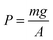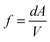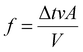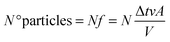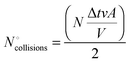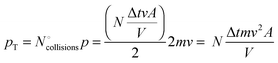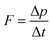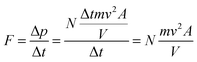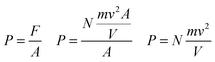Natural laws and ontological reflections: the textual and didactic implications of the presentation of Boyle's law in general chemistry
Waldo
Quiroz
* and
Cristian Merino
Rubilar
Pontificia Universidad Católica de Valparaíso – Instituto de Química, Campus Curauma, Avenida Universidad 330, Valparaíso, Valparaíso 2373223, Chile. E-mail: waldo.quiroz@ucv.cl
First published on 5th June 2015
Abstract
This study develops a tool to identify errors in the presentation of natural laws based on the epistemology and ontology of the Scientific Realism of Mario Bunge. The tool is able to identify errors of different types: (1) epistemological, in which the law is incorrectly presented as data correlation instead of as a pattern of causality; (2) semantic, in which natural law is presented as a mathematical statement that relates variables but with an absence of ambiguous material reference; (3) deterministic, in which the relationship of natural variables is presented but with no causality statement; and (4) mechanistic, in which a causality statement is presented with the absence of an explanatory mechanism. In this work, Boyle's law was used as an example of the applicability of the instrument. In this case, we found errors in most of the university textbooks that we analyzed. Most of the errors arose from the disconnection between the symbolic and microscopic levels. The presentations of Boyle's law in general chemistry are given in textbooks that include illustrations based in a macroscopic perspective, in which the macroscopic compression mechanism is completely disconnected from the microscopic collision mechanism. This disconnection results in the incorrect presentation of gas pressure as the cause and gas volume as the effect.
Introduction
There has been widespread confusion regarding the concepts of correlation, hypothesis, law and theory, not only in the general population but also among science teachers (Siddiquee and Ikeda, 2012; Faikhamta, 2013) and high school chemistry textbooks (Abd-El-Khalick, et al., 2008). This confusion exists due to differences as much in terms of classes as of hierarchy.In our opinion, the best definition of these concepts, at least within the natural sciences, comes from the philosophical system proposed and developed by Mario Bunge in his famous treatise (Bunge, 1974a, 1974b; Bunge, 1979; Bunge, 1983a, 1983b; Bunge, 1985; Bunge, 1989). He established that all laws are scientific hypotheses but that not all scientific hypotheses are laws. Scientific laws are scientific hypotheses that comply with the requirements of all scientific hypotheses which include having
• A material reference;
• A foundation in scientific theory; and
• Empirical testability.
However, laws must also comply with the following additional requirements:
• They present a causal relationship;
• They present favorable empirical evidence; and
• They propose a proven mechanism connecting causes and effects (Bunge, 2000; Bunge, 2012).
The relationship between correlation and law arises from the concept of evidence. Because a law is a causal relationship, the data on the magnitude of measured variables must be correlated and fit the mathematical models that give the law formal structure, which means that behind all law, there must be correlations between the pieces of evidence that fit the law in question. The inverse is not necessarily the case: behind a data correlation, there is not necessarily a law because we might be observing a false correlation or a relationship between two variables that are indirectly connected through a third (Bunge, 2000).
A scientific theory is a system of scientific hypotheses, some of which could be laws. As such, it can be said that the relationship between theory and hypothesis is systemic (Bunge, 1997). Laws are a subset of the category of scientific hypotheses. In terms of class, although theories and the hypotheses and laws that compose them exist on the plane of ideas, correlations exist on the plane of observations and measurements, i.e., on the plane of empirical facts.
The function of an experiment is therefore to test a law, but its aim is not to generate the law (Popper, 2008) or to establish causes and effects. The latter requires proposing mechanisms within the framework of a theory and verifying each of its stages.
Presentation of natural laws
The recognition of our absence of knowledge of what a scientific law is and what such a law represents is a relatively recent phenomenon that remains under discussion. As such, the results of this discussion have not yet trickled down to the educational context. As a result, superior status is still conferred on laws above theories (Lederman, et al., 2002).Therefore, the principal objective of this research is to apply the philosophical system of Mario Bunge to develop an instrument for detecting errors in the presentation of scientific laws within the framework of science education material. We applied the instrument to Boyle's law.
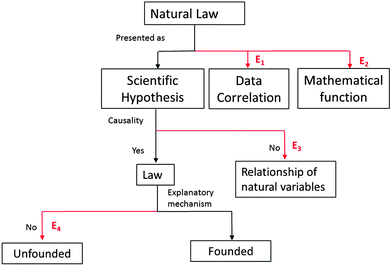
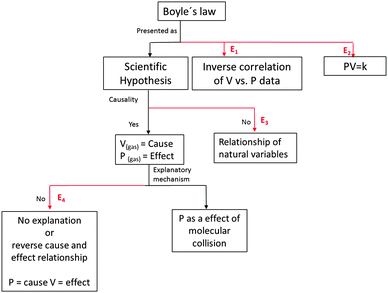
Considering the requirements of Bunge's philosophical system for scientific hypothesis, in general and for natural laws in particular, we could propose an instrument to detect errors in the presentation of natural laws in 4 categories. The error detection scheme is shown in Fig. 1.
