The analysis of the changes in integration of nature of science into Turkish high school chemistry textbooks: is there any development?
Sevgi
Aydin
*a and
Selma
Tortumlu†
*b
aSecondary Science and Mathematics Education, College of Education, Yuzuncu Yil University, Van, Turkey. E-mail: sevgi.aydin45@hotmail.com; Fax: +90 4322251368; Tel: +90 4322251751
bEducational Science Institute, Yuzuncu Yil University, Van, Turkey
First published on 22nd June 2015
Abstract
To attain the goal of scientific literacy, the nature of science (NOS) is one of the areas that should be addressed. In many countries, the training of scientifically literate generations is a fundamental aim of science education, as a result there is an emphasis on NOS in science curricula and curricular materials. Textbooks prepared to teach curricula should incorporate aspects of NOS for all grades. In Turkey, the secondary science curricula were reformed in 2013. In this document analysis, aspects of NOS were included, the approach adopted for integrating aspects of NOS (i.e., explicit-reflective, implicit, and historical), and content-embeddedness of integration were analyzed in the reform-based and old high school chemistry textbooks published and provided by the National Ministry of Education. The results revealed that from the 9th to the 12th grade, the number of NOS aspects mentioned in the textbooks decreased. The most frequently cited aspects were the tentative nature of scientific knowledge, the empirical basis of science, and the difference between observation and inference. However, models in science, creativity and imagination were NOS aspects that were overlooked. Regarding the approach, contrary to the suggestion of the literature, the implicit approach was employed frequently. Finally, NOS aspects were provided in a content-embedded way in the 9th and 10th grades whereas they were presented mostly in a content-generic way in the 12th grade. Only the 9th grade textbook provided two NOS activities for teaching NOS. Although some changes have been made regarding teaching NOS, some important parts are missing. In light of the results, we suggest that all NOS aspects should be integrated into textbooks in an explicit-reflective way. Additionally, for teaching NOS, explicit-reflective activities should be offered in textbooks.
Introduction
What is science? There is no single right answer to this question. Different scholars have described science by highlighting its different aspects (e.g., scientific inquiry, scientists' characteristics, and the nature of scientific knowledge). Regarding science, ‘the nature of science’ (NOS) is an important concept that should be addressed. McComas et al. (1998) viewed NOS as an amalgam of the sociology, history, psychology, and philosophy of science. NOS is about the features of scientific knowledge, how scientists accumulate scientific knowledge, the methods scientists use, and the relationship between science, technology, and society.All around the world (e.g., Great Britain, The Netherlands, the United States, Turkey, Chile, and South Africa) scientific literacy has been a targeted outcome of science education (Dillon, 2009; Cofré et al., 2014). Although there is no single definition of the scientific literacy construct, it can be described as “the knowledge and understanding of scientific concepts and processes required for personal decision making, participation in civic and cultural affairs, and economic productivity” (National Research Council [NRC], 1996, p. 22). In daily life, people hear news about nuclear energy, genetically modified foods, the green house effect, and acid rain. In order to be a scientifically literate citizen, one should have adequate knowledge about NOS and scientific knowledge (McComas, 1998; Bartos and Lederman, 2014; Cofré et al., 2014). Hence, NOS has been an important focuss of science curricula in many countries (e.g., in the USA; American Association for the Advancement of Science [AAAS], 1993; NRC, 1996, 2011; in Turkey National Ministry of Education, [NME], 2013a; in Chile Cofré et al., 2014, etc.). In this qualitative research paper, we have focused on how NOS has been integrated into high school chemistry textbooks (from the 9th to the 12th grades) in Turkey. Additionally, we have also studied how NOS has been integrated into recently written textbooks in the second year of the reform of secondary science curricula.
Literature review
NOS and teaching NOS
In the related literature (McComas, 1998; Lederman et al., 2002; Irzik and Nola, 2011) different ideas have been presented about the aspects of NOS and which aspects of NOS should be taught in science courses. However, the aspects of NOS accepted in general are: the tentative nature of scientific knowledge, the empirical basis of science, the inferential/theoretical nature of scientific knowledge, scientific methods, subjectivity in science, the role of creativity and the imagination of scientists in scientific inquiry, the relationship between theory and law, the socio-cultural embeddedness of science, the morality of science, the interdisciplinary nature of science, the difference between observation and inference, the relationship between science and technology, the use of models in science, and science as a solitary pursuit. Detailed explanations of these aspects are provided in Table 1.| Aspects of NOS | Explanation |
|---|---|
| Scientific method | There is no single method that all scientists use. It may change according the field and the research questions focused on. |
| Empirical basis of science | Scientific knowledge is accumulated through the collection of data. Scientists support their claims with the data collected. |
| Inferential/theoretical | Developments in science occur owing to the data collected (i.e., through observations and experiments conducted). Additionally, interpretation of the theoretical knowledge and the inferences made are sources of the developments. |
| Tentative nature of scientific knowledge | Scientific knowledge may be replaced with new knowledge in light of the collection of new data or the re-interpretation of existing data (e.g., changes in the atomic models from Dalton to modern atomic theory). |
| Subjectivity | Complete objectivity may not be possible in deciding which type of data will be collected, how the data will be collected, which part of the data will be used, and how the data will be interpreted. |
| Creativity and imagination in science | The creativity and imagination of scientists are important in all steps of the scientific inquiry (e.g., Kekule' s imagination of the benzene ring). |
| Theories/laws | Theories and laws have different natures. Theories do not turn into laws with adequate evidence. Theories have an explanatory nature whereas laws have a descriptive nature that describes what happens under certain circumstances. |
| Socio-cultural embeddedness | The culture in which scientist live, ethnicity, beliefs that they have may influence scientists' interpretation. |
| Observation and inference | Observation and inference are different concepts. Observation is the description of the observed phenomenon or the event whereas the inference includes interpretation of the observed things. |
| Science and technology | Science and technology are different fields. Science aims at understanding the nature whereas technology focuses on application and making life easier. |
| The use of models in science | Models are utilized in science to make understanding and interpretation easier. Models do not represent the real structure. |
| Science is a solitary pursuit | Scientists generally study together while forming research questions, collecting and analyzing data. |
| Science is a human endeavor | When scientists make an investigation, they share their study with the public. In this process, peers review their investigation. Different scientists take part in this process. |
| Moral aspects of science | Science is neutral. It does not focus on the use of the scientific information regarding moral issues (e.g., genetically modified foods vs. treatment of genetic disease and producing nuclear energy vs. the atomic bomb). |
| Interdisciplinary nature of science | There are no clear-cut compartments of science. Fields are related to each other. |
Many ideas about science, scientists, and scientific knowledge have been presented in the news, newspapers, social media, and in science textbooks. Research has revealed that the information provided by textbooks and other media include many misconceptions about what science is, how scientists work, and what NOS is (Abd-El-Khalick et al., 2008; Irez, 2009). It is not only the people who write about science and scientific processes in the newspapers or textbooks, but also science teachers, college professors, and pre-service teachers who also have misconceptions about these aspects (Lederman, 2007). Therefore, it is assumed that if NOS, its aspects, and the features of science are taught to students from the early stages of science education, students would have a better understanding of science, scientists, and their work.
