‘Drugs, religion and chemistry in Tanzania’: an interactive seminar for chemistry students
Malcolm S.
Buchanan
Faculty of Natural and Applied Sciences, St. John’s University of Tanzania, Dodoma, P.O. Box 47, Tanzania, East Africa. E-mail: mbuchanan@sjut.ac.tz
First published on 17th April 2015
Abstract
Most Tanzanian Higher Education Institutes do not have the materials and technology to give students a significant practical experience in the sciences. In 2013 Tanzania was rated 159th out of 187 countries for ‘human development’ (United Nations Development Program 2014 Report). In order to supplement their current, limited practical experience, a culturally relevant, interactive seminar which makes the chemical sciences real to the world of Tanzanians was developed. This was achieved via a Natural Product Drug Discovery seminar during which Tanzanian students were able to appreciate how Tanzanian culture is connected with the fundamentals and applications of the chemical sciences (in this case natural product drug discovery to combat diseases prevalent in Tanzania). Post-seminar evaluation and, observation of student behaviour and chemistry staff feedback supported the value of this seminar. An interactive seminar such as this provides an innovative method of chemical education, useful to motivate final year students and provide them with new ideas before they go into their communities to teach chemistry.
Introduction
The ‘general’ situation within University Chemistry Department laboratories in Tanzania is that there is only basic equipment and materials (e.g. flasks, beakers, separating funnels, measuring cylinders, weighing balances, water baths, routine chemicals and solvents) to allow ‘fundamental’ experiments, such as the reaction of cinnamaldehyde with acetone (aldol condensation), the extraction of caffeine from tea leaves, and the preparation of alum {KAl(SO4)2·12H2O} from aluminium metal. The recent experience of Czieslik and Barke (2013) supports these observations. However, this means that more demanding experiments which develop students' HOCS (Higher Order Cognitive Skills) (Zoller and Pushkin, 2007) cannot be carried out and it is a challenge to conduct meaningful undergraduate research projects (Jansen-van Vuuren et al., 2013). To illustrate this point (as far as the author is aware) there is currently no functioning NMR spectrometer in the whole of East Africa (Matlin and Abegaz, 2011). C. N. R. Rao in a 2011 Nature Chemistry paper highlighted the diverging agony and hope which chemistry generates in less-developed countries: ‘hope’ that it may provide solutions to many of the problems, but ‘agony’ because of the lack of expertise and poor infrastructure (Rao, 2011).Likewise, a Nature editorial in November 2012 stressed the need for Western donors, non-governmental aid organizations and African governments alike to invest in post-secondary education in Africa (Nature Editorial, 2012). “Science needs Africa as much as Africa needs science” and African talent needs to be developed to participate as a scientific peer. Investing in skills in people is an admirable model to follow, but this education needs to be contextualized for African culture living in global times. Even now, decades after the end of colonialism, African educational institutions are still very much shaped by European education (Kanu, 2007). African culture has been devalued, resulting in an identity crisis in many and trouble relating to the world they live in. And so the chemical sciences often appear irrelevant to the world students live in.
Jegede (1997) has written widely on the issues around science education in Africa, in particular Western science being counter cultural to African mode of thought. Table 1 shows Jegede's attempt to summarize the distinguishing characteristics of the two worlds of thought about nature. He also has the valuable insight that teaching science within a traditional African worldview must start with their cultural attitudes towards, and local knowledge about, their environment. Beasley (2005) recognized that meaningful learning within a blend of social, cultural and intellectual forces, is based on the assumption that students' worldviews are aligned with those of the chemistry as it is taught. There is a shared understanding of the culture of classrooms between teachers and students using a social constructivist design which emphasizes inquiry. A new emphasis in course design was introduced requiring an approach summarised as ‘context to concept’ rather than ‘concept to exercise’. This context to concept approach requires teachers to engage students in authentic real-world experiences with the context as the starting point for learning (Anthony et al., 1998). Beasley (2005) explains that learning in context builds upon two recognised principles of learning: student motivation and student prior knowledge and experience. Teaching in context also requires strategies that enhance engagement and exploration by students of issues, ideas and concepts surrounding the context under investigation.
| African mode of thought | Western science |
|---|---|
| Anthropomorphic | Mechanistic, exact and hypothesis driven |
| Monistic-vitalistic and metaphysical | Seeks empirical laws, principles, generalisation and theories |
| Based on cosmology interwoven with traditional religion | Public property, divorced from religion |
| Orally communicated | Primarily documented via print |
| The elder's repository of knowledge is truth not to be challenged | Truth is tentative and challengeable by all |
| Learning is a communal activity | Learning is an individual enterprise |
A Chemistry Education Research and Practice editorial in 2012 highlighted the significance of cultural differences to chemistry learning (Taber, 2012). Another very recent article “Chemistry Education Research Trends: 2004–2013” in the same journal stressed the need for more chemistry education research in areas which include culture and philosophy (Teo et al., 2014).
Creativity is required in order to bring traditional African culture into education along with the realities and needs of living in a global community.