(1) Epistemological: natural laws are presented as scientific hypotheses. An error in this point is to present a natural law as a data correlation (E1) or as an induction, i.e., as more than an intellectual creation.
(2) Semantic: variables in natural laws are presented as being related to the material nature of reality. An error in this point is to present a natural law as a mathematical equation that relates variables (E2) to non-existent or erroneous material references.
(3) Deterministic: natural law is presented as pattern of causality. An error in this point is to present the natural law as only a relationship of natural variables without specifying the cause and effect relationship (E3).
(4) Mechanistic: the pattern of causality of the law is based on a mechanism within the framework of a scientific theory. The absence of an explanatory mechanism renders the choice of the cause or effect arbitrary and unfounded (E4).
It is important to note that this conception of natural law in Bunge's philosophical system includes both mechanistic laws, such as Newton's laws, which connect a cause with an effect, and non-mechanistic or stochastic laws, such as Mendel's laws, in which one cause (parental cross) can generate multiple combinations of effects (phenotype distributions) but with different probabilities.
In Table 1, the analysis of three scientific laws using the tool of Fig. 1 is presented. As seen in Table 1, this tool can be applied without distinction to causal laws, such as the Lambert–Beer law, or to stochastic laws, such as the segregation law of Mendel. Furthermore, it applies equally to laws of physics, chemistry or biology, even if they are not presented in mathematical language. All of these laws exhibit a pattern of causality, a mechanism and a theory into which they are embedded.
| Law | Cause | Effects | Mechanism | Theory |
|---|---|---|---|---|
| Segregation law (Mendel) | Parental cross | Phenotype distribution | Allele pairs separate during gamete formation and randomly unite at fertilization. | Genetic theory |
| Periodic law | Atomic number | Properties of the elements at regular intervals of 2, 8, 18, and 32 | Periodicity of atomic properties results from the arrangement of electrons in atomic orbitals. “s” orbitals can have a maximum of 2 electrons, “p orbitals can have a maximum of 6 electrons, and “d” orbitals a maximum of 10 electrons. | Quantum theory |
| A = ∈ × I × C (Lambert–Beer) | C = concentration | A = absorbance | Increasing the concentration of an analyte generates higher probability of absorption of light beam, which is generated by the electronic transition of atoms or molecules. | Electromagnetic/orbital molecular theory |
In the case of the law of segregation, for example, the absence of a pattern of causality between the parental cross as the cause and the combinations of phenotypes as the effect would generate a presentation of the segregation law as a mere statistical correlation. Moreover, the absence of the allele recombination mechanism would render descendant phenotypes purely fortuitous and unfounded, thus failing to explain why some phenotypes are more likely than others. In the case of the periodic law, the absence of the concept of the orbital and of the Pauli Exclusion Principle would generate a presentation of the periodic law as a mere temporal mathematical series. Finally, for the Lambert–Beer law, the connection with the photo-physical mechanisms of absorption and electronic transition allow the concentration as the cause and the absorbance as the effect to be established, making it clear that it is a law and not merely a linear regression or a straight line equation.
Certainly, the analysis of the mechanisms and theories in which a law is inserted is not sufficiently simple to be summarized in a table. This analysis should include not only disciplinary aspects of science but also a semantic analysis based on a clear logic and ontology. In the case of chemistry, its theories and laws pertain mainly to the microscopic world.
Understanding the microscopic world is always challenging (Harrison and Treagust, 2003). Chemical knowledge can be represented through the famous triplet of the macro, submicro, and symbolic levels. Connection among these levels has become essential for research in chemical education (Talanquer, 2011). It was reported that students encountered great difficulties in relating the macroscopic and submicroscopic levels of matter because they represented the concepts of matter through properties that could be observed, which were closer to the dimensions of the macroscopic world than to the corpuscular world (Arellano, et al., 2014). Research in the field of chemistry education has provided possible reasons for these difficulties. For example, regarding the math skills of students, those who present weaknesses in math skills are considered to encounter greater difficulties in learning chemistry. It was reported that students feel “abandoned by science” because of the abstract nature of the content (Coll, et al., 2006).
Ontologically, Boyle's law is one of the most complex laws due to the relationships of causality connecting reality at the microscopic level (particle collisions) to that at the macroscopic level (gas pressure). One of the main causes of students making errors in learning Boyle's law is mistranslation between the macroscopic and the microscopic external representations (Kang, et al., 2008).
For all of the above reasons, to apply our tool, Boyle's law is used as an example because it is mathematically one of the simplest laws, incorporating only 2 variables, and it is a law that is studied in all secondary education science curricula within the school system.