Approaches for teaching NOS
In terms of how NOS is taught, there are three different approaches for teaching NOS, namely, explicit-reflective, implicit, and historical approaches. The explicit-reflective approach assumes that learners learn about NOS by participating in scientific activities through which learners design, collect data, analyze and interpret, and share the results with peers. After the activity, teachers should discuss NOS aspects included in the activity explicitly through the participation of learners (Abd-El-Khalick and Lederman, 2000). In contrast to the first approach, the implicit approach assumes that learners learn about NOS by participating in inquiry activities. In other words, an explicit discussion on the aspects of NOS, the nature of scientific inquiry, and how scientists work is not used in the implicit approach. Finally, in the historical approach, NOS is addressed by the use of interesting cases which have happened in the history of science, the story of the discoveries, and the inventions discovered by coincidence. In order to teach NOS through the historical approach, teachers need to be knowledgeable about the history of science (HOS) and the interesting stories of discoveries (Abd-El-Khalick and Lederman, 2000). Research on teaching NOS has revealed that the explicit-reflective approach results in a better understanding of NOS than the implicit one (Khishfe and Abd-El-Khalick, 2002; Lederman, 2007).Content relation of NOS
Another important aspect of teaching NOS that deserves attention is the content relation of NOS. There are two ways of teaching NOS regarding the content relation, namely, content-embedded and content generic (Lederman, 2007). The former involves the teaching of NOS aspects by integrating the aspects into the topic taught. For instance, to teach about the tentative nature of scientific knowledge, the topic of atomic models (i.e., stressing the tentative nature of scientific knowledge with modifications and changes from Dalton's theory to modern atomic theory) is very suitable. To emphasize the tentative nature of scientific knowledge, this topic can be integrated into the topic of atomic theories (i.e., the content from the chemistry curriculum) to form the basis for teaching about the way in which scientific knowledge can be changed or modified by the use of new knowledge or by the re-interpretation of existing knowledge. The content-generic way of teaching involves teaching NOS aspects without integrating them into the topic taught. There are many content-generic activities in the literature (e.g., Tricky Tracks by Lederman and Abd-El-Khalick, 1998; The Great Fossil Find by Randak and Kimmel, 1999), which can be used to teach NOS with content-free activities. Literature has stated that both ways are effective in teaching NOS (Lederman, 2007).In order for teachers to teach NOS and its aspects by the use of the approaches summarized above, teachers should be proficient in using them. Teachers have to be equipped with knowledge about NOS, how to teach it, and learners' difficulties and misconceptions about NOS. However, studies have clearly shown that science teachers do not have the required knowledge for teaching NOS (Hanuscin et al., 2011; Cofré et al., 2014). It is clear that teachers should be encouraged to teach NOS by supporting their NOS knowledge and pedagogy for teaching NOS through pre-service teacher education and in-service training activities in which the explicit-reflective teaching approach is emphasized (Lederman, 2007). Even if teachers are trained to teach NOS, research has revealed that teachers still have obstacles in teaching NOS (Abd-El-Khalick et al., 1998; Hanuscin et al., 2011). To minimize the obstacles teachers face regarding NOS aspects, teaching NOS, and useful strategies and historical cases should be provided in science curriculum documents and science textbooks explicitly. In this way, science teachers would have examples of how to integrate NOS into their science classes, and have activities to apply in the class, this will have the potential of increasing teachers' NOS integration into teaching. Additionally, attention should be paid to integrate NOS and give examples of NOS activities and HOS cases (Lederman, 2007; Aydın et al., 2013) not only in the education of teachers and their professional development activities but also in written documents (i.e., science curricula documents and textbooks). Thus, with this point in mind, this research was focused on analyzing high school chemistry textbooks (from the 9th grade to the 12th grade, four books in total) with regard to NOS teaching. Textbooks have two types of influence on learners: direct and indirect influence (Irez, 2009). The former is the case when learners use the textbook on his/her own. The indirect influence occurs when teachers utilize textbooks to organize their teaching. Likewise, textbooks are essential sources for teachers in designing their teaching. Therefore, textbooks should be equipped with essential aspects and examples for teaching and learning NOS in order to raise scientifically literate citizens.
Research on NOS integration into textbooks and curriculum documents
There have been some studies on teacher training for integrating NOS into science courses in the USA (e.g., Akerson et al., 2007; Akerson et al., 2009; Hanuscin et al., 2011). However, in countries where much less money is invested on teacher education professional development projects, the curriculum materials and textbooks are vital sources for teachers who need support for integrating NOS into their teaching. Therefore, the quality of these materials and textbooks plays an important role in determining the quality of science teaching and learning. In this part of the literature review, research focusing on NOS in curriculum materials and textbooks is presented.Abd-El-Khalick and his colleagues (2008) stated that one of the main reasons for inadequate understanding of NOS by learners and teachers is the poor quality of science textbooks. “In the case of NOS, the impact of textbooks gains significance because very few, if any, commercially viable science textbooks have been recently designed specifically to help pre-college students develop informed NOS conceptions as emphasized in current science education reform documents” (p. 836). Therefore, examining how and to what extend NOS is integrated into science textbooks would increase the pressure on both authors and publishers to pay more attention to NOS (Abd-El-Khalick et al., 2008). To fill this gap in the literature, Abd-El-Khalick and his colleagues analyzed 14 high school chemistry textbooks used frequently in the US (i.e., five of them had series between one decade to four decades). In this study, the researchers analyzed the ‘Scientific Method’, ‘The Structure of the Atom and Atomic Models’, and ‘The Kinetic Molecular Theory’ parts of the textbooks rather than analyzing the entire books. Results revealed that textbooks characterized scientific method, theories and laws, and the role of creativity and imagination not in accord with recent NOS related research in science education. Additionally, 12 of the analyzed books did not mention socio-cultural embeddedness of science at all. Another noticeable result was that there was no clear development regarding NOS integration in the last 40 years.