This article is divided into three parts. Firstly, the design of the seminar is explained, followed by a description of the contents and presentation of the seminar. It concludes with an evaluation of the seminar.
Seminar design: drugs, religion and chemistry in Tanzania
Bearing the above challenges in mind, an interactive seminar for chemistry students was developed. These students are studying for a BSc Ed. (chemistry major) in preparation to teach in schools around Tanzania. The seminar is not intended to replace any practical laboratory experience, but rather to supplement this activity in a location where more advanced experiments are limited. Moreover, the seminar goals (general objectives) are intended to: (1) help Tanzanian students relate the chemical sciences to the world they live in (i.e. provide them with an ‘authentic learning experience’) (Lombardi, 2007); (2) enrich Tanzanian students' learning experience in the chemical sciences by conducting an engaging and motivating seminar; (3) stimulate Tanzanian students to think critically about how the chemical sciences have been applied in this seminar; and (4) equip students entering the teaching profession with ideas, data and information that they can use in teaching chemistry to their students.Thought in a similar direction has been described using traditional dyeing in Portugal (Alves et al., 2014). Student awareness was raised in the relationship between science, culture and daily life and the project promoted a greater interest in the sciences.
In this project a seminar was prepared in the area of Natural Product Drug Discovery (Newman and Cragg, 2010a): the aim was for Tanzanian students to learn the interconnectedness between Tanzanian culture (traditional medicine, religion), the fundamentals of the chemical sciences (atoms, molecules, molecular interactions) and how this could be applied to an authentic situation in life, in this case natural product drug discovery to combat diseases including those prevalent in Tanzania (Fig. 1).
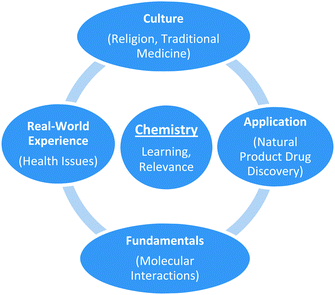 | ||
| Fig. 1 A graphical diagram illustrating the linking of different areas of knowledge to increase chemistry learning and relevance in the drugs, religion and chemistry seminar. | ||
In order to reach the seminar aim, topics and activities were chosen in order to aid learning the interconnectedness and make the seminar interactive and interesting. This was guided by the principle that purposeful learning occurs when student's worldviews are consistent with the chemistry that is being taught (Beasley, 2005). The following are the main chemical concepts which the topics and activities exposed the students to: (i) natural products (secondary metabolites) (ii) molecular interactions (intermolecular and bonding forces) (iii) drug targets (protein structure) (iv) drug–target binding (v) chemical structures and functional groups (vi) 3D molecular shapes and structures.
The seminar design follows the five principles of curriculum design developed by Meyers and Nulty (2009), guided by the Biggs' 3P model of learning and teaching. These maximize the quality of student learning outcomes in courses by providing students with teaching and learning materials, tasks and experience which:
(1) “are authentic real-world and relevant;
(2) are constructive, sequential and interlinked;
(3) require students to use and engage with progressively higher order cognitive processes;
(4) are all aligned with each other and the desired learning outcomes (‘the specific objectives in this seminar’); and
(5) provide challenge, interest and motivation to learn.”
Seminar description: drugs, religion and chemistry in Tanzania
The content of the seminar is as follows:• Seminar introduction
• Preliminary quiz
• Main presentation
• Hands-on activity
• Discussion groups
• Concluding comments
• Post-seminar evaluation
Seminar introduction
The seminar begins by outlining the current difficulties facing most Tanzanian students such as poorly equipped and maintained laboratories and chemical education very much from a Western construct often having little relevance to their lives. After the problem is explained, the student's attention is drawn to the goals of the seminar which are described in detail above. These are fundamentally to understand how the chemical sciences fit within their culture and connecting this to real-life issues faced by Tanzania and also the rest of the world. The main focus of the interactive seminar will be on Natural Product Drug Discovery. This is a topic that most Tanzanians can relate to because of their interest in traditional medicine and the fact that killer diseases are prevalent in Tanzania (Strangeland et al., 2008; WHO, 2014a). The aim and specific objectives for the seminar are introduced:(2) To recognise that medicinal natural products from endemic plants could potentially be used to combat life-threatening diseases prevalent in Tanzania.
(3) To realize that molecular interactions are fundamental to drug action in biological systems.
(4) To associate that molecular interactions are subject to the ‘laws of nature’ and God is a rational explanation as the ultimate force behind the ‘laws of nature’.
Preliminary quiz
The Quiz includes closed-ended questions and is intended, to initiate student thinking on the topic, preparing students for the seminar ahead. The questions are:• What is a natural product?
• What are Africa's three most life threatening diseases?
• Name a compound isolated from an African plant which has significant medicinal value?
• List three different types of intermolecular force?
• Can religion and chemistry be connected (yes or no)?
