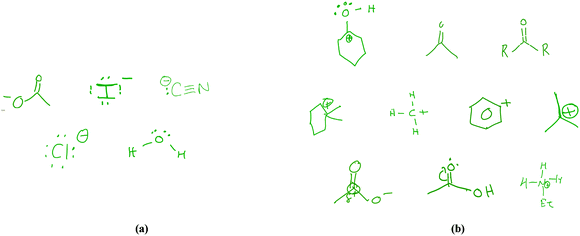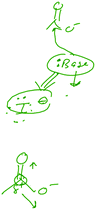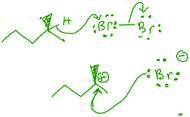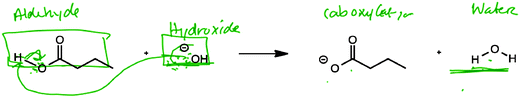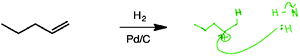Organic chemistry students' ideas about nucleophiles and electrophiles: the role of charges and mechanisms
Mary E.
Anzovino
and
Stacey
Lowery Bretz
*
Miami University, Department of Chemistry & Biochemistry, Oxford, OH, USA. E-mail: bretzsl@miamioh.edu
First published on 3rd July 2015
Abstract
Organic chemistry students struggle with reaction mechanisms and the electron-pushing formalism (EPF) used by practicing organic chemists. Faculty have identified an understanding of nucleophiles and electrophiles as one conceptual prerequisite to mastery of the EPF, but little is known about organic chemistry students' knowledge of nucleophiles and electrophiles. This research explored the ideas held by second-semester organic chemistry students about nucleophiles and electrophiles, finding that these students prioritize structure over function, relying primarily on charges to define and identify such species, both in general and in the context of specific chemical reactions. Contrary to faculty who view knowledge of nucleophiles and electrophiles as prerequisite to learning mechanisms and EPF, students demonstrated that they needed to know the mechanism of a reaction before they were able to assess whether the reaction involved nucleophiles and electrophiles or not.
Introduction and background
Organic chemistry has a well documented history of being a challenging subject for students. Undergraduate students are prone to developing a wide variety of alternative conceptions, many of which persist to and beyond graduation. Additionally, students often struggle to develop expert-like ways of thinking about the material, which leads to difficulty in applying prior knowledge and understanding new concepts. These difficulties have been chronicled in the organic chemistry education research (OCER) literature.Alternative conceptions
A wide variety of alternative conceptions have been documented among organic chemistry students. For example, polarity is a poorly-understood concept: students believe that bond polarity is caused simply by the presence of an electronegative atom in a molecule, with no regard for the relative electronegativities of atoms involved in a particular bond (Taagepera and Noori, 2000). A similar way of thinking has been exhibited by students who claim that functional group determines acid strength without considering the emergent nature of acid strength as a function of a combination of factors (McClary and Bretz, 2012).Students also hold incorrect ideas that arise from incorrect or inappropriate reliance on rote memorization. Henderleiter, et al. (2001) reported that students in their study generated lists of atoms that could participate in hydrogen bonding containing incorrect entries, such as chlorine, that likely arose from such a reliance on memorization. They also believed that hydrogen bonding can be induced in hydrocarbons or that it is a type of covalent bond. This type of faulty recall has been demonstrated in studies asking students to predict the products of reactions of alkenes; alternative conceptions in this realm include that alcohol products are produced when an alkyl halide and strong base are reacted in an alcoholic solvent, and that ether, ketone, and aldehyde products are produced by reacting water with an alkene in the presence of acid (Şendur, 2012).
Thought processes for concepts related to organic chemistry
Understanding students' thought processes has been the focus of other OCER studies. Acid strength is one concept related to reactivity in organic chemistry, and Bhattacharyya (2006) has shown that even graduate students invoke unsophisticated mental models of acidity, relying primarily on bond polarization to explain “weakening” of the bond between the acidic hydrogen and the atom to which it is bonded. McClary and Talanquer (2011) identified a set of heuristics that undergraduate students used in tasks asking them to rank various organic compounds based on acid strength. The majority of students relied on lexicographic heuristic methods, one prominent example of which involved resonance structures and how they contribute to strength of an acid via stabilization of a conjugate base. Although students often correctly attributed a trend in acid strength to this factor, they were not able to articulate how or why a conjugate base having more resonance forms would be more stable.Domin et al. (2008) asked students to categorize organic compounds that differed on three significant features: functional group (alcohol or ketone), stereochemical configuration at the carbon adjacent to the functional group, and whether the compounds were linear hexanols/hexanones or cyclic ones. Students could sort the compounds into any two groups they wished. Most students used functional group as the critical attribute by which to classify the molecules, but struggled to articulate why. It is possible that the students were simply grouping the compounds based on patterns of symbols rather than considering their functional behaviors. In another research study, students were asked to select a set of reagents likely to effect a given transformation in a multiple-choice item. The students typically resorted to one of three strategies: attribute substitution (looking for a familiar reagent set rather than considering the mechanism of reaction between given reactants and each set of reagents), fluency processing (predominant focus on familiar features), and intuitive associations (“X does Y”) (Graulich, 2015).
Reaction mechanisms and related concepts
When chemistry majors were presented with multiple-choice items asking them to choose the most likely product given a set of reagents and reaction conditions, the students chose answers by considering product stability rather than evaluating the feasibility of the mechanisms that would lead to the possible products (Rushton et al., 2008). Surprisingly, these undergraduate chemistry majors, who were within one semester of graduation, did not consider reaction mechanisms to be important for product prediction. Grove et al. (2012) reported similar findings for students enrolled in introductory organic chemistry who failed to use mechanistic thinking to predict the products of reactions – even when explicitly directed to do so. Graduate students in chemistry who were asked to generate mechanisms for organic chemistry transformations simply drew arrows that would result in electron pair and atom placement consistent with the products, without considering the chemical feasibility of the steps they proposed (Bhattacharyya and Bodner, 2005). In a separate study, undergraduate chemistry majors enrolled in organic chemistry encountered a variety of barriers to using the curved arrow formalism in a meaningful way when engaging in mechanistic tasks (providing mechanisms for transformations, given starting materials and products), including an inability to apply information (for example, mentioning the trivalent nature of boron but failing to apply it to evaluate the reactivity of NaBH4) and gaps in content understanding (for example, using a very weak base in a deprotonation step) (Ferguson and Bodner, 2008). All of these reports on students' understanding of mechanistic thinking suggest that the curved arrows that hold so much meaning for organic chemists are essentially little more than symbolic decorations to the students, who persist in viewing the mastery of organic chemistry as a Herculean task of memorization rather than one of process-oriented thinking and deep conceptual understanding (Grove and Bretz, 2010, 2012).To investigate the lack of alignment between faculty and student perspectives on the usefulness and meaning of the arrows and associated electron-pushing formalism (EPF), Bhattacharyya (2013) surveyed faculty, asking them to identify concepts which must be mastered before a student could develop fluency with the EPF. Among the conceptual prerequisites identified were electronegativity, bond polarity, Lewis acid–base theory, and identification of nucleophilic and electrophilic sites or functional groups in organic molecules. Subsequent work reported that senior chemistry majors held a tenuous grasp of the relationship between structure and nucleophilic/electrophilic functionality mere weeks prior to graduation (DeFever et al., 2015). Cartrette and Mayo (2011) previously discovered that students often considered the relationships between nucleophiles/electrophiles and bases/acids only when explicitly prompted to do so. Furthermore, these students struggled to apply the appropriate model of acid–base behavior (i.e., Lewis) and often attempted to incorrectly apply the labels of nucleophile and electrophile in Brønsted–Lowry acid–base reactions. More recently, Cruz-Ramírez de Arellano and Towns (2014) reported a variety of gaps in student understanding surrounding alkyl halide reactions, including failure to recognize strong bases (e.g. methoxide) or weak bases (e.g. iodide) as such, as well as the failure to understand the concerted or step-wise nature of SN2 or SN1 reactions.