Similar to Abd-El-Khalick and his colleagues (2008), Niaz and Maza (2011) examined the introductory chapters of 75 general chemistry textbooks with regard to nine NOS aspects (e.g., the tentative nature of scientific knowledge, the theory-law relation, there is no single scientific method, the role of imagination and creativity in science, etc.). The results showed that not enough attention had been paid to NOS. For instance, almost 95% of the books analyzed did not mention that theories and laws have a different nature, and that with more evidence theories do not become laws. Likewise, 85% of the books did not provide any information regarding the fact that scientists might interpret the same data in different ways. The NOS aspects presented satisfactorily were the tentative nature of scientific knowledge (17%) and the role of creativity and imagination in science (16%). The tentative nature of scientific knowledge aspect of NOS was the most frequently mentioned one with 39%. Given the fact that this study analyzed only the introductory chapter of the textbooks, the results do not provide any information about how and to what extend the rest of the books addressed NOS aspects.
In another study, Irez (2009) examined five 10th grade Turkish high school biology textbooks. In all the textbooks analyzed, scientists were represented as objective, curious, and good observers. ‘Science is a solitary pursuit’ was mentioned in only one of the books analyzed. Furthermore, none of the books stated the tentative nature of scientific knowledge and socio-cultural embeddedness of science. However, all of the books mentioned that science is empirical. Another noteworthy result was that all of the books stated that ‘there is a single scientific method that scientists follow step by step’ and ‘theories become law with adequate evidence’, which are not consistent with informed NOS understanding. Irez (2009) discussed that those misconceptions detected in the textbooks are one of the main reasons for the misconceptions that teachers and learners have about NOS. Finally, the researcher emphasized that NOS was not integrated into all of the chapters rather it was mentioned in some parts of the textbooks.
Finally, in addition to textbooks in some of the studies researchers focused on professional journals regarding representation of NOS and its aspects. Aydın et al. (2013) studied papers published in The Science Teacher journal (i.e., NSTA journal for high school teachers). 65 papers published from 1995 and 2010, were examined regarding the NOS aspects, the content-embeddedness of them, and the approaches used (e.g., explicit-reflective). The most frequently mentioned NOS aspect was ‘science as a human endeavor’ (27 articles out of 65). The ‘Tentative nature of science’ (24 articles) and ‘socio-cultural embeddedness of science’ (24 articles) were also included more than other aspects (e.g., science and technology relation, the difference between observation and inference, and the relation between theory and law). 28 of the articles mentioned NOS in the explicit-reflective way. The history of science (HOS) was used in 19 of the papers. Regarding the content-embeddedness, 27 of the articles focused on embedded NOS aspects into a topic whereas 38 of them mentioned NOS in a content-generic way. Researchers stated that less frequently mentioned NOS aspects would be problematic for teachers to integrate into their teaching.
The significance of the study
Textbooks are cheap and are the most-readily available sources for both learners and teachers (Abd-El-Khalick et al., 2008; Irez, 2009).“Among others, as resulting in classroom practices that often times closely mirrored the sequences and structures in textbooks or preparatory coursework. For NOS and SI [scientific inquiry], no such referent or document exists. Having subject matter experts, or even those charged with informing the inclusion of NOS and SI in reform documents and curricula, explicate their own knowledge structures might also prove efficacious in helping develop coherent conceptions in teachers, and hopefully, their students (Bartos and Lederman, 2014, p. 1178).”
Previous research has examined how NOS was integrated into some chapters (i.e., ‘Scientific Method’, ‘The Structure of the Atom and Atomic Models’, and ‘The Kinetic Molecular Theory’) of high schools chemistry books (Abd-El-Khalick et al., 2008), the introductory chapter of college general chemistry textbooks (Niaz and Maza, 2011), and 10th grade high school biology textbooks (Irez, 2009). However, none of them analyzed all of the book chapters, which makes generalization of the results of the study of these books impossible. Furthermore, McComas and Olson (1998) stated that analysis of the curriculum documents “in languages other than English and particularly from non-Western cultures” (p. 51) should be done. To fill this gap, we focused on high school chemistry textbooks (from the 9th to the 12th grades) and analyzed them with regards to NOS aspects mentioned, the approaches used for addressing them (i.e., explicit, implicit, and historical), and the content relation of them (i.e., content-embedded and content-generic) in the whole books used all around the country. We think that, with this study we will be able to address McComas and Olson's (1998) call. Finally, this study provides valuable data about the big picture of NOS integration into all chapters of high school chemistry textbooks from the 9th to the 12th grades.
In Turkey, a national curriculum is used all around the country for all courses in K-12 in order to provide the same opportunities for all learners in the country. The National Ministry of Education (NME) is responsible for preparing the national curriculum. The NME also provides the textbooks for all learners free for all courses. During the textbook writing and publishing process, first, the NME requests that textbooks are written. Then, science educators write them and submit the textbook to the writing commission in which many experts (i.e., including science teachers and experts from measurement in education, etc.) working for NME examine the textbooks submitted. After a thorough examination, the textbooks receiving the highest scores for each grade are distributed to learners all around Turkey for free. All learners and teachers utilize the textbooks distributed by NME. Therefore, the textbooks used by all learners should reflect NOS appropriately and necessarily. As in other countries in the world, raising scientifically literate citizens is a major goal of science education in Turkey, which makes NOS integration into the books obligatory.
In 2013, NME made some alterations to the high school science curricula which highlighted developing science process skills and scientific literacy. Teachers started to utilize the new curricula in the 2013–2014 academic year (i.e., fall semester) for the 9th grade. Then in the following academic year, 2014–2015, they started to utilize the 10th grade curriculum and new textbooks. It was the second year of the reform. In 2016, the 11th grade curriculum and the textbook will be utilized in the schools. In 2017, all the grades will be using the new curricula and the new textbooks. In other words, the reform is being carried out in stages. To check to what extent the reform addresses the scientific literacy regarding NOS, it would be worthy to compare the new (i.e., 9th and 10th grade textbooks) and the old ones (i.e., 11th and 12th grade textbooks) in terms of NOS aspects issued, the approaches used, and to what extent the aspects were mentioned.
The research questions guiding the study were:
(1) Which aspects of NOS were emphasized in high school chemistry textbooks in Turkey?
(2) Which approaches were utilized to integrate NOS aspects into the Turkish chemistry textbooks?
(3) How are NOS aspects presented in Turkish textbooks regarding the content embeddedness?
(4) How do chemistry textbooks prepared in light of the reform-based and old curricula differ in terms of addressing NOS?