Analysis of Boyle's law
In the work of DeBerg in 1995 (de Berg, 1995), a historical analysis of Boyle's law was performed. In this work, it was clearly established that the development of an understanding of the properties of air was absolutely necessary before any law could be established. Air volume changes were an issue that generated several concerns, and different ideas were proposed before Boyle's law. For example, in 1653, Henry Power proposed that the changes in air volume on the Torricellian apparatus be considered a measurement of the air's elasticity, with spring properties. It is clear that at this stage, Henry Power did not associate the elasticity of air with the pressure of air.One of the most interesting historical facts in the work of De Berg is related to experiments of Power and Towneley. In these experiments, they measured the elasticity of different trapped volumes of air by comparing the height of mercury above the bowl in an experimental tube with the normal barometric height at altitudes of 800 and 1800 feet.
Surprisingly, although it could be inferred that pressure is inversely proportional to volume from their results, they made no such statement. It is clear that results at different altitudes were probably sufficient to give them the intuitive relationship between the pressure and volume of air. However, this intuition lacked rigorous verification.
From a historical perspective, rigorous verification over a wider range of pressures was provided by the work of Robert Boyle in September 1661. For a time, Boyle's first experiments were related to air compression, and by adding increasing amounts of mercury, Boyle's results confirmed the hypothesis that the greater the weight that leans upon the air, the greater is its spring (pressure). Subsequently, Boyle performed a second set of experiments related to air expansion. According to De Berg, “It appears that Boyle was not certain that expansion of air would follow the same quantitative law as compression of air”, which is a clear historical fact. To believe Boyle's hypothesis, it appears that Boyle used the table of results for the compression of air to confirm the inverse proportion hypothesis rather than to show that the hypothesis naturally emerged from the data (de Berg, 1995).
Strictly speaking, Boyle's research tested the idea that P and V are inversely proportional, (West, 1999) and it is clear that according to De Berg, Boyle's hypothesis emerged before their experiments. However, based on these same historical data, we believe that the status of the law remained unresolved at that time, and the reason is simple: at that time, there was no way to establish the cause and effect relationship because of the particle collision mechanism, which required accepting that the atomic-molecular theory of matter is incompatible with the Aristotelian view of time. Epistemologically, the basis and mechanism of this hypothesis was not known until the hypothesis was inserted into the scientific kinetic theory of gases; therefore, its status as a law emerged later.
Boyle's law is a law of the natural sciences because it complies with the requirements detailed above. The empirical data that support the law fit its inverse trend, which was one of the main contributions of Boyle, who measured volumes and pressures of gases of different types (West, 1999). The law also forms part of the scientific theory of gases, indicating that the basis of the law is a theory in which the cause and effect are connected via a mechanism within the framework of the model of atomic/molecular collisions.
The causal relationship of Boyle's law is as follows:
| PV = k, |
The model that supports the law also assumes that the interactions between the particles and the volume of each individual particle are negligible. For this reason, experiments conducted to verify the law have used noble or inert gases at low pressures to approximate experimental conditions for the model in the best possible manner Smith, 1971.
In general, chemistry texts that teach Boyle's law have explained this quite correctly. However, there is one question that most textbooks omit with regard to Boyle's law, which is the focus of this study: what are the cause and effect in Boyle's law?
The question is complex because most textbooks approach the subject with the example of occluded gas cylinders. The typical experiment uses occluded gases with mercury, in which the increase in the amount of mercury generates greater gas compression, thus decreasing its volume.
Examining how the cause and effect in a law are stipulated, it can be seen that it is not arbitrary or a mere whim of the researcher who proposes to assign causes and effects to a law. There are two possibilities in the case of Boyle's law:
(a) The pressure of the gas is the cause, and the volume of the gas is the effect; or
(b) The volume of the gas is the cause, and the pressure of the gas is the effect
In university chemistry textbooks, it can be seen that these options are distributed heterogeneously, and it is therefore of interest to clarify why the second option is in fact correct.
The confusion between pressure as cause and volume as effect: compression vs. collision
It is common sense to think, based on certain everyday experiences, that as a gas is pressured, its volume decreases. The concept of “compressibility” is widely used in textbooks and is a concept that references the process of decreasing the volume of a gas by applying external pressure. Based on this process, the pressure is designated as the cause of the decrease in volume.In the framework of the theory of physics and macroscopic properties, this designation is correct. The pressure on a piston is the cause, the effect of which is to move the piston due to the subsequent causal relationships associated with the theory of classical physics and not the molecular kinetic theory of gases. This relationship can be represented as:
| F → P |
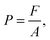 where F if the force applied to the piston, P is the pressure, and A is the area over which the pressure is exerted. We can ask ourselves where this force originates, and in the case of piston experiments and Boyle's experiments, the force arises from the action of a body with mass m and from the action of gravity g on the body, by the following expression:
where F if the force applied to the piston, P is the pressure, and A is the area over which the pressure is exerted. We can ask ourselves where this force originates, and in the case of piston experiments and Boyle's experiments, the force arises from the action of a body with mass m and from the action of gravity g on the body, by the following expression:| F = mg |
Therefore, additional pressure greater than atmospheric pressure on an occluded gas in a cylinder that supports a body of mass m, as shown in Fig. 2, is:
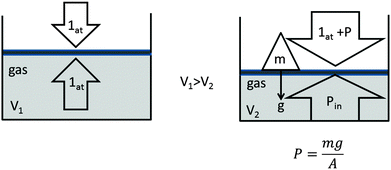 | ||
| Fig. 2 Compression of a gas due to external pressure and the corresponding effect of a decrease in volume. The increase in internal gas pressure is the final effect. | ||
Work is then generated by the movement of the piston via the following equation:
| W = Fd, |
In the absence of friction, the piston moves until the external pressure exerted on the piston (1 atmosphere + P) is equal to the internal pressure exerted by the gas (Pin). Therefore, we have two pressures: the internal pressure, Pin, of the gas associated with Boyle's law and the theory of gas kinetics; and the external pressure on the piston associated with classical macroscopic Newtonian physics.