Although several studies have examined students' use of mechanisms and the EPF, only a few have begun to examine the prerequisite conceptual underpinnings such as organic chemistry students' understandings of the structure and properties of nucleophiles and electrophiles. This manuscript reports the findings of a study designed to investigate students' ideas about essential characteristics of nucleophiles and electrophiles, as well as how to identify them in reactions.
Theoretical framework
As demonstrated by previous research, organic chemistry is a subject in which students frequently rely on rote memorization, often to their detriment. Rote learning occurs when students simple memorize new information without considering how it relates to knowledge they already possess. The goal of organic chemistry educators is not to encourage memorization, but rather to foster meaningful learning, in which the learner makes significant connections between new information and prior knowledge (Bretz, 2001; Novak, 2002; Grove and Bretz, 2012). Meaningful learning, which is the opposite of rote memorization, has three prerequisite conditions: relevant prior knowledge (the student must already possess knowledge that can be related in a non-trivial manner to the new information), meaningful material (the new information must be relevant to other knowledge, with substantial concepts and relationships between those concepts), and learner's choice (the student must deliberately choose to relate the new information to his or her prior knowledge) (Novak, 2010). The objective of this study was to explore what ideas students have engaged with in a meaningful way, rather than simply memorized. To that end, a qualitative interview methodology was employed, which allowed for a semi-structured dialogue between researcher and student, including follow-up questions to more deeply probe student ideas. This characterization of student knowledge about nucleophiles and electrophiles provides an important first step toward designing pedagogical and/or curricular improvements grounded in research.Methods
Goals and research questions
The ideas that organic chemistry students hold about the structure and properties of nucleophiles and electrophiles have not been previously investigated. This research investigated students' general ideas about these concepts, as well as how students applied those ideas in the context of explaining specific organic chemistry reactions. The research questions addressed by this manuscript are(1) How do organic chemistry students define nucleophiles and electrophiles? What essential characteristics suggest a chemical species is either a nucleophile or an electrophile?
(2) How do organic chemistry students identify nucleophiles and electrophiles in specific organic chemistry reactions?
Sample and setting
This study was conducted at a medium-sized public research university in the midwestern United States. Participants were recruited from two second-semester organic chemistry lecture courses, typically taken by undergraduate science majors in the second year of their undergraduate curriculum. Purposeful sampling (Patton, 2002) resulted in 11 interviews; four participants were enrolled in the course intended for chemistry and biochemistry majors, while seven were enrolled in the course intended for students in other science majors. The nonmajors' course sample consisted primarily of chemical engineering majors. The sample included students who had earned a range of grades (from As to Cs) in previous courses (first-year university chemistry I and II, as well as organic chemistry I). The students in this study had been taught each of the reaction types explored in this study, including the electron-pushing mechanism for each reaction, with the exception of alkene hydrogenation. The students had learned that hydrogen and a metal catalyst transform an alkene to an alkane, but had not been explicitly taught an electron-pushing mechanism for this reaction.To provide some context for the findings, the textbook used for the nonmajors' course (Klein, 2012) was reviewed for relevant content and a concept map (Novak and Gowin, 1984) was created by the authors to depict the information contained in the textbook regarding nucleophiles and electrophiles (Fig. 1). The nonmajors' textbook was Organic Chemistry, by Klein (2012) and the textbook for the majors course was Organic Chemistry, by Bruice (2011). The resulting concept map, which is included here to describe the content coverage in the students' textbook, actually contained very few characteristic cross-links indicative of a rich, connected knowledge structure. Rather, the concept map made of the textbook information resembles a ladder with nucleophiles on one side merely contrasted against electrophiles on the other side. For each “side,” there is one descriptor for nucleophile and one for electrophile. The two chapters that focused primarily on nucleophiles, electrophiles, and fundamental reactions thereof introduced and discussed these terms focusing mainly on describing their characteristics (the “rungs” in the middle of the ladder): charge, orbitals, electron pairs, and their relationship to both Brønsted–Lowry and Lewis acids and bases. The concept map is organized such that the characteristics closer to the top pertain more to the structure of each type of species, e.g., what kind of charge a nucleophile has. Those toward the bottom of the map include concepts related to function, with the Brønsted–Lowry line serving as the point of transition between the two. The distinction between Brønsted–Lowry bases and nucleophiles is an issue of function (what the species is reacting with), whereas the distinction between Brønsted–Lowry acids and electrophiles is structural (i.e., whether the structure contains an acidic proton or not). The absence of any links between each of the characteristics was somewhat surprising. Connections between the characteristics were not prevalent in the text, resulting in a somewhat fragmented, isolated presentation of structural and functional considerations. While nucleophiles and electrophiles were contrasted against one another in terms of each characteristic one at a time, there was no substantive discussion of how each of these characteristics comes together to form a conceptual whole. (Comparisons between the students' ideas and the author-constructed concept map are the subject of a future manuscript.)
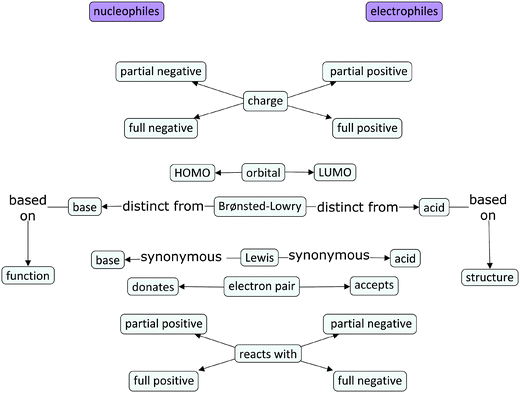 | ||
| Fig. 1 Author-generated concept map based on the nonmajors' course textbook presentation of nucleophile and electrophile characteristics. | ||
The first author of this manuscript also observed the course lectures in which these chapters were introduced. The instructors similarly emphasized the distinction between nucleophiles and Brønsted–Lowry bases. Additionally, polarizability was mentioned as an important feature to consider for nucleophiles: the more polarizable an atom is, the more nucleophilic it is, all other factors being approximately equal. Examples presented in class typically relied on surface features (e.g., lone pairs) for identification of nucleophiles, with electrophiles being simply the other starting material in a reaction.