Methodology
Research design
This study is qualitative in nature and is the content analysis of Turkish high school chemistry textbooks (Merriam, 2009). The high school chemistry textbooks were analyzed with regard to NOS integration.Data collection and analysis
First, high school chemistry textbooks for 9th to 12th grades were obtained from a high school. Then, we started the analysis of the textbooks received. When we were confronted with an NOS aspect addressed in the textbook, we coded that part with regard to four dimensions, namely; (1) the NOS aspects, (2) the approach, (3) content-embeddedness, and (4) the degree to which the textbook mentioned the NOS aspect. In this way, we collected the raw data. Then, by using the raw data, we formed tables, calculated percentages, and drew graphs to better interpret the results. In other words, data analysis of the study included four parts, namely, the aspects of NOS addressed (see Table 1 in the literature review part and Table 2), the content-embeddedness (i.e., content embedded vs. content generic) (Table 3), the approaches used for addressing NOS aspects (i.e., explicit-reflective, implicit, and historical), and the comprehensiveness of addressing NOS aspects in the chemistry textbooks. The details regarding the coding are provided in the following paragraphs.| Aspects from NSTA document | Additional aspects |
|---|---|
| Tentative nature of scientific knowledge | Observation-inference |
| Scientific method | Relation between science and technology |
| Creativity and imagination in science | Model use in science |
| Theories and laws | Science is a solitary pursuit |
| Socio-cultural embeddedness of science | Moral aspects of science |
|
Empirical bases of science
Subjectivity Inferential/theoretical |
Interdisciplinary nature of science
Science is a human endeavor |
| Content-embeddedness | Explanation |
|---|---|
| Content-embedded | NOS aspect is embedded into the topic covered. For instance, tentative NOS can be addressed well in the atomic theories from Dalton to modern atomic theory by the use of cases showing that scientific knowledge can be changed by the use of new knowledge or re-interpretation of the existent one. |
| Content-generic | Addressing NOS aspects without using the content taught. For instance, using ‘old or young lady’ picture to teach subjectivity in science aspect of NOS. |
First, to code the textbooks, we utilized the NOS aspects stated in the National Science Teacher Association (NSTA, 2000) document and additional aspects of NOS (Aydın et al., 2013). All aspects used for coding are provided in Table 2.
Second, we coded the NOS aspects mentioned in the books regarding the content embeddedness (Table 3).
Third, the parts addressing NOS aspects were also coded regarding the approach used (Table 4).
| Approaches | Explanation |
|---|---|
| Explicit-reflective | For teaching NOS, activities and/or scientific inquiry are used. After that, a whole class discussion about the NOS aspects addressed in the activity are discussed explicitly rather than assuming that learners simply understand the aspects focused in the activity. |
| Implicit | It assumes that learners learn NOS aspects by participating in scientific processes such as observation, data collection, and analysis of the data. Teacher neither provides explicit explanation nor holds a whole class discussion on the aspects. |
| Historical | The use of interesting cases that occurred in the history of science. For instance, Rutherford's gold foil experiment is a good example of the difference between observation (i.e., very low percent of the alpha particles scattered) and inference (i.e., there should be a small and dense nucleus with positive charge in the atom). |
As seen from Table 4, in the explicit-reflective approach, there should be activities for teaching NOS aspects and then NOS aspects should be discussed explicitly through the participation of learners. However, when we analyzed the textbooks, we realized that only two activities were provided in the 9th grade textbook. The other authors did not present any activities; rather, they provided some examples from HOS or examples with some information about the NOS aspects. Therefore, when we used the explicit-reflective approach in this study, we had to modify its meaning. When we used the explicit-reflective approach, the reader should understand that the textbook authors explicitly mention the NOS aspect, provided explanation, and an example for addressing that aspect. Similarly, when the implicit approach was used, it meant that the NOS aspect(s) was mentioned implicitly without stating the NOS aspect clearly and explicitly. Those were required modifications due to the lack of activities that Turkish chemistry textbooks focused on.
Regarding the approaches mentioned in the textbooks, when we started the analysis, we realized that hybrid approaches existed in the textbooks (e.g., explicit-reflective and historical approach or implicit and historical one). Therefore, we added them into our codings.
Finally, the data were coded regarding the comprehensiveness of addressing NOS aspects, which is related to what extent textbooks provide information about the NOS aspects. With the help of the rubric presented by Abd-El-Khalick and his colleagues (2008); we coded the data (Table 5). Abd-El-Khalick et al. (2008) used negative scores for the textbook parts that make learners develop alternative conceptions about NOS in textbooks. However, due to the fact that we did not detect any part that may cause alternative conceptions about NOS in the textbooks analyzed, there was no negative score in the rubric we utilized.
| Scores | Category | Explanation |
|---|---|---|
| 1 point | Mentioned | NOS aspects were described but not supported with an example from chemistry, and/or history of chemistry. |
| 2 points | Satisfactory | NOS aspects were described and the explanation was enriched with the necessary examples from chemistry and/or from history of chemistry. |
Before coding independently, researchers formed the tables for all dimensions and discussed their meanings. Both researchers coded the textbooks independently by the use of the descriptions of the aspects, approaches, content-relations, and completeness for one chapter in the 9th grade chemistry textbook. After coding the data for a unit independently, they compared and contrasted the codings. The interrater reliability was 0.84 (Miles and Huberman, 1994). Small differences were discussed. Then, both researchers coded another chapter independently and compared the coding. The comparison of the coding showed that they coded almost in the same way. The interrater reliability was calculated as 0.96 (Miles and Huberman, 1994). Very little minor issues were discussed again. Yet another chapter was coded independently and a comparison was done. In the third cycle, the coding was identical for aspects, approaches, and content-relation, and completeness. Then, the second author coded the rest of the 10th, 11th, and 12th grade textbooks. All of the data received were used to form tables. To help readers to follow the data coding, details for the procedure are summarized in Table 6 for all of the dimensions focused on.
