Translating across macroscopic, submicroscopic, and symbolic levels: the role of instructor facilitation in an inquiry-oriented physical chemistry class†
Nicole
Becker
*a,
Courtney
Stanford
a,
Marcy
Towns
b and
Renee
Cole
a
aDepartment of Chemistry, University of Iowa, USA. E-mail: nicole-becker@uiowa.edu
bDepartment of Chemistry, Purdue University, USA
First published on 9th July 2015
Abstract
In physical chemistry classrooms, mathematical and graphical representations are critical tools for reasoning about chemical phenomena. However, there is abundant evidence that to be successful in understanding complex thermodynamics topics, students must go beyond rote mathematical problem solving in order to connect their understanding of mathematical and graphical representations to the macroscopic and submicroscopic phenomena they represent. Though traditional curricular materials such as textbooks may provide little support for coordinating information across macroscopic, submicroscopic, and symbolic levels, instructor facilitation of classroom discussions offers a promising route towards supporting students' reasoning. Here, we report a case study of classroom reasoning in a POGIL (process-oriented guided inquiry learning) instructional context that examines how the class coordinated macroscopic, submicroscopic, and symbolic ideas through classroom discourse. Using an analytical approach based on Toulmin's model of argumentation and the inquiry-oriented discursive moves framework, we discuss the prevalence of macroscopic, submicroscopic and symbolic-level ideas in classroom reasoning and we discuss how instructor facilitation strategies promoted reasoning with macroscopic, submicroscopic, and symbolic levels of representation. We describe one sequence of instructor facilitation moves that we believe promoted translation across levels in whole class discussion.
Introduction
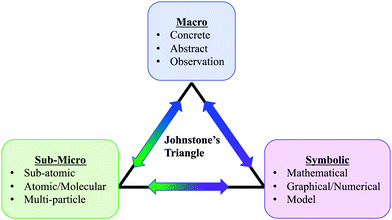
Student learning in thermodynamics has long been viewed as highly dependent on students' mathematical proficiency (Nicoll and Francisco, 2001; Derrick and Derrick, 2002; Hahn and Polik, 2004). However, there is growing evidence that mathematical proficiency is perhaps necessary, but not sufficient for understanding thermodynamics topics. Despite years of preparation in mathematics and other science courses, even advanced students in upper-division physical chemistry courses may fail to develop coherent understandings of foundational thermodynamics concepts (Thomas and Schwenz, 1998; Carson and Watson, 1999; Bennett and Sözbilir, 2007; Bain et al., 2014; Nilsson and Niedderer, 2014). Even students who are successful according to course metrics and are well-prepared in terms of their mathematical background may use algorithmic approaches to successfully solve problems and may not grasp what those expressions mean at a fundamental level (Hadfield and Wieman, 2010; Becker and Towns, 2012). This is problematic because the ability to use mathematical representations as tools to predict and explain chemical phenomena is a key disciplinary practice (Kozma et al., 2000; Kozma and Russell, 2005).Some have suggested that in order to develop a robust understanding of foundational chemistry concepts, students must be able to connect their understanding of the symbolic level to the macroscopic (observable) and submicroscopic levels (Bain et al., 2014; Hernández et al., 2014). In fact, over twenty years ago Johnstone (1991) highlighted the importance of what he termed “multilevel thought”. According to Johnstone, reasoning about chemistry concepts requires coordination across three distinct types of knowledge, forming a triplet relationship: the “macro” level, which addresses visible and macroscopic attributes such as density or volume; the “symbolic” level, which involves the formulae and equations by which chemical substances and their changes are represented; and lastly, the “submicro” level, which addresses the behavior of submicroscopic entities, such as atoms and molecules. This last level is of critical importance as it is used to explain macroscopic phenomena. These “levels” of the chemistry triplet relationship have been interpreted in a variety of ways, from levels of thought (Jaber and BouJaoude, 2012) to levels at which chemical ideas can be represented for teaching (Gabel, 1999). In part these variations in how the triplet relationship has been defined reflect shifts in theories of learning chemistry over the three decades since the introduction of Johnstone's model (Talanquer, 2011; Taber, 2013).
Johnstone's original perspective centered on the idea that the macro, submicro, and symbolic “levels” represented levels of thought, an interpretation framed by an information–processing model of learning (Taber, 2013). According to this perspective, working memory capacity is a key limitation to cognition and learning. Though working memory certainly influences how individuals process and recall random information, the ideas used to solve chemistry problems are seldom random and can often be “chunked” together (Baddeley, 2003) or anchored to what students already know (Ausubel, 1963; Novak, 2002). Furthermore, experts and novices alike routinely use external resources, such as calculators, pen and paper, or computers as resources to “store” information externally while solving problems, permitting many of the limits of working memory to be overcome (Kozma et al., 2000).
By contrast, sociocultural theories of learning suggest that interaction with instructors or more knowledgeable peers can support students in tasks that would otherwise be too complex for them to complete alone (Vygotsky, 1978). With respect to the triplet relationship, for instance, instructors may scaffold students' reasoning by modeling ways of coordinating macro, submicro and symbolic information (Taber, 2013) or may provide hints and prompts to enable students to make those connections themselves. Carefully designed instructional materials may also provide support for students' reasoning. Gradual reduction of instructional scaffolds over time may promote students to develop more independence as their own conceptual frameworks become better integrated (Vygotsky, 1978).
Our own interpretation of the triplet relationship is consistent with a sociocultural perspective on learning (Vygotsky, 1978; Wenger, 1998). We view macro-, submicro-, and symbolic-level representations as resources for communicating chemistry knowledge (Kozma et al., 2000). By using such resources with the support of instructors or more experienced peers, students may gain deeper understanding of discipline-appropriate ways of engaging with these representations than they might on their own (Vygotsky, 1978; Wenger, 1998). Instructors and curricular materials play a critical role in supporting students' reasoning with levels of representation in classroom learning environments. Thus, we turn the next portion of our discussion toward work that has explored the ways in which collaborative learning environments and instructional materials support students' reasoning within the triplet-relationship framework.
Levels of representation and curricular materials in physical chemistry
At the K-12 level, there are a number of curricular approaches designed to help students understand chemical data and phenomena in terms of all three levels of the chemistry triplet (e.g. see Levy and Wilensky, 2009; Jaber and BouJaoude, 2012). However, in most undergraduate chemistry curricula, especially in advanced courses like physical chemistry, support for this skill may be largely implicit. Evidence thus far suggests that curricular materials in physical chemistry, such as textbooks and laboratory activities, may provide relatively little support for students reasoning with macro, submicro, and symbolic ideas. For instance, Nyachwaya and Wood (2014) found that the most widely-used physical chemistry textbooks largely omit explicit discussion of how mathematical representations relate to the macroscopic or submicroscopic levels. They analyzed representations used in 12 common physical chemistry textbooks published in the United States and found that between 81 and 100% of the representations in the texts addressed only the symbolic level. Most commonly, these symbolic representations were mathematical and graphical representations. The use of multiple representations to highlight relationships across macro, symbolic, and submicro levels was minimal and occurred in fewer than 1% of figures in all texts. The heavy use of symbolic representations may not be surprising considering the highly mathematical nature of the discipline (Tsaparlis, 2007; Tsaparlis and Finlayson, 2014); however, given the evidence that even advanced students may use mathematical expressions algorithmically (Bain et al., 2014) the lack of explicit attention to coordinating across levels in curricular resources is problematic.The laboratory component of physical chemistry courses may similarly provide limited support for students' ability to coordinate across levels of the chemistry triplet. An expert may believe that laboratory activities requiring students to observe macroscopic phenomena, to construct symbolic representations, and to explain trends in data would surely promote coordination of ideas across levels of representation, but this may be overly optimistic. For instance, Hernandez and colleagues (2014) developed a laboratory activity on adiabatic and isothermal gas expansion. The activity was designed using a model-based learning approach (Justi and Gilbert, 2002) and asked students to express an initial model for the relationship between temperature and time for gas compression or expansion. Students were prompted to test their initial model by creating an observed temperature-versus-time graph for the system. Students were prompted to examine the differences between the observed and predicted models, then revise their predictions and extend their revised model to a new relationship (pressure versus time). To evaluate the effect of this activity on students' reasoning, Hernandez et al. characterized the models and rationales students used to account for observable (macroscopic) changes during classroom discussion and in their writing. They found that when students worked on the laboratory activity in small groups without instructor intervention, they typically used mathematical relationships algorithmically and struggled to revise their symbolic-level models to account for their experimental observations. Hernandez et al. also observed that on their own, students did not connect observed phenomena to particulate-level models, despite prompts within the laboratory activity that asked students to provide a detailed rationale for the graphs they made – even though the prompts might lead an expert to believe that students would use particulate-level explanations.