We can now see that Boyle's law refers to only the internal pressure and not the external pressure, whereas chemistry textbooks refer to both pressures indifferently, which is a conceptual mistake in the context of Boyle's law. One defense of the use of pistons as the example would be to state that the pressure “P” is understood to be a necessary experimental procedure for generating a decrease in volume (compressibility) as an effect and that the increase in internal pressure “Pin” is understood to be a final effect.
These are undoubtedly the processes that occur, and conceptually, this usage is not erroneous, provided that a distinction is made between the two pressures, the external and the internal, and between the two levels of reality, the macroscopic and microscopic, which are explained and detailed further below.
Propositions that confuse the pressure and force exerted on the piston and the resulting pressure on the internal wall of the cylinder are mistaken not only because they refer to different levels or reality aspects but also because they are incomplete because it is not necessary to apply force to modify volume. For example, if the external pressure is less than the internal pressure of the cylinder, as shown in Fig. 3, the volume expands to decrease the internal pressure to equal the external pressure. In this case, the equation 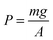 does not apply; thus, the causal relationships for explaining the external pressure are not the same in the two cases described herein because it is the internal pressure, a phenomenon of microscopic origin, that moves the piston in the latter.
does not apply; thus, the causal relationships for explaining the external pressure are not the same in the two cases described herein because it is the internal pressure, a phenomenon of microscopic origin, that moves the piston in the latter.
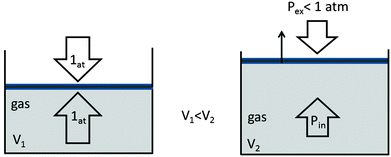 | ||
| Fig. 3 Expansion of a gas due to decreased external pressure and the corresponding effect of a decrease in internal pressure. | ||
In summary, in both Fig. 1 and 3, there are two pressures: internal and external. The first is directly connected to Boyle's law and the molecular kinetic theory of gases and the second is connected to classical macroscopic Newtonian physics.
Boyle's law and atomic/molecular collisions: causal mechanism
With regard to the connection between internal pressure and internal volume in Boyle's law, it can be said that internal pressure is a macroscopic variable, but it is an effect of the microscopic processes associated with particle collisions, on the atomic/molecular scale, with the wall of the cylinder, as shown in Fig. 4.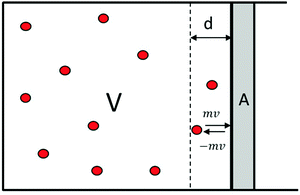 | ||
| Fig. 4 Atoms/molecules collide with the wall of a piston with area A. Red circles represent atom/molecule particles. | ||
The mathematical expressions consider a cylinder with volume V and a piston with area A at one end. The particles moving around within the cylinder have momentum p, which is equal to:
| p = mv |
If we follow the path of a particle that collides with the piston, it will travel a distance d, and only the particles close to the piston, as shown by the dotted line in Fig. 4, will collide. This critical distance is denoted as “d”, which is the distance covered before colliding. The distance d can be expressed in terms of time before collision (Δt) and velocity (v), as follows:
| d = Δtv |
Thus, we have a fraction (f) of the total volume of the cylinder occupied by the particles that collide with the piston, which can be defined as:
of the total number of particles “N”, a fraction “f“is within reach of the piston wall to exert pressure upon it. Assuming homogenous distribution of the gas particles over the total volume of the cylinder, the proportion of particles close to the piston is equal to the proportion of the total volume close to the piston. Therefore, the number of particles, “N° particles”, that will collide with the piston wall can be calculated as follows:
where Δt is time before collision, v is particle velocity, A is piston area, and V is the total volume of the cylinder.