Data collection and analysis
Each student was interviewed once, approximately two-thirds of the way through the semester; an interview typically lasted about 60 minutes. Audio recordings and students' writing/drawings were collected using a Livescribe smartpen (Linenberger and Bretz, 2012; Livescribe, Inc.). A video camera was used to record student gestures that were subsequently used to annotate the transcripts (e.g., clarification of students' use of “this” or “that” while pointing at a particular reaction). The research protocol for this study was approved by the Institutional Review Board. Each student provided informed consent prior to the interview and received a $20 amazon.com gift card as compensation for completing the interview. Pseudonyms are used to refer to the students in this manuscript.Interviews were transcribed verbatim and data was managed using NVivo 10 for Mac (QSR International Pty Ltd, 2014). The interview transcripts were open coded based on themes appearing in the data, using the constant comparative method (Lincoln and Guba, 1985; Strauss and Corbin, 2008). In addition to open coding, the success of students in (a) classifying reactions as involving (or not) a nucleophile and electrophile and (b) identifying nucleophiles and electrophiles when the specific reaction was judged by the student to include them, were also examined.
Description of interview prompts
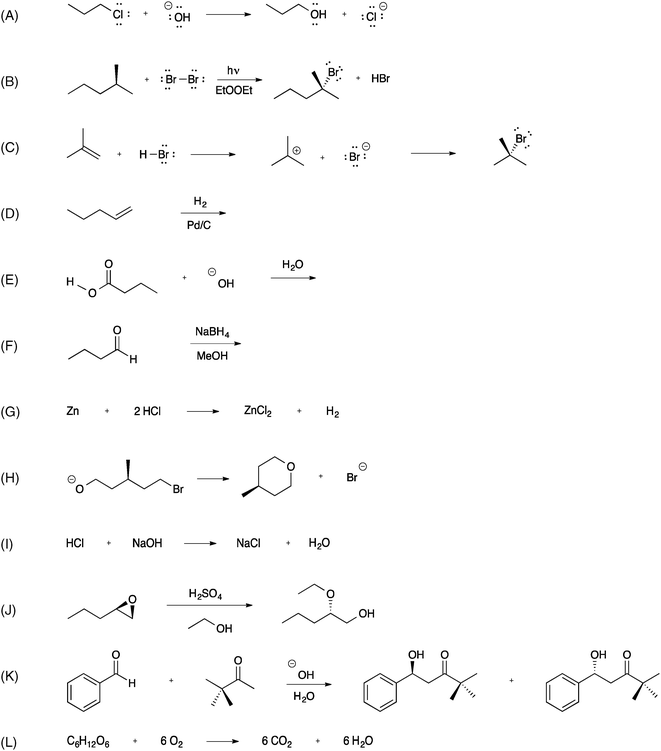
The interviews followed a semi-structured interview protocol; the flexibility of this technique allowed for deeper investigation of students' ideas as they emerged during the interview. The interviews began by asking each student a series of open-ended questions regarding both the definitions and defining characteristics of nucleophiles and electrophiles. Students were asked to provide examples of each and to discuss the relationships between nucleophiles/electrophiles. Students were also asked to discuss their ideas about what, if any, relationships existed between nucleophiles, electrophiles, acids, and bases. After sharing their prior knowledge regarding nucleophiles and electrophiles, students were presented with a variety of reactions (Fig. 2), in sets of three, and asked to assess them in light of the following questions: (1) Does the reaction involve a nucleophile and/or an electrophile? How can you tell? (2) If so, what is the nucleophile? What is the electrophile? How can you tell?The twelve reactions in Fig. 2 were purposefully chosen to embody a number of important criteria. First, a wide variety of reactions typically encountered in the two semesters of undergraduate organic chemistry were included (reactions A–F, H, J, K). The representations of reactions A–C explicitly included lone pairs of electrons, in order to explore if students would respond to those depictions differently than reactions without explicit lone pairs (reactions D–L). Redox, neutralization, and combustion reactions are typically encountered in first-year university chemistry (G, I, L); these were included to investigate whether students applied organic ideas such as nucleophilicity to previously-learned material. Reactions for which students did not have a mechanistic frame of reference (D) or which did not follow an ionic mechanism (B) also provided the possibility of revealing interesting details of students' thinking. Reactions D–F included their respective products for six of the interviews; in hopes of learning about the students' thinking when products of the reaction were not provided, these reactions were edited to omit that information for the final five interviews. All students evaluated reactions A–F. The first interviews in which students were asked about all twelve reactions led to the interview being too long. Therefore, subsequent interviewees evaluated either reactions G–I or J–L. The number of students who assessed each reaction can be found in Table 1.
| Reaction | Reaction description | # Students who viewed reaction | # Students who correctly evaluated reaction | # Students who Identified nucleophile & electrophile | |||
|---|---|---|---|---|---|---|---|
| Both correct | Incorrect | ||||||
| Consistent with own incorrect mechanism | Identified nucleophile without electrophile | Identified electrophile without nucleophile | |||||
| a Reaction involves a nucleophile and an electrophile. | |||||||
| Aa | SN2 | 11 | 11 | 6 | 4 | 1 | 0 |
| B | Alkane bromination | 11 | 2 | — | 8 | 1 | 0 |
| Ca | Alkene hydrohalogenation | 11 | 11 | 5 | 0 | 1 | 0 |
| D | Alkene hydrogenation | 11 | 3 | — | 2 | 0 | 0 |
| E | Carboxylic acid deprotonation | 11 | 2 | — | 2 | 2 | 0 |
| Fa | Borohydride reduction | 11 | 9 | 4 | 2 | 3 | 1 |
| G | Redox | 6 | 3 | — | 1 | 1 | 0 |
| Ha | Intramolecular SN2 | 6 | 6 | 1 | 2 | 1 | 0 |
| I | Acid–base neutralization | 6 | 1 | — | 0 | 1 | 0 |
| Ja | Epoxide opening | 8 | 6 | 2 | 3 | 0 | 0 |
| Ka | Aldol addition | 8 | 7 | 2 | 0 | 2 | 0 |
| L | Combustion | 8 | 4 | — | 0 | 0 | 0 |
Five organic chemistry faculty were asked to critique the collective set of reactions, as well as to indicate which reactions they deemed to involve nucleophile(s) and electrophile(s). There was unanimous agreement by the faculty on all points except one. Of note, there was some disagreement about the classification of reactions that involve a proton transfer between a Brønsted acid and Brønsted base (e.g., reaction E). Although the definition of Lewis base (electron-pair donor) technically subsumes that of Brønsted–Lowry base, and therefore the terms nucleophile and electrophile could apply to the latter class of reactions, these reactions are typically described as acid–base, rather than nucleophile–electrophile. Previous research has revealed that organic chemistry students' failure to distinguish between nucleophilic and Brønsted–Lowry basic behaviors indicates a lack of process-oriented thinking (Strickland et al., 2010). Because it is precisely this type of process-oriented thinking that is of interest in the present study, reactions/steps involving proton transfers were considered to not involve a nucleophile or an electrophile for the purposes of this research.