Instructor facilitation and the triplet relationship
Hernandez and colleagues found that while the structure of the laboratory activity did little to promote students' understanding of data across macro, submicro, and symbolic levels, whole-class discussion about the laboratory activity provided more support. They observed that during whole-class discussions the instructor's interaction with the class seemed to promote more explicit discussions of the relationships among macroscopic, submicroscopic, and symbolic levels. This was especially true in instances where the instructor pressed students to generate submicroscopic explanations to account for macro-level changes. The authors suggest that the instructor's orchestration of whole-class discussions through the use of discursive interactions played a key role in creating the kind of environment in which students felt comfortable sharing reasoning and evaluating each others' justifications. While analyzing the nature and scope of instructor discursive moves was beyond the scope of this particular study, the authors suggest instructor facilitation as a promising route towards helping students connect levels of representation.In high school and general chemistry contexts, instructor facilitation has also been shown to play a critical role in supporting students' reasoning with macro, submicro, and symbolic ideas. For instance, Stieff et al. (2013) analyzed the way that instructor facilitation and whole-class discussion contributed to “levels confusion” as five high-school chemistry classes reasoned about phase change. Specifically, these researchers conducted a micro-analysis of classroom discourse in multiple high school chemistry classrooms in order to explore how instructor facilitation moves supported (or constrained) the class's attempts to negotiate joint understandings about the features of macro, submicro, and symbolic levels of representation and the relationships between them. They adapted Lidar's and colleagues' (2006) framework for characterizing instructors' use of epistemological moves, that is, moves that communicate to students which ideas and relationships are valid and which are not. Stieff and colleagues observed that confirming moves, those that affirm the appropriateness of a student's contribution, and reorienting moves that direct the class towards more canonical ideas were most supportive of students' reasoning with the triplet relationship. However, their analysis also demonstrated how instructors' use of certain technical definitions and heuristics may contribute to “levels confusion” by implicitly referring to multiple levels at once.
Warfa et al. (2014) also used Lidar's epistemological-moves framework to examine the role of instructor facilitation in an undergraduate general chemistry POGIL context. From an analysis of classroom discourse during an activity on solution chemistry, the authors also observed that confirming moves and reorienting moves that prompted students to explore alternative explanations were most supportive of students' reasoning. However, Warfa and colleagues found that these moves were only effective when used in dialogical discourse in which students contributed to the discussion. The same moves were largely ineffective when used in monological discourse in which the instructor lectured or posed rhetorical questions that she then addressed.
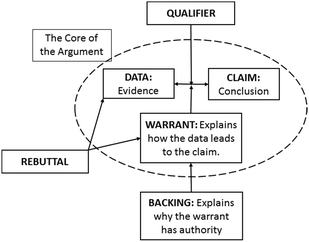
In another POGIL general chemistry context, Kulatunga and Lewis (2013) examined the ways in which peer leaders' verbal interactions supported small group argumentation. They analyzed student reasoning using Toulmin's model of argumentation (1958) in order to examine the ways in which students built chemistry knowledge by connecting claims about chemical and physical properties to evidence and reasoning. Kulatunga and Lewis categorized peer leaders' verbal behaviors using a framework developed by Gillies (2008). They observed that peer leaders' use of questioning techniques and probing/clarifying behaviors prompted students to provide evidence for their assertions (data, according to Toulmin's model) and to elaborate on the relationship of evidence to claim (warrant, according to Toulmin's model). In particular, the warrants identified by Kulatunga involved explicit discussion of relationships between concepts and scientific principles. To an expert, reasoning for many foundational chemistry concepts requires explanation at the atomic-molecular level. While the focus of this study was not on the extent to which instructor facilitation supported coordination across macro, submicro, and symbolic levels, we believe instructor facilitation strategies that prompt students to explain their thinking and explain relationships between claim and evidence may lead to greater elaboration of relationship between levels of representation.
In summary, these studies suggest that even though texts and laboratory activities may offer limited support for students' reasoning with the triplet relationship, effective instructor facilitation of collaborative learning activities may have a positive impact on students' ability to coordinate information across macro, submicro, and symbolic levels. However, the challenge here is that lecture approaches to instruction offer few opportunities for instructors to engage with students as they are learning. Such collaboration is far more likely to occur in classrooms using engaged learning pedagogies.
The process-oriented guided inquiry learning approach
The process-oriented guided inquiry learning (POGIL) approach used in the Warfa et al. (2014) and Kulatunga and Lewis (2013) studies is an increasingly popular approach to engaging students in collaborative activity (Eberlein et al., 2008). POGIL materials (workbooks and instructor guides) are available for high school and general chemistry courses as well as for advanced undergraduate courses such as thermodynamics (Spencer, et al., 2004).As we have noted, there is some evidence that engaged learning approaches such as POGIL may contribute towards improved reasoning with macroscopic, submicroscopic and symbolic-level ideas (Warfa et al., 2014). However, to date, there have been no studies that evaluate the impact of approaches such as POGIL on student reasoning in advanced chemistry courses such as physical chemistry. Given that the ability to coordinate symbolic (especially mathematical) ideas to macro and submicro levels remains a considerable barrier to students' success in physical chemistry, and that current curricular materials may provide little support for these skills, it is important to understand the nature and mechanism of instructor facilitation in engaged learning approaches. Without detailed characterizations of how engaged-learning approaches like POGIL support or constrain students' reasoning with levels of representation, our limited understanding of effective instructor facilitation strategies will remain a barrier towards effectively using engaged learning approaches.
To address these challenges, we present a case study of classroom reasoning in a POGIL physical chemistry class. This represents a strand of analysis separate from the data set reported on in Becker et al. (2013). In our prior work in this classroom context, we found that students often misapplied aspects of submicroscopic systems during small-group discussions of the POGIL workbook activities (Becker, et al., 2013). Hence an important function of the instructor, and more broadly of whole class discussions, was to scaffold the class's reasoning towards more normative ways of reasoning using particulate-level ideas. While our prior work focused on the class's reasoning with particulate (submicroscopic) information, our intent here is to explore how the class coordinated submicroscopic, symbolic, and macroscopic information. Specifically, we address the following research questions:
Research questions
• To what extent does the class reason using concepts from macroscopic, submicroscopic, and symbolic levels?• What role do instructor discursive strategies play in supporting the class's efforts to coordinate macroscopic, submicroscopic, and symbolic levels?
To address these questions, we coordinated three analytical frameworks. First, we used Toulmin's model of argumentation to examine how the class related ideas through collaborative discussion (Cole et al., 2012). Next, we characterized argument components in terms of the level of representation they addressed (macroscopic, submicroscopic, or symbolic). Third, we classified instructor discursive moves in whole-class discussion using an analytical framework known as the Inquiry Oriented Discursive Moves framework (Rasmussen, et al., 2008). By examining the interplay between instructor discursive moves and classroom reasoning at different levels of representation, our aim was to identify specific features of classroom interactions that promote reasoning across macro, submicro and symbolic levels.
Methods
Participants and setting
The data for this report are drawn from a case study of classroom learning in an upper-division undergraduate physical chemistry course at a midwestern comprehensive university in the United States. Fifteen undergraduate chemistry majors were enrolled in the course. All participants were chemistry majors in their third or fourth year of undergraduate study. Institutional Review Board approval for this research was obtained and all students provided informed consent as research participants.The class was taught using the Process Oriented Guided Inquiry Learning (POGIL) approach and instructional materials (Spencer et al., 2004). The design of the POGIL thermodynamics materials is intended to help students develop conceptual understanding of the theoretical models that are the foundation of thermodynamics. A typical POGIL thermodynamics activity begins with an introduction to a “model”, that is, information from graphs, tabular data, text, diagrams, etc. In the initial phase of a POGIL activity (exploration), students respond to questions that direct them to consider various aspects of the information in the model. These questions are primarily intended to support interpretation and application of the information but may also require the use of prior knowledge. The second phase of the POGIL activities (concept development), includes questions that require students to synthesize or analyze information (Moog and Spencer, 2008). In classroom implementation of the POGIL approach, the exploratory phase and critical thinking questions are often used as the basis for small group and whole class discussion. The third phase of the learning cycle (application) involves further questions that require students to extend their understandings to new contexts. Application questions may be used in homework activities or in further discussion.