Of this number, half of the particles move toward the piston to collide with it and the other half move away; thus, the number of particles that collide with the piston, “ ”, will be half the number of particles close to the piston:
”, will be half the number of particles close to the piston:
If we calculate the change in momentum “pT” of all of the particles that collide with the piston, we obtain the following:
The macroscopic concept of force “F” is based on Newton's second law, which expresses the change in momentum (Δp) as a function of time (Δt), as follows:
This expression connects the microscopic world of atomic/molecular collisions with their macroscopic effects. Thus, because the particle mass “m” and gas temperature “T” are constant, we obtain the following expression for the force “F” exerted on the piston:
In this expression, we have the variables of force “F”, particle mass “m” velocity “v”, piston area “A” and cylinder volume “V”, but we are yet to include gas pressure “P”. Recalling the expression for force/pressure we have:
The expression above connects P and V in a gaseous and molar microscopic phenomenon. Under constant temperature conditions and in a purely gaseous environment, the total number of particles N can be considered constant, as can the mass of each of the particles and the velocity v as the average velocity of the moles of the particles present, which is the same as stating that the energy of the system remains constant, as stated by Sharma (1982) (Sharma, 1982). Therefore, in Boyle's law, the factor k has physical significance in the following form:
PV = k![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = Nmv2 k = Nmv2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (temperature and moles are constant) (temperature and moles are constant) |
Ontological and epistemological reflections on Boyle's law
That Boyle's law has a formal mathematical structure does not mean that it is a merely formal statement. Formalism indicates that it is possible to use the advantages of logic/mathematics to make, for example, precise predictions and to use language that is less subjective than the predictive structure of more commonly used language. However, the variables involved must be addressed adequately with their respective natural or material references. P and V in Boyle's law are macroscopic properties of, in this case, a gaseous system occluded within a cylinder. Ontologically, the pressure in Boyle's law does not refer to external pressure because external pressure can even be generated by a solid but not by a gas.Committing this mistake would be like considering the volume of a standard pipette to be the cause of the increased absorbance in the Lambert–Beer law:
| A = ∈lc |
In this expression, A is the absorbance generated, which is directly proportional to the concentration C of a chromophore in a solution. Undoubtedly, to prepare a solution with concentration C, a pipette will be used several times to take an aliquot of a standard solution, but this procedure is an experimental procedure and not a causal process because it could be performed in another way.
It should also be understood that the determinism connecting the cause and effect cannot be explained or based outside of the framework of a theory. It is impossible to explain or form a basis of Boyle's law outside the framework of the kinetic theory of gases. Without kinetic theory, we can only describe Boyle's law but cannot explain its causal relationships; therefore, we run the risk of incorrectly assigning its causes and effects.
General chemistry textbook analysis for the presentation of Boyle's law
The evaluation of general chemistry textbooks provides an overview of what the textbooks' authors consider to be essential for teaching this topic. This study evaluated ontological, epistemological and didactic aspects of textbooks relative to Boyle's law. Our first analysis started with a general didactic image analysis. The general chemistry textbooks selected for our study are described in Table 2.| Textbook | Editorial | Author | Year | Pages | ID |
|---|---|---|---|---|---|
| Química general 5 edn | McGraw-Hill | Ebbing, D. | 1998 | 183–185 | L1 |
| Química 7 edn | McGraw-Hill | Chang, R., College, W. | 2002 | 159–162 | L2 |
| Química 9 edn | McGraw-Hill | Chang, R. | 2007 | 177–178 | L3 |
| Principios de química 3 edn | Panamericana | Atkins, P., Jones, L. | 2006 | 131–132 | L4 |
| Principios de química 5 edn | Panamericana | Atkins, P., Jones, L. | 2012 | 134–139 | L5 |
| Chemistry & Chemical Reactivity 2 edn | Harcourt college Pub | Kotz, J., Purcell | 1991 | 458–460 | L6 |
| Química y reactividad Química 5 edn | Thomson international | Kotz, J., Treichel, P. | 2003 | 475–477 | L7 |
| Química y reactividad Química 6 edn | Cengage learning | Kotz, J., Treichel, P., Weaver, G. | 2005 | 475–477 | L8 |
| Conceptos básicos de Química | Compañía editorial continental | Sherman, A., Sherman, S., Russikokk, L. | 1999 | 275–276 | L9 |
| Química general | McGraw-Hill | Longo, F. | 1975 | 105–104 | L10 |
| Química general 3 edn | McGraw-Hill | Whitten, K., Gailey, K., Davis, R. | 1998 | 264–265 | L11 |
| Química general 5 edn | McGraw-Hill | Whitten, K., Gailey, K., Davis, R. | 1999 | 395–397 | L12 |
| Química general 8 edn | Cengage learning | Whitten, K., Davis, R., Peck, M., Stanley, G. | 2008 | 404–406 | L13 |
| Química general 8 edn | Prentice hall | Petrucci, R., Harwood, W., Herring, F. | 2003 | 181–183 | L14 |
General chemistry images in books: didactic analysis
It is an empirical fact that general chemistry textbooks require considerable space for illustrations. This work addresses questions such as the following. How are verbal and visual information correlated? Do illustrations facilitate the understanding and recall of information? Do illustrations provide support for the logical construction of a law or do they reaffirm errors? What use is made of illustrations in textbooks? What types of references exist between the text and illustrations? The texts analyzed were selected according to the Niaz and Fernandez criteria (Niaz and Fernández, 2008). Availability of textbooks in our university or nearby libraries;(a) Inclusion of recent textbooks;
(b) Inclusion of textbooks with various editions, demonstrating their acceptance by the science education community;
(c) Consultations with colleagues in different parts of the world, revealing that the various textbooks selected for this study are used for translations; and
(d) Various studies published in science education journals using these textbooks.
(1) Evocation. Reference is made to a fact of everyday experience or a concept that is assumed to be known by the student.
(2) Definition. The meaning of a new term is introduced in its theoretical context.
(3) Application. An example is given that extends and consolidates a definition.