Results and discussion
The first phase of the interview provided information regarding students' ideas about definitions and examples of nucleophiles and electrophiles. The themes discussed in this section arose from the open coding of data from this interview phase.Student definitions
“Nucleophiles donate electron pairs, they're negative, and electrophiles, positive, they're the opposite.” (Ross, second year biomedical engineering major)
Four of the students considered the possibility that partial charges could also signify either a nucleophile (negative) or an electrophile (positive), as exemplified by Paul (second year chemical engineering major):
“So nucleophiles, um, want the nucleus, they love the nucleus, so they have, they're electron dense, so they would have a minus charge, or partial minus, and then electrophiles are just the opposite, they want electrons, they're electron-deficient, so they would have partial positive or a completely positive charge.”
Chandler's (second year biochemistry major) definitions combined the ideas of charge and function:
“I think of nucleophiles as being nucleus-loving, and, um, since, and I believe a nucleus has a positive charge, so I'm assuming that nucleophiles are looking for a positive charge to attack, um, and an electrophile is electron-loving, so, it's looking for, since the electron has a negative charge, it's looking for a negative charge to attack.”
Rather than focusing on the charge of a nucleophile itself, or an electrophile itself, Chandler focused on what charges each type of species would react with.
Interviewer: “So you said it's [a nucleophile] usually negative, it doesn't have to be, can you think of any other defining characteristics, for nucleophiles? Like what a certain species has to have in order to be a nucleophile?
Barry: “A lone pair.”
Interviewer: “So, the lone pair is the thing that makes you look at something and go, yeah, this is a nucleophile.”
Barry: “Yeah, and the negative charge is more, it's just a strong nucleophile or not.”
Barry was able to distinguish between what he considered to be an essential feature of a nucleophile (a lone pair of electrons) and a feature that is often present (a negative charge) but not required. His ideas are not quite correct, however, because electrons in σ and π bonds can also serve as nucleophiles. Despite his fairly sophisticated view of nucleophiles, he struggled when asked to define or provide defining characteristics of electrophiles:
“An electrophile… not sure, now that I think about it. I mean, it would have to… hmm. I don't think it has to have a positive charge. I'm not really sure… I mean, I know positive charges are like usually a sign for them, that, like I know it's an electrophile and that's what it, it will be attacked by the nucleophile, but, I don't know if that's a defining characteristic of electrophiles, either.”
“[A nucleophile is] a molecule with lone pairs, or just anything that it can donate in order to form a bond to another, um, to another compound.” (Joey, second year biology major)
“Electrophile will have a positive charge. Um… the ability to accept electrons.” (Monica, first year chemical engineering major)
It is important to note, however, that even students whose definitions contained both structural and functional aspects, such as Monica, did not explicitly connect those two ideas, i.e., how structural features led to particular functional behaviors. Phoebe (second year zoology major) was the only student to demonstrate a strong understanding of defining features of both nucleophiles and electrophiles:
“It [a nucleophile] needs to have a, either like a lone pair of electrons or, uh, a double bond. It needs to have, like, um, electrons that can be donated, um, like to an electrophile in order to react as a nucleophile.”
Although she also mentioned charge in her response, to Phoebe, the essential characteristic of a nucleophile is related to its function rather than simply its structure. She was also the only student to mention orbitals in her definition of electrophiles:
“They [electrophiles], um, sometimes, uh, will have a positive charge, which indicates that they're electron deficient. Um, but, they don't necessarily need to have a positive charge, they just need to, um, have, like an orbital that can be, um, like an empty orbital, um, that can take the electrons, uh, of a nucleophile.”
Similar to her definition of a nucleophile, Phoebe's ideas about what makes a chemical species an electrophile were related to function; it must be able to accept electrons, which is possible only if there is an empty orbital present. The scarcity of orbitals in the students' ideas was not surprising, given that frontier molecular orbital theory is mentioned briefly in the textbooks but not particularly emphasized by the instructors, except in the specific context of cycloaddition reactions such as the Diels–Alder.
“I don't remember if it was for electrophiles or nucleophiles, but I think it was electrophiles, so this probably doesn't answer your question, but, one of them had, it helps if they're small and polarizable.”
His comments suggest that he simply remembered “polarizable” as being a desirable characteristic for one of the two terms of interest and did not comprehend what it actually means for something to be polarizable, particularly since he associated polarizability with smaller size, when in fact, larger atoms are more polarizable.
Phoebe struggled to use the concept of polarizability in her definitions in a different way:
Phoebe: “I think, um, it also makes a difference if, um, the molecule is, like polarizable. So if it's more polarizable, then it would be a stronger nucleophile?”
Interviewer: “What does it mean for something to be polarizable?”
Phoebe: “Uh, like, in a larger molecule, um, if there's a more electronegative atom, then the electron density will be pulled, um, towards that atom, so there's one area that's, uh, more positive charged, and one area that's more negatively charged, on a molecule.”
Phoebe's definition of polarizability indicated that she confused it with the polarization of electron density in σ bonds via induction, a concept that is actually more relevant in considering electrophiles than nucleophiles. The confusion experienced by Paul and Phoebe suggests that they simply memorized this term rather than learning to apply it in a meaningful way. Because of this memorization, polarizability was not a helpful idea for them.
Students' examples of nucleophiles and electrophiles
Students were asked to provide an example of a nucleophile and one of an electrophile (Fig. 3). (Only unique contributions are included in the figure.) Two students gave verbal descriptions of examples, but provided no drawings.Four students chose chloride as their nucleophile example, citing its lone pairs and/or negative charge when asked to provide justification for their choice:
“so a nucleophile has lone pairs, these lone pairs are very receptive to attacking electrophiles…“ (Joey)
Interviewer: “Can you give me an example of a nucleophile?”
Rachel (second year chemical engineering major): “Um, C-L minus.”
Interviewer: “And what makes that a nucleophile?”
Rachel: “It has a formal charge of a negative, um, negative one.”
All of the students' examples of nucleophiles were valid, as they all possessed a lone pair of electrons that could be used to form a new bond to a partially positive atom. Although seven of the students mentioned some kind of functional component of their definitions of nucleophiles, when it came to justifying their examples, most emphasized a structural feature (e.g. the lone pair of electrons) rather than talking about the function of the species. Ross, Barry, and Jack did cite function, and in this task, specifically discussed connections between structure and function, as exemplified by Jack's (third year chemical engineering major) comments:
Interviewer: “So, so what makes I minus a nucleophile?”
Jack: “the electrons it can donate, that I know it has around it, so… and the minus charge, so I know it's willing to give away, or willing to donate, I should say, um, electrons.”
In this case, Jack explicitly connected a structural feature (negative charge) to its reactivity (donating electrons).
The students' examples of electrophiles, on the other hand, were more varied, both in terms of identity and correctness. Some students provided a perfectly reasonable example, citing a positive charge as the primary criterion. After drawing a tert-butyl carbocation, Phoebe said “yeah, something with a positive charge. Um, would be an electrophile.” Notably, her reason for this example did not incorporate her earlier comment about electrophiles having an empty orbital into which electrons can be accepted. This example demonstrates that even if students can articulate a sophisticated, detailed understanding, they don't always draw upon that understanding to its full capability when engaged in a specific task.
Just as the students struggled to explain defining characteristics of electrophiles, they found it similarly difficult to provide examples of this type of chemical species. For example, Barry selected his example of an electrophile, [EtNH3]+, purely because of its charge:
Barry: “Yeah, Um, a nitrogen, with 3 Hs, and like [mumbles] like a ethyl group, so the nitrogen would be an electrophile.”