The POGIL physical chemistry class in this study typically spent one-half to one-third of each class period engaged in small group discussion. Typically, the instructor provided an introduction to a new topic, after which students would work on critical-thinking questions from the POGIL thermodynamics workbook. During small-group work, the instructor generally monitored student groups, assisting as needed. In whole-class discussions, the instructor often initiated discussion of critical-thinking questions by asking students to report on their groups' reasoning. The class would then engage in more general discussion of the concepts and models related to the questions.
Data collection
Video recordings of classroom interactions served as the primary source of data. In total, we recorded twelve class periods across a five-week period that began the second month of the spring semester. Each class period was 50 minutes long. Content covered during this period is summarized in Table 1.| Day | Class period | Topic |
|---|---|---|
| 1 | 2/2 | Work (T1) |
| 2 | 2/4 | First law of thermodynamics (T2) |
| 3 | 2/6 | Enthalpy (T3) |
| 4 | 2/9 | Enthalpy (cont.) (T3, T3a) |
| 5 | 2/11 | Heat capacity (T4) |
| 6 | 2/13 | Heat capacity (cont.); temperature dependence of enthalpy of reaction (T4, T5) |
| 7 | 2/16 | Temperature dependence of enthalpy (cont.); continued; entropy (T5, T6) |
| 8 | 2/18 | Entropy (T6) |
| 9 | 2/20 | Entropy changes as a function of temperature (T7) |
| 10 | 2/23 | Entropy changes as a function of temperature; third law of thermodynamics (T7, T8) |
| 11 | 2/25 | Gibbs and Helmholtz energy (T9) |
| 12 | 3/2 | Gibbs energy as a function of temp and pressure (T10) |
Two cameras were used to record classroom activity; the first recorded the instructor and class during whole-class discussions and an overview of the classroom during small-group activity. The second camera recorded the focus group throughout the class period. We selected one group of four students for observation; this group's membership remained stable for the duration of the semester and all members of the group routinely contributed to small group discussion. Copies of student work (focus group only) were obtained at the end of the semester.
Data analysis
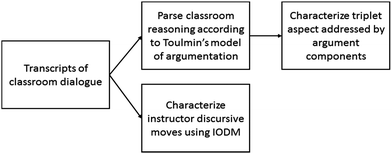
We conducted two strands of analysis in order to (1) examine the roles of macroscopic, submicroscopic, and symbolic-level ideas in classroom reasoning and (2) explore ways in which the instructor supported students' reasoning with these ideas. Fig. 1 illustrates our approach.We analyzed transcripts of classroom dialogue and characterized each statement either as one of the components of Toulmin's model or as non-argumentative discourse (e.g. off-topic, procedural information, etc.). Next, we created a log of argumentation activity for each class period, paraphrasing lengthier statements. Condensing the data in this way allowed us to more easily compare and contrast the class's reasoning across the data set. Subsequent analyses of argumentation logs were conducted in tandem with a review of transcript and video data in order to maximize our understanding of the context of each argument.
To establish a reliable application of Toulmin's framework, the research team (described in Becker et al., 2013) collaboratively analyzed a portion of the whole-class data. Subsequently, individual members of the team coded the remainder of the data set. To verify reliability of our analysis, a minimum of two raters discussed each argumentation log and resolved all discrepancies prior to further analysis. Full code definitions for this analysis are provided in Appendix A.
To examine reliability of this phase of our analysis, two of the authors independently coded six of the 24 argumentation logs (∼25% of the data) and compared codes for individual argument components. Initial percent agreement between the two raters was 75% and all discrepancies between the raters were reconciled through discussion of coding approaches. Code definitions were refined in response to the discussion of differences (see Appendix B for complete lists of codes and code definitions).
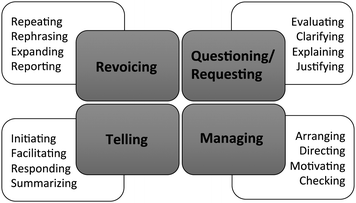
We identified the inquiry-oriented discursive moves (IODM) framework as an analytical lens because it provides a way to characterize instructor discursive moves and their role in creating and sustaining inquiry-oriented learning environments (Rasmussen, et al., 2008). Originally developed in an undergraduate mathematics context, the IODM framework describes four types of instructor discursive moves and their role in advancing classroom discourse and reasoning. As illustrated in Fig. 4, the four main classifications in this framework include questioning/requesting, revoicing, managing, and telling. Each of these moves is further parsed into four sub-categories. To illustrate, the revoicing move occurs when one speaker re-states or rephrases a contribution from a second speaker. Sub-categories of revoicing indicate the specific function of the revoicing move and include (I) repeating, (II) rephrasing, (III) expanding, and (IV) reporting. Revoicing moves are commonly used to attribute ideas to students, to refine contributions made by students, and to highlight discipline-appropriate explanations.
The questioning/requesting discursive move includes questions used to identify students' understanding of key concepts. The four sub-categories, (I) evaluating, (II) clarifying, (III) explaining, and (IV) justifying, are aimed at eliciting increasingly more complex information, from declarative knowledge to conditional knowledge. Questioning moves are important tools for instructors because they provide a mechanism for eliciting student reasoning.
The telling discursive move is used to provide information or respond to student questions. While less prevalent in interactive pedagogies than in lecture contexts, telling moves are important techniques that may be used to provide direct evaluative feedback or to introduce new information to the class. Lastly, the managing discursive move relates to classroom management strategies and the way instructors orchestrate student activity. Managing moves may be aimed at arranging, directing, motivating, or checking students' progress. Overall, managing moves represent a mechanism for pacing the class.
We used a deductive coding approach to characterize each instructor utterance using the sub-categories in the IODM framework (see Appendix C for further discussion of each sub-code). To establish a reliable application of the categories in the IODM framework, two members of the research team individually analyzed transcripts for three class periods, or approximately 25% of the data set. Initial percent agreement between the two raters was 80% and all discrepancies between the raters were reconciled through discussion.
The final stage in our analysis was to map instructor discursive moves to representational levels in the class's reasoning. We identified instructor discursive moves (if any) that corresponded to argument-types in order to examine the extent to which instructor-facilitated arguments addressed levels of the triplet relationship.
Findings
Our goal in this analysis was to identify ways in which instructor facilitation, in an undergraduate POGIL physical chemistry class, contributed to reasoning with macroscopic, submicroscopic, and symbolic levels of representation. To begin our discussion, we describe the prevalence of macro, submicro, and symbolic ideas in whole-class discussion and small-group activity. In particular, we observed that small-group discussions were considerably more focused on symbolic-level reasoning than was whole-class discussion. Overall, small-group reasoning included very few appeals to submicroscopic ideas. As we will illustrate, the higher prevalence of symbolic-only arguments in small-group discussion is the product of multiple factors, including students' difficulties in coordinating all three types of information, and the supportive role of some instructor facilitation strategies in whole-class conversation.In the second part of our discussion, we review our analysis of instructor discursive strategies and their role in supporting the class's reasoning with macroscopic, submicroscopic, and symbolic representations. We show a pattern of interaction that supports “connected” reasoning, that is, reasoning that explicitly interprets information at multiple levels of the chemistry triplet. We also discuss instances in which richer facilitation strategies may have contributed to a more elaborate network of connections among macro, submicro, and symbolic levels.
