller, R. A. Wessling and B. Singaram, Angew. Chem., Int. Ed., 2007, 46, 6457–6459 CrossRef CAS PubMed
.
- Z. Sharrett, S. Gamsey, J. Fat, D. Cunningham-Bryant, R. A. Wessling and B. Singaram, Tetrahedron Lett., 2007, 48, 5125–5129 CrossRef CAS
.
- J. N. Camara, J. T. Suri, F. E. Cappuccio, R. A. Wessling and B. Singaram, Tetrahedron Lett., 2002, 43, 1139–1141 CrossRef CAS
.
- D. B. Cordes and B. Singaram, Pure Appl. Chem., 2012, 84, 2183–2202 CrossRef CAS
.
- A. R. Lippert, G. C. Van de Bittner and C. J. Chang, Acc. Chem. Res., 2011, 44, 793–804 CrossRef CAS PubMed
.
- J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef CAS PubMed
.
- S. D. Bull, M. G. Davidson, J. M. van den Elsen, J. S. Fossey, A. T. Jenkins, Y. B. Jiang, Y. Kubo, F. Marken, K. Sakurai, J. Zhao and T. D. James, Acc. Chem. Res., 2013, 46, 312–326 CrossRef CAS PubMed
.
- L. Feng, L. Wu and X. Qu, Adv. Mater., 2013, 25, 168–186 CrossRef CAS PubMed
.
- F. He, Y. Tang, M. Yu, S. Wang, Y. Li and D. Zhu, Adv. Funct. Mater., 2006, 16, 91–94 CrossRef CAS
.
- G. Springsteen and B. Wang, Chem. Commun., 2001, 1608–1609 RSC
.
- W. M. J. Ma, M. P. Pereira Morais, F. D'Hooge, J. M. H. van den Elsen, J. P. L. Cox, T. D. James and J. S. Fossey, Chem. Commun., 2009, 532–534 RSC
.
- J. W. Tomsho and S. J. Benkovic, J. Org. Chem., 2012, 77, 2098–2106 CrossRef CAS PubMed
.
- A. Sikora, J. Zielonka, M. Lopez, J. Joseph and B. Kalyanaraman, Free Radical Biol. Med., 2009, 47, 1401–1407 CrossRef CAS PubMed
.
- J. Zielonka, A. Sikora, M. Hardy, J. Joseph, B. P. Dranka and B. Kalyanaraman, Chem. Res. Toxicol., 2012, 25, 1793–1799 CrossRef CAS PubMed
.
- X. Sun, Q. Xu, G. Kim, S. E. Flower, J. P. Lowe, J. Yoon, J. S. Fossey, X. Qian, S. D. Bull and T. D. James, Chem. Sci., 2014, 5, 3368 RSC
.
- X. Sun, S. Y. Xu, S. E. Flower, J. S. Fossey, X. Qian and T. D. James, Chem. Commun., 2013, 49, 8311–8313 RSC
.
- J. W. Reed, H. H. Ho and W. L. Jolly, J. Am. Chem. Soc., 1974, 96, 1248–1249 CrossRef CAS
.
- M. Abo, Y. Urano, K. Hanaoka, T. Terai, T. Komatsu and T. Nagano, J. Am. Chem. Soc., 2011, 133, 10629–10637 CrossRef CAS PubMed
.
- Y. Yang, S. K. Seidlits, M. M. Adams, V. M. Lynch, C. E. Schmidt, E. V. Anslyn and J. B. Shear, J. Am. Chem. Soc., 2010, 132, 13114–13116 CrossRef CAS PubMed
.
- Z. Xu, S. K. Kim, S. J. Han, C. Lee, G. Kociok-Kohn, T. D. James and J. Yoon, Eur. J. Org. Chem., 2009, 2009, 3058–3065 CrossRef
.
- Y. Kubo, M. Yamamoto, M. Ikeda, M. Takeuchi, S. Shinkai, S. Yamaguchi and K. Tamao, Angew. Chem., Int. Ed., 2003, 42, 2036–2040 CrossRef CAS PubMed
.
- J. S. Fossey and W. D. G. Brittain, Org. Chem. Front., 2015, 2, 101–105 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4sc03983a |
| This journal is © The Royal Society of Chemistry 2015 |