Interviewer: “And what makes the nitrogen an electrophile?”
Barry: “Hmm. I'm not really sure. I mean, I know positive charges are like usually a sign for them, that, like I know it's an electrophile and that's what it, it will be attacked by the nucleophile, but, I don't know if that's a defining characteristic of electrophiles, either.”
His response was consistent with his definition (an uncertain declaration that positive charges are usually indicative of electrophiles) but only reinforced that he did not have a firm grasp on what is required for a species to react as an electrophile. His thinking was limited to considering structural features rather than also considering the function of such a species.
Jack provided another example of structural-feature-focused reasoning, offering his example of an electrophile in the context of a reaction (Fig. 4), even though this reaction would not occur as Jack has drawn it:
Jack: “It does, um, there's an electrophile in this step here. An electrophilic carbon, is what I'll say it is.”
Interviewer: “And what makes that carbon electrophilic?”
Jack: “first of all because of the oxygens that are attached, so that's drawing, it's kind of like drawing a charge away, which makes this like a partially positive, um, so induction effects, um sterics play a role into it as well. Or electronic things, sorry.”
Jack's response is multifaceted. First, it tells us that he focused on the partially positive charge of the carbon atom, a feature of the atom, rather than considering how the species overall would react. The acetate anion would most likely not react with an anion (in this case iodide), but rather would act as a nucleophile or Brønsted base. As he continued to try to explain why the carbon would be partially positive and therefore electrophilic, he seemed to be simply listing what might be considered “buzzwords of organic chemistry:” sterics, inductive effects, and electronic effects, rather than showing an actual understanding.
With the exception of Phoebe, the students' justifications for their examples emphasized structural features (e.g. lone pairs for nucleophiles, positive charges for electrophiles), as was consistent with the particular defining characteristics they had stated previously. Ross was the only student to connect structure and function when justifying his electrophile choice (phenyl cation):
“because then the nucleophile will attack the positive charge.”
Although, he was able to make the connection between a structural aspect and functional behavior of his example, Ross failed to consider the fact that the cation he proposed as an electrophile was unlikely to form in the first place, suggesting a lack of deeper understanding.
Students' evaluation of reactions for nucleophiles and electrophiles
Students experienced varying degrees of success with the tasks of (a) evaluating reactions as to whether or not they involved nucleophiles and electrophiles, and identifying (b) the nucleophile and (c) the electrophile for each reaction they deemed to involve those species (Table 1). All students successfully identified reactions A and C as identifying both a nucleophile and an electrophile. Although these reactions explicitly included lone pairs, students did not explain their responses using that feature. Rather, both reactions involve steps that are representative of the type “nucleophilic attack,” one of four types of mechanistic steps students learned (the others being proton transfer, loss of leaving group, and carbocation rearrangement) (Klein, 2012). Monica justified her identification of the hydroxide as a nucleophile in reaction A:“This [hydroxide] is the nucleophile, it's the attacking, nucleophilic attack, so that must be the nucleophile, it's the one attacking.”
If nucleophilic attack is taking place, the reaction must involve a nucleophile.
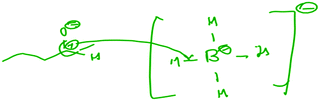
The students were generally more successful in evaluating the reactions that did involve a nucleophile and electrophile (correctly stating such) than in assessing the reactions that did not. For those reactions that did not involve a nucleophile and electrophile, students' difficulties manifested themselves in a variety of ways. First, many students proposed incorrect reaction mechanisms, and subsequently identified a nucleophile and electrophile based on a structural feature of one or more species present in their mechanisms. For example, for reaction B (a radical monobromination of an alkane), 8 students proposed an ionic mechanism and all 8 students subsequently identified the negatively charged species as the nucleophile and the positively charged species as the electrophile (Fig. 5). Monica explained her reasoning:
Monica: “The nucleophile would be this lone, negatively charged bromine [sic], and then the electrophile would be this carbon with the positive charge.”
Interviewer: “And how did you make those assignments?”
Monica: “This one it's more obvious because this one has a positive charge and this one has a negative charge.”
Although the students' mechanisms varied (some students proposed a mechanism involving a carbanion and a monatomic bromonium ion for reaction B), the tendency to identify the nucleophile and electrophile based on charges was unanimous among those students who provided an ionic mechanism. By contrast, the two students who successfully determined that this reaction did not involve a nucleophile and an electrophile focused on structural features of the starting materials, as exemplified by Rachel:
“Because, well here the B-R-2, those are shared equally, because it's a diatomic molecule, so there's not really like induction going on, to give a negative, a net negative or a positive charge, here same thing, these are all carbons and hydrogens.”
For Rachel, the lack of significant partial charges anywhere in the reagents signified that this reaction was of a different class than those involving nucleophiles and electrophiles, because she could not envision generating charged species that would be required for those labels to be applicable.
The other primary difficulty students confronted with reactions not involving a nucleophile and electrophile was conflation between the concepts of Brønsted acid–base and nucleophile/electrophile. For example, consider how Gunther (second year microbiology major) described reaction I:
“Um, I'd say that these with the, um, with the negative charges are the nucleophile, but like I don't see how, you could, like, I don't know if you could have two nucleophiles in a reaction and two electrophiles in a reaction.”
In this case, Gunther's limited view that a nucleophile must be negatively charged created confusion as he searched for what species in the reactants could in fact carry a negative charge. He failed to consider the fact that the hydroxide was acting as a Brønsted base. Likewise, Reaction E also involved a Brønsted acid–base proton transfer, but in this case the acid was a carboxylic acid. Students who saw this reaction with the product were able to ascertain that the hydroxide removes the proton from the acid, but some still classified it as a nucleophile-electrophile reaction (Fig. 6):
“And when I say this [hydroxide] is attacking this [carboxylic acid, which he mistakenly refers to as an aldehyde], I mean the uh uh electrons are attacking this hydrogen and making water. And so yes, there's a nucleophile. And I believe this [hydroxide] is a nucleophile. Which by default makes this [carboxylic acid] the electrophile of the reaction.” (Jack)
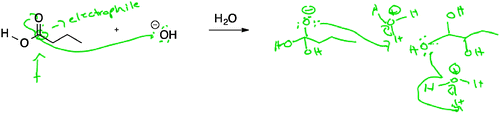
This failure to distinguish between nucleophiles and Brønsted bases has been previously reported in the literature (Strickland et al., 2010), and was, in fact, the only reaction about which the organic chemistry experts disagreed, as noted above. To further explore how students differentiated between the two, the interview protocol was modified to provide only the reactants (and not the products) for reaction E (as well as for reactions D and F). Subsequently, yet another conflation between nucleophiles/electrophiles and acids/bases was observed for students who were asked to evaluate reaction E in the absence of products (Fig. 7). These students often failed to recognize the acidic nature of the carboxylic acid, focusing solely on the partially positive carbonyl carbon:
“This carbon is an electrophile, because this O [in carbonyl] is more, oxygen is more electro, is more electronegative, so it's gonna pull electron density of this bond upwards, so there'll be a partial positive charge, and then O–H can act as a nucleophile, and, because it has a minus charge, and it's fairly small, so it can attack the carbonyl carbon.” (Paul)
Paul's comments suggest that he looks for bond polarization and charges to make assignments of nucleophile and electrophile. The focus on the partially positive nature of the carbonyl carbon atom among these students is not surprising, given that a significant fraction of the second semester course is devoted to the various functional group transformations that can occur at this electrophilic site. However, the students were so focused on recognizing the carbonyl group, that they failed to recognize that the carbonyl here is not a simple aldehyde or ketone, but rather part of a larger functional group with very different reactivity.