Trends in whole class and small group argumentation
To address our first research question (To what extent does the class reason using concepts from macroscopic, submicroscopic, and symbolic levels?), we characterized each argument component and overall argument in terms of the representational level addressed. Overall, we found that the most prevalent type of argument in both whole-class and small-group discussion dealt with only one aspect of the triplet relationship at a time. For instance, in whole-class discussion, macroscopic-only arguments constituted 20% of the total 114 arguments made, while symbolic-only arguments made up 30%. In comparison, 25% addressed relationships between symbolic and macroscopic ideas (macro-symbolic argument), while 11% addressed relationships between macroscopic and submicroscopic levels (macro-submicro argument). Rarely did the class address only submicro ideas or all three levels of representation simultaneously (macro-micro-symbolic arguments). Fig. 5 summarizes the prevalence of argument types in whole-class and small-group discussions. Note that for the purpose of our analysis, arguments including references to “other” information (such as system/surrounding distinctions or reversibility) in addition to macroscopic information were characterized as macroscopic-only since our intent was to examine how the class coordinated information from the levels of the triplet relationship. We did not include arguments that addressed only “other” information in Fig. 5, though these constituted approximately 7.5% of total arguments.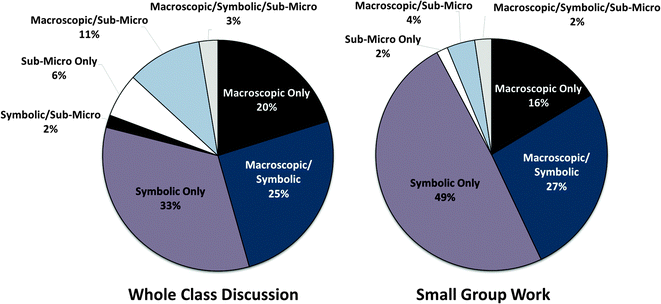 | ||
| Fig. 5 Percentage of whole class arguments (N = 114) and small group arguments (N = 128) and level of representation addressed over the entirety of the data collection period. | ||
As illustrated by Fig. 5, the focus group constructed a higher proportion of symbolic-only arguments (49% of arguments in small-group work versus 33% in whole-class discussion). The focus group also made fewer references to the submicro level overall (8% of small group arguments, versus 22% in whole class discussion).
Upon examining argument types by class period, it became clear that the nature of the content covered in whole-class discussions had influenced students' use of representational levels. For instance, on the first day of data collection the class discussed the macroscopic constructs of work and heat transfer. The resulting whole-class discussion was entirely macroscopic. In contrast, on the final day of data collection, in which the class discussed Gibbs' energy, a more abstract concept, the class's reasoning was predominantly symbolic in both small-group and whole-class discussions. Fig. 6 summarizes the prevalence of argument types by class period.
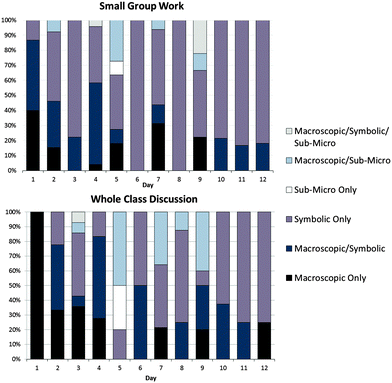 | ||
| Fig. 6 Percentage of whole class arguments and small group arguments and level of representation addressed by class period. | ||
While small-group discussions generally mirrored trends in whole-class discussion, there were some noticeable differences. For instance, our focus group addressed submicroscopic ideas during only four class periods, but whole-class discussion addressed the submicroscopic during 11 class periods. This observation is consistent with our finding that overall, the focus group addressed the submicroscopic level less often than the whole class did.
We also observed that the focus group spent more periods on entirely symbolic reasoning than the entire class did in whole-class discussion. Symbolic-only arguments typically arose as the focus group worked with mathematical expressions and balanced chemical equations in derivations and calculations. These arguments commonly featured discussions of the meaning of mathematical representations, or descriptions of the procedure used to obtain a calculated value, as warrants and backings. To illustrate, consider an example in which the focus group discussed the following critical-thinking question:
Use these data [provided in Model 1] and Hess's law to calculate ΔrH° for the following reaction:
Reaction 1: H2O (l) → H2O (g)
Information provided in Model 1 before the critical thinking question:
Reaction 2: H2 (g) + 1/2O2 (g) → H2O (l) ΔrH° = −285.83 kJ mol−1
Reaction 3: H2 (g) + 1/2O2 (g) → H2O (g) ΔrH° = −241.82 kJ mol−1
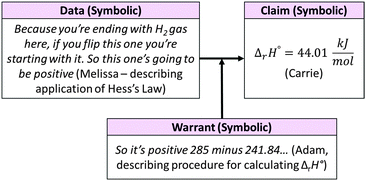
Fig. 7 summarizes the focus group's reasoning in terms of the components of Toulmin's model. Note that in the figures illustrating examples of classroom discourse, italicized text indicates a direct quotation from the transcript.
As was typical in response to questions that asked students to calculate a value, the group's reasoning focused on the symbolic and procedural aspects of the task. In this exchange, Carrie initiated discussion by sharing the value she obtained for ΔrH° (claim). To corroborate her value, the other group members compared their manipulations of reactions 2 and 3 (data) and discussed how they determined the enthalpy change for reaction 1 by subtracting ΔrH° (reaction 2) from ΔrH° (reaction 3). Describing the mathematical procedure allowed the group to negotiate a common interpretation of ΔrH° in this context (change in enthalpy for the reactions as written). Note that the focus group did not consider whether their answer seemed reasonable by discussing the meaning of ΔrH° in terms of macroscopic or submicroscopic levels. That is, they did not identify that a positive value of ΔrH° indicated an endothermic reaction or evaluate whether the value would be considered reasonable for this system. This is not surprising given that they were not explicitly asked to do so by the question prompt.
The whole-class discussion that followed centered largely on the reasonableness of the answer rather than the procedure used to obtain the value (Fig. 8). The instructor initiated whole-class discussion by asking, “What is the heat of reaction?” Carrie, the designated spokesperson for the focus group on that day, shared their value of ΔrH° for reaction 1. The instructor briefly explained to the class how this value could be obtained, commenting, “If I leave my 285.83 and I add the other reaction, so this is for going from H2O liquid to H2O gas,” as she wrote the solution on the board. She then prompted the class to characterize the reaction as endothermic or exothermic, essentially asking them to use Carrie's calculated value of ΔrH° to make a new claim about the meaning of the value at the macroscopic level.
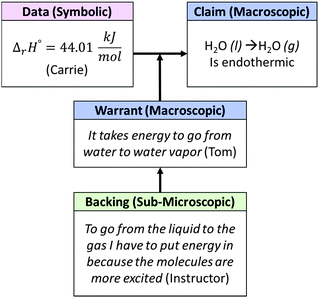 | ||
| Fig. 8 Whole class macro-symbolic-submicro argument addressing the interpretation of a calculated value of ΔrH°. | ||
Here, the instructor re-framed the class's discussion of the POGIL workbook exercise, priming them to consider whether the reaction would be considered endothermic or exothermic in addition to thinking about how the value could be obtained. She also pushed the class to consider the appropriateness of the calculated quantity, a feature that was not an explicit component in the workbook, by asking, “Does this make sense?” In elaborating on Tom's contribution, the instructor also affirmed Tom's statement that “it takes energy to go from water to water vapor”. In particular, she added that water molecules in the gas phase are “more excited,” a statement we interpreted as addressing molecular-level motion or energy. The overall argument constructed by the class (Fig. 8) provides a model of how to connect macroscopic, submicroscopic, and symbolic information in order to evaluate the reasonableness of a calculated quantity.
In the previous example the small group did not consider on their own what a macroscopic quantity meant, in large part because this was not part of the question prompt. In contrast, critical-thinking questions that asked students to explain their reasoning did typically elicit more discussion of relationships between macroscopic-, submicroscopic-, and symbolic-level ideas. However, even in arguments that appealed to multiple aspects of the triplet relationship, there was evidence that students struggled to make appropriate connections among these levels. Consider, for example, the focus group's discussion of a critical-thinking question on enthalpy (Fig. 9). Students were asked to predict the change that would occur in the volume of the cylinder if two different reactions were carried out. The group's reasoning appealed to both macroscopic and symbolic ideas.
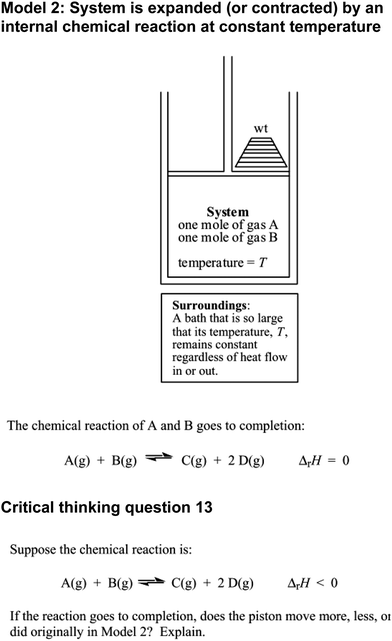 | ||
| Fig. 9 Model 2 and Critical Thinking Question 13 from ChemActivity 3A in the POGIL Workbook (reproduced with permission) (Spencer et al., 2004). | ||
Adam initiated the group's discussion by asserting that the piston would “move less” than it would during the reaction included in Model 2. His contributions suggest that he interpreted ΔrH < 0 as meaning that heat is transferred from the system to the surroundings. Since he believed heat would be “released” from the system to the surroundings, Adam predicted that the temperature of the system would decrease (reasoning summarized in Fig. 10).
