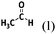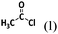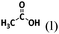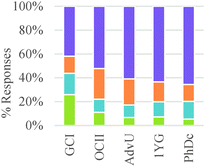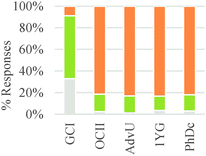Mapping students' conceptual modes when thinking about chemical reactions used to make a desired product
M. L.
Weinrich
and
V.
Talanquer
*
Department of Chemistry and Biochemistry, University of Arizona, Tucson, AZ 85721, USA. E-mail: vicente@u.arizona.edu
First published on 28th April 2015
Abstract
The central goal of this qualitative research study was to uncover major implicit assumptions that students with different levels of training in the discipline apply when thinking and making decisions about chemical reactions used to make a desired product. In particular, we elicited different ways of conceptualizing why chemical reactions happen (chemical causality), how these processes occur (chemical mechanism), and how they can be controlled (chemical control). In each of these areas we characterized conceptual modes with different explanatory power and explored how they were applied by participants when facing different types of questions. Our findings suggest potential paths in the development of understanding about chemical reactions in the context of making specific substances. Our study also highlights the benefits of analyzing students' understanding not only by focusing on implicit cognitive elements, but by using disciplinary crosscutting concepts as lenses of analysis.
Introduction
In recent years there has been an increased interest in developing learning progressions of core science ideas with the promise of improving instruction, curriculum, and assessment. These progressions are “descriptions of successively more sophisticated ways of thinking about a topic that can follow one another as children learn about and investigate a topic over a broad span of time” (NRC, 2007, p. 219). They focus on the development of coherent scientific knowledge and practices over time, as opposed to focusing on isolated facts or pieces of information students should know (Smith et al., 2006). They are important because they provide coherence for the development of curriculum, instruction, and assessment in a way that is based not only on what students should know but on students' actual ideas, helping learners move toward more expert ways of thinking (Krajcik, 2012). Learning progressions for many different topics in the sciences have been developed, but there is debate about what exactly a learning progression is, how progress can be characterized, and how learning progressions should be developed (Duschl et al., 2011).In order to develop useful learning progressions we need to better understand how students' conceptualize and reason about core ideas at various stages of training in a discipline. A detailed analysis of the implicit assumptions and reasoning strategies used by students at different educational levels is needed to support such development work (Sevian and Talanquer, 2014). We have thus sought to explore and map the ways of thinking that students exhibit when reasoning through diverse tasks in core areas in chemistry. In this contribution, we focus on student thinking about chemical reactions used with the intention of making a desired product. Chemical reactions can be used to attain different goals, from making a substance to removing it from a system, and such goals are likely to influence how someone thinks about the process. For example, thinking about how to isolate the product is critical in the making of a substance, but not necessarily in other situations. Our study thus contributes to the knowledge base on student thinking about chemical processes in different contexts.
Several research studies have explored student understanding and misconceptions of chemical reactions and how and why reactions occur (Andersson, 1990; Zoller, 1990; Ahtee and Varjola, 1998; Kraft et al., 2010; Grove et al., 2012; Maeyer and Talanquer, 2013; Sendur and Toprak, 2013; Bhattacharyya, 2014; de Arellano and Towns, 2014). Most of this research has focused on students' explicit knowledge. However, implicit cognitive elements seem to have a major effect on learning (Talanquer, 2006; Bhattacharyya, 2014; Taber, 2014). For example, previous research on students' ideas about chemical mechanisms has indicated that often students consider chemical reactions as occurring through adding or mixing together molecules without a detailed model of what could be occurring during the reaction (Andersson, 1990; Ahtee and Varjola, 1998). Additionally, students often focus on surface features when reasoning through a mechanism and do not attribute meaning to the symbols used to represent changes in chemical structure during a reaction (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Kraft et al., 2010; Grove et al., 2012). A better characterization of these cognitive elements could facilitate “the design of studies to test out teaching approaches that can recruit the most suitable implicit knowledge elements to support learning of canonical chemical ideas.” (Taber, 2014, p. 447). Thus, the central goal of this study was to explore the answers to these research questions:
• How do students at different stages of training in chemistry build explanations and make decisions about the feasibility of chemical reactions that are used to make intended products?
• What do these explanations and decisions reveal about common implicit ways of thinking about why and how chemical reactions happen and how to control them?
The results of our study provide a basis upon which actual learning progressions of student understanding of chemical reactions in relevant contexts may be built.
Theoretical framework
Our studies of student reasoning in chemistry have been guided by research in science education and in cognitive and development psychology suggesting that human thinking relies on implicit cognitive elements that guide prediction, explanation, and decision-making (Talanquer, 2013a; Taber, 2014). A variety of implicit constructs have been described in the research literature, including cognitive constraints (Keil, 1990), core knowledge (Spelke and Kinzler, 2007), implicit presuppositions (Vosniadou, 1994), ontological beliefs (Chi, 2008), phenomenological primitives (diSessa, 1993), intuitive rules (Stavy and Tirosh, 2000), and fast and frugal heuristics (Todd and Gigerenzer, 2000). Many of these cognitive elements can be thought of as implicit assumptions that people make about the nature and behavior of the entities and processes with which they interact. The extent to which a person's assumptions in a given domain constitute either a fragmented collection of knowledge pieces or a more coherent schema likely varies with the knowledge domain under consideration and the prior knowledge and experiences of each individual (Brown and Hammer, 2008; Vosniadou et al., 2008).When people interact with an object or event, a variety of implicit and explicit cognitive elements are triggered by perceptual and language cues (Baillargeon et al., 2009; Gelman, 2009). Recognition memory, associative thinking, analogical reasoning, and metaphorical linking help individuals classify the entity or phenomenon as belonging to a certain category within or across knowledge domains (Vosniadou and Ortony, 1989; Bowdle and Gentner, 2005). For example, when a student first listens to the description of an electron as a small particle, the mind likely categorizes electrons as “solid objects” and implicitly ascribes particular properties to them. The student will thus likely assume that electrons are rigid and impenetrable objects which move in continuous trajectories. How we categorize entities and phenomena has major repercussions about how we reason with and about them (Chi, 2008).
The assumptions that people make about the properties and behaviors of the members of a given category act as cognitive constraints that guide and support, but also constrict their reasoning. These cognitive constraints help us make decisions about what behaviors are possible or not and about what variables are most relevant in determining behavior. These cognitive elements give rise to dynamic but constrained knowledge systems that allow us to generate plausible explanations and make quick decisions when facing a specific task in a particular context (Sloman, 1996; Brown and Hammer, 2008). They allow us to make reasonable, adaptive inferences about the world given limited time and knowledge. They often generate acceptable answers with little effort, but sometimes lead to severe and systematic biases and errors (Keil, 1990; Hatano and Inagaki, 2000).
Paying close attention to the implicit categorization decisions made by students about the nature of relevant entities or phenomena can provide invaluable information about the underlying assumptions that guide their thinking. Nevertheless, different contextual factors and past experiences may affect the features of an object or event that are more salient to an individual at different times or in different contexts, potentially triggering different implicit assumptions in each case. From this perspective, a single individual can exhibit different ways of conceptualizing a system or phenomenon depending on the situation (Mortimer, 1995). These different ways of thinking are often intertwined with different ways of speaking about what is observed or analyzed (Mortimer, 2001). In this paper we use the term “conceptual modes” to refer to the different manners in which a given entity, system, or phenomenon seem to be conceptualized by an individual in different situations or by different individuals with diverse backgrounds. A given conceptual mode is likely supported by a set of interrelated implicit assumptions about the system under consideration.
The extent to which a given conceptual mode is a productive reasoning tool often depends on the situation. For example, one can expect people to think of and talk about “heat” in different ways in different contexts (Mortimer et al., 2014). A person may ask her child to close the car windows to keep the heat in during a cold day. This way of talking implies thinking of “heat” as a substance that can be stored or contained within an object, a conceptualization that is rather common and productive in communicating with people in daily life. The same person, however, could conceptualize heat as a form of energy transfer when participating in a chemistry class. The ability to switch from one conceptual mode to another in the proper context is likely to depend on expertise in the relevant domain (Gupta et al., 2010). Novice science students have been shown to persistently think of heat as a substance and struggle to conceptualize it as a dynamic process (Slotta et al., 1995). Eliciting the conceptual modes that individuals with different levels of training commonly apply in different contexts can help us characterize how understanding progresses in a particular area.