Main presentation
The main presentation has the following structure:• Introduction
• Traditional African thought
• Life threatening diseases in Tanzania
• Drugs from African biota
• Fundamentals of the chemical sciences
• The chemistry behind drug action
• Relevant connections
• Conclusion
• Key references
• Acknowledgements
The presentation is interspersed with rhetorical questions to allow the students to engage with the material, and to think and process the information given. A summary of the PowerPoint presentation content is as follows:
Following the ‘Introduction’ (above), five relevant areas were then discussed: (i) traditional African thought, (ii) life threatening diseases in Tanzania, (iii) drugs from African biota, (iv) the fundamentals of the chemical sciences, (v) and the chemistry behind drug action. The latter two are discussed within Appendix A.
“That metaphysical understanding in traditional African thought so neatly dovetails with the regular understanding of physical nature that the two understandings ought to be seen as forming one continuous order of understanding.”
In African thought the metaphysical concepts of being, knowing and identity join (or fit) together well with the normal understanding of the physical world that they are seen as one continuous order of understanding. African Traditional thought is well positioned to respect the principle of unity of inquiry i.e. considering data from the various fields of inquiry including metaphysics (Menkiti, 2004b).
(1) HIV/AIDS is the world's leading infectious killer, an infectious viral disease caused by human immunodeficiency virus (HIV) (WHO, 2014b). In 2012 in Tanzania 1.5 million (3.3% of population) were living with HIV and 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people died (UNAIDS, 2014).
000 people died (UNAIDS, 2014).
(2) Tuberculosis (TB) is the second greatest infectious killer worldwide and is an infectious bacterial disease caused by Mycobacterium tuberculosis (WHO, 2014c). It is the leading killer of people living with HIV/AIDS causing a quarter of these deaths.
(3) Malaria is an infectious protozoal disease caused by Plasmodium sp. transmitted to people through the bites of infected Anopheles mosquitoes (WHO, 2014d). Malaria deaths are mostly among children with one child dying every minute from malaria in Africa. In 2012 there was an estimated 207 million cases of malaria with 627![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people dying from the disease. Malaria mortality rate globally has dropped by 42% since 2000. For Tanzania in 2012 (Tanzania 2012 population ca. 47.8 million) there were approximately 2.1 million cases of malaria and 7650 deaths (WHO, 2014e; WHO, 2014f).
000 people dying from the disease. Malaria mortality rate globally has dropped by 42% since 2000. For Tanzania in 2012 (Tanzania 2012 population ca. 47.8 million) there were approximately 2.1 million cases of malaria and 7650 deaths (WHO, 2014e; WHO, 2014f).
• Tanzania has a rich traditional medicine knowledge that can be used to find new medicines
• There are life threatening diseases in Tanzania which can potentially be combated by natural compounds from endemic medicinal plants
• Molecular interactions are fundamental to drug action in biological systems
• Molecular interactions are subject to the ‘laws of nature’ for example electromagnetic force
– The ‘laws of nature’ are governed by constants (such as Planck's Constant, charge and masses of electrons, protons etc.)
– The actual values of these constants are unexplained by science, but must have precise values for the universe to work
• The laws of nature provide a universe which is ordered, consistent and so knowable
• The religious African sees God (or spirits) as the ultimate cause behind the laws of nature
Where do the laws of nature come from? Why do the laws of nature take the form they do? (Metaphysical questions). For the religious African everything occurring under God's direction is a reasonable explanation with God being behind the mathematical form in which the ‘laws of nature’ are expressed.
• Chemistry touches every area of our daily lives
– applied here to natural product drug discovery
• Chemistry connects with culture
– traditional medicine and religion
• A holistic approach to knowledge appeals to traditional African thought
Therefore, and not only for the African, if we are working towards completeness in understanding then we should seek continuity between the physical and the metaphysical. It would be strange indeed if these two were out of step.
Hands-on activity
The hands-on activity is to give students the opportunity to be actively engaged in the seminar and promote critical thinking skills. The activities are as follows:• Observation and handling of artemisinin and penicillin G molecular models together with identifying structural features
• Observation and handling of protein structure (α-helix and β-pleated sheet molecular models and pictures of tertiary and quaternary structure)
• Building amino acids and linking them together (primary protein structure)
• Building the molecular model of captopril
Discussion groups
Students break into groups of four to discuss some open-ended questions (small group learning). This gives an opportunity for shared learning and to encourage creative thought. Tanzanian culture is community-based and very relational and most students therefore value any group work (cf.Table 1). The following are the questions supplied for discussion:• In this seminar what was new or most interesting for you?
• In what way has this seminar raised your awareness and understanding of the role and value of the chemical sciences?
• How are the different aspects of today's seminar connected?