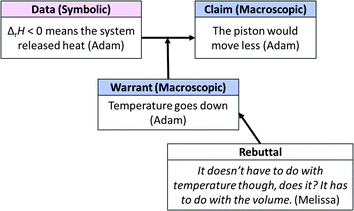 | ||
| Fig. 10 Small group macro-symbolic argument in response to question in Fig. 9. | ||
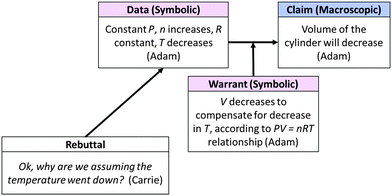
Another member of the group, Melissa, contested Adam's prediction that the temperature would decrease, commenting, “It doesn't have to do with the temperature, does it? It has to do with the volume.” In response, Adam clarified what he had meant by “the piston would move less”; he meant that the volume of the cylinder would decrease. As support for this modified claim, Adam reiterated that temperature would decrease (as per his earlier reasoning that ΔrH < 0 means that the system released heat) and explained how according to the ideal gas law equation, volume would decrease to compensate for a decrease in temperature (argument summarized in Fig. 11).
As Adam responded to further questions from the group, it became evident that he understood temperature as a “measure of the heat that’s there”. Since he believed that heat would be “lost” by the system (presumably to the surroundings) he assumed that the amount of heat “in” the system would be reduced and thus the temperature of the system would decrease, an invalid assumption. Despite his attempts to explain his reasoning, the other group members remained confused (perhaps by his incorrect assertion that temperature would decrease, as evidenced by Carrie's rebuttal) and continued to question him. The argumentation that followed is shown in Fig. 12.
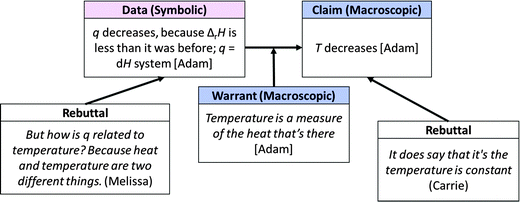 | ||
| Fig. 12 Small group macro-symbolic argument in response to prompt in Fig. 9. | ||
Carrie seemed to recognize that the constant-temperature surroundings described in Model 2 might affect the temperature of the system. However, the group could not agree as to whether the constant-temperature surroundings applied to Question 13 or whether, as Adam suggested, the scenarios in Model 2 and in Question 13 were “totally different things”. Eventually, the instructor noticed the group's difficulty and intervened. However, her intervention seemed to confuse the group further and the students eventually moved on to the next question without arriving at a consensus.
For this question, an expert would point out that temperature would be expected to remain constant as a result of the external water bath. Since the cylinder and the bath are assumed to be in thermal equilibrium, the heat produced by the reaction would be absorbed by the surroundings. The group did not arrive at this conclusion, perhaps because they struggled with multiple aspects of this problem: First, they incorrectly interpreted the symbolic-level expression ΔrH < 0; they became confused as to how the concepts heat (q), temperature (T), and enthalpy change ΔrH were related to one another; and they did not identify the heat bath as being relevant to the problem. These difficulties left the group unable to connect their understanding of the symbolic representations used in Fig. 14 to their understanding of macroscopic energy changes. We present this illustration to highlight the fact that that although the frequency data in Fig. 5 and 6 suggest that the focus group did construct arguments appealing to multiple levels of the chemistry triplet, their reasoning was not without difficulty.
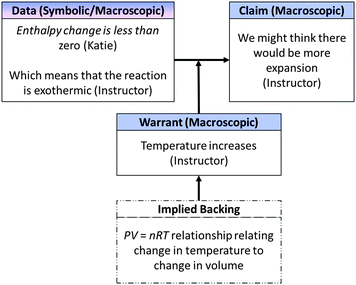 | ||
| Fig. 13 Whole class macroscopic-symbolic argument in response to critical thinking question in Fig. 9. | ||
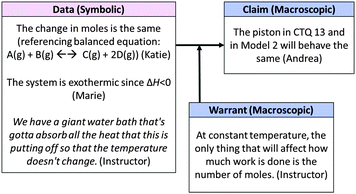 | ||
| Fig. 14 Whole class macroscopic-symbolic argument in response to critical-thinking question in Fig. 9. | ||
The instructor initiated the whole-class discussion that followed by asking students to compare the chemical reaction in Model 2 with the reaction in CTQ 13 (Fig. 9).
Instructor: What's the difference between these two scenarios?
Katie: Enthalpy changes, but the moles are the same. It's [ΔrH] just less than.
Instructor: Right, so here I've got an exothermic reaction, so what's, what I predict would happen to the temperature of the system? Temperature would increase. So, we might think there'd be more expansion, but what did we set up in our experimental conditions?
Here, the instructor re-framed Katie's comment that enthalpy change would be less than zero to mean that the reaction in Model 2 is exothermic (a macroscopic characterization of the system). The instructor also noted that temperature might be expected to increase in this scenario. The implicit reasoning connecting the expansion of the cylinder with an increase in temperature was the ideal gas relationship, PV = nRT, an expression that had been used earlier in the class period and that remained written on the whiteboard during the class's discussion. This relationship was not explicitly referenced in whole-class discussion, perhaps because the instructor assumed students understood this relationship from an earlier discussion. The class's reasoning is summarized in terms of the components of Toulmin's model of argumentation in Fig. 13.
Returning to the prompt for Question 13, the instructor questioned the class again: “So, we might think there'd be more expansion, but what did we set up in our experimental conditions?” To which another student, Marie, responded, “The temperature's constant.” Expanding on Marie's idea that temperature would be held constant by the water bath, the instructor elaborated, saying that the water bath would absorb the heat released by the reaction. She also noted that since the number of moles was the same in each of the two scenarios, the only thing that would affect the amount of work done would be a temperature change. Since temperature would be held constant by the water bath, no work or change in volume could take place. The class's reasoning is summarized in Fig. 14.
Interestingly, in this exchange the instructor framed movement of the piston as a function of the work done by the system. She did not explicitly reference the mathematical relationship between work and change in volume (w = −PΔV), but rather used a qualitative statement of the relationship between work and change in volume. Given that a previous question asked students to predict the sign of work involved in an expansion, it seems plausible that she might have believed this information to be understood by all members of the class. However, the focus group did not consider the role of work in this process and instead used the ideal gas law, PV = nRT, to predict the piston's behavior. Since the focus group struggled to relate the question to other macroscopic constructs such as heat and temperature, it is not clear whether the relationship of work to change in volume would have been obvious to all students.
Though both whole-class and small-group arguments in this example addressed both macro and symbolic levels, it is clear that the two types of discussion played very different roles in supporting students' reasoning. Small group work served as a space where students could engage in more in-depth discussion of mathematical procedures, terminology, and meaning of mathematical symbolism, while whole-class discussion served as a space in which the instructor could model discipline-appropriate reasoning. An important role of the instructor in this exchange was to direct the class to consider the relevance of a particular piece of evidence that the focus group had overlooked. That is, she directed the class to consider the impact of the constant-temperature water bath, a macroscopic constraint on the system. By elaborating on student contributions, she modeled an appropriate interpretation of the symbolic representation ΔrH < 0, an expression that had been challenging for the focus group.
In whole-class discussion, instructor facilitation contributed to greater elaboration of relationships among macro, submicro, and symbolic-level information than typically occurred in small group reasoning. In the next section, we discuss the prevalence and role of particular instructor facilitation strategies in supporting the class's reasoning with macro, submicro, and symbolic ideas.
Prevalence of inquiry-oriented discursive moves in whole-class discussion
To address our second research question (What role do instructor discursive strategies play in supporting the class's efforts to coordinate macroscopic, submicroscopic, and symbolic levels?) we used the inquiry-oriented discursive moves (IODM) framework to characterize the instructor facilitation strategies in whole-class discussion. In the following section, we illustrate the role played by each type of facilitation strategy in the IODM framework. We then discuss patterns in instructor facilitation and its role in supporting students' reasoning with macroscopic, submicroscopic, and symbolic ideas. Code definitions and examples of each move in the IODM framework (including managing) are included in the appendices.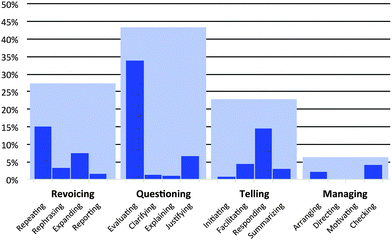 | ||
| Fig. 15 Percentages of various discursive moves used by the instructor during whole class discussion (total number of discursive moves = 797). | ||
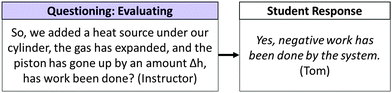
The majority of questioning moves were evaluating questions. Typically, evaluating questions were used to check for understanding or to focus the class's attention on a specific aspect of a problem. To illustrate, consider the discussion of a question that asked students to predict whether work would (or would not) be done if a piston-cylinder setup were heated. Fig. 16 illustrates the instructor's use of an evaluating question and the student response.