“So it's my understanding that this [bromide] is the nucleophile, this [carbocation] is the electrophile. The nucleophile lone pair attacks the electrophile. And it gives you that [product].” (Ross)
“Uh, the electrophile is going to be this here, with the positive charge, because it's going to be accepting, um, lone pairs, from the nucleophile, which is here, on the bromine [sic].” (Joey)
“Yes, it does, involve a nucleophile and electrophile, um, I can tell just because of the positive and negative charge.” (Barry)
Regardless of whether a reaction in Fig. 2 included charged species or not, the most frequent student response by far was to first propose a mechanism for the reaction in order to subsequently consider the potential involvement of nucleophiles or electrophiles. In total, the 11 students provided 108 unique reaction evaluations. They proposed mechanisms for 77, or 71%, of those evaluations. Contrary to previous research in which students explicitly ignored directions to use mechanistic thinking (Grove et al., 2012), the students in this study not only attempted to use mechanistic reasoning without being told to do so, but seemed to consider it essential to the task at hand. This finding provides further evidence for the proposition by Graulich (2014) that “students need to experience an actual benefit from using curved-arrow notation as a problem solving tool.” After generating a mechanism, students proceeded to search for the features they had identified in the first phase of the interview (e.g. charges, donating/accepting electrons) in their proposed mechanisms.
The dependence upon mechanisms, and yet the difficulty in generating correct mechanisms for the reactions led many students to generate mechanisms that solely accounted for where the atoms ended up in the products without ever considering the chemical feasibility of their steps. Subsequently, these students were then able to identify nucleophiles and electrophiles consistent with their incorrect mechanisms (column 6 in Table 1).
Students' dependence upon mechanisms in order to evaluate the presence or absence of nucleophiles and electrophiles was especially prevalent for reactions that proceed via a non-ionic mechanism. Students tended to propose incorrect, ionic mechanisms for these reactions, as previously discussed regarding Monica's mechanism in Fig. 3 for the radical halogenation of an alkane. Similarly, four students proposed an SN1 mechanism for reaction A (e.g., Fig. 8) and subsequently identified the nucleophile and electrophile as hydroxide and the 1-propyl cation, respectively, as exemplified by Ross' comments:
Ross: “This [hydroxide] is a nucleophile, I'm pretty certain about that.”
Interviewer: “And why is that?”
Ross: “Um… its lone pairs, it's a Lewis base, and, I think I just, I think I just remember that, that it was a strong nucleophile, um, you know, just from something that I, we went over. And then, so I guess that would make this [carbocation] an electrophile, because it attacks it, and then the bond is formed...”
Ross' reason for identifying the nucleophile in this reaction is memorization, although he also used the lone pairs on hydroxide to justify his choice. His explanation for identifying the electrophile in this reaction, however, was completely dependent upon the mechanism he proposed: whatever is being 'attacked' by the nucleophile must be the electrophile. Given students' well-documented difficulties with using the curved arrow notation to provide reaction mechanisms, this kind of reasoning can be quite problematic. Indeed, Ross' mechanism is incorrect and thus he identifies a nonexistent intermediate as the electrophile in this reaction. Ross provides an example of students who identified a nucleophile and/or an electrophile consistent with their own incorrect mechanism (column 6 in Table 1). This phenomenon also occurred in student responses to reaction H, another SN2 reaction for which two students provided an SN1 mechanism and identified a nucleophile and electrophile consistent with such a mechanism.
The relationship between knowing the mechanism and being able to identify the nucleophile and electrophile in a reaction was so persistent for some students that in the absence of a mechanism and/or reaction products to guide them in proposing a mechanism, they were unable to even engage in the task of evaluating whether there was a nucleophile or electrophile involved in a given reaction. This limitation was largely related to the idea that charges are important and particularly prevalent for reactions with uncharged starting materials, as evidenced by Barry's uncertainty regarding whether reaction B involved a nucleophile and electrophile or not:
“I don't know if it might be a nucleophile and the negative charge just might not be there, but… there's also no positive charge anywhere here.”
Gunther's remarks about reaction K provide further evidence that students relied on the reaction mechanism for identifying the nucleophile and electrophile:
Gunther: “Yeah, I mean, that's sort of basis that like anytime there's a negative charge it's gonna be a nucleophile.”
Interviewer: “And what about electrophiles?”
Gunther: “The positive charges.”
To Gunther, anything with a charge could be classified as either a nucleophile (if it was negative) or an electrophile (if it was positive). Rather than using mechanism to think about the function of the starting materials or intermediates, these students merely used mechanisms to access charged species, which they could then classify based on their simple definitions.
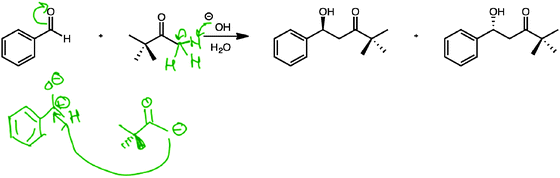
Phoebe's evaluation of reaction K (Fig. 9) was an exception in that she considered both structural and functional issues:
“The O–H, uh, could, could deprotonate that carbon (alpha carbon of ketone), and then the electrons would go to the carbon, um, and then so, it would… … so, because the electrons moved, um, onto the oxygen (in the aldehyde), the carbon (in the aldehyde), um, would be electron deficient, and then, since this carbon (enolate) was deprotonated, um, it would then be able to form a bond, um, to this positively charged carbon, um, so then, that would make, uh, this molecule (aldehyde, in zwitterionic resonance form) the, um, electrophile, and this molecule (enolate) the nucleophile.”
Although charge was important to her evaluation, she also considered the possibility of bond formation, suggesting that what happens (function) was also part of her justification for identifying the nucleophile and electrophile as she did.
As noted in the methods section, five students were asked to examine reactions D–F without products (for a total of 15 unique evaluations). In four of these instances, the students' first decisions were to identify the product(s). For reaction D, students were generally able to recall having memorized an important structural change occurs for the reactants under the given conditions:
“I know like I know this does reduce double bonds to like single bond.” (Barry)
“When I originally look at this reaction, I kind of just think of it, I don't really think of the mechanism, I just think of the hydrogens just adding to each end.” (Monica)
However, when it came to ascertaining whether the reaction involved a nucleophile and/or an electrophile, some students proposed a mechanism analogous to hydrohalogenation of an alkene (Fig. 10):
“Well, I think they do add one at a time, maybe. So the first one is going to add to the less substituted side, 'cause the positive charge wants to be on a secondary carbon as opposed to a primary. And then you know if it's H-2, and if this guy were to add first, then the pair would go here, and then this hydrogen would have a pair of electrons, which then it would be a nucleophile, and then it would attack.”