Concluding comments
Students are given the opportunity to comment or ask a question. The instructor then concludes by emphasizing the ‘relevant connections’ and their compatibility with traditional African thought.Seminar evaluation: drugs, religion and chemistry in Tanzania
Evaluation methods
After the initial running of the seminar an evaluation was conducted of the seminar's effectiveness in raising interest, awareness and understanding in the role and value of the chemical sciences (i.e. meeting the goals). This was an ethnographic study (Hunter et al., 2007) using qualitative methodology, where final year BSc Ed students majoring in chemistry at St. John's University of Tanzania were the first group to experience this seminar. This is a 3 year degree which includes not only science courses, but also education courses, as well as compulsory courses in development studies, communication skills and computer applications. Chemistry major students have chemistry courses in each of the 3 years. However, prior to this seminar, as far as I am aware, students have no formal studies in natural products. There were 30 out of a potential 56 students who voluntarily participated in this seminar.The research project was conducted under the St. John's University of Tanzania (SJUT) “Regulations on Research”. The implementation of these regulations is the responsibility of the SJUT Internal Review Committee (IRC). This project was examined by the IRC which determines whether ethical issues are being identified and managed appropriately. Ethical clearance was granted. Informed consent was obtained from the student and staff participants. All evaluations were anonymised and data stored in a safe place.
Firstly, initial understanding of the seminar's content was assessed by the following question: “Based on what you have learnt in this seminar, describe briefly (maximum 100 words) the function and value of the chemical sciences as they connect with different areas of life and science.”
Secondly, the seminar's relevance to the student was assessed from the following question: “In under 50 words briefly describe the relevance and/or irrelevance of the seminar content to your life in Tanzania.”
Thirdly, student behavior was monitored during and after the seminar to assess growing awareness and enthusiasm for the chemical sciences by several measures as follows:
• Spontaneous feedback on the seminar was noted
• Six months after the seminar the following question was asked: “For you, what was the most significant feature or learning point from the ‘Drugs, Religion and Chemistry in Tanzania’ seminar which you attended 6 months ago?”
Finally, two chemistry teaching staff were present during the initial running of the seminar and feedback from them was received. With these staff members a review of which ideas from the seminar could be used to improve normal lectures and teaching classes was also performed.
Evaluation results
For the students responses a content analysis procedure was followed. In other words, the qualitative data is treated in a quantitative way, by coding and counting data. Using this method the responses to the questions identified response categories to which each student's response was matched.For the first question 37% of students responded by giving the function and value of the chemical sciences in drug discovery. E.g.:
“The chemical sciences are important as they help to understand and design different structures which are of medicinal importance…They also help to know about the biochemical reactions in the body and how a drug interacts with the body.”
“The chemical sciences make people aware of the relationship between existing drugs and traditional medicine…and finding out the chemistry behind those drugs.”
Another 33% focused on giving examples of the chemical sciences involvement in different areas of life (medicine, agriculture, technology, etc.). E.g.:
“The chemical sciences play a fundamental role in drug invention to combat various diseases, agrochemicals which are used in agriculture, food preservatives, and understanding the chemical reactions which are taking place in our bodies.”
The remaining students generally gave vague answers.
In the second question the relevance that 70% of students focused on was in the area of Tanzanian traditional medicine being linked to drug discovery and the need for drugs to treat Tanzania's life-threatening diseases. E.g.:
“The seminar was relevant to me since it has raised my awareness on the relationship between traditional medicine and existing drugs.”
“This seminar is relevant to my life in Tanzania as it has provided me an understanding on how Tanzania is rich in medicinal plants which are traditionally used by many people and these can produce drugs that can be very useful in curing disease.”
“The relevance of the seminar content to my life in Tanzania is that it helps me understand the significance of African traditional medicine related to my country Tanzania, and in relation to the discovery and synthesis of new drugs.”
From student observation, seminar discussion and comments the following was noted. It was fascinating for the students to use the molecular models to build chemical structures and understand structures better. This was a very rare experience and their enthusiasm was obvious as they persevered building models, boldly asked for help and using their mobile phones took photographs of one another. It was clear from several students that they were excited about the potential of Tanzanian plants (‘Tanzanian biodiversity’) to yield useful medicinal compounds bearing in mind the rich traditional knowledge. Some appeared motivated to discover more from Tanzania's natural resources. Furthermore, the comment was made that we need to conserve the environment to protect Tanzania's medicinal plants. Some said the most interesting part of the seminar was seeing the connections between the chemical sciences and religion with one student commenting that it would help him as a teacher. This is important as people are often troubled and need help making connections between science and their religious belief. Science and religion do not need to be seen as enemies. Taber (2014) has notably said “tolerating ignorance is perhaps the true enemy of science, religion, and education”. Lastly for some students the small-molecule binding to biomolecular target was what interested them.
For the question supplied six months subsequent to conducting the seminar there were two predominant responses. Firstly, 45% of students focused around their increased awareness for the potential of Tanzanian medicinal plants being used in drug discovery for the treatment of diseases (and the role of chemistry in this). E.g.:
“Drugs can be extracted from natural sources by the application of chemistry.”
“The importance of natural sources available in Tanzania which can be used towards the synthesis of drugs to cure us.”
The second main response was from 27.5% of students who said a relationship was learnt between, drugs, religion and chemistry. E.g.:
“The most significant feature was to understand the relationship between chemistry, drugs and religion.”