Different conceptual modes can be expected to have different explanatory power. The explanatory power may be judged based on the extent to which a given conceptualization allows individuals to propose generalizable mechanisms to describe, explain, and predict properties and phenomena. For example, thinking of heat as a substance may help us explain why a cold object becomes hotter when in contact with a hot object (e.g., heat is transferred from one object to another). However, this conceptual mode does not help us explain how heat transfer actually occurs or predict its effects under different conditions. According to Vosniadou (2013) “learning science requires the ability to understand that the same phenomenon can be explained from different perspectives and some of these perspectives have greater explanatory power than others” (p. 22).
Chemical thinking
Our research is also influenced by a particular perspective on chemistry education that focuses on helping students understand “chemical thinking” and use it in productive ways to build explanations, generate predictions, and make decisions in relevant contexts (Talanquer and Pollard, 2010; Sevian and Talanquer, 2014). Chemical thinking results from the integration of chemical knowledge and practices with the intent of analyzing, synthesizing, and transforming matter for practical purposes (Sevian and Talanquer, 2014). The type of reasoning that students are expected to develop can be organized around six major disciplinary crosscutting concepts that provide responses to essential questions in the discipline:• Chemical identity: How do we identify chemical substances?
• Structure–property relationships: How do we predict properties of substances?
• Chemical causality: Why do chemical processes occur?
• Chemical mechanism: How do chemical processes occur?
• Chemical control: How do we control chemical processes?
• Benefits-costs-risks: How do we evaluate impacts of chemical processes?
These disciplinary crosscutting concepts provided lenses through which we have analyzed students' ideas about chemical reactions. We were particularly interested in characterizing student thinking in the areas of chemical causality, chemical mechanism, and chemical control, and sought to identify dominant conceptual modes of individuals with different levels of training in the discipline. Very few research studies provide insights into chemistry student thinking using a similar analytical focus (Andersson, 1986; Hatzinikita et al., 2005; Talanquer, 2010; Ngai et al., 2014; Stains and Sevian, 2014).
Methodology
Context and participants
Participants in our study attended a large research-intensive state university in the southwestern United States. During the time of data collection there were approximately 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 undergraduate and graduate students in attendance at this university. The student body was 52% female and 48% male. The ethnic diversity was 56% Caucasian, 20% Hispanic, 22% other minorities, and 2% unknown.
000 undergraduate and graduate students in attendance at this university. The student body was 52% female and 48% male. The ethnic diversity was 56% Caucasian, 20% Hispanic, 22% other minorities, and 2% unknown.
We recruited students from a range of educational stages in order to capture diverse modes of thinking through semi-structured interviews. Our goal was to map the range of conceptual modes applied by individuals with diverse chemistry backgrounds to think about chemical reactions used with the intention of making desired products. Each study participant was labeled using two letters: the first letter represented their educational level and the second letter differentiated students within each group. For example, I-A was the first general chemistry student who was interviewed. These different groups were:
• GCI: first-semester general chemistry (n = 16, I-A to I-P),
• OCII: second-semester organic chemistry (n = 15, O-A to O-O),
• AdvU: advanced undergraduate students (n = 9, U-A to U-I),
• 1YG: first-year graduate students (n = 14, G-A to G-N),
• PhDc: PhD candidates (n = 16, named C-A to C-P).
The general chemistry students were recruited toward the beginning of the semester in order to capture student thinking with minimal instruction in college chemistry. The organic chemistry students were recruited from five different sections of second semester organic chemistry towards the end of the course. The advanced undergraduates came from a senior level course co-enrolled with graduate students which focused on writing organic mechanisms. Data were collected within the last four weeks of the course to capture student thinking toward the end of their undergraduate career. The first-year graduate students were recruited from a college chemistry teaching course at the beginning of graduate school and represented diverse backgrounds and research interests in chemistry and biochemistry. The PhD candidates were recruited from a list of chemistry and biochemistry students who had passed their oral exam and were in either their third, fourth, fifth, or sixth year of graduate school.
Instrument and data collection
Individual semi-structured interviews were used to explore students' thinking in the area of interest. The interview began with a question asking the students to generally define what is important in a chemical synthesis. We used the term “chemical synthesis” in our research instrument to represent chemical reactions used to make a desired product. Then, the participants were asked three different types of problems:• to compare the easiness of chemical reactions. (4 questions)
• to design the synthesis of a compound. (3 questions)
• to evaluate the feasibility of a proposed reaction. (1 question)
The wording for these questions is shown in Table 1 and the compounds and chemical reactions are presented in Table 2.
| Type | Wording |
|---|---|
|
Compare
Evaluate |
Which compound is easier to synthesize? (list of chemical reactions) |
| Design |
You have been asked to synthesize: (compound)
Devise a strategy to successfully synthesize the compound above using the following resources Elements: H2(g), Li(s), Al(s), N2(g), O2(g), Compounds: H2O(l), NaOH(aq), HCl(aq), AlCl3(s), NH3(g), LiH(s), CH3CH3(g), CH3CH2OH(l), CH3CN(l), |
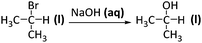
| Evaluate |
A student proposed the following synthesis (representation of chemical reaction)
Evaluate the feasibility of this synthesis |
We designed these qualitative tasks to involve science practices such as explaining, predicting, evaluating, and designing which are major aspects of chemists' work (Sevian and Talanquer, 2014). Additionally, these questions were created to be open-ended and could be approached in both intuitive and academic ways. In order to identify a range of ways of thinking about chemical reactions we asked questions with diverse degrees of difficulty. We expected that novice students would struggle with the more difficult questions, but be compelled to use any cognitive resources available to provide a potential answer. On the other hand, we expected more expert students to approach the simpler questions in a more sophisticated manner and even be critical of the proposed reactions. For example, question two asked students to compare the easiness of three different processes. Given the nature of these reactions, we anticipated that novices might focus their attention on explicit features of the representations such as relative size of molecules and different stoichiometric coefficients. We considered that more advanced students might rather focus on implicit features such as bond strength, or enthalpy and entropy of reaction. These reactions are presented in a format that students may encounter in a general chemistry course but are also very complex and have an extensive research history and importance in the fuel industry (Davis and Occelli, 2007). Thus, the selected reactions created opportunities for different types of analyses.
Questions that asked students to design a way to make a compound included substances with simple composition and structures. However, associated options had different levels of complexity. Again, we sought to open opportunities for experts to express specialized knowledge and for novices to apply existing cognitive resources to unknown situations. Evaluation questions were designed to have participants adopt a more critical stance, seeking to explore how they used their understandings in judging the feasibility of the proposed processes, some of which (e.g., Q4) represented unrealistic ways to produce a desired product. Prior to implementation of this study, questions were piloted with an introductory student, a graduate student, and a professor to test them for readability, understandability, and appropriateness. Feedback from these pilot interviews was used to create the final version used in this study.
The interviews began with instructions to the students indicating that we were not interested in whether they provided right or wrong answers but instead how they were thinking through the problems. Students were provided with a periodic table and paper to write on, and asked to think aloud. If a participant did not start talking or became quite during an interview, they were asked to share their thoughts. When prompted, these students were able to express their ideas out loud. We did not encounter any cases where we could not elicit rich responses after prompting. During the interview additional questions were posed in order to explicitly explore participants' thinking about the feasibility of the chemical reaction they chose or proposed, how the reaction might proceed to form the products, and why the chemical reaction could or could not happen. Each interview lasted approximately 20–60 minutes and was audio recorded and transcribed. Artifacts, such as participant's drawings, were also collected. This research project received approval from the Human Subjects Protection Program at our institution. Student volunteers were assured confidentially of their responses and were not offered any incentives for their participation. At the end of the interview, the interviewer addressed any questions a participant had about the study.