Even though Monica claimed she didn't really consider the mechanism, upon further consideration, she proposed one to help her determine whether the reaction involved a nucleophile and/or an electrophile.
For Ross, this seemed to be because of his reliance on hydroxide's instrinsic quality of being a nucleophile:
“Well there's hydroxide again, so, I'm gonna go ahead and say that's a nucleophile. I pretty strongly believe that it is. Um… I think this is a case where it's [hydroxide] acting as a base, just to deprotonate this. So this [hydroxide] is going to attack the hydrogen, and then that bond is going to the oxygen… I think this [hydroxide] is a nucleophile, pretty much sticking to that, um… just because I think I memorized it as a nucleophile.” (Ross, Reaction E)
“I'm drawn to this, thinking this [hydroxide] is a nucleophile, just because I think I have that ingrained.” (Ross, Reaction K)
Both of these statements arose while evaluating reactions in which hydroxide serves as a Brønsted base rather than a nucleophile, and even though he also acknowledged this behavior for reaction E, he did not draw any distinction between being a nucleophile and being a base based on function.
Monica decided that reaction F involved a nucleophile but not an electrophile as well:
“It's [hydride] like substituting in, like it's, yeah. Whereas this carbon here (carbonyl carbon), used to have two C–O bonds, now it has a C–H, another C–H bond, and only one C–O bond, so I would consider I guess, by the way it acts, I would call it [hydride] a nucleophile.
It does not even occur to Monica to search for an electrophile once she's identified and justified the nucleophile in this reaction. She was not alone in this regard (six other students exhibited similar disregard for electrophiles), suggesting that there is a disproportionate amount of emphasis placed on nucleophiles relative to electrophiles in instruction.
“[The positively charged carbon is an electrophile] because it's unstable, like the carbocation is unstable, and, so it wants the electrons to stabilize it.”
Although he drew an incorrect mechanism, Gunther's assessment of the carbonyl carbon was accurate; that site is electrophilic. However, he failed to identify the borohydride as the nucleophile reacting with that electrophile.
“Just because general chemistry doesn't, like we didn't learn about that in general chemistry, so I think they're more specific to organic reactions.” (Gunther, reaction G)
“This just makes me think of AP chemistry at like, in high school again, where we generally, it's like, I don't know if it's redox reaction, or acid base reaction, um, but you just, I was just kinda told what occurs. And I was never, I never associated nucleophiles or electrophiles the being in this type of reaction.” (Jack, reaction G)
Although Gunther and Jack were successful in evaluating reaction G, their characterizations were not supported by sound chemical reasoning, but rather by the erroneous perspective that different topics and courses in chemistry must be unrelated to one another.
Other students tried to fit these reactions into a framework of nucleophiles and electrophiles:
“There might be some OH bonds, but I would assume that the carbons are the ones bonded to the oxygens, based on that I would say that this [sugar molecule] could be the electrophile, um, because this [oxygen atom(s)] is gonna have the partial negative charge, which is gonna pull electrons away from these carbons, leaving a partial positive charge on there, and then if O 2 , um, how's O 2 … I guess then this [O 2 ] would be the nucleophile, with like maybe a very small negati-, or uum, very small partial, partial positive charge on one of the oxygens versus a very small partial negative charge on the other one.” (Chandler)
Chandler used the limited information he had about the structure of one of the reactants to infer that there were likely bonds present between carbon atoms and oxygen atoms, which would inherently be polarized, a feature he recognizes as indicative of electrophiles. As a result, the other reactant must be the nucleophile, and he justified that identification by concluding there must be a partial negative charge on one of the oxygen atoms. However, he failed to address the implications of there also being a partial negative charge (or several) in the sugar molecule, which he has identified as the electrophile, and a partial positive charge in the diatomic oxygen, which he has identified as the nucleophile.
Conclusions
Although students discussed both structure (charges, lone electron pairs) and function (donating or accepting electrons) in their definitions of nucleophiles and electrophiles (research question 1), the search for structural features in reactants and mechanisms clearly dominated students' thinking when evaluating a reaction as to whether it involved a nucleophile and an electrophile (research question 2). This dependence upon structural features often led students to erroneously evaluate reactions as to whether or not they involved nucleophiles and electrophiles. The students' first step in evaluating reactions was to propose a mechanism, and their identification of the nucleophile and electrophile was based on that mechanism. Hence if the mechanism was incorrect, the students identified nonexistent intermediates as nucleophiles or electrophiles, irrespective of the chemical feasibility of the mechanism. The prevalence of this outcome suggests that students engage in rote memorization of what features are related to nucleophilic and electrophilic behavior, rather than try to more deeply comprehend the relationships between those features and functionality. In many cases, students did not consider the reactive properties of the starting materials correctly, either. One significant impediment to students' success in evaluating the reactions involved a conflation of the ideas of Brønsted acids/bases and nucleophiles/electrophiles. Distinguishing between the two pairs of concepts requires consideration of function, which the students did not exhibit as much and therefore, they struggled with the tasks they were asked to complete. The multiple instances in which students evaluated hydroxide demonstrated their tendency to classify that ion as a nucleophile, even where the products of a Brønsted–Lowry acid–base proton transfer were provided (e.g., reaction E for some students). Where such products were omitted, students failed to even consider the possibility of a proton transfer and instead proposed a mechanism in which the hydroxide acted as a nucleophile on the carbonyl carbon of the carboxylic acid. However, this type of response may be influenced by other factors, such as the inability to identify the carboxylic acid as a good proton donor. The point in time at which students were interviewed likely influenced their responses, given that midway through the second semester of organic chemistry, they were learning a wide variety of reactions whose mechanisms proceed via nucleophilic attack on a carbonyl carbon, so that kind of reaction is likely to be at the front of their minds. The tendency of some students to automatically discard the notion of using what they deemed to be organic chemistry vocabulary to discuss reactions learned in general chemistry also suggests a lack of meaningful learning; these students did not even attempt to relate new information (the concepts of nucleophile and electrophile) to prior knowledge (e.g. redox reactions).Implications for teaching and research
The findings reported in this manuscript suggest a sort of chicken-and-egg problem: faculty feel that students need to have a firm grasp of the concepts of nucleophilicity and electrophilicity (among others) before they can truly understand reaction mechanisms and the EPF. However, students feel they need to know the mechanism of a reaction in order to evaluate whether the species involved are nucleophiles or electrophiles. Although the sample in this study was small, the ubiquity of student reliance on mechanisms to evaluate for the presence of nucleophiles and electrophiles suggests that these students and the faculty surveyed previously have opposite ideas about the order in which understanding of nucleophiles, electrophiles, and reaction mechanisms should be built. It is therefore quite possible that there exists a misalignment between teaching and learning; further study of this issue is warranted. The language used by faculty in the instructional setting is critical; discussing the ideas of nucleophiles and electrophiles in the context of function first, rather than overly emphasizing the structural features when identifying a nucleophile or electrophile, could help students overcome the limitations to their ideas. For example, if faculty merely teach “hydroxide is a good base” when discussing acid–base reactions and then teach “hydroxide is a good nucleophile” when discussing nucleophile/electrophile reactions, without discussing the thought processes involved in deciding which process is involved in a particular reaction, students are left to rely on memorization. However, discussing hydroxide from a function-first perspective may help students avoid recall in favor of meaningful concept application as a strategy when evaluating reactions.A rearranged curriculum focusing on reaction mechanisms was recently reported by Flynn and Ogilvie (2015). This curriculum provides explicit instruction in the use of the EPF first, followed by learning reactions that are grouped by nucleophile or electrophile type (e.g., π electrophiles with a leaving group). Seven of their sixteen units use π species as the common denominator, with four of those seven sections pertaining to π electrophiles and the remaining three sections discussing π nucleophiles. Obviously π systems can undergo a wide variety of reactions, so it is crucial that students be able to use both inherent characteristics (structure) as well as contextual clues to suggest function (what else is reacting?) and to classify these systems as nucleophilic, electrophilic, or neither (as would be the case in reactions involving radicals, for example). Such an emphasis on categorizing reactions based on reactivity behavior could be a solution to some of the student difficulties reported from the present study.