| Aspects | Approaches | Content-relation | Short excerpt from the textbooks | |
|---|---|---|---|---|
| Tentativeness | Explicit-reflective | Content-embedded | 2 | “Sometimes, it is necessary to change knowledge or model due to accumulation of the new knowledge. In this topic, you will learn how the knowledge about the atom has changed in light of the new information accumulated.” (Altun and Tümay, 2013, 9th grade, p. 55) |
| Science is a solitary pursuit | Explicit-reflective | Content-embedded | 2 | “As you may remember, J. J. Thomson stated that atoms have a spherical shape and have particles with a negative charge surrounded by particles with a positive charge. Is that the real structure of the atom? A research group lead by one of Thomson's students Ernest Rutherford studied the structure of the atom. (Altun and Tümay, 2013, 9th grade, p. 75) |
| Empirical | Explicit-reflective | Content-generic | 1 | “The difference between Dalton's and Democritus's theory is that Democritus's idea is a philosophical view and a non-empirical one whereas Dalton's idea is a hypothesis based on scientific evidence and necessitates empirical evidence…” (Dursun et al., 2013, 10th grade, p. 17). |
| Science-technology | Implicit | Content-generic | 1 | “The discovery of the steam engine, also known as the industrial revolution, resulted in changes in people's way of living (from agriculture to industry). The discovery happened owing to the development in the thermodynamic field of science. Hence, science influenced technology and society…”. (Kavak, 2013, 11th grade, p. 44) |
| Empirical | Implicit | Content-embedded | 2 | “The plane of polarization is turned by optically active compounds, cannot be determined by the examination of the structure of the substance, rather experimentally. Most of the biological molecules have enantiomeric structure. For instance, glucose and galactose have enantiomeric structure.” (NME, 2013b, 12th grade, p. 151) |
| Observation and inference | Implicit-historical | Content-embedded | 1 | “If the galaxy moves away, the wavelength of the light decreases, the frequency of the light increases, and the spectral lines move to red side. Russian meteorologist and mathematician Alexander Friedmann realized the expansion of the universe in 1922.” (NME, 2013b, 12th grade, p. 17) |
| Theories/laws | Explicit-historical | Content-embedded | 2 | “How does Dalton's atomic theory explain law od conservation of mass and law of conservation of Mass?… As you can understand the information presented above, scientific theories and laws are different regarding both their meaning and function. They do not have hierarchical relations. They do not turn into other. Laws are the descriptions of the events observed in the nature. Theories, on the other hand, are the explanation how those events occur… (Altun and Tümay, 2013, 9th grade, p. 68) |
| Inferential/theoretical | Implicit | Content-embedded | 1 | “The existence of the charged particles in the atom, likewise the experiments and observations that show the existence of the atom, was understood by the use of evidence gathered through indirect ways.” (Altun and Tümay, 2013, 9th grade, p. 70) |
| Interdisciplinary & human endeavor | Implicit | Content-embedded | 1 | “In 1803, by conducting research, the British chemist and teacher John Dalton used the atom concept after Democritus. In 1869, the Russian chemist Dmitri Mendeleev developed a periodic table for the elements known…. In 1911, physicist Ernest Rutherford discovered the nucleus model of atom.” (Altun and Tümay, 2013, 9th grade, p. 52) |
| Use of models | Explicit, historical | Content-embedded | 2 | “As you can understand from the Dalton, Thompson, Rutherford, Bohr, and Modern Atomic models that represent the atom, those models help us understand scientists” theories about the atom. Models play an important role in science just as theories do. Models developed in chemistry are useful especially in understanding what happens in the sub-microscopic level.” (Altun and Tümay, 2013, 9th grade, p. 96) |
| Creativity and imagination | Implicit | Content-embedded | 1 | “Alfred Werner explained the structure of the of the compounds that transition metals form with neutral molecules and ions when bond concept had not been known well. He received Nobel Prize owing to that research” (Kavak, 2013, 11th grade, p. 181) |
| Human endeavor | Implicit | Content-embedded | 1 | “Kinetic Theory that explains the behaviour of the gases was firstly proposed by Bernoulli. Then Clausius, Maxwell, Boltzmann, and van der Walls developed it”. (Altun and Tümay, 2013, 9th grade, p. 242) |
| Subjectivity | Implicit | Content-embedded | 1 | “His interest in hot air balloons directed Charles to examine the effect of temperature on the volume of a gas”. (Dursun et al., 2013, 10th grade, p. 193) |
In this way, all of the chapters in the chemistry textbooks were analyzed and presented in the results part of the study.
Results
In this part of the study, the results of the analysis focusing on how NOS was integrated into the high school chemistry textbooks are presented with regard to the aspects of NOS (i.e., research question 1, i.e., creativity and imagination in science) and the quality of the integration, the approaches (i.e., explicit-reflective, implicit, and historical) used to integrate NOS (i.e., research question 2), the content relation of NOS (i.e., research question 3) (i.e., content-embedded and content generic). For the 4th research question focusing on the differences between the new textbooks (for 9th and 10th grades) prepared in light of the reforms and old textbooks (for 11th and 12th grades), the results are presented after presenting the results for aspects integrated, comparisons of the new and old textbooks are provided.The distribution of NOS aspects through the grades
The results of the analysis are presented in Table 7.| Grade | The number of the aspects addressed | The frequency of the NOS aspects addressed |
|---|---|---|
| 9 | 11 | 59 |
| 10 | 9 | 30 |
| 11 | 7 | 16 |
| 12 | 5 | 12 |
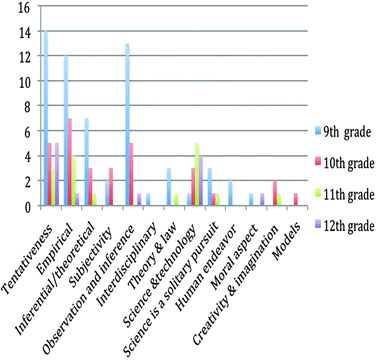
As the table shows, the number of NOS aspects issued in the textbooks decreases from the 9th to the 12th grade. Regarding the number of aspects addressed, the 9th grade textbook integrated 11 different aspects of NOS (i.e., out of 15 aspects provided in Tables 1 and 2) whereas the 12th grade textbook addressed only 5 aspects. Regarding the frequency of the aspects stated in the textbooks, a similar tendency was observed. The frequency of the NOS aspect issued decreased from 59 to 12 from the 9th grade to the 12th grade. A detailed further analysis was done to determine which aspects were addressed in different grades (Fig. 1).
As Fig. 1 shows, some of the aspects of NOS, for instance, tentativeness of scientific knowledge, empirical NOS, the difference between observation and inference were stressed mostly. However, models used in science, the moral aspects of science, creativity and imagination in science aspects were the ones that were mentioned in very few part of the textbooks analysed.
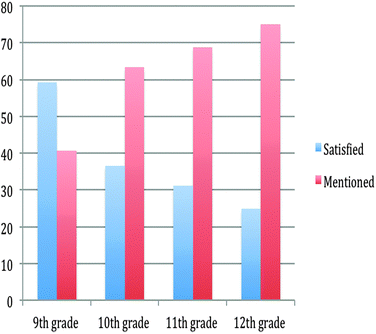
Regarding the aspects of NOS integrated into the textbooks, we were also interested in seeing the quality of the integration (Fig. 2).
As Fig. 2 shows, only the 9th grade textbook included more parts that addressed NOS issues satisfactory than the parts just mentioning NOS aspects. In the other grades, for instance, the 12th grade textbook issued NOS aspects 12 times. However, nine of them only mentioned the aspect rather than providing detailed explanations or examples from science and HOS. In three cases, the authors specified the NOS aspect and provided examples related to teaching the aspect, which was coded as ‘satisfied’.