For the chemistry staff input a discourse analysis procedure was followed where patterns of speech were looked for while the seminar was discussed. The feedback from the two chemistry staff members was that the seminar was clear, interesting and relevant. More specifically it was relevant because of the significance of traditional medicine in Tanzania and its application to drug discovery. Furthermore, the hands-on approach was an added attribute for students to build chemical structures. The chemistry staff indicated that the seminar gives students an appetite to discover the chemical constituents from Tanzanian plants and conduct bioactivity tests.
A review suggested that some of the presentation content could be used in the departmental Medicinal Chemistry course (for example the African natural product drugs) and it can also be used as a seminar to supplement laboratory practicals, especially when there is a delay to starting the practicals. Lastly it was suggested that interactive seminars could be developed for other areas of chemistry. This will help students see chemistry from another perspective (non-theoretical), motivate them more as chemists and stimulate creativity when they go to teach in their communities.
Conclusions
Essentially, this original seminar aims to foster an awareness of the interconnectedness of Tanzanian culture (traditional medicine and religious belief), the fundamentals of the chemical sciences (molecular interactions) and relevant application of the chemical sciences (natural product drug discovery). In other words it gives a creative and powerful entry into a richer understanding of the way in which a cultural reality can be probed and enhanced by science. The pedagogy, linking relevant knowledge areas, is a significant change from current general practice. An evaluation indicated that the seminar was clear, interesting and relevant for Tanzanian students. The use of molecular models was meaningful and helpful in enabling the students to visualize molecules in 3-D. The seminar will provide inspiration to final year students and give them new ideas as they go into their communities to be teachers. This educational method has the potential to be applied to other areas of chemistry and also introduced to other universities in Tanzania and indeed institutions further afield.An approach to knowledge which is inclusive of other forms of knowledge outside science, including religion, appeals to traditional African thought. From personal experience, including responses from this seminar, traditional African medicine is still foundational to Tanzanian culture. African education would do well to be contextualized for African culture living in global times and this seminar is an attempt to do so.
Appendix A
Introduction
This explains five key terms in the seminar. The following are the two remaining which are not included in the article text:Chemistry: the study of matter and its properties, the changes that matter undergoes, and the energy associated with those changes. Chemistry is often called the central science, because a basic knowledge of chemistry is essential for students of biology, physics, geology, medicine, engineering, ecology and many, many other subjects. It plays a pivotal role in science and technology and in many facets of everyday life. Chemistry is central to life and our way of life (e.g. pharmaceutical drugs in medicine; insecticides and herbicides in agriculture; the silicon chip in computers; enzymes, DNA and RNA in the human body; tannins in tea and caffeine in coffee).
Silberberg, M. S., (2010), Principles of General Chemistry, 2nd edn, McGraw Hill, New York, p. 2.
Natural Products: organic compounds that are formed by living systems. Primary metabolites are the carbohydrates, proteins, fats and nucleic acids found in all living organisms and play a central role in growth, development and reproduction. Secondary metabolites are characteristic of a limited range of species and are classified as follows: polyketides and fatty acids, terpenoids and steroids, phenylpropanoids, alkaloids and specialized secondary metabolites.
Hanson, J. R., (2003), Natural Products: The Secondary Metabolites, Cambridge: Royal Society of Chemistry, pp. 1–3.
Fundamentals of the Chemical Sciences
Drugs are compounds that interact with a biological system to produce a biological response. Therefore, a key fundamental chemical concept for drug–target binding is ‘molecular interactions’ (Blackman et al., 2008; Silberberg, 2010). There are forces within and between molecules. Bonding (intramolecular) forces are relatively strong because they involve larger charges closer together. Intermolecular forces are relatively weak because they usually involve smaller charges that are further apart. Both bonding (intramolecular) forces and intermolecular forces arise from electrostatic interactions between charges. Bonding forces influence the chemical properties of a substance: ionic bonding is between cations and anions, covalent bonding is between nuclei and electron pairs, metallic bonding is between metal cations and delocalized valence electrons. Non-bonding (intermolecular) forces influence the physical properties of a substance. For large molecules there are also weak intramolecular forces between different parts of the same molecule.In ‘usual’ order of decreasing strength the intermolecular forces are: ion–ion (attraction between oppositely charged ions in separate ‘molecules’), ion–dipole (attraction between an ion and a polar molecule), hydrogen bond (occurs between a lone pair of electrons on a small, electronegative atom, mainly N, O or F, and a hydrogen bonded to an electronegative atom), dipole–dipole or Keesom (attraction between the negatively charged end of a polar molecule and the positively charged ends of neighboring polar molecules), ion-induced dipole (attraction between ion charge and polarizable electron cloud), dipole-induced dipole or Debye (attraction between dipole charge and polarizable electron cloud), dispersion or London (attraction between the negatively and positively charged ends of electron clouds in neighboring molecules). All interactions between molecules are mixtures of some of these forces. The strength of intermolecular force depends on the following factors: dipole moment of a polar molecule, the charge of an ion, the angle of alignment between species, the distance separating species, polarizability of the atom or molecule and frequency of electronic distribution fluctuation within species.