Data analysis
Transcripts of the interviews were carefully read and tentatively coded to identify major themes. An iterative process was employed where code categories and themes were constantly revisited, rethought, and compared as the open coding process occurred (Charmaz, 2006). Through this constant comparison process we noticed underlying similarities between groups of codes and uncovered common patterns of reasoning. The web application Dedoose was utilized to perform and organize the codes. Participants' drawings were analyzed in conjunction with the transcripts. Constant discussion and reflection involving two researchers was used to ensure reliability in the analysis of the data. One researcher analyzed all transcripts and the second researcher separately analyzed a randomly selected set of student answers to prompts (100 of the 639 answers to prompts, 16%). There was 88% agreement in the coding of these two researchers for the selected student answers.Interview transcripts were analyzed to elicit students' implicit assumptions and major conceptual modes in the response for each prompt. We defined conceptual modes as the different ways in which an entity, system, or phenomenon seemed to be conceptualized by participants; a given conceptual mode may rely on several assumptions. Implicit assumptions were inferred from students' justifications and explanations by analyzing the types of cues participants paid attention to, the verbal predicates that they used, and the nature of the claims that they made (see Appendix). This approach to inferring implicit knowledge elements has been used by a variety of authors (Keil, 1979; diSessa, 1993; Slotta et al., 1995). For example, if a student paid attention to the number of atoms that made up a product and claimed that the fewer the atoms the easier the chemical process would be because less energy was required to put atoms together, we inferred that this student assumed that the fewer components were needed to generate a substance the more feasible the process would be. This type of thinking has been elicited in previous studies and it has been associated with a conceptualization (or conceptual mode) of chemical reactions as reassembling processes in which different parts have to be put together in an effortful manner, similarly to how macroscopic composite objects are assembled (Maeyer and Talanquer, 2013). As part of our analysis, we kept track of the frequency with which elicited conceptual modes manifested in each of the responses provided by all study participants.
We also made judgments about the explanatory power of the different conceptual modes that were identified. For example, some participants thought of chemical reactions as direct assembling processes that required the action of external agents to happen. Others conceived chemical reactions as processes that were constrained by internal factors, such as the likelihood of some particles colliding with each other. Although these two conceptual modes may be productive in particular contexts, the latter conceptualization has greater explanatory power as it is likely to be productive in a wider variety of situations (and it more closely resembles an accepted chemical explanation). In those cases in which students expressed conceptual modes that resembled chemical ways of thinking, we also characterized how appropriately such conceptualizations were applied to a scenario. We coded explanations as “spurious” when they involved incorrect, overgeneralized, or inappropriately applied ideas.
Findings
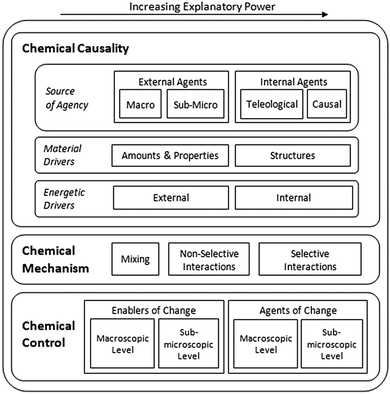
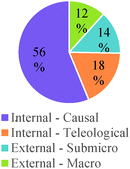
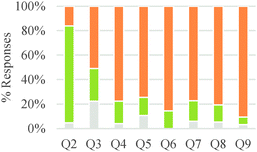
The participants in this study expressed a variety of ways of thinking about chemical reactions and generating chemical products during the interviews. Nevertheless, several common conceptual modes emerged from our analysis of different responses. Major findings are described in the following subsections and a summary diagram is presented in Fig. 1. In this diagram, conceptual modes are organized using the crosscutting concepts of chemical causality (Why do reactions occur?), chemical mechanism (How do reactions occur?), and chemical control (How do we control chemical processes?) as core categories of analysis. Additionally, conceptual modes within each of these three major groups are arranged in order of increasing explanatory power from left to right.Chemical causality
The analyses of students' responses elicited diverse ideas about what causes chemical reactions to occur (chemical causality). We classified these conceptual modes into three major groups: (a) Source of agency, which describes the types of agents thought to drive chemical processes; (b) Material drivers, which focuses on the types of features of chemical substances used to make predictions or build explanations about chemical reactivity; and (c) Energetic drivers, which describes assumptions about the role of energy in chemical reactions. The frequency with which these different conceptual modes manifested in students' responses across educational levels and types of questions is summarized in Tables 3–5.External agents – macro effects. Some students mainly referred to external agents acting at the macroscopic scale (e.g., temperature, pressure, human actions) as the cause of chemical reactions. These students tended to focus on the effect of these agents on the starting materials in a chemical reaction (e.g., changes in state of matter). The following interview excerpt illustrates this conceptual mode:
O-K Q3: I'd say perhaps you'd first want to form the lithium, aluminum solid by combining the two together as a mixture of solids and melting them, melt them together to the appropriate um area where they create one phase and are together, mixed… and allow them to cool down to be a single solid in a single solid phase, and… perhaps add hydrogen gas into the mixture as they're liquid to pressurize the hydrogen gas into a liquid combination of the two metals to create the lithium aluminum hydride and then after that of course let them combine and cool down so you have a big solid mix of lithium aluminum hydride.
In this case, the student focused on describing the actions and externally-induced changes that would be needed to form the desired product from the starting materials. Only a small fraction of the participants in our study (12% of instances in this category) expressed this type of conceptual mode.
External agents – sub-micro effects. In some cases (14% of instances), students described external agents as causing changes to substances at the submicroscopic scale. For example, some students focused on the effect of supplied heat (external agent) on bond formation (submicroscopic process):
Interviewer: what else makes this reaction easier than the other two reactions?
G-K Q2: all you're forming are carbon–hydrogen bonds instead of carbon–carbon bonds
Interviewer: why is forming carbon–hydrogen bonds easier than forming carbon–carbon bonds?
G-K Q2: delta H requires lower, so less energy is required to be put in to form the bond
This example illustrates the combination of intuitive ideas about what is needed to induce a chemical change (e.g., heating up a system) and academic knowledge about submicroscopic changes during a chemical reaction (e.g., formation of bonds).
Internal agents – teleological. Students who applied this conceptual mode (18% of instances in this category) talked as if chemical reactions were caused by internal agents (e.g., atoms, molecules, substances) that had particular purposes, needs, or wants. The following excerpt is representative of this category:
Interviewer: you said the O minus is unhappy, can you tell me more about why it is unhappy?
U-H Q7: yea because if, I'm going to draw it, so here it had two lone pairs and a double bond, here it has one double bond still two lone pairs which is only five electrons in its valence shell but it wants six so it, it wants another bond to equal it's happy valence.
This teleological way of talking about chemical entities and processes has been elicited by a variety of authors (Taber and Watts, 2000; Taber and Adbo, 2013; Talanquer, 2013b).
Internal agents – causal. In most instances (56%), study participants described chemical processes as driven by internal features of the entities involved that affected their interactions. The nature of the main characteristics that were considered is described in the following sections (i.e., material and energetic drivers), but the following interview excerpts illustrate this type of causal reasoning:
G-M Q4: it would definitely be exothermic and I guess that would have to do with the bonds formed being lower in energy than the bonds already existing.
U-B Q7: amides are pretty stable like more stable in comparison to acyl chloride […] you'd have electron withdrawing effects so it would pull charges away from the carbon and make it even more electropositive than, and vulnerable, especially under basic conditions, attack from anything that's electronegative around
In these examples, graduate student M identified differences in bond energy as the main driver of the reaction. On the other hand, the advanced undergraduate B used relative structural stability, providing a causal mechanism to justify the answer.
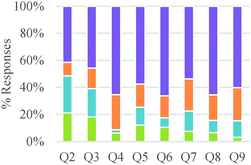
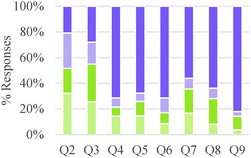
As shown in Table 3, a majority of participants built causal explanations based on properties of internal agents. However, the relative frequency of different conceptual modes related to “source of agency” varied with level of training in the discipline. General chemistry students were more likely to invoke the action of external agents than other groups of students. Teleological claims were more frequently made by students at intermediate educational levels. Causal explanations were more common among graduate students. In general, our results suggest a gradual shift from focusing on external agents acting at the macroscopic level to internal agents interacting at the submicroscopic level. Our findings also indicate that the type of question had an influence on the conceptual modes that were deployed. For example, the presence of vastly different stoichiometric coefficients in the chemical reactions depicted in Question 2 (see Table 2) seemed to have led more students to consider external agents as drivers of the reaction (e.g., more heat or human effort needed to affect larger amounts of substance). Alternatively, the presence of fluorine in one of the reactions included in Question 4 cued more teleological explanations based on the needs or desires of this highly electronegative atom to reach a desired state.