(4) Description. The description refers to events or everyday circumstances that are assumed to be known to the reader and that provide a necessary context.
(5) Interpretation. Explanatory passages are provided in which theoretical concepts are used to describe the relationship between experimental events.
(6) Problematization. There are no rhetorical questions that cannot be solved with the concepts already defined.
(7) Inoperative. The text does not provide any figures that can be used to apply knowledge but must merely be observed.
(8) Elementary operation. The text contains elements of universal representation, sketches, dimensions, etc.
(9) Syntactic. The text contains elements, the use of which requires knowledge of specific rules, vectors, electric circuits, etc.
(10) Connotative. The text describes the contents without mentioning their correspondence to the elements included in the illustration. These relationships are considered to be obvious to the reader.
(11) Denotative. The text establishes the correspondence between the elements of illustration and the content represented.
(12) Synoptic. The text describes the correspondence between the elements of illustration and the represented content and establishes the conditions under which the relationships between the elements included in the figure represent the relationships between the content so that the image and text form an indivisible unit.
(13) No tags. The illustration does not contain any text.
(14) Nominative. There are letters or words that identify some of the elements of the illustration.
(15) Relational. Text is provided that describes the relationships between the elements of the illustration. An example would be an explanation of a drawing from the previous page.
Scores were defined according to Niaz and Maza (2011) (Niaz and Maza, 2011).
(a) Satisfactory (S): Treatment of the subject in the textbook is considered to be satisfactory if the criteria are described and examples are provided to illustrate various topics.
(b) Mention (M): A simple mention of the criteria with little elaboration and no examples.
(c) No mention (N): No mention of the issues involved in the criteria, constituting an ‘uninformed’ perspective.
Textbooks were scored according to the following criteria: S = 2 points; M = 1 point; and N = 0 point. The evaluation of the illustrations of 14 textbooks, according to the criteria and scores previously described, is presented in Table 3.
| No. | Functiona | Functionalitya | Relationshipa | Verbal labela | Pointsb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||
| a Criteria. (1) Evocation. Reference is made to a fact of everyday experience or a concept that is assumed to be known by the student. (2) Definition. The meaning of a new term is introduced in its theoretical context. (3) Application. An example is given that extends and consolidates a definition. (4) Description. The description refers to events or everyday circumstances that are assumed to be known to the reader and that provide a necessary context. (5) Interpretation. Explanatory passages are provided in which theoretical concepts are used to describe the relationship between experimental events. (6) Problematization. There are no rhetorical questions that cannot be solved with the concepts already defined. (7) Inoperative. The text does not provide any usable item, and we can only observe. (8) Elementary operation. The text contains elements of universal representation, sketches, dimensions, etc. (9) Syntactic. The text contains elements, the use of which requires knowledge of specific rules, vectors, electric circuits, etc. (10) Connotative. The text describes the contents without mentioning their correspondence to the elements included in the illustration. These relationships are considered to be obvious to the reader. (11) Denotative. The text establishes the correspondence between the elements of illustration and the content represented. (12) Synoptic. The text describes the correspondence between the elements of illustration and the represented content and establishes the conditions under which the relationships between the elements included in the figure represent the relationships between the content so that the image and text form an indivisible unit. (13) No tags. The illustration does not contain any text. (14) Nominative. There are letters or words that identify some of the elements of the illustration. (15) Relational. Text is provided that describes the relationships between the elements of the illustration. An example would be an explanation of a drawing from the previous page. b Points: S = 2, M = 1, N = 0. | ||||||||||||||||
| L1 | M | S | M | N | M | N | N | S | S | N | S | N | N | S | N | 13 |
| L2 | N | N | S | S | M | N | N | S | S | M | M | N | N | S | N | 13 |
| L3 | N | N | S | S | M | N | N | S | S | M | M | N | N | S | N | 13 |
| L4 | N | S | M | S | S | N | N | S | S | N | S | S | N | S | S | 19 |
| L5 | N | S | M | S | S | N | N | S | S | N | S | S | N | S | S | 19 |
| L6 | M | N | M | N | N | M | N | M | N | M | S | S | N | S | S | 13 |
| L7 | S | N | M | N | N | M | N | M | N | M | S | S | N | S | S | 14 |
| L8 | S | N | M | N | N | M | N | M | N | M | S | S | N | S | S | 14 |
| L9 | N | N | N | N | N | N | S | N | N | S | N | N | N | M | N | 5 |
| L10 | N | M | M | N | N | N | M | M | N | N | M | M | S | M | N | 9 |
| L11 | N | S | M | M | M | N | N | S | M | N | S | S | N | S | S | 16 |
| L12 | S | S | M | M | M | N | N | S | M | N | S | S | N | S | S | 18 |
| L13 | S | S | M | M | M | N | N | S | M | N | S | S | N | S | S | 18 |
| L14 | N | S | M | M | M | N | N | S | M | N | S | S | N | S | S | 16 |
According to the criteria and score classification previously described, each criterion was studied individually and quantitatively. The results are given in Table 4.