Examination of the approaches to address NOS aspects in textbooks
In the literature review part of the study, it was stated that NOS aspects are presented in three different ways, namely, explicit-reflective, implicit, and historical approaches. The analysis of the approaches used to integrate NOS aspects into the chemistry textbooks are presented in Table 8.| Approaches | 9th grade | 10th grade | 11th grade | 12th grade |
|---|---|---|---|---|
| Explicit-reflective | 10 | 8 | 0 | 0 |
| Implicit | 23 | 18 | 8 | 4 |
| Historical | 8 | 1 | 0 | 2 |
| Historical-implicit | 18 | 2 | 8 | 6 |
| Historical-explicit-reflective | 0 | 1 | 0 | 0 |
| Total | 59 | 30 | 16 | 12 |
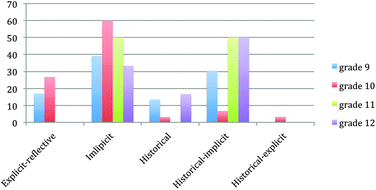
To understand the analysis better, the data were calculated as a percentage. In Fig. 3, the same data are presented as a percentage.
In general, the most preferred approach was the implicit one. Likewise, the historical-implicit approach was utilized frequently. In the 9th and 10th grade books, the explicit-reflective approach was used 17% and 23%, respectively. However, the 11th and 12th grade textbooks did not employ the explicit-reflective approach. Additionally, the historical approach was not utilized very much.
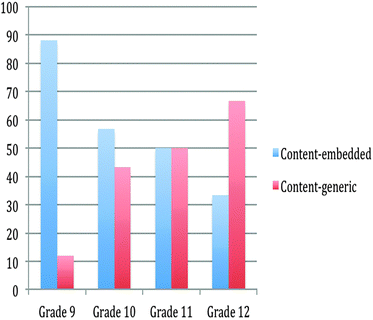
The analysis of the content relation of NOS aspects in the textbooks
The NOS aspects may be presented either by embedding the aspect into the topic taught (i.e., content-embedded) or without embedding it to the topic taught (i.e., content-generic). The analysis of the textbooks regarding content relations is summarized in Fig. 4.The analysis showed a great variation in relating NOS and the topics taught. In the 9th grade textbook, NOS aspects were mostly integrated into the topic covered. On the contrary, NOS aspects were mostly integrated into the textbooks without embedding them into the topic in the 12th grade. In the 10th and 11th grade textbooks, the percentages of the content-embedded and content-generic NOS integration are relatively close.
Discussion, conclusions, and implications
This study focussed on NOS integration into high school chemistry textbooks and revealed that textbooks showed a great variation regarding the number of NOS aspects mentioned and the way in which they are integrated (i.e., explicit–implicit and content-generic vs. content-generic). The results presented are discussed in the sequence of the NOS aspects, the approaches of integrating NOS, and the content relation order.First, the 9th grade textbook was the one that had the highest number of NOS aspects integrated (i.e., 11 aspects out of 15). The 10th grade textbook included 9 NOS aspects. The number of NOS aspects which appeared in the textbooks decreased with an increase in the grade up to the 12th grade (i.e., 5 aspects out of 15). The variation in the attention paid to NOS may be related to the authors' interest in NOS. These four textbooks were written by different groups of authors who may or may not be interested in NOS. In the study of the curricular documents of different countries regarding NOS integration, McComas and Olson (1998) stated that the authors' interests had a large influence on how and to what extend NOS was emphasized in the curricular materials. Therefore, the differences regarding NOS aspects included and the frequency of integrating NOS aspects may be related to the authors' interest in NOS.
The difference in the number of NOS aspects issued may also be related to the national curriculum documents. Şardağ et al. (2014) analyzed the new high school (grade 9 to 12) physics, chemistry, and biology curriculum standard documents regarding NOS integration and revealed that some of the NOS aspects (e.g., inferential-theoretical, there is no single scientific method, creativity and imagination in science, and socio-cultural embeddedness of science) were not included in the standard documents at all. However, some of the aspects were emphasized very frequently (e.g., the tentative nature of scientific knowledge). When we compared the results of both studies, similarities regarding the missing aspects and the aspects cited frequently were noticed. In both studies, the most frequently emphasized NOS aspect was the tentative nature of scientific knowledge. The NOS aspects that were missing both in the curricular documents and textbooks were revealed as the use of models in science, creativity and imagination in science, and the moral aspects of science. Similar results were revealed in the analysis of the Australian, US, British, and other countries' science curriculum documents by McComas and Olson (1998). In another study in which the introductory chapter of the general chemistry textbooks were analysed Niaz and Maza (2011) showed that the difference between theories and laws were only emphasized by 4 out of the 75 textbooks analyzed. Additionally, the subjectivity in science aspect was one of the missing aspects in the college textbooks. Finally, they stated that textbooks did not fully address NOS in the college chemistry textbooks.
Regarding the decrease in the numbers from the 9th to the 12th grade, another possible reason may be that more stress is placed on NOS in the reformed-based chemistry curriculum. As we stated in the significance of the study part, high school chemistry and other science curricula were modified in 2013 in Turkey. At the beginning of the curriculum document for high school chemistry, it was stated that NOS is one of the important aspects of educating scientifically literature citizens (National Ministry of Education, [NME], 2013a). In the document, five objectives were provided for NOS and four objectives for understanding the nature of scientific knowledge (NME, 2013a, p. 2). Therefore, more explicit NOS stress in the new curricula may make textbooks authors integrate more aspects in the textbooks. However, NOS was not stressed very much in the old curriculum documents, which may explain why the old textbooks include less NOS aspects.
Yet another factor may be the nature and the context of the units covered in the different grades. When we looked at the curricula and the textbooks for the content and the context of the units, we realized that it might be easier for authors to integrate NOS aspects into some units than in others. For instance, there are more accessible examples for integrating NOS aspects into the science of chemistry (from alchemy to chemistry) and the periodic table unit (in the 9th grade curriculum) than into the equilibrium or organic chemistry units (in the 11th and 12th grades curricula). The nature and the context of the topic may also be a factor explaining the difference.