In this example, the instructor used an evaluating question to initiate discussion and focus the class's attention on a particular aspect of the problem. In response to this question, Tom predicted that work has been done by the system once the system was heated and that the height of the piston had increased by a distance, h. He did not immediately provide evidence for his assertion.
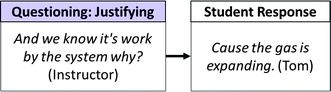
While the evaluating move was useful in checking whether students could make an appropriate prediction, explaining and justifying questions were more useful in eliciting student reasoning. Explaining questions aimed at broadly eliciting student reasoning, however tentative (e.g. “Ok, so what are some first impressions? You don't have to be right. Just kind of what are your first impressions about what's going on here.”). Justifying questions were more targeted than evaluating questions in that they typically required students to provide evidence and reasoning for an assertion. That is, the instructor used justifying questions to elicit data, warrants, and backings for student-generated claims. Fig. 17 illustrates a typical justifying question in which the instructor asked a student (Tom) to provide evidence for his assertion that work would be done by the system.
The instructor used additional questioning moves to elaborate the relationship of gas expansion to the sign convention for work.
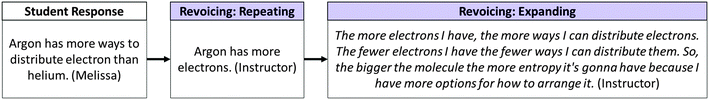
By repeating Melissa's statement that argon has more ways to distribute electrons, the instructor highlighted the appropriateness of her response (O'Connor and Michaels, 1993) while avoiding direct evaluation (e.g. “yes, that's right”). The instructor then used a revoicing/expanding move to elaborate upon Melissa's idea: bigger molecules have more electrons, more electrons mean more ways to arrange them, and hence bigger molecules have more entropy. Expanding on Melissa's idea in this way focused attention on the relationship between sub-atomic structure (submicroscopic level) and a macroscopic construct (entropy). In other instances, the expanding move was used to rephrase student contributions in order to translate them to more scientifically appropriate language.
In addition to evaluating student responses, telling moves were used to summarize ideas, point to next steps for problem solving (telling/summarizing), or suggest that students consider information they may have overlooked (telling/facilitating). As such, telling moves were sometimes useful in directing the class's attention to particular aspects of the triplet relationship.
The questioning → revoicing/repeating → revoicing/expanding facilitation pattern (QRE)
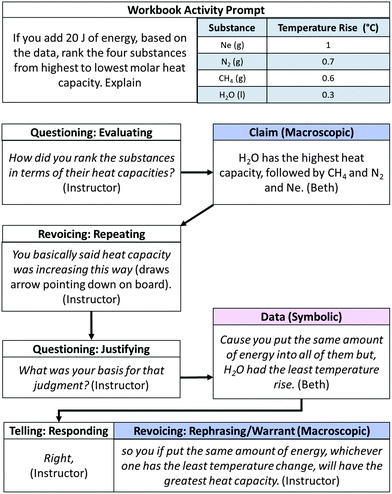
The instructor discursive moves described in the previous section were rarely used independently. Rather, they were used in combination in order to orchestrate classroom discourse. By coordinating our analysis under the IODM framework with our analysis of representational level addressed by classroom reasoning, we identified a distinct pattern of facilitation moves that we believe supported the class's understanding of information as it pertained to the entire chemistry triplet. This pattern involved the instructor's scaffolding of classroom reasoning using a sequence of questioning moves (e.g. evaluating, explaining, and justifying) followed by revoicing/repeating and revoicing/elaborating moves. We refer to this pattern as questioning → revoicing/repeating → revoicing/expanding (QRE for brevity). Overall, the QRE pattern of instructor discursive moves co-occurred with the majority of arguments that addressed two or more levels of representation (68%).To illustrate this pattern, consider the class's reasoning about a critical thinking question from a unit on heat capacity as summarized in Fig. 20. Arrows indicate the sequential flow of conversation.
In this example of the QRE pattern, the instructor used an evaluating question to elicit a prediction of heat capacity ranking for the substances given in the question prompt. A student responded with a macroscopic claim, stating that water has the highest heat capacity, followed by CH4, N2, and finally Ne. The instructor then asked a justifying question, “What was your basis for that judgment,” to prompt students to elaborate their reasoning for this claim. Beth's response elaborated the relationship between the macroscopic claim (ordering of heat capacities) to symbolic data (tabulated values) by explaining her interpretation of the data: “Cause you put in the same amount of energy into all of them, but the H2O had the least temperature rise.” Next, the instructor evaluated the student's answer, acknowledging she was correct in her interpretation of the table, before rephrasing Beth's response to highlight the link between the macroscopic claim and the symbolic interpretation of the information provided by the workbook.
In this example, the student's response to an evaluating question was immediately followed by revoicing/repeating and revoicing/elaborating moves. In addition to this pattern, we also observed a variation in which the instructor used a sequence of questions (rather than a single question) in conjunction with the QRE pattern to guide students towards appropriate claims, evidence, and reasoning. This type of extended QRE exchange commonly occurred in instances in which there was evidence that the class struggled to identify appropriate evidence or reasoning for their claims. An example of an extended QRE pattern is shown in Fig. 21.
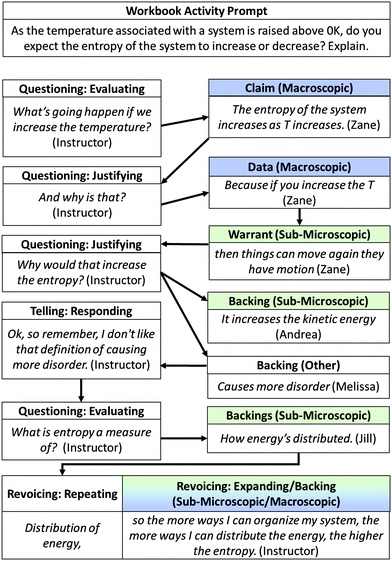 | ||
| Fig. 21 Illustration of questioning → revoicing/repeating → revoicing/expanding (QRE) pattern in whole-class discussion and macro/submicro argument. | ||
Here, the instructor initiated discussion of the POGIL activity through an evaluating question and then pressed, with a subsequent justifying question, for further reasoning for the claim that entropy increases with increasing T. In response, Zane elaborated, saying that he believed increasing the temperature of the system would increase the movement of the particles. He also alluded to the fact that all materials are solids at absolute zero and that increasing temperature allows particles to overcome intermolecular forces and move from their fixed solid-state positions. This idea had been discussed earlier in the class period.
The instructor continued questioning the students, asking, “Why would that increase the entropy?” Two other students simultaneously offered suggestions: Andrea commented that increasing the temperature increases the kinetic energy of the particles. The instructor, perhaps suggesting to the class that this was not the answer sought, did not comment on this idea. Melissa's response, that increasing temperature would increase the disorder in the system, was followed by a direct evaluation (telling move) in which the instructor clarified, saying that “disorder” is not a discipline-appropriate way of describing entropy. This too had been discussed earlier in the class period (See Fig. 19).
The instructor continued questioning the class, focusing students' attention on the definition of entropy introduced during the previous class period by asking, “What's entropy a measure of?” Jill responded that she considered entropy to be a measure of how energy is distributed. The instructor highlighted Jill's contribution by repeating it and expanded on the idea, noting that the number of ways of organizing a system is the scientific definition of entropy. Her contribution helped provide the missing link between Zane's comment on the movement of particles and the claim that entropy would increase if temperature increased.