| Criteria | Classificationa | |||||
|---|---|---|---|---|---|---|
| N (%) | M (%) | S (%) | ||||
| a Classification: S, Satisfactory; M, mention; N, no mention. | ||||||
| 1 | 8 | (57.1) | 2 | (14.3) | 4 | (28.6) |
| 2 | 6 | (42.9) | 1 | (7.1) | 7 | (50.0) |
| 3 | 1 | (7.1) | 11 | (78.6) | 2 | (14.3) |
| 4 | 6 | (42.9) | 4 | (28.6) | 4 | (28.6) |
| 5 | 5 | (35.7) | 7 | (50.0) | 2 | (14.3) |
| 6 | 11 | (78.6) | 3 | (21.4) | 0 | (0.0) |
| 7 | 12 | (85.7) | 1 | (7.1) | 1 | (7.1) |
| 8 | 1 | (7.1) | 4 | (28.6) | 9 | (64.3) |
| 9 | 5 | (35.7) | 4 | (28.6) | 5 | (35.7) |
| 10 | 8 | (57.1) | 5 | (35.7) | 1 | (7.1) |
| 11 | 1 | (7.1) | 3 | (21.4) | 10 | (71.4) |
| 12 | 4 | (28.6) | 1 | (7.1) | 9 | (64.3) |
| 13 | 13 | (92.9) | 0 | (0.0) | 1 | (7.1) |
| 14 | 0 | (0.0) | 2 | (14.3) | 12 | (85.7) |
| 15 | 5 | (35.7) | 0 | (0.0) | 9 | (64.3) |
Philosophical errors in the presentation of Boyle's law
The difficulties in the presentation of Boyle's law arise not only from didactic problems but also from philosophical problems. These problems are related to how the law is presented, the pattern of causality that is presented, the law's ontological reference and the law's explanatory mechanism. Fig. 5 shows a chart of the types of errors in the presentation of Boyle's law, applying our instrument presented in Fig. 1. A more detailed explanation of the types of errors in the presentation of Boyle's law is presented in Table 5.| Error type | Description |
|---|---|
| E1 | This error is epistemological: it arises when Boyle's law is presented as a correlation of data obtained by measurements of pressure and volume. The origin of this error is an empirical conception of a scientific law resulting from an inductive process. |
| E2 | This error is semantic: it arises when Boyle's law is presented as a mere relationship of proportionality between two variables, without any reference to natural variables. This presentation is based on the conception of a scientific law as a mere mathematical expression. |
| E3 | This error involves determinism: it arises when Boyle's law is presented as a relationship of volume and pressure but without specifying which is the cause and which is the effect. This error is generated by ignoring the deterministic nature of natural laws. |
| E4 | This error involves mechanism: it arises by omission or ignorance of the foundation of the causal mechanism of the atomic/molecular collision, which connects a smaller gas volume to a greater probability of atomic/molecular collision with the internal wall, generating higher gas pressure as a final effect. |
The chart in Fig. 5 was applied to the analysis of the 14 general chemistry textbooks described in Table 1. The results for the detection of errors in the texts and illustrations of the textbooks are shown in Table 6.
| No. | Error | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| T = Text error; I = Illustration error. | ||||
| 1 | TI | TI | ||
| 2 | T | TI | ||
| 3 | TI | |||
| 4 | I | |||
| 5 | T | TI | ||
| 6 | T | TI | ||
| 7 | T | TI | ||
| 8 | T | TI | ||
| 9 | TI | |||
| 10 | I | TI | TI | |
| 11 | I | TI | TI | |
| 12 | TI | TI | TI | |
| 13 | TI | TI | TI | |
| 14 | T | TI | ||
L1: The text presents a title of “empirical gas law”, reflecting an inductive definition of the concept of a law (E1). Subsequently, the text states, “The volume of a gas at a given temperature varies inversely with the pressure applied”, which refers to the concept of a gas compression mechanism. Thus, in this text, the external pressure is presented as the cause and the volume of gas is presented as the effect, without any reference to particle collision mechanisms (E4).
Fig. 5.6 in L1 is consistent with the errors in the text presenting the volume as the dependent variable (effect) and the pressure on the gas as the independent variable (cause) and thus disconnected from the theory and mechanism of particle collision (E4). Moreover, the text presents Boyle's law as a correlation of measured values of pressure and volume, according to the empiricist view of a law (E1).
L2: The text includes the following sentence: “The pressure of a fixed amount of a gas held at constant temperature is inversely proportional to the volume of gas”, which communicates a relationship of variables with no pattern of causality (E3), and with no explanatory mechanism (E4).
Fig. 5.6 in L2 highlights the pressure on the plunger with a large arrow (compression). However, no reference is made to the gas pressure exerted on the wall, so the mechanism of particle collisions is not present (E4).
In Fig. 5.7 in L2, the P vs. V graphic is shown. In this graphic, the gas pressure (P) is the dependent variable and the gas volume is the independent variable, in agreement with Boyle's law. However, in the label of the same figure, the mechanism erroneously refers to compressibility, as indicated by pressure P exerted on the gas labeled as the cause and gas volume V as the effect, so the compression mechanism is used instead of the collision mechanism (E4).
L3 is the newest edition of L2.