The chemistry behind drug action
It is important to know the molecular basis of disease – medical students must learn about the huge number of processes that enable the human body to function and appreciate the role that chemistry plays in many of them. Four important aspects in drug design are described followed by two examples of small molecule drugs and their biological targets (Patrick, 2009, pp. 1–5).(1) The name molecular body is given because a molecular view of the human body would reveal an amazing array of chemical reactions which keep the body healthy and functioning. Therefore, drugs are chemicals entering a reaction chamber in which they will interact and have specific effects on drug targets in the body. The body is made up of cells and different drugs act on molecular targets at different locations in the cell
(2) The main drug targets are proteins (enzymes, receptors, transport proteins) and nucleic acids (DNA, RNA). Carbohydrates and lipids can also be drug targets. Drug targets are large molecules e.g. carboxypeptidase B enzyme has Mw 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 compared with a small molecule drug e.g. penicillin G with Mw 334.
300 compared with a small molecule drug e.g. penicillin G with Mw 334.
(3) Binding is the interaction of a drug with a molecular target. Binding usually takes place at a specific area of the macromolecule known as the binding site. Typically this takes the form of a hollow or a canyon on the surface of the macromolecule allowing the drug to sink into the body of the larger molecule. Some drugs react with the binding site (e.g. a covalent bond is formed), but most drugs interact through weaker forms of interaction (intermolecular bonds as described previously). These intermolecular interactions can be formed and broken again unlike the covalent bond. The length of time a drug binds to its target depends on the number of intermolecular bonds involved and the relative strength of intermolecular binding forces. Both the target binding site and drug have ‘functional groups’ and a ‘carbon skeleton’ which play a role in binding. The term pharmacodynamics is the study of how drugs interact with their targets through binding interactions.
(4) Protein Structure is important to understand in order to understand drug action on proteins (Patrick, 2009, pp. 17–29). The vast majority of drugs used in medicine, are targeted on proteins such as receptors, enzymes and transport proteins. There are four levels of protein structure: primary structure (order of amino acids linked through peptide bonds), secondary structure (regions of ordered structure: α-helix, β-pleated sheet, β-turn), tertiary structure (overall 3D shape), and tertiary structure (arrangement of subunits). Secondary, tertiary and quaternary structures are formed to maximise favourable intramolecular and intermolecular bonds, and to minimize unfavourable interactions. Amino acids with polar residues are favoured on the outer surface of a protein because this allows hydrogen bonding interactions with water. Amino acids with non-polar residues are favoured within the protein because this maximizes van der Waals and hydrophobic interactions.
The first example is inhibitors of angiotensin-converting enzyme (ACE), a membrane bound enzyme (Patrick, 2009, pp. 285–291). ACE is a member of a group of enzymes called zinc metalloproteinases where the zinc ion is present as a co-factor. Zinc ion is important to binding and mechanism catalyzing the hydrolysis of a dipeptide fragment from the end of angiotensin I (decapeptide) to angiotensin II (octapeptide). Angiotensin II is a hormone that causes blood vessels to constrict resulting in a rise in blood pressure. ACE inhibitors are potential anti-hypertensive agents because they inhibit the production of angiotensin II. Captopril was the first non-peptide ACE inhibitor (IC50 23 nM). Enalapril (IC50 1.2 nM) is the ether ester prodrug of enalaprilate and is absorbed more easily from the gut (esterase enzymes do the conversion).
Captopril was not designed based on any knowledge of the three-dimensional structure of ACE (ACE could not be isolated), but on an assumed mechanistic homology to carboxypeptidase A. The active site contains a charged arginine (Arg-145) and a zinc ion which are both crucial in binding the substrate (angiotensin I) – this was assumed from CPB-A. The zinc ion plays a crucial role in the hydrolysis of the natural substrate by polarizing the carbonyl and making the amide more susceptible to hydrolysis. There are also hydrophobic pockets called S1 and S1′ pockets (hydrophobic pocket is a binding site that contains mostly hydrophobic amino acids). In captopril the proline carboxylate (ionised) interacts with arginine on ACE (ion–ion forces) and the thiol interacts with the zinc ion (thiol gave stronger affinity than carboxylate and acts as a bio-isostere for carboxylate) (ion–dipole forces). A methyl group fits in the S1′ hydrophobic pocket and increases the binding affinity with weak hydrophobic interactions caused by induced dipoles (dispersion/Debye forces). The stereochemistry of the methyl group is very significant because the opposite enantiomer has 100 fold less activity. However, captopril has side-effects the most common are rashes and loss of taste, which are thought to be associated with the thiol group. Therefore, researchers worked to find an ACE inhibitor as potent as captopril, but which lacked the thiol group.
Removing the thiol meant reintroducing the far weaker binding carboxylate group. Therefore, groups with extra binding interactions in the active site were needed to compensate for the poorer zinc ligand. To compensate extra binding interactions with an amine and a phenylethyl were introduced. The amine group mimics the NH of the amide in the natural substrate and has hydrogen bonding with the active site. A phenyl ethyl fits into the S1 pocket and has good hydrophobic binding to the enzyme (dispersion/Debye forces). The opposite stereochemistry to that shown for the phenylethyl gives a 700 fold drop in activity. The structure is named enalaprilate and enalapril is the ethyl ester prodrug of enalaprilate and is used clinically.