Amounts and properties of components as drivers. In some instances (25% of responses where material drivers were considered), students focused on either the amounts or the properties of specific components to make judgments about the feasibility of chemical reactions. For example, some students considered that the smaller the amounts of substances needed to carry out a reaction (based on reaction stoichiometry) the easier or more feasible the process would be:
O-C Q2: It [first reaction] has like the least number of compounds that you would need, like you would need less like H2and CO to actually make those products than you would need on the other ones
As illustrated by this excerpt, a focus on amount of substance was often guided by intuitive ideas about chemical reactions as direct assembling processes (Maeyer and Talanquer, 2013). Nevertheless, attention to amount of substance was also prompted by academic knowledge about the relationship between entropy of reaction and changes in the number of moles of materials present before and after a process:
C-G Q2: I would say methane
Interviewer: and why?
C-G Q2: 4…2… just looking at number of moles there's not as, there's, on all of the reactions there's more moles on this side than this side but here there is a smaller gradient than here
Interviewer: and then why would the smaller gradient of moles make the first reaction easier?
C-G Q2: um entropy
Interviewer: ok, can you tell me more about entropy?
C-G Q2: um as far as for gas it favors going from less moles to more moles
In some situations students relied on known properties of specific components to make and justify their predictions. For example, students often viewed the presence of highly electronegative atoms as indicative of high reactivity (and thus a more favored chemical process):
O-A Q4: …like HCl is a strong acid but I would assume that HF is a stronger acid because it's closer to the top
Interviewer: do you have any ideas as to why it's more acidic?
O-A Q4: because of the trends on the periodic table, it's more electronegative
In most cases, students who adopted this conceptual mode seemed to conceptualize reactions as processes that would be easier to carry out if fewer components were involved and if these components had higher values on particular properties.
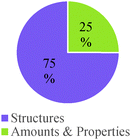
Structure of particles as reaction drivers. A majority of the responses that invoked a “material driver” focused on some sort of structural feature of reactants or products (75% of instances; see Table 4). The basic assumption seemed to be that some structural features were more favorable or desirable than others. This conceptual mode cued a variety of chemical concepts and ideas such as the octet rule, bond strength, structural stability, and structural reactivity.
Some students seemed to adopt an octet framework (Taber, 2013) to think about why reactions happened. These students used teleological arguments based on the idea that atoms wanted or needed a full outer shell of electrons in order to become more stable. When describing the octet as a cause of reactions, students often focused on the fact that atoms in the product fulfilled the octet rule and ignored the electronic structure of starting materials. For example, one student stated:
I-L Q6: it's always looking for that [octet], so you know, apparently even if it already has it
This student explicitly commented that the search for an octet of electrons was the major driver of the reaction even when such an electronic structure was already present in the reactants.
Other students paid attention to the relative bond strength in reactants and products (assuming that systems with stronger bonds were more favored). The following excerpt illustrates this type of thinking:
C-L Q9: if you add to this you are breaking a carbon–carbon double bond, carbon–oxygen double bond and leaving the carbon–carbon double bond but in the other case the carbon double bond, carbon–oxygen double bond remains intact as you break the carbon–carbon double bond so maybe going off the strength of the bonds, we can say the second reaction favors formation of the more stable product.
Some students made judgments about the relative stability or reactivity of the entities involved based on structural features. As illustrated by the following excerpt, these students often used known rules about the stability or reactivity of different types of functional groups to make decisions:
C-M Q8: I'm actually very confused so I know it's hard to form geminal diols, they are not stable. They tend to release water and just go to the, it's not easy to form geminal diols
In this case, the participant made judgments about the feasibility of making a product based on a rule about the stability of geminal diols. Other participants, however, built mechanistic justifications based on the analysis of how structural features affected the distribution of charge in different molecules:
C-K Q7: [acyl chlorides] are less stable than amides, and that's usually in part because the amides have a, this lone pair on the nitrogen which can donate to the system making it very very stable due to resonance and even though you can have it in chlorines because they, you can argue that they have lone pairs right, their electronegativity, their inductive effect wins over this resonance factor
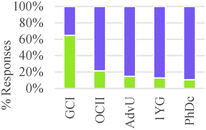
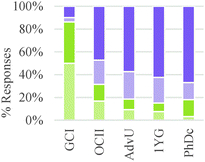
As shown in Table 4, study participants mostly relied on structural features to build explanations and make decisions related to generating a product. This trend, however, was the inverse for general chemistry students who tended to rely on amounts and properties of components to make their decisions.
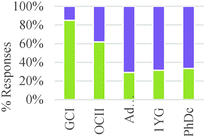
These results suggest a rather sharp progression from focusing on the amounts of materials required to make a product to paying attention to implicit structural features of the particles involved in the chemical reaction. Nevertheless, ability to correctly or productively apply these structural characteristics may evolve more gradually. As shown in Fig. 2, graduate students were able to build more valid explanations about the effect of structure on chemical reactivity than undergraduate students in our sample. In general, the type of question had a minor influence on the type of conceptual mode that was applied in this category. The exception was again Question 2 in which the major salient difference between reactions was the amount of different reactants needed to form a product.
Energy as an external driver. Some study participants talked of chemical reactions as requiring external energy input to induce a change (44% of instances in the “energetic drivers” category). Energy was needed to, for example, combine amounts of materials, make bonds, or overcome energy barriers. Consider the following interview excerpt:
I-G Q2: methane would be easier to synthesize simply because, first of all, you're using less reactant, second of all because you are using less reactant it takes less energy to react between the two in order to make the methane and water that comes out of the reaction
In this case, the student intuitively assumed that less energy was needed to combine smaller numbers or amounts of reactants. Other students considered bond formation as an effortful process that demanded energy input. The following excerpt illustrates this way of thinking:
Interviewer: what else makes this reaction easier than the other two reactions?
G-K Q2: all you're forming are carbon–hydrogen bonds instead of carbon–carbon bonds
Interviewer: why is forming carbon–hydrogen bonds easier than forming carbon–carbon bonds?
G-K Q2: delta H requires lower, so less energy is required to be put in to form the bond
This student combined academic concepts with intuitive assumptions about bond formation to build an explanation. These types of “hybrid” responses were common in explanations involving energy considerations.
Some students related the feasibility of a reaction with activation energy. However, these participants often talked about an activation barrier as an obstacle that had to be overcome or reduced to obtain the desired product rather than as a kinetic barrier that determined the rate of reaction. The following excerpt is illustrative of this manner of talking:
C-D Q2: the lower activation energy will probably be better, easier to synthesize […] because from your reactant to product you need to overcome activation energy which is the energy barrier um so if the energy barrier is really high and then it is probably difficult to achieve
Energy as an internal driver. In more than half of the instances (56%) in which energy considerations were taken into account to build explanations or make decisions, participants seemed to conceptualize energy as an internal property of the system. These students tended to focus on energy differences (e.g., bond energy, enthalpy, Gibbs free energy) between reactants and products, using these differences to make reactivity claims as illustrated by this example:
C-K Q2: you have four CH bonds and two waters being formed which you have to take into account also for your overall delta H so you have three bonds of hydrogen which should be weaker than the CH bonds, so you are forming stronger bonds as your products formed. That should drive the reaction to your products.
For this student, the chemical process was driven by the formation of stronger bonds and the corresponding change in enthalpy.
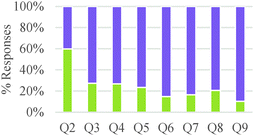
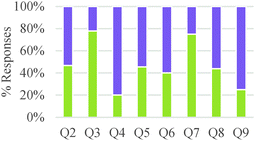
As shown in Table 5, a larger fraction of study participants who invoked energetic factors considered energy as an internal rather than as an external driver. However, thinking of chemical reactions as driven by external energy inputs was dominant among general chemistry and organic chemistry students. Although graduate students also made references to external energy needs, it was often linked to activation energy considerations and applied in valid ways. Our findings suggest a gradual progression in understanding of the role of energy in chemical reactions among undergraduate students, from thinking of energy as a required input to induce change to conceptualizing internal energy differences between reactants and products as indicators of chemical reactivity. Nevertheless, the nature of a question may have a strong influence on the conceptual mode that is applied. In our study, questions that asked students to design a process (Q3,5,7) tended to elicit more references to energy as an external driver, whereas questions that asked students to compare reactions (Q2,4,8,9) elicited more talk about internal energy differences as drivers of chemical reactions.