In this vignette, the instructor's use of the QRE facilitating pattern allowed her to scaffold students' reasoning about the question through her use of increasingly targeted questions, which directed the class towards a particular submicroscopic definition. Her use of revoicing at the conclusion of the exchange modeled appropriate connections between a macroscopic idea (entropy) and submicroscopic information (particle motion). We consider this type of facilitation critically important because, as the highly scaffolded nature of this exchange suggests, making connections across representational levels was challenging for the class. As illustrated by our previous examples of small-group reasoning, there were numerous instances in which the small group did not construct appropriate relationships among macro, submicro, and symbolic-level ideas on their own. Therefore, we believe building on students' ideas to model appropriate reasoning across aspects of the chemistry triplet is a critical mechanism for supporting students' own reasoning.
While the QRE pattern was useful in supporting the class's efforts to use information at macro, submicro, and symbolic levels, there were also instances in which the instructor missed opportunities to connect a concept to a particular level. In the example shown in Fig. 22, students were prompted to determine the relationships for how Gibbs energy changes with respect to temperature and pressure. As is often the case in physical chemistry texts, the information in this prompt was presented at a strictly symbolic level and the question required only a symbolic response.
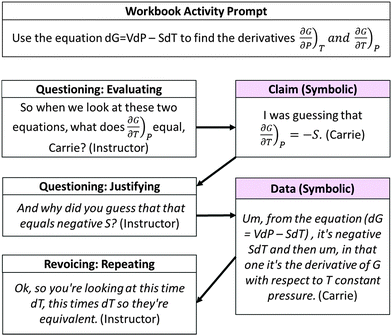 | ||
| Fig. 22 Illustration of missed opportunity in which revoicing does not contribute towards greater elaboration of relationships between macro, submicro, and symbolic levels. | ||
The reasoning presented by Carrie for her group's claim that 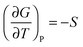 included a description of the mathematical manipulation they used to obtain the relationship. The instructor used questioning and repeating moves to elicit Carrie's reasoning. However, she did not expand on Carrie's contribution to connect the equations to the physical processes. Even though Carrie said she was guessing, she still arrived at the correct answer. The instructor did not prompt the class to further discuss the importance of this derivation or its predictive/explanatory power.
included a description of the mathematical manipulation they used to obtain the relationship. The instructor used questioning and repeating moves to elicit Carrie's reasoning. However, she did not expand on Carrie's contribution to connect the equations to the physical processes. Even though Carrie said she was guessing, she still arrived at the correct answer. The instructor did not prompt the class to further discuss the importance of this derivation or its predictive/explanatory power.
Carrie's correct answer may have led the instructor to assume the students understood the derivation and how it related to other concepts such as thermodynamic favorability. In fact, our analysis of subsequent classroom use of this mathematical expression yielded little evidence that students understood why finding the derivatives might be useful or that they could apply them in appropriate contexts. Rather, we observed that even with instructor intervention, students struggled to use thermodynamic values and the construct of Gibbs energy to predict the thermodynamic favorability of processes at different temperatures. We believe this pattern of behavior further supports the notion that students need to be prompted to connect the symbolic to other levels of representation, particularly when working with abstract concepts such as Gibbs energy.
Limitations
The findings reported here represent a case study of how one instructor facilitated her class's reasoning with macroscopic, submicroscopic, and symbolic ideas via a post hoc analysis. It must be noted that the ways in which students engage in reasoning and argumentative practices are highly dependent on classroom cultures and the ways in which instructors facilitate classroom discourse (Berland, 2011). With that in mind, we make no claims as to the representativeness of this instructor's approach to implementing the POGIL activities. In our own research, we have collected similar data sets in a second classroom with a different instructor and will report on a comparison of reasoning across classrooms in a separate report.A second limitation is that we characterized ideas that were used in classroom reasoning but that were not closely aligned with these categories as “other” information. “Other” ideas included the concept of conservation of energy, the concept of reversibility, and definitions of system versus surroundings. While a closer examination of the role that these “other” ideas played was beyond the scope of our own work, a closer analysis might give deeper understanding of students' collaborative sense-making in thermodynamics contexts, as concepts such as these also play key roles in students' understanding of thermodynamics.
Conclusions and implications
In this study, we observed that during small-group work, students spent substantial time negotiating mathematical procedures and understanding of symbolic representations. This was reflected in a higher proportion of symbolic-only arguments than was found in whole-class discussion. This type of reasoning is to some extent necessary since, as we show, a sufficient understanding of mathematical representations and the meaning they convey was sometimes assumed in whole class discussion (e.g. see example in Fig. 13). While small-group work provided space for students to negotiate meanings of terms and symbols, students did not always construct appropriate connections across macroscopic, submicroscopic, and symbolic ideas on their own. In fact, we saw that the focus group addressed the submicroscopic level far less frequently than did whole-class discussions, suggesting there may have been confusion as to which aspects of the submicroscopic level were related to the symbolic problems they were attempting to solve.Hence, we believe instructor facilitation that helps students to connect their understandings of macro, submicro, and symbolic ideas is critical in supporting students' reasoning with thermodynamics concepts. In our classroom setting, whole-class discussions provided opportunities for the instructor to guide students towards more appropriate understandings of relationships between macroscopic, submicroscopic, and symbolic ideas. Certain patterns of instructor facilitation, such as the questioning, revoicing/repeating → revoicing/expanding (QRE) pattern, were particularly useful in generating more explicit discussion of relationships among levels of representation. In the QRE pattern, the instructor questioned students in order to elicit their thinking and revoiced student contributions in order to translate across levels of representation. In contrast to other patterns of facilitation, such as questioning alone or questioning and telling, the use of revoicing enabled the instructor to synthesize student contributions and build on student ideas while attributing ownership of the ideas to students.
Given these observations, we suggest that it is critical that learning environments provide explicit opportunities for students to negotiate connections between domains of knowledge. We consider this function of whole-class discussion to be especially important because we observed instances in which the small group occasionally focused exclusively on symbolic-level ideas or neglected to determine whether calculated answers' made sense at the macroscopic and submicroscopic levels. Many textbooks (and likely many faculty) seem to assume that students will grasp the meanings of equations and variables and use them with proficiency after instructors have presented or defined them once. Our analysis suggests that this is clearly not the case and that students require substantial support.
Acknowledgements
This work was supported by the National Science Foundation under grants #0816792, #0817467, and #0816948. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. We are grateful for the contributions of Chris Rasmussen, Megan Wawro, and George Sweeney, who played key roles in the development of the methodologies and in inter-rater reliability analysis for our work with Toulmin's model of argumentation. We thank Jacob Byers for his assistance with inter-rater reliability analyses. We also wish to thank the physical chemistry students who took part in this study.References
- Ausubel D. P., (1963), The psychology of meaningful verbal learning, New York: Grune & Stratton.
- Baddeley A., (2003), Working memory: looking back and looking forward, Nat. Rev. Neurosci., 4, 829–839, DOI: http://10.1038/nrn1201.
- Bain K., Moon A., Mack M. R. and Towns M. H., (2014), A review of research on the teaching and learning of thermodynamics at the university level, Chem. Educ. Res. Pract., 15, 320–335, DOI: 10.1039/C4RP00011K.
- Becker N. M., (2012), Social aspects of classroom learning: results of a discourse analysis in an inquiry-oriented physical chemistry class, Doctoral dissertation, Purdue University, retrieved from ProQuest Dissertations and Theses (UMI No. 3544338).
- Becker N. M. and Towns M., (2012), Students' understanding of mathematical expressions in physical chemistry contexts: an analysis using Sherin's symbolic forms, Chem. Educ. Res. Pract., 13, 209–220, DOI: 10.1039/C2RP00003B.
- Becker N. M., Rasmussen C., Sweeney G., Wawro M., Towns M. and Cole R. S., (2013), Reasoning using particulate nature of matter: an example of a sociochemical norm in a university-level physical chemistry class, Chem. Educ. Res. Pract., 14, 81–94, DOI: 10.1039/C2RP20085F.
- Bennett J. M. and Sözbilir M., (2007), A study of Turkish chemistry undergraduates' understanding of entropy, J. Chem. Educ., 84, 1204, DOI: http://10.1021/ed084p1204.
- Berland L. K., (2011), Explaining variation in how classroom communities adapt the practice of scientific argumentation, J. Learn. Sci., 20, 625–664, DOI: http://10.1080/10508406.2011.591718.
- Bowers J. S. and Nickerson S., (2001), Identifying cyclic patterns of interaction to study individual and collective learning, Math. Think. Learn., 3, 1–28, DOI: http://10.1207/S15327833MTL0301_01.
- Carson E. and Watson J. R., (1999), Undergraduate students' understanding of enthalpy change, Univ. Chem. Educ., 3, 46–51.