The second example is artemisinin and related antimalarial drugs (Patrick, 2009, pp. 292–297). Artemisia annua has been a Chinese herbal medicine for malaria since 340 AD. In 1972 artemisinin (or qinghaosu) was isolated as the active component. There was great excitement because it was found to be active against the particularly dangerous chloroquine-resistant Plasmodium falciparum and also acted more quickly against chloroquine-sensitive strains. The multicyclic structure of artemisinin contains seven asymmetric centres and an unusual and unstable looking trioxane ring that includes an endoperoxide group. However, despite the unstable appearance of the molecule it is stable to light and heat.
Artemisinin has a totally different mechanism of action from the quinine-based drugs and has therefore proved effective against chloroquine-resistant strains of malaria. Structure–activity relationship (SAR) studies reveal that although the lactone is not essential for biological activity the endoperoxide is. Thus, dihydroartemisinin, artemether, arteether, deoxoartemisinin and sodium artesunate are all more active than artemisinin, but deoxyartemisinin and deoxodeoxyartemisinin are poorly active. The endoperoxide is the pharmacophore. Currently artemisinin, artemether and sodium artesunate are used clinically as components of Artemisinin Combination Therapy (ACT). The endoperoxide group acts as a ‘molecular trigger’ causing severe damage in the parasite cell leading to its death. The artemisinin molecule is activated by Fe2+ ions to produce two possible radical species. Further reactions take place to generate a series of cytotoxic free radicals and reactive electrophiles which alkylate, cross-link, and oxidize vital biomolecules within the parasite. Parasite cell death is the result.
Acknowledgements
The author is grateful for financial support from the Royal Society of Chemistry Educational Initiatives Fund. The author thanks Ross Jansen-van Vuuren (University of Queensland, Australia) for useful discussions and for providing helpful comments and suggestions. Thanks are also due to Albin Lyimo, Dominic Parmena and Cliff Studman (all St. John's University of Tanzania) for their comments and suggestions and to Peter O' Loghlin for help with proof reading.Notes and references
- altMD, (2014), http://www.altmd.com/Articles/Traditional-African-Medicine–Encyclopedia-of-Alte, accessed October 2014.
- Alves H., Manhita A., Barrocas Dias C. and Ferreira T., (2014), Traditional Dyeing – An Education Approach, Chem. Educ. Res. Pract., 15, 610–619.
- Anthony S., Mernitz H., Spencer B., Gutwill J., Kegley S. and Molinaro M., (1998), The ChemLinks and ModularChem Consortia: using active and context-based learning to teach students how chemistry is actually done, J. Chem. Educ., 75(3), 322–324.
- Appendino G. and Pollastro F., (2010), Plants: Revamping the Oldest Source of Medicines with Modern Science, in Buss A. D. and Butler M. S. (ed.), Natural Product Chemistry for Drug Discovery, Cambridge, UK: RSC Publishing, pp. 156–157.
- Beasley W., (2005), Teacher and Student Learning in Chemistry: Contrasts and Contradictions, Chem. Educ. Int., 6(1), 1–11, http://www.iupac.org/publications/cei/vol6/09_Beasley.pdf, accessed November 2014.
- Beutler J. A., Cragg G. M., Newman D. J., (2012), The National Cancer Institute and Natural Product-Based Drug Discovery in Africa, in Chibale K., Davis-Coleman M. and Masimirembwa C. (ed.), Drug Discovery in Africa, Heidelberg: Springer-Verlag, pp. 29–52.
- Blackman A., Bottle S. E., Schmid S., Mocerino M. and Wille U., (2008), Chemistry, Brisbane: John Wiley & Sons Australia, Ltd, pp. 225–337.
- CIA World Factbook, (2014), http://https://www.cia.gov/library/publications/the-world-factbook/geos/tz.html, accessed June 2014.
- Czieslik W. and Barke H.-D., (2013), Science Needs Africa as Much as Africa Needs Science (1). A Case in Tanzania, Afr. J. Chem. Educ., 3(1), 94.
- Hunter A-B., Laursen S. L. and Seymour E., (2007), Becoming a Scientist: The Role of Undergraduate Research in Students' Cognitive, Personal, and Professional Development, Sci. Educ., 91(1), 36–74.
- Jansen-van Vuuren R. D., Buchanan M. S. and McKenzie R. H., (2013), Connecting Resources for Tertiary Chemical Education, J. Chem. Educ., 90, 1325–1332.
- Jegede O. J., (1997), School Science and the Development of Scientific Culture: a review of contemporary science education in Africa, Int. J. Sci. Educ., 19(1), 1–20.
- Kanu Y., (2007), Tradition and Educational Reconstruction in Africa in Postcolonial and Global Times: The Case Study for Sierra Leone, Afr. Stud. Quart., 9(3), http://asq.africa.ufl.edu/files/Kanu-Vol9Issue3.pdf, accessed July 2014.