Chemical mechanism
Our analysis of the data also uncovered students' ideas about how reactions could proceed. Study participants considered what could happen between the starting material and the product during the chemical reaction in different ways. Three major conceptual modes about chemical mechanisms were elicited from the data: (a) Mixing components; (b) Non-selective interactions; and (c) Selective interactions. The frequency with which these different conceptual modes manifested in students' responses across educational levels and types of questions is summarized in Table 6.I-K Q3: how would the product form? Hydrogen is gas, lithium solid, H2O is liquid… ok what I'm looking at is like a math way of doing it like H2plus H2… Li and then H2O from what I know if I have like X to the fourth…
This student looked for the specific components that would have to be added in a simple mathematical way to get the desired number of atoms of each type in the product.
I-A Q1: Well considering how, you know, compounds are formed by bonds of the atoms, I would assume at least that the compounds would need to be formed somehow by like possibly heating them up or is it, it might be cooling them down to attach them to each other to basically create bonds in order for them to stay together
In this case, the student recognized that different components would have to bond to each other to form a product, but talked about the process in a rather static way. Contrast this description with the following excerpt:
I-H Q2: that one [hexane] might be easier because there would be more of a probability of them hitting each other since atoms are tiny and even though it happens all the time the probability of it happening is… it happens, so maybe that one might be easier to make just because there are more things to throw together and hope they stick
This student also provided a generic, non-selective description of interactions between components, but conceptualized reactions as dynamic processes resulting from random collisions.
O-G Q3: ok well I know that if something is positively charged it's going to go toward, it's going to gravitate toward something that's negatively charged […]
Interviewer: so do you have anything positively or negatively charged here?
O-G Q3: well this lithium is positively charged because it's an alkaline earth metal I think, I think it appears in either the first or second column right so aluminum, that's a metal, chloride's a halide, so then… or halogen I mean, it's negatively charged, this is positively charged
Sequential stories. In other instances, students created sequential stories of how a reaction could happen by chaining together the properties and actions of specific entities and building a step-by-step story of the chemical mechanism. The following excerpt exemplifies this way of conceptualizing a chemical process (for the production of acetamide from acetyl chloride and ammonia):
O-J Q7: So NH3adds and then the double bond on the oxygen goes up and makes um oxygen anion and then the Cl is still attached so it's a tetrahedral intermediate and then it goes based on stability… so because Cl is the weaker link it's the one that when the electron pair wants to go back down and make a double bond again, Cl will get kicked out because NH3is much more stable
Constrained sequential stories. Some study participants described chemical mechanisms as sequential stories guided and constrained by particular atomic or molecular features of the particles involved, such as their electron density. As shown in the excerpt below, in these instances students made decisions about actions and interactions based on judgments about preferred distributions of charge, attractions between specific reaction centers, and steric interactions between different species:
Interviewer: ok so if you took that acid chloride and the NH3and reacted them together, how would they form the product?
U-E Q7: Because the carbonyl is partially positive at the carbon and partially negative here your NH3with the lone pair would attack here at the carbon and then you would get a tertiary intermediate with that there and you would have your NH3, your chloride… and then… oh and this would be positive missed that, and when this collapses back down to the carbonyl the chloride would leave and you would have this here and then something would come along and deprotonate the amine… or the… NH3 plus here, at least that's the way I would picture it happening with these materials.
Interviewer: ok and then at the beginning you said that the acid chloride is the most reactive of these materials, do you know why the chloride is the most reactive?
U-E Q7: the chloride is the most… it's able to be a good leaving group when you get to this tertiary intermediate and then also because of electron withdrawing. This chloride is pulling electron density away from this carbon making it even more electropositive or well not electropositive but partially positively charged making the attack more likely.
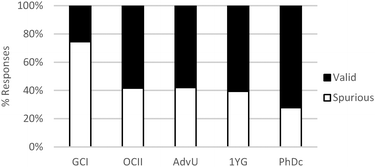
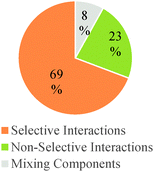
As shown in Table 6, in the majority of instances in which study participants expressed ideas about chemical mechanism they built explanations and justifications based on selective interactions. However, there was a sharp difference between general chemistry students and the other sets of participants. The more novice students mostly relied on ideas categorized within the conceptual modes of “mixing components” or “non-selective interactions.” These findings suggest that students' conceptualizations of chemical mechanism undergo major changes during the first two years of college chemistry (general and organic chemistry) in the US. Nevertheless, our results also suggest that the ability to build valid chemical mechanisms based on selective interactions increases gradually with training in the discipline (see Fig. 3).
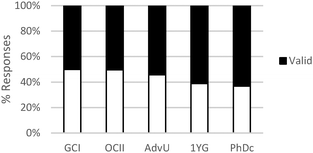 | ||
| Fig. 3 Relative frequency of spurious and valid explanations across different educational levels related to the construction of chemical mechanisms based on selective interactions. | ||
Students did not always consider a chemical mechanism when thinking about these problems and were only directly asked to explain how the product formed in questions three, five, and seven during the interview. However, many students considered the chemical mechanism to be a useful tool to answer the different questions even when they were not explicitly asked to do so. Questions 2 and 3 (see Table 6) were more likely to trigger ideas about mixing components or non-selective interactions than other questions. These two questions asked students to think about reactions for classes of substances that were not typically discussed in the chemistry classes of our study participants.
Chemical control
In addition to thinking about why and how chemical reactions happened, participants in this study considered how they could use reaction conditions to control the outcome of a chemical reaction. Students discussed the physical conditions (such as temperature and pressure) and chemical conditions (such as solvents, catalysts, and additives) they viewed as necessary for generating a product. Two major conceptual modes about chemical control were elicited from the data: (a) Reaction conditions as agents of change; and (b) Reaction conditions as enablers of change. Agents or enablers were both described as affecting processes at either the macroscopic or the sub-microscopic levels. The frequency with which these conceptual modes manifested in students' responses across educational levels and types of questions is summarized in Table 7.O-K Q3: I'd say perhaps you'd first want to form the lithium, aluminum solid by combining the two together as a mixture of solids and melting them, melt them together to the appropriate um area where they create one phase and are together, mixed… and allow them to cool down to be a single solid in a single solid phase, and… perhaps add hydrogen gas into the mixture as they're liquid to pressurize the hydrogen gas into a liquid combination of the two metals to create the lithium aluminum hydride and then after that of course let them combine and cool down so you have a big solid mix of lithium aluminum hydride.
This student assumed that a series of phase changes were needed to generate the product. Temperature and pressure were then used to produce the macroscopic changes required to directly transform reactants into products.
Other students referred to the manipulation of external conditions (e.g., cooling, heating) to effect changes at the submicroscopic level (e.g., chemical bonding). Sometimes pressure and temperature were used to force chemical bonds to form, as illustrated by the following excerpt:
Interviewer: …then you were talking about forming carbon–hydrogen bonds versus forming carbon–carbon bonds
O-K Q2: certainly
Interviewer: can you tell me a little bit more about?
O-K Q2: the carbon hydrogen bonds are weaker and therefore less difficult to get to form using temperatures and pressures you need to force the gases into each other so that they will react and reactions will occur where the carbon–carbon bonds being much stronger bonds will be more difficult, the conditions would need to be more difficult, strenuous I suppose
Interviewer: ok, so a stronger bond
O-K Q2: would be more difficult to form
This student expressed the idea that bond formation was a difficult process that necessitated an outside agent (pressure and temperature) to force it to happen. In a few cases students focused on chemical conditions as agents for chemical control. For example,
I-N Q3: HCl is an acid, and acids have a tendency to break things down, so maybe the HCl works better at breaking apart the covalent bonds so that you just separate what you need.
In this case, the participant thought of acids as active agents capable of breaking chemical bonds.
C-E Q2: let's see… so from my perspective I would think a more stable compound would be easier to synthesize because from the energy diagram, so you always, it's always favorable to go from a higher energy compound or higher energy state to a lower energy state, but I don't know for sure the energy state of these three compounds or which one is more stable, for me I would like to choose the last one because it's liquid
Interviewer: ok
C-E Q2: but I would think this all depend on what kind of condition you are choosing like temperature or catalyst
For this student the outcome of the reaction was governed by the energetic stability of different substances but was facilitated or hindered by different reaction conditions. The analysis in this case remained at the macroscopic level, while in other instances students considered how system conditions could affect the properties and behavior of submicroscopic entities:
C-F Q5: NH2that's electron donating making this one not quite electrophilic… but the H minus… how likely?… I think the reaction could be easy, maybe use LAH [LiAlH4] that would be a low temperature [reaction] and I think that's easy to do because the H minus attack here should be very easy
In this case, the student considered that the structure of the compounds would determine the outcome of the process but identified physical conditions that would affect the reactivity of specific species. Chemical reaction conditions were considered in a similar fashion:
O-L Q6: ok um cause SN1 one creates a carbocation, so polar protic solvents help the carbocation be stable through, because of the dipoles
In this case, the solvent was seen as an entity that stabilized a desired intermediate species.