Second, regarding approaches used in integrating NOS into the textbooks, results showed that explicit-reflective approaches were used less than the others. Specifically the old ones (i.e., 11th and 12th grade textbooks) did not utilize the explicit-reflective approach at all, which contradicts the literature's suggestion for NOS teaching. The 9th and 10th grade new textbooks utilized ther explicit-reflective approach to some extent. Research has revealed that the explicit-reflective approach is more useful for developing NOS understanding than the implicit approach (Abd-El-Khalick and Lederman, 2000; Khishfe and Abd-El-Khalick, 2002; Lederman, 2007). The results presented in this study are similar to the results revealed by Şardağ et al. (2014) who analyzed secondary science curriculum documents with regard to NOS. Likewise, NOS aspects were not mentioned in the documents explicitly. Curriculum documents play a guide role for textbook authors in terms of the topics covered, NOS aspects integrated, and the objectives focused on. Therefore, implicit NOS emphasis in the curriculum document may result in implicit NOS integration into science textbooks. Given the fact that teachers' inadequate NOS understanding and teaching NOS repertoire, the use of the implicit approach for embedding NOS into textbooks will not be beneficial for teachers (Lederman, 2007). Similarly, in the study analyzing the paper published in The Science Teacher (i.e., a NSTA journal) Aydın et al. (2013) revealed that 43% (i.e., 28 articles out of 65) of the analyzed NOS paper used the explicit-reflective approach for addressing NOS aspects. In this study, the results showed that none of the textbooks provided activities for teaching NOS, which may be an important limitation for the teaching of NOS in chemistry classes. To support teachers to enrich their instructional strategy repertoire, textbooks should include them. Although the US teachers have a great opportunity to have that repertoire because of NSTA professional journals, teachers who work in different countries may not have that opportunity. Another important result received in this study was the use of the hybrid approach in the textbooks. The historical approach was utilized with the explicit-reflective and implicit approaches. Lin and Chen (2002) stated that the use of interesting cases occurring in the HOS helps learners to enrich their NOS understanding and provides an opportunity for learners to talk about NOS aspects. Although the 9th grade textbook included HOS examples and the hybrid use of HOS, the 10th grade textbook did not have enough HOS cases. In light of this point, more examples from the HOS should be integrated into the reform-based textbooks for both teachers to use in their class and for learners who may read them on their own.
Third, regarding the content embeddedness of NOS, both methods are useful for teaching and learning NOS (Khishfe and Lederman, 2006). In this study, results revealed that in the 9th and 10th grade textbooks NOS was frequently integrated in a content-embedded way whereas in the 12th grade textbook NOS was presented in a content-generic way. In the 11th grade, both ways were used equally. In the analysis of the articles published in The Science Teacher, results showed that authors of the practitioner papers preferred to present NOS aspects in a content-embedded way (%60 of the articles) (Aydın et al., 2013). The analysis of curriculum documents showed that NOS was integrated into the document by integrating the aspects into the topic covered (Şardağ et al., 2014) Although the literature has suggested using both strategies, we think that embedding NOS aspects into the topic taught may be more helpful for learners and teachers to see the aspects in the context of the science and scientific inquiry. The new textbook authors preferred to integrate NOS aspects by relating them to the topic. The integration of NOS in textbooks is vital because textbooks are easily accessible and commonly utilized sources for both learners and teachers. Therefore, it is important to integrate NOS into all topics covered in textbooks. We think that when NOS is included in science teaching and learning, learners become more interested in science. Sometimes we, as science educators, are posed the question “why do we have to learn all of the [atomic] theories rather than learning the correct one?” which indicates an inadequate understanding of NOS. If textbooks were to include NOS aspects satisfactorily, we think that teachers would probably mention tentative NOS and other aspects of NOS more often, and learners would stop asking that question and start to appreciate Rutherford's creativity in planning the alpha particle experiment and Bohr calculations, and develop a more positive attitude towards science.
To conclude, the new Turkish high school chemistry textbooks showed some good changes regarding the number of NOS aspects issued, the use of the explicit-reflective method, and the use of HOS examples. However, more NOS integration regarding the aspects is still needed. Additionally, the new reform-based textbooks should include more NOS activities for teachers to implement in class. Activities that help learners to understand NOS should be part of the textbooks. Given the usefulness of the explicit emphasis of NOS in the curricular materials (Hanuscin et al., 2011), “curricular materials that model and support the implementation of instruction explicitly emphasizing these dynamic conceptions of NOS and SI [scientific inquiry] may likewise facilitate their [teachers'] translation into practice” (Bartos and Lederman, 2014, p. 1178). Moreover, HOS cases would enriched textbooks. The real experiences that scientists have had in the past are real and enlightening examples both for learners and teachers. In terms of the approach used, the explicit-reflective approach is suggested by the literature. However, the new textbooks provided more implicit NOS aspects than the explicit ones. Authors and publishers should be careful about this point and make necessary changes to offer more explicit ones. Finally, regarding the content-embeddedness, the authors of new textbooks embedded NOS aspects to the topics covered, which may be useful for learners to see the connection between the aspects and the chemistry topics that they learn. New textbooks have shown very good development but some more work is necessary regarding the quality of integrating NOS into textbooks as well (i.e., the explicit reflective NOS activities, HOS examples, etc.).
In this research, we studied the integration of NOS into the high school chemistry textbooks used in Turkey. The results presented are limited to the textbooks analyzed. Therefore, how and to what extend chemistry teachers use them in class is still a question, which is one of the limitations of this study. The enacted curriculum should be focused because teachers are not very enthusiastic to emphasize NOS especially when the education system is dominated by high stake exams. It is not very realistic to reach the goal of educating scientifically literate citizens by only teaching science content. Therefore, teachers' use of the HOS examples and other activities should be examined. Additionally, the difficulties that teachers face with integrating NOS into their teaching should be focused on.
In the science textbooks, science and the scientists' nature, how science and society influence each other, and science and technology relations should be accurately presented (Koseoglu et al., 2003). To attain the goal, as Niaz and Maza (2011) and Abd-El-Khalick et al. (2008) stated, not only researchers but also textbook authors and publishers should understand the importance of the emphasis on NOS. The authors and publishers should be informed about the role of NOS in educating scientifically literature generations. To achieve that, the groups (i.e., researchers, curriculum makers, authors, and publishers) should come together and share their ideas and concerns about the topic. Furthermore, to increase the quality of science textbooks, the criteria list should include NOS integration, which increases the probability of attracting authors and publishers' attention on NOS and its integration into the textbooks.
Notes and references
- Abd-El-Khalick F. and Lederman N. G., (2000), Improving science teachers' conceptions of nature of science: a critical review of the literature, Int. J. Sci. Educ., 22(7), 665–701.
- Abd-El-Khalick F., Bell R. L. and Lederman N. G., (1998), The nature of science and instructional practice: making the unnatural natural, Sci. Educ., 82(4), 417–436.
- Abd-El-Khalick F., Waters M. and Le A., (2008), Representations of nature of science in high school chemistry textbooks over the past four decades, J. Res. Sci. Teach., 45, 835–855, DOI: http://10.1002/tea.20226.
- Akerson V. L., Hanson D. L. and Cullen T. A., (2007), The influence of guided inquiry and explicit instruction on K-6 teachers' views of nature of science, J. Sci. Teacher Educ., 18, 751–772, DOI: http://10.1007/s10972-007-9065-4.