- Cole R., Becker N. M., Towns M., Sweeney G., Wawro M. and Rasmussen C., (2012), Adapting a methodology from mathematics education research to chemistry education research: documenting collective activity, Int. J. Sci. Math. Educ., 10, 193–211, DOI: http://10.1007/s10763-011-9284-1.
- Corbin J. and Strauss A., (2008), Basics of qualitative research: techniques and procedures for developing grounded theory, 3rd edn, Thousand Oaks, CA: Sage Publications.
- Derrick M. E. and Derrick F. W., (2002), Predictors of success in physical chemistry, J. Chem. Educ., 79, 1013, DOI: http://10.1021/ed079p1013.
- Eberlein T., Kampmeier J., Minderhout V., Moog R. S., Platt T., Varma-Nelson P. and White H. B., (2008), Pedagogies of engagement in science: a comparison of PBL, POGIL, and PLTL, Biochem. Mol. Biol. Educ., 36, 262–273, DOI: http://10.1002/bmb.20204.
- Erduran S., Simon S. and Osborne J., (2004), TAPping into argumentation: developments in the application of Toulmin's argument pattern for studying science discourse, Sci. Educ., 88, 915–933, DOI: http://10.1002/sce.20012.
- Gabel D., (1999), Improving teaching and learning through chemistry education research: a look to the future, J. Chem. Educ., 76, 548, DOI: http://10.1021/ed076p548.
- Gillies A. P. R. M., (2008), Teachers' and students' verbal behaviours during cooperative learning, in Gillies A. P. R. M., Ashman P. A. F. and Terwel P. J. (ed.), The Teacher's Role in Implementing Cooperative Learning in the Classroom, US: Springer, pp. 238–257.
- Hadfield L. C. and Wieman C. E., (2010), Student interpretations of equations related to the First Law of Thermodynamic, J. Chem. Educ., 87, 750–755, DOI: http://10.1021/ed1001625.
- Hahn K. E. and Polik W. F., (2004), Factors influencing success in physical chemistry, J. Chem. Educ., 81, 567–572, DOI: http://10.1021/ed081p567.
- Hernández G. E., Criswell B. A., Kirk N. J., Sauder D. G. and Rushton G. T., (2014), Pushing for particulate level models of adiabatic and isothermal processes in upper-level chemistry courses: a qualitative study, Chem. Educ. Res. Pract., 15, 354–365, DOI: 10.1039/C4RP00008K.
- Jaber L. Z. and BouJaoude S., (2012), A macro–micro–symbolic teaching to promote relational understanding of chemical reactions, Int. J. Sci. Educ., 34, 973–998, DOI: http://10.1080/09500693.2011.569959.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Learn., 7(2), 75–83, DOI: http://10.1111/j.1365-2729.1991.tb00230.x.
- Justi R. S. and Gilbert J. K., (2002), Modelling, teachers' views on the nature of modelling, and implications for the education of modellers, Int. J. Sci. Educ., 24, 369–387, DOI: http://10.1080/09500690110110142.
- Kozma R. and Russell J., (2005), Students becoming chemists: developing representational competence, in Gilbert J. K. (ed.), Visualization in Science Education, Dordrecht, The Netherlands: Springer, pp. 121–145.
- Kozma R., Chin E., Russell J. and Marx N., (2000), The roles of representations and tools in the chemistry laboratory and their implications for chemistry learning, J. Learn. Sci., 9, 105–143.
- Kulatunga U. and Lewis J. E., (2013), Exploration of peer leader verbal behaviors as they intervene with small groups in college general chemistry, Chem. Educ. Res. Pract., 14, 576–588, DOI: 10.1039/C3RP00081H.
- Kulatunga U., Moog R. S. and Lewis J. E., (2013), Argumentation and participation patterns in general chemistry peer-led sessions, J. Res. Sci. Teach., 50, 1207–1231, DOI: http://10.1002/tea.21107.
- Levy S. T. and Wilensky U., (2009), Crossing levels and representations: the connected chemistry (CC1) curriculum, J. Sci. Educ. Technol., 18, 224–242, DOI: http://10.1007/s10956-009-9152-8.
- Lidar M., Lundqvist E. and Östman L., (2006), Teaching and learning in the science classroom: the interplay between teachers' epistemological moves and students' practical epistemology, Sci. Educ., 90, 148–163, DOI: http://10.1002/sce.20092.
- Mehan H., (1979), “What time is it, Denise?”: asking known information questions in classroom discourse, Theory Pract., 18, 285–294.
- Moog R. S. and Spencer J. N., (2008), POGIL: an overview, in Process Oriented Guided Inquiry Learning (POGIL), American Chemical Society, vol. 994, pp. 1–13, DOI: http://10.1021/bk-2008-0994.ch001.
- Nicoll G. and Francisco J. S., (2001), An investigation of the factors influencing student performance in physical chemistry, J. Chem. Educ., 78, 99–102, DOI: http://10.1021/ed078p99.
- Nilsson T. and Niedderer H., (2014), Undergraduate students' conceptions of enthalpy, enthalpy change and related concepts, Chem. Educ. Res. Pract., 15, 336–353, DOI: 10.1039/C2RP20135F.
- Novak J. D., (2002), Meaningful learning: the essential factor for conceptual change in limited or inappropriate propositional hierarchies leading to empowerment of learners, Sci. Educ., 86, 548–571, DOI: http://10.1002/sce.10032.
- Nyachwaya J. M. and Wood N. B., (2014), Evaluation of chemical representations in physical chemistry textbooks, Chem. Educ. Res. Pract., 15, 720–728, DOI: 10.1039/C4RP00113C.
- O'Connor M. C. and Michaels S., (1993), Aligning academic task and participation status through revoicing: analysis of a classroom discourse strategy, Anthropol. Educ. Quart., 24, 318–335.
- O'Connor M. C. and Michaels S., (1996), Shifting participant frameworks: orchestrating thinking practices in group discussion, in Discourse, learning, and schooling, London: Cambridge University Press.
- Rasmussen C. and Stephan M., (2008), A methodology for documenting collective activity, in Kelly A. E. and Lesh R. (ed.), Design research in education, Mahwah, NJ: Erlbaum, pp. 195–215.
- Rasmussen C., Kwon O. and Marrongelle K., (2008), A framework for interpreting inquiry-oriented teaching, paper presented at the Eleventh Conference on Research in Undergraduate Mathematics Education, San Diego, CA.
- Spencer J. N., Moog R. S. and Farrell J. J., (2004), Physical chemistry: guided-inquiry thermodynamics, Boston, MA: Houghton Mifflin.
- Stieff M., Ryu M. and Yip J. C., (2013), Speaking across levels – generating and addressing levels confusion in discourse, Chem. Educ. Res. Pract., 14, 376–389, DOI: 10.1039/C3RP20158A.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14, 156–168, DOI: 10.1039/C3RP00012E.
- Talanquer V., (2011), Macro, submicro, and symbolic: the many faces of the chemistry “triplet,” Int. J. Sci. Educ., 33, 179–195, DOI: http://10.1080/09500690903386435.
- Thomas P. L. and Schwenz R. W., (1998), College physical chemistry students' conceptions of equilibrium and fundamental thermodynamics, J. Res. Sci. Teach., 35, 1151–1160.
- Toulmin S., (1958), The uses of argument, Cambridge, MA: Cambridge University Press.
- Tsaparlis G., (2007), Teaching and learning physical chemistry: a review of educational research, Advances in Teaching Physical Chemistry, Washington, DC: American Chemical Society, vol. 10, pp. 75–112.
- Tsaparlis G. and Finlayson O. E., (2014), Physical chemistry education: its multiple facets and aspects, Chem. Educ. Res. Pract., 15, 257–265, DOI: 10.1039/C4RP90006E.
- Vygotsky L., (1978), in Cole M., John-Steiner V., Scribner S. and Souberman, E., (ed.), Mind in society: the development of higher psychological processes, 14th edn, Cambridge, MA: Harvard University Press.
- Warfa A.-R. M., Roehrig G. H., Schneider J. L. and Nyachwaya J., (2014), Role of teacher-initiated discourses in students' development of representational fluency in chemistry: a case study, J. Chem. Educ., 91, 784–792, DOI: http://10.1021/ed4005547.
- Wenger E., (1998), Communities of practice: learning, meaning, and identity, New York: Cambridge University Press.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5rp00064e |
| This journal is © The Royal Society of Chemistry 2015 |