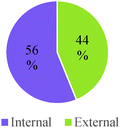
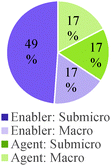
Overall, students in our sample tended to discuss reaction conditions as enablers of change more often than as agents of change (Table 7). They also more frequently discussed chemical control effects at a submicroscopic level than at a macroscopic level. However, this pattern of reasoning was reversed for general chemistry students and seemed to change gradually with increasing training in the discipline. Attention to physical conditions was also dominant in the lower educational level while consideration of chemical conditions was more prevalent in the higher educational levels. As was the case with other categories of analysis, students' responses to Questions 2 and 3 were characterized by a greater frequency of conceptual modes with weaker explanatory power.
Discussion
The central goal of this research study was to uncover major conceptual modes that students apply when thinking and making decisions about chemical reactions and generating specific chemical products. In particular, we elicited different ways of conceptualizing why chemical reactions happen (chemical causality), how these processes occur (chemical mechanism), and how they can be controlled (chemical control). In each of these areas we characterized conceptual modes with different explanatory power (see Fig. 1) and explored how they were applied by students when facing different types of questions.Although our qualitative study involved a small number of students at different levels of training in the discipline, our findings suggest potential progression paths in the understanding of chemical reactions and the preparation of substances. Some conceptual modes were more frequently applied by students in particular groups, which indicates that these ways of thinking may be more common at certain educational stages. It is unlikely that all students will follow the same trajectory as they grow in their understanding of any subject. Thus, we do not claim that chemistry students will develop a specific sequence of conceptual modes as they progress in their understanding. However, we can use our results to hypothesize conceptual modes that likely become more dominant as students advance in their studies. These hypotheses are in alignment with our research findings but need to be further validated through additional studies:
(a) Novice chemistry students are likely to conceptualize reactions as macroscopic assembling processes. Thus, they will assume that making a compound demands effort through the action of external agents that can transform the reactants into the desired products. These transformations will likely involve physical changes, such as mixing the components in the right proportions and changing their state of matter to facilitate the mixing process (e.g., melting solids or condensing gases so that they can mix more easily). A process will be judged to be easier when smaller numbers and amounts of components need to be transformed and assembled. This way of thinking has core elements in common with what Andersson (1986) identified as an experiential gestalt of causation and with force dynamics accounts of causation in human reasoning (Talmy, 1988; Pinker, 2007). A variety of studies have shown that interpreting chemical reactions as mixing or simple association processes is common among novice chemistry students (Andersson, 1990; Ahtee and Varjola, 1998).
(b) Exposure to chemical concepts and ideas, such as atom, molecule, chemical bond, likely helps students develop a sense of mechanism for how new substances are formed at the submicroscopic level (e.g., atoms aggregate, bonds are formed). Nevertheless, implicit assumptions about the need for external intervention may remain unchanged. Consequently, students may still judge that direct action and energy investment are needed to induce the desired processes at the submicroscopic scale. Different studies have shown that reliance on the action of external agents to explain physical and chemical change is common among students at various educational levels (Hatzinikita et al., 2005; Stains and Sevian, 2014).
(c) The transition from focusing on external agents to focusing on internal agents as drivers of chemical change may be mediated and potentially aided, by the development of teleological accounts of chemical reactions. As students become aware of properties of atoms and molecules used to explain and predict reactivity (e.g., electron configuration, electronegativity, polarity), they may think of these properties as intrinsic characteristics that determine particles' needs or wants to achieve a more desirable state (e.g., acquire an octet of electrons, become more stable). This type of teleological thinking has been elicited in different students (Taber, 2013; Taber and Adbo, 2013) and has been shown to persist with training in the discipline (Talanquer, 2013b).
(d) Initial mechanistic conceptualizations of chemical reactions at the submicroscopic scale will likely be based on non-selective interactions between reacting particles (e.g., generic views of atoms colliding and bonding with each other). As attention to structural features develops, students may start thinking of mechanisms based on selective interactions between entities with different electrical charge or any other property thought to determine reactivity (e.g., polarity, electronegativity). However, it is probable that students will switch conceptual modes from one type of problem to another, depending on the particular cues that are more salient to them based on prior knowledge, recent experiences, and explicit features of the reactions under analysis. The challenges that students face in learning to build mechanistic accounts based on constrained sequential stories have been described by several authors (Bhattacharyya and Bodner, 2005; Bhattacharyya, 2014).
(e) Exposure to chemical concepts and ideas in introductory general and organic chemistry courses likely shifts many students' attention from compositional (i.e., types and amounts of atoms involved) to structural features of reactants and products in making judgments about chemical reactivity. However, the ability to use structural characteristics to build valid explanations and make reasonable predictions may develop gradually over many years of specialized training in the discipline. This latter finding is consistent with results from studies on students' thinking about structure–property relationships (Cooper et al., 2012, 2013).
(f) Students' attention to structural features and ability to use them in productive ways may develop more rapidly than their understanding of internal energy transformations and transfer during chemical processes. Students may recognize that energy differences between reactants and products act as drivers of chemical reactions, but struggle to develop appropriate mechanistic models connecting material and energetic changes in a system.
(g) Students' judgments about the feasibility of a chemical process to create a product are likely to merge ideas about the extent (thermodynamics) and the rate (kinetics) of chemical reactions. Differentiation of factors that determine whether a reaction is product-favored or reactant-favored versus whether a reaction is fast or slow can be expected to be challenging to students at all educational levels. In general, structural and energetic considerations are likely to be dominant in student reasoning about chemical reactions over time-related issues (which were minimally present in our study participants' thoughts).
Our findings suggest that development in the understanding of some ideas may occur at a slower pace in some areas than in others. For example, dominant assumptions about source of agency in groups of students at different educational stages seemed to gradually switch from external agents to internal agents that acted in a teleological way to internal agents that acted causally. There was a more drastic switch of assumptions related to how reactions happened, going from mechanisms mostly based on mixing or non-selective interactions at the general chemistry level to mechanisms that mostly invoked selective interactions for students who finished organic chemistry. Differences after this educational level did not seem to involve a major switch in conceptual mode but increased ability to apply chemical thinking in valid ways.
Our results also indicate that the same student may apply different conceptual modes depending on the type of question or problem under consideration. For example, questions that confront students with substances or types of reactions that are less familiar to an individual, such as questions 2 and 3 in our study, may elicit conceptual modes of weaker explanatory power. Similarly, the nature of a task may affect how students' conceptualize causality and mechanism. In our case, questions that asked students to design processes triggered more explanations based on external energy drivers than questions that required them to make comparisons between different chemical reactions. Questions that required the comparison of chemical reactions involving molecules of different sizes and reacting in vastly different amounts (e.g., question 2) led more students to pay attention to explicit rather than implicit differences between the processes. In general, our study points to the need for further investigations about how the nature of questions and probes affects the cueing of different conceptual modes.
Given the qualitative nature of our study, one should be cautious with generalizations. Our study involved a small number of study participants who were not necessarily representative of the targeted populations. Additionally, we explored student thinking through the analysis of answers which were created on the spot during an interview. Thus our data may not have captured students' actual level of understanding, but rather their ability to generate answers from salient contextual cues and available cognitive resources under conditions of limited time. Moreover, our inferences about student thinking were derived from the analysis of students' expressed ideas through talk and writing. Our interpretations may thus be biased by our own beliefs about how people think. Nevertheless, we consider that our study provides important insights into how student understanding about chemical reactions and making specific chemical products may progress with training in the discipline.
Implications
The results of our study highlight and describe different conceptual modes that chemistry students may apply to build explanations and make decisions related to how and why chemical reactions occur, and how to control them. Our findings elicit implicit ways of thinking that may support or hinder students' progress in the understanding of a core chemistry practice. Recognizing that our students' reasoning may be guided by tacit views about causality, mechanism, and control that drastically differ from those that support productive chemical thinking may help us devise more effective strategies to support their learning.Traditional teaching approaches in the chemistry classroom commonly focus on increasing students' explicit knowledge base and correcting explicit misunderstandings. Little time, if any, is dedicated to engage students in the analysis and discussion of the implicit assumptions chemists make about the nature of chemical entities and phenomena (Talanquer, 2015). For example, we do not help students recognize different causal mechanisms, from direct causal chains to emergent processes (Chi, 2005; Grotzer, 2003), that may be at play in different chemical systems. We rarely open spaces for students to build or evaluate different models for explaining chemical properties and reactions. Nevertheless, research in science education indicates that making explicit the different mechanisms that may be responsible for natural phenomena (Chi et al., 2012) and engaging students in modeling practices (Clement and Rea-Ramirez, 2008) significantly increase student understanding.