- Akerson V. L., Cullen T. A. and Hanson D. L., (2009), Fostering a community of practice through a professional development program to improve elementary teachers views of nature of science and teaching practice, J. Res. Sci. Teach., 46, 1090–1113, DOI: http://10.1002/tea.20303.
- Altun Y. and Tümay H., (2013), Secondary Level Chemistry 9th Grade, Ankara: Sözcü Publishing.
- American Association for the Advancement of Science [AAAS], (1993), Benchmarks for science literacy: a project 2061 report, New York, NY: Oxford University Press.
- Aydın S., Demirdöğen B., Muslu N. and Hanuscin D. L., (2013), Professional Journals as a Source of PCK for Teaching Nature of Science: An Examination of Articles Published in the Science Teacher (an NSTA Journal) 1996–2010, J. Sci. Teacher Educ., 24, 977–997.
- Bartos S. A. and Lederman N. G., (2014), Teachers' knowledge structures for nature of science and scientific inquiry: conceptions and classroom practice, J. Res. Sci. Teach., 51(9), 1150–1184, DOI: http://10.1002/tea.21168.
- Cofré H., Vergara C., Lederman N. G., Lederman J. S., Santibáñez D., Jiménez J. and Yankovic M., (2014), Improving Chilean in-service elementary teachers' understanding of nature of science using self-contained NOS and content-embedded mini-courses, J. Sci. Teacher Educ., 25, 759–783, DOI: http://10.1007/s10972-014-9399-7.
- Dillon J., (2009), On scientific literacy and curriculum reform, Int. J. Environ. Sci. Educ., 4(3), 201–213.
- Dursun M. F., Gülbay I., Çetin S., Tek U., Özkoç F. F. and Güntut M., (2013), Secondary Level Chemistry 10th Grade, Ankara: National Ministry of Education.
- Hanuscin D. L., Lee M. H. and Akerson V. L., (2011), Elementary teachers' pedagogical content knowledge for teaching the nature of science, Sci. Educ., 95(1), 145–167, DOI: http://10.1002/sce.20404.
- Irez S., (2009), Nature of science as depicted in Turkish biology textbooks, Sci. Educ., 93, 422–447, DOI: http://10.1002/sce.20305.
- Irzik G. and Nola R., (2011), A family resemblance approach to the nature of science for science education, Sci. Educ., 20, 591–607, DOI: http://10.1007/s11191-010-9293-4.
- Kavak N., (2013), Secondary Level Chemistry 11th Grade, Ankara: Mega Publishing.
- Khishfe R. and Abd-El-Khalick F. S., (2002), Influence of explicit and reflective versus implicit inquiry-oriented instruction on sixth graders' views of nature of science, J. Res. Sci. Teach., 39(7), 551–578, DOI: http://10.1002/tea.10036.
- Khishfe R. and Lederman N., (2006), Teaching nature of science within a controversial topic: integrated versus nonintegrated, J. Res. Sci. Teach., 43(4), 377–394, DOI: http://10.1002/tea.20137.
- Koseoglu F., Atasoy B., Akkus H., Budak E., Kadayifci H. and Tasdelen U., (2003), How should be a textbook for a constructivist learning environment? Ankara: Asil Publisher.
- Lederman N. G., (2007), Nature of science: past, present, and future, in Abell S. K. and Lederman N. G. (ed.), Handbook of research on science education, Mahwah, NJ: Erlbaum, pp. 831–879.
- Lederman N. G. and Abd-El-Khalick F., (1998), Avoiding denatured science: activities that promote understandings of the nature of science, in McComas W. F. (ed.), The nature of science in science education: rationales and strategies, Dordrecht, The Netherlands: Kluwer Academic Publishers, pp. 83–126.
- Lederman N. G., Abd-El-Khalick F., Bell R. L. and Schwartz R., (2002), Views of nature of science questionnaire (VNOS): toward valid and meaningful assessment of learners' conceptions of nature of science, J. Res. Sci. Teach., 39(6), 497–521, DOI: http://10.1002/tea.10034.
- Lin H. and Chen C., (2002), Promoting preservice chemistry teachers' understanding about the nature of science through history, J. Res. Sci. Teach., 39(9), 773–792, DOI: http://10.1002/tea.10045.
- McComas W. F., (1998), The Principal Elements of the Nature of Science: Dispelling the Myths, in McComas W. F. (ed.), The Nature of Science in Science Education: Rationales and Strategies, The Netherlands: Kluwer Academic Publishers.
- McComas W. F. and Olson J. K., (1998), The Nature of Science in International Science Education Standards Documents, in McComas W. F. (ed.), The Nature of Science in Science Education: Rationales and Strategies, The Netherlands: Kluwer Academic Publishers, pp. 41–52.
- McComas W. F., Clough M. P. and Almazroa H., (1998), The Role and Character of the Nature of Science in Science Education, in McComas W. F. (ed.), The Nature of Science in Science Education: Rationales and Strategies, The Netherlands: Kluwer Academic Publishers, pp. 3–39.
- Merriam S. B., (2009), Qualitative research: a guide to design and implementation, San Francisco: John Wiley and Sons.
- Miles M. B. and Huberman A. M., (1994), Qualitative data analysis: an expanded sourcebook, 2nd edn, Thousand Oaks, CA: Sage.
- National Research Council [NRC], (1996), National Science Education Standards, Washington, DC: National Academies Press.
- National Research Council [NRC], (2011), A Framework for K-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas, Washington, DC: National Academies Press.
- National Science Teachers Association [NSTA], (2000), NSTA position statement on the nature of science, retrieved August 18, 2014, from http://www.nsta.org/about/positions/natureofscience.aspx.
- Niaz M. and Maza A., (2011), Nature of Science in General Chemistry Textbooks, Springer Briefs in Education, vol. 2, pp. 1–37.
- NME, (2013a), Secondary Chemistry Curricula, (for grades 9, 10, 11, and 12), Ankara.
- NME, (2013b), Secondary Level Chemistry Grade 12, Ankara: National Ministry of Education.
- Randak S. and Kimmel M., (1999), The Great Fossil Find. ENSI (Evolution & the Nature of Science Institutes), available: http://www.indiana.edu/%E2%88%BCensiweb/lessons/gr.fs.fd.html, retrieved 20 September 2014.
- Şardağ M., Aydın S., Kalender N., Tortumlu, N., Çiftçi, M., Perihanoğlu S., (2014), The Integration of Nature of Science in the New Secondary Physics, Chemistry, and Biology Curricula, Educ. Sci., 39, 233–248, DOI: http://10.15390/EB.2014.3069.
Footnote |
| † This study was conducted as part of the master's thesis of Selma Tortumlu. |
| This journal is © The Royal Society of Chemistry 2015 |