The students in this study had substantial experience with reaction mechanisms by the time they were interviewed, being roughly 75% of their way through the full year of organic chemistry. It is possible that their experience was leading them to place heavy emphasis on mechanisms as a way to identify nucleophiles and electrophiles in reactions, because they knew (or at least knew they had been taught) the mechanisms for all but one of the reactions in Fig. 2. (The students were not taught an electron-pushing mechanism for the hydrogenation of an alkene.) Comparing the response patterns of these students to those of students who are much earlier in their organic chemistry learning (e.g., midway through first semester) could provide valuable information about the development of this relationship in students' minds.
Acknowledgements
This material is based upon work supported by the Volwiler Distinguished Research Professorship at Miami University (SLB).References
- Bhattacharyya G., (2006), Practitioner development in organic chemistry: How graduate students conceptualize organic acids, Chem. Educ. Res. Pract., 7(4), 240–247.
- Bhattacharyya G., (2013), From source to sink: Mechanistic reasoning using the electron-pushing formalism, J. Chem. Educ., 90(10), 1282–1289.
- Bhattacharyya G. and Bodner G. M., (2005), “It gets me to the product”: How students propose organic mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Bretz S. L., (2001), Novak's theory of education: Human constructivism and meaningful learning, J. Chem. Educ., 78(8), 1107.
- Bruice P. Y., (2011), Organic Chemistry, 6th edn, Upper Saddle River, NJ: Prentice-Hall.
- Cartrette D. P. and Mayo P. M., (2011), Students' understanding of acids/bases in organic chemistry contexts, Chem. Educ. Res. Pract., 12(1), 29–39.
- Cruz-Ramírez de Arellano D. and Towns M. H., (2014), Students' understanding of alkyl halide reactions in undergraduate organic chemistry, Chem. Educ. Res. Pract., 15, 501–515.
- DeFever R. S., Bruce H. and Bhattacharyya G., (2015), Mental rolodexing: Senior chemistry majors' understanding of chemical and physical properties, J. Chem. Educ., 92(3), 415–426.
- Domin D. S., Al-Masum M. and Mensah J., (2008), Students' categorizations of organic compounds, Chem. Educ. Res. Pract., 9(2), 114.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9(2), 102–113.
- Flynn A. B. and Ogilvie W. W., (2015), Mechanisms before reactions: A mechanistic approach to the organic chemistry curriculum based on patterns of electron flow, J. Chem. Educ., 92, 803–810.
- Graulich N., (2014), The tip of the iceberg in organic chemistry classes: How do students deal with the invisible?, Chem. Educ. Res. Pract., 16, 9–21.
- Graulich N., (2015), Intuitive judgments govern students' answering patterns in multiple-choice exercises in organic chemistry, J. Chem. Educ., 92(2), 205–211.
- Grove N. P. and Bretz S. L., (2010), Perry's scheme of intellectual and epistemological development as a framework for describing student difficulties in learning organic chemistry, Chem. Educ. Res. Pract., 11(3), 207.
- Grove N. P. and Bretz S. L., (2012), A continuum of learning: From rote memorization to meaningful learning in organic chemistry, Chem. Educ. Res. Pract., 13(3), 201.
- Grove N. P., Cooper M. M. and Rush K. M., (2012), Decorating with arrows: Toward the development of representational competence in organic chemistry, J. Chem. Educ., 89(7), 844–849.
- Henderleiter J., Smart R., Anderson J. and Elian O., (2001), How do organic chemistry students understand and apply hydrogen bonding?, J. Chem. Educ., 78(8), 1126.
- Klein D. R., (2012), Organic Chemistry, 1st edn, Hoboken, NJ: John Wiley & Sons, Inc.
- Lincoln Y. S. and Guba E. G., (1985), Naturalistic Inquiry, Newbury Park, CA: Sage Publications, Inc.
- Linenberger K. J. and Bretz S. L., (2012), A novel technology to investigate students' understandings of enzyme representations, J. Coll. Sci. Teach., 42(1), 45–49.
- Livescribe, Inc., Livescribe: Never miss a word. http://www.livescribe.com/en-us/.
- McClary L. M. and Bretz S. L., (2012), Development and assessment of a diagnostic tool to identify organic chemistry students' alternative conceptions related to acid strength, Int. J. Sci. Educ., 34(15), 2317–2341.
- McClary L. and Talanquer V., (2011), Heuristic reasoning in chemistry: Making decisions about acid strength, Int. J. Sci. Educ., 33(10), 1433–1454.
- Novak J. D., (2002), Meaningful learning: The essential factor for conceptual change in limited or inappropriate propositional hierarchies leading to empowerment of learners, Sci. Educ., 86(4), 548–571.
- Novak J. D., (2010), Learning, Creating, and Using Knowledge, 2nd edn, New York, NY: Taylor & Francis.
- Novak J. D. and Gowin D. B., (1984), Learning How to Learn, Cambridge: Cambridge University Press.
- Patton M. Q., (2002), Qualitative Research & Evaluation Methods, 3rd edn, Thousand Oaks, CA: Sage Publications, Inc.
- QSR International Pty Ltd, (2014), NVivo qualitative data analysis software (Version 10).
- Rushton G. T., Hardy R. C., Gwaltney K. P. and Lewis S. E., (2008), Alternative conceptions of organic chemistry topics among fourth year chemistry students, Chem. Educ. Res. Pract., 9(2), 122.
- Şendur G., (2012), Prospective science teachers' misconceptions in organic chemistry: The case of alkenes, Turk. Sci. Educ., 9(3), 160–185.
- Strauss A. and Corbin J., (2008), Basics of Qualitative Research, 2nd edn, Thousand Oaks, CA: Sage Publications, Inc.
- Strickland A. M., Kraft A. and Bhattacharyya G., (2010), What happens when representations fail to represent? Graduate students' mental models of organic chemistry diagrams, Chem. Educ. Res. Pract., 11(4), 293–301.
- Taagepera M. and Noori S., (2000), Mapping students' thinking patterns in learning organic chemistry by the use of knowledge space theory, J. Chem. Educ., 77(9), 1224–1229.
| This journal is © The Royal Society of Chemistry 2015 |