Our study also highlights the benefits of analyzing students' understanding not only by focusing on implicit cognitive elements (Taber, 2014), but by using disciplinary crosscutting concepts as lenses of analysis. Existing research in chemistry education tends to be conducted using disciplinary topics (e.g., atomic structure, chemical bonding) as analytical guides (Kind, 2004). The goal is to uncover how students think about specific chemical concepts and how to improve their understanding in those areas. This type of research needs to be complemented by research that helps us understand students' ideas about overarching concepts, such as chemical causality and chemical mechanism, that may strongly influence how students reason about different chemistry topics (Sevian and Talanquer, 2014). Recent studies on students' thinking about chemical identity (Ngai et al., 2014) and structure-property relationships (Talanquer, 2008; Cooper et al., 2013; Maeyer and Talanquer, 2013) illustrate this approach and, together with the present study, help build a solid foundation for the future development of actual learning progressions that target core ideas and practices in chemistry.
Appendix
In this appendix we present example quotations and codes that were used to identify the types of cues participants paid attention to, the verbal predicates that they used, and the nature of the claims that they made (Table 8).| Quotation | Code | Conceptual mode |
|---|---|---|
| Causality – source of agency | ||
| O-K Q3: I'd say perhaps you'd first want to form the lithium, aluminum solid bycombining the two together as a mixture of solids and melting them, melt them together to the appropriate um area where they create one phase and are together, mixed… and allow them to cool down to be a single solid in a single solid phase, and…perhaps add hydrogen gas into the mixture as they're liquid to pressurize the hydrogen gas into a liquid combinationof the two metals to create the lithium aluminum hydride and then after that of course let them combine and cool down so you have a big solid mix of lithium aluminum hydride. | Combining, mixing, melting as agent | External agents – macro effects |
|
Interviewer: what else makes this reaction easier than the other two reactions?
G-K Q2: all you're forming are carbon–hydrogen bonds instead of carbon–carbon bonds Interviewer: why is forming carbon–hydrogen bonds easier than forming carbon–carbon bonds? G-K Q2: delta H requires lower, soless energy is required to be put into form the bond |
Energy input as agent | External agents – sub-micro effects |
|
Interviewer: you said the O minus is unhappy, can you tell me more about why it is unhappy?
U-H Q7: yea because if, I'm going to draw it, so here it had two lone pairs and a double bond, here it has one double bond still two lone pairs which is only five electrons in its valence shellbut it wants six so it, it wants another bond to equal it's happy valence. |
Wants, happiness as agent | Internal agents – teleological |
| G-M Q4: it would definitely be exothermic and I guess that would have to do with thebonds formed being lower in energy than the bonds already existing. | Energy of bonds as agent | Internal agents – causal. |
| Causality – material drivers | ||
| O-C Q2: It [first reaction] has like the least number of compounds that you would need, like you would need less like H2 and CO to actually make those products than you would need on the other ones | Numbers of compounds as driver | Amounts and properties of components as drivers |
| I-L Q6: it'salways looking for that [octet], so you know, apparently even if it already has it | Octet as driver | Structure of particles as reaction drivers |
| C-K Q7: [acyl chlorides] are less stable than amides, and that's usually in part because the amides have a, this lone pair on the nitrogen which can donate to the system making it very very stable due to resonance and even though you can have it in chlorines because they, you can argue that they have lone pairs right, their electronegativity, their inductive effect wins over this resonance factor | Electronic structure as driver | Structure of particles as reaction drivers |
| Causality – energetic drivers | ||
| I-G Q2: methane would be easier to synthesize simply because, first of all, you're using less reactant, second of all because you are using less reactantit takes less energy to react between the two in order to make the methaneand water that comes out of the reaction | Input of energy as driver | Energy as an external driver |
| C-K Q2: you have four CH bonds and two waters being formed which you have to take into account also for your overall delta H so you have three bonds of hydrogen which should be weaker than the CH bonds, soyou are forming stronger bonds as your products formed.That should drive the reaction to your products. | Bond energy as driver | Energy as an internal driver |
| Mechanism | ||
| I-K Q3: how would the product form? Hydrogen is gas, lithium solid, H2O is liquid… ok what I'm looking at islike a math way of doing it like H2plus H2…Li and then H2Ofrom what I know if I have like X to the fourth… | Adding symbols | Mixing components |
| I-H Q2: that one [hexane] might be easier because there would be more of a probability of them hitting each other since atoms are tiny and even though it happens all the time the probability of it happening is… it happens, so maybe that one might be easier to make just because there are more things to throw together and hope they stick | Random collisions | Non-selective interactions |
| O-G Q3: ok well I know thatif something is positively charged it's going to go toward, it's going to gravitate toward something that's negatively charged[…] | Selective attractions | Selective interactions |
| Chemical control | ||
|
Interviewer: …then you were talking about forming the carbon–hydrogen bonds versus forming carbon–carbon bonds
O-K Q2: certainly Interviewer: can you tell me a little bit more about? O-K Q2: the carbon hydrogen bonds are weaker and therefore less difficult to get to form using temperatures and pressuresyou need to force the gases into each other so that they will reactand reactions will occur where the carbon–carbon bonds being much stronger bonds will be more difficult, the conditions would need to be more difficult, strenuous I suppose |
Temperature and pressure used to force bond formation | Reaction conditions as agents of change |
| O-L Q6: ok um cause SN1 one creates a carbocation, so polar protic solvents help the carbocation be stable through, because of the dipoles | Solvent helps carbocation stability | Reaction conditions as enablers of change. |
Acknowledgements
The authors wish to acknowledge the funding sources, US National Science Foundation awards 1222624 and 1221494, that support our work. Any opinions, conclusions, or recommendations expressed in this paper are those of the authors, and do not necessarily reflect the views of the funding sources.Notes and references
- Ahtee M. and Varjola I. (1998). Students' understanding of chemical reaction, Int. J. Sci. Educ., 20, 305–316.
- Andersson B., (1986), The experimental gestalt of causation: a common core to pupils preconceptions in science, Eur. J. Sci. Educ., 8(2), 155–171.
- Andersson, B., (1990), Pupils' conceptions of matter and its transformations (age 12–16), Stud. Sci. Educ., 18, 53–85.
- Baillargeon R., Li J., Ng W. and Yuan S., (2009), An account of infants' physical reasoning, in Woodward A. and Needham A. (ed.), Learning and the infant mind, New York: Oxford University Press, pp. 66–116.
- Bhattacharyya G., (2014), Trials and tribulations: student approaches and difficulties with proposing mechanisms using the electron-pushing formalism, Chem. Educ. Res. Pract., 15(4), 594–609.
- Bhattacharyya G. and Bodner G. M., (2005), “It gets me to the product”: How students propose organic mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Bowdle B. F. and Gentner D., (2005), The career of metaphor, Psychol. Rev., 112(1), 193–216.
- Brown D. E. and Hammer D., (2008), Conceptual change in physics, in Vosniadou S. (ed.), International handbook of research on conceptual change, New York: Routledge, pp. 127–154.
- Charmaz K., (2006), Constructing grounded theory: a practical guide through qualitative analysis, Thousand Oaks: Sage.
- Chi M. T. H., (2005), Commonsense conceptions of emergent processes: why some misconceptions are robust, J. Learn. Sci., 14(2), 161–199.
- Chi M. T. H., (2008), Three kinds of conceptual change: belief revision, mental model transformation, and ontological shift, in Vosniadou S. (ed.), International handbook of research on conceptual change, New York: Routledge, pp. 61–82.
- Chi M. T. H., Roscoe R. D., Slotta J. D., Roy M. and Chase C. C., (2012), Misconceived causal explanations for emergent processes, Cognit. Sci., 36(1), 1–61.
- Clement J. J. and Rea-Ramirez M. A. (ed.), (2008), Model based learning and instruction in science, London: Springer.
- Cooper, M. M., Underwood, S. M. and Hilley, C. Z., (2012), Development and validation of the implicit information from Lewis structures instrument (IILSI): do students connect structures with properties? Chem. Educ. Res. Pract., 12, 195–200.