- Lombardi M. M., (2007), Authentic Learning for the 21st Century: An Overview, EDUCAUSE Learning Initiative, 1–12, http://alicechristie.org/classes/530/EduCause.pdf, accessed July 2014.
- Matlin S. A. and Abegaz B. M., (2011), Chemistry for Development, in Garcia-Martinez J. and Serrano-Torregrosa E. (ed.), The Chemical Element: Chemistry's Contribution to Our Global Future, 1st edn, Weinheim: Wiley-VCH Verlag & Co. KGaA, pp. 1–70.
- Mbiti J. S., (1994), African Religions and Philosophy, 2nd edn, Johannesburg: Heinemann Publishers Ltd., pp. 1–5.
- Menkiti I. A., (2004a), Physical and Metaphysical Understanding: Nature, Agency, and Causation in African Traditional Thought, in Brown L. E. (ed.), African Philosophy: New and Traditional Perspectives, New York: Oxford University Press, Inc., p. 108.
- Menkiti I. A., (2004b), Physical and Metaphysical Understanding: Nature, Agency, and Causation in African Traditional Thought, in Brown, L. E. (ed.), African Philosophy: New and Traditional Perspectives, New York: Oxford University Press, Inc., p. 121.
- Meyers N. M. and Nulty D. D., (2009), How to Use (Five) Curriculum Design Principles to Align Authentic Learning Environments, Assessment, Students' Approaches to Thinking and Learning Outcomes, Assess. Eval. High. Educ., 34(5), 565–577.
- Nature Editorial, (2012), Science Aid: Donors and African Governments Must Invest in Advanced Science and Maths Education, Nature, 491, 159–160.
- Newman D. J. and Cragg G. M., (2010a), Natural Products as Drugs and Leads to Drugs: The Historical Perspective, in Buss A. D. and Butler M. S. (ed.), Natural Product Chemistry for Drug Discovery, Cambridge, UK: RSC Publishing, pp. 3–27.
- Newman D. J. and Cragg G. M., (2010b), Natural Products as Drugs and Leads to Drugs: The Historical Perspective, in Buss A. D. and Butler M. S. (ed.), Natural Product Chemistry for Drug Discovery, Cambridge, UK: RSC Publishing, pp. 23–24.
- Oxford Dictionaries, (2014), http://oxforddictionaries.com/definition/english/metaphysics, accessed June 2014.
- Patrick G. L., (2009), An Introduction to Medicinal Chemistry, 4th edn, Oxford: Oxford University Press, pp. 1–5.
- Polkinghorne J., (1994), Science and Christian Belief: Theological reflections of a bottom-up thinker, London: SPCK, pp. 9–10.
- Rao C. N. R., (2011), The Two Faces of Chemistry in the Developing World, Nat. Chem., 3, 678–680.
- Silberberg M. S., (2010), Principles of General Chemistry, 2nd edn, New York: McGraw Hill, pp. 368–375.
- Strangeland T., Dhillion S. S. and Reksten H., (2008), Recognition and Development of Traditional Medicine in Tanzania, J. Ethnopharmacol., 117, 290–299.
- Taber K. S., (2012), The International Dimension of Chemistry Education Research and Practice, Chem. Educ. Res. Pract., 13, 398–400.
- Taber K. S., (2014), Science vs. Religion: What Scientists Really Think, by Elaine Howard Eckland, Oxford University Press: Oxford, UK, 2010, ISBN 9780195392982 (Book Review), Sci. Ed., 98(1), 182–184.
- Teo T. W., Goh M. T. and Yeo L. W., (2014), Chemistry Education Research Trends: 2004–2013, Chem. Educ. Res. Pract., 15, 470–487.
- UNAIDS, (2014), http://www.unaids.org/en/regionscountries/countries/unitedrepublicoftanzania/, accessed June 2014.
- World Health Organization, (2014a), http://www.who.int/mediacentre/factsheets/2003/fs134/en/, accessed July 2014.
- World Health Organization, (2014b), http://www.who.int/mediacentre/factsheets/fs360/en/, accessed June 2014.
- World Health Organization, (2014c), http://www.who.int/mediacentre/factsheets/fs104/en/, accessed June 2014.
- World Health Organization, (2014d), http://www.who.int/mediacentre/factsheets/fs094/en/, accessed June 2014.
- World Health Organization, (2014e), http://www.who.int/malaria/publications/country-profiles/profile_tz2_en.pdf (MAINLAND), accessed June 2014.
- World Health Organization, (2014f), http://www.who.int/malaria/publications/country-profiles/profile_tz1_en.pdf (ZANZIBAR), accessed June 2014.
- Zoller U. and Pushkin D., (2007), Matching Higher-Order Cognitive Skills (HOCS) Promotion Goals with Problem-Based Laboratory Practice in a Freshman Organic Chemistry Course, Chem. Educ. Res. Pract., 8, 153–171.
| This journal is © The Royal Society of Chemistry 2015 |