- Cooper M. M., Corley L. H. and Underwood S. M., (2013), An investigation of college chemistry students' understanding of structure–property relationships, J. Res. Sci. Teach., 50, 699–721.
- Davis B. H. and Occelli M. L. (2007), Fischer-Tropsch synthesis, catalyst and catalysis, Boston: Elsevier.
- de Arellano D. C. R. and Towns M. H., (2014), Students' understanding of alkyl halide reactions in undergraduate organic chemistry, Chem. Educ. Res. Pract., 15(4), 501–515.
- diSessa A. A., (1993), Toward an epistemology of physics, Cognit. Instr., 10(2/3), 165–255.
- Duschl R., Maeng S. and Sezen A., (2011), Learning progressions and teaching sequences: a review and analysis, Stud. Sci. Educ., 47(2), 123–182.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9(2), 102–113.
- Gelman S. A., (2009), Learning from others: children's construction of concepts, Annu. Rev. Psychol., 60, 115–140.
- Grotzer T. A., (2003), Learning to understand the forms of causality implicit in scientifically accepted explanations, Stud. Sci. Educ., 39, 1–74.
- Grove N., Cooper M. and Rush K., (2012), Decorating with arrows: toward the development of representational competence in organic chemistry, J. Chem. Educ., 89, 844–849.
- Gupta A., Hammer D. and Redish E. F., (2010), The case for dynamic models of learners' ontologies in physics, J. Learn. Sci., 19, 285–321.
- Hatano G. and Inagaki K., (2000), Domain-specific constraints on conceptual development, Int. J. Behav. Dev., 24(3), 267–275.
- Hatzinikita V., Koulaidis V. and Hatzinikitas A., (2005), Modeling pupils' understanding and explanations concerning changes in matter. Res. Sci. Educ., 35, 471–495.
- Keil F. C., (1979), Semantic and conceptual development: an ontological perspective, Cambridge, MA: Harvard University Press.
- Keil F. C., (1990), Constraints on constraints: Surveying the epigenetic landscape, Cognit. Sci., 14(1), 135–168.
- Kind V., (2004), Beyond appearances: students' misconceptions about basic chemical ideas, 2nd edn, London: Royal Society of Chemistry.
- Kraft A., Strickland A. M. and Bhattacharyya G., (2010), Reasonable reasoning: multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11(4), 281–292.
- Krajcik J. S., (2012), The importance, cautions and future of learning progression research, in Alonzo A. C. and Gotwals A. W. (ed.), Learning progressions in science: current challenges and future directions, Rotterdam: Sense Publishers, pp. 27–36.
- Maeyer J. and Talanquer V., (2013), Making predictions about chemical reactivity: Assumptions and heuristics, J. Res. Sci. Teach., 50(6), 748–767.
- Mortimer E. F., (1995), Conceptual change or conceptual profile change? Sci. Educ., 4(3), 267–285.
- Mortimer E. F., (2001), Perfil conceptual: Formas de pensar y hablar em las classes de ciencias [Conceptual profile: modes of thinking and ways of speaking in science classrooms], Infancia y Aprendizaje, 24(4), 475–490.
- Mortimer E. F., Scott P., Ribeiro do Amaral E. M. and El-Hani C. N., (2014), Conceptual profiles: theoretical-methodological bases of a research program, in Mortimer E. F. and El-Hani C. N. (ed.), Conceptual profiles: a theory of teaching and learning scientific concepts, Dordrecht: Springer, pp. 3–33.
- National Research Council (NRC), (2007), Taking science to school: learning and teaching science in grades K-8, Washington, D.C.: National Academies Press.
- Ngai C., Sevian H. and Talanquer V., (2014), What is this substance? What makes it different? Mapping progression in students' assumptions about chemical identity, Int. J. Sci. Educ., 36, 2438–2461.
- Pinker S., (2007), The stuff of thought: language as a window into human nature, New York: Penguin Group.
- Sendur G. and Toprak M., (2013), The role of conceptual change texts to improve students' understanding of alkenes, Chem. Educ. Res. Pract., 14(4), 431–449.
- Sevian H. and Talanquer V., (2014), Rethinking chemistry: a learning progression on chemical thinking, Chem. Educ. Res. Pract., 15(1), 10–23.
- Sloman S. A., (1996), The empirical case for two systems of reasoning, Psychol. Bull., 119(1), 3–22.
- Slotta J. D., Chi M. T. H. and Joram E., (1995), Assessing students' misclassifications of physics concepts: An ontological basis for conceptual change, Cognit. Instr., 13, 373–400.
- Smith C., Wiser M., Anderson C. and Krajcik J., (2006), Implications of research on children's learning for standards and assessment: a proposed learning progression for matter and atomic-molecular theory, Measurement, 14(1&2), 1–98.
- Spelke E. S. and Kinzler K. D., (2007), Core knowledge, Dev. Sci., 10(1), 89–96.
- Stains M. and Sevian H., (2014), Uncovering implicit assumptions: a large-scale study on students' mental models of diffusion, Res. Sci. Educ., DOI: http://10.1007/s11165-014-9450-x.
- Stavy R. and Tirosh D., (2000), How students (mis-)understand science and mathematics: intuitive rules, New York: Teachers College Press.
- Taber K. S., (2013), A common core to chemical conceptions: Learners' conceptions of chemical stability, change and bonding, in Tsaparlis G. and Sevian H. (ed.), Concepts of matter in science education, Dordrecht: Springer, pp. 391–418.
- Taber K. S., (2014), The significance of implicit knowledge for learning and teaching chemistry, Chem. Educ. Res. Pract., 15(4), 447–461.
- Taber K. and Adbo K., (2013), Developing chemical understanding in the explanatory vacuum: Swedish high school students' use of an anthropomorphic conceptual framework to make sense of chemical phenomena, in Tsaparlis G. and Sevian H. (ed.), Concepts of matter in science education, Dordrecht: Springer, pp. 347–372.
- Taber K. S. and Watts M., (2000), Learners' explanations for chemical phenomena, Chem. Educ. Res. Pract., 1(3), 329–353.
- Talanquer V., (2006), Commonsense chemistry: a model for understanding students' alternative conceptions, J. Chem. Educ., 83(5), 811–816.
- Talanquer V., (2008), Students' predictions about the sensory properties of chemical compounds: additive versus emergent frameworks, Sci. Educ., 92(1), 96–114.
- Talanquer V., (2010), Exploring dominant types of explanations built by general chemistry students, Int. J. Sci. Educ., 32 (18), 2393–2412.
- Talanquer V., (2013a), How do students reason about chemical substances and reactions? in Tsaparlis G. and Sevian H. (ed.), Concepts of matter in science education, Dordrecht: Springer, pp. 331–346.
- Talanquer V., (2013b), When atoms want, J. Chem. Educ., 90(11), 1419–1424.
- Talanquer V., (2015), Threshold concepts in chemistry: the critical role of implicit schemas, J. Chem. Educ., 92(1) 3–9.
- Talanquer V. and Pollard J. (2010), Let's teach how we think instead of what we know, Chem. Educ. Res. Pract., 11(2), 74–83.
- Talmy L., (1988), Force dynamics in language and cognition, Cognit. Sci., 12(1), 49–100.
- Todd P. M. and Gigerenzer G., (2000), Precis of simple heuristics that make us smart, J. Behav. Brain Sci., 23(5), 727–780.
- Vosniadou S., (1994), Capturing and modeling the process of conceptual change, Learn. Instr., 4(1), 45–69.
- Vosniadou S., (2013), Conceptual change in learning and instruction: the framework theory approach, in Vosniadou S. (ed.), International handbook of research on conceptual change, 2nd edn, New York: Routledge, pp. 11–30.
- Vosniadou S. and Ortony A., (1989), Similarity and analogical reasoning: a synthesis, in Vosniadou S. and Ortony A. (ed.), Similarity and analogical reasoning, New York: Cambridge University Press, pp. 1–17.
- Vosniadou S., Vamvakoussi X. and Skopeliti I., (2008), The framework theory approach to the problem of conceptual change, in Vosniadou S. (ed.), International handbook of research on conceptual change, New York: Routledge, pp. 3–34.
- Zoller U., (1990), Students misunderstandings and misconceptions in college freshman chemistry (general and organic), J. Res. Sci. Teach., 27(10), 1053–1065.
| This journal is © The Royal Society of Chemistry 2015 |