The text includes the following sentence: “Insofar as the pressure increases, the volume occupied by the gas decreases. Conversely, if the applied pressure decreases, the volume occupied by the gas increases”. This explanation represents a type 4 error (E4) because it communicates a pattern of causality but ignores the particle collision mechanism. Moreover, the compressibility mechanism is considered, so the causality pattern refers to the pressure exerted on the piston instead of the gas volume as the cause.
The following can also be found in the text:
“The pressure of a fixed amount of gas at constant temperature is inversely proportional to the volume of gas”. Here, Boyle's law is presented as a relationship of variables but with no pattern of causality (E3) and without any reference to a mechanism of collision (E4).
The presentation of figures was unchanged from that of the previous edition a (L2), so the errors were exactly the same (E4).
L4: In Atkins' third Spanish edition, no errors could be found in the text, which clearly differentiated gas pressure relative to the pressure exerted on the plunger, assigned volume as the cause and gas pressure as the effect and described the law using the mechanism of molecular collision. It established the correct connection between the compression experiments that Boyle performed with gas with the ultimate effect of increasing the gas pressure.
The text includes the following sentence “The pressure of a given amount of gas at constant temperature is inversely proportional to the volume which is confined”. This sentence states the proportionality between variables, which could be considered a type 3 error. However, in the text, the incomplete definition was completed according to Boyle's law as follows:
“The law implies that if we compress a constant amount of gas at constant temperature to half its volume, for example, 0.5 L to 1 L, gas pressure will double”. Establishing the mechanism properly without errors, according to the correct pattern of causality of Boyle's law, finally connected the law to the particle collision mechanism.
“Our molecular model is compatible with Boyle's law. When a gas is compressed, its molecules are confined to a smaller volume. The volume of container means more and more molecules collide with the same area of the walls in a given time interval”. In this text, all of the requirements for presenting a scientific law were fulfilled according to our proposed instrument.
Fig. 4.10 in L4 presents the appropriate reference to the gas pressure on the inner wall of the piston, with an arrow clearly indicating gas particles colliding with the inner wall. However, in Fig. 4.8, the explanation became incorrect, stating that “Boyle's Law expresses the effect of pressure on the volume” (E4), attributing inverse causality. Then, it is stated that “when the pressure of a gas sample increases, the volume decreases”, which makes the same error (E4).
L5: Inexplicably, in the fifth edition of Atkins (2012), all explanatory mechanisms are eliminated and Boyle's law is presented as neither a simple relationship of variables without specifying a pattern of causality (E3) nor an explanatory mechanism of particle collision (E4).
The figures present the same problem as in the previous edition. The P vs. V graphic axis refers to pressure as the dependent variable and volume as the independent variable. However, the legend of the figure refers to gas compression and external pressure and not to gas pressure (E4).
L6: In Kotz (1991), Boyle's law is presented without a pattern of causality; for example, the text includes the following sentence: “the pressure and the volume are inversely proportional”, which does not express a causal pattern (E3). In contrast, it states that “the volume of a confined gas is inversely proportional to the pressure exerted on the gas”, which conveys the compressibility mechanism instead of the particle collision mechanism (E4).
The legend for Fig. 12.2 in L6 is misleading because it states that “a syringe containing some air was sealed and then a lead shot was added to the baker at the top of the barrel. As the mass of lead increased, thereby increasing the pressure on the air in the syringe, the air was compressed”. Here, we have a presentation of the mechanism of gas compressibility, but it is still an incomplete presentation of Boyle's law because the mechanism does not end with the gas pressure increase.
The graph associated with Fig. 12.2 in L6 is misleading because volume (1/V) is related to the y axis and with the dependent variable, the effect, contrary to Boyle's law, in which it is the cause (E4).
Fig. 12.3 in L6 uses the example of marshmallows: “some marshmallows are placed in a flask. The air is withdrawn from the flask using a vacuum pump”. The explanation for the relationship expressed in Fig. 12.3 is as follows: “the air in the marshmallows expands as the pressure lowered, causing the marshmallows to expand”.
One of the interesting aspects of this explanation is that it does not refer to the air pressure inside the marshmallow but refers to the pressure outside the marshmallow. Clearly, after a vacuum is created, the pressure outside the marshmallow becomes less than the air pressure inside the marshmallow. In this manner, the mechanism involves the marshmallow volume increasing (cause) until its internal pressure equals the external pressure (effect). This mechanism is not considered in the figure (E4).
L7: In Kotz (2005), it is stated that “The volume of a fixed amount of gas at given temperature is inversely proportional to the pressure of the gas”. Here, Boyle's law is presented as a relationship of variables with no pattern of causality (E3) or explanatory mechanism (E4). However, in a second phrase, it states that “the gas volume decreases when the pressure increases”. In this sentence, Boyle's law is presented as a pattern of causality, but the pressure is not specified; instead, the sentence refers to the pressure exerted on the piston or the pressure of the gas (E4).
The text presents 3 figures. The first, Fig. 12.2 in L7, depicts the process of inflating a bicycle with a hand pump. The text states that in this case, the pressure increase is perceived by pushing the plunger pump. It is interesting to note that it is understood that the net effect is an increase in the gas pressure exerted on the piston. In this respect, the pattern of causality is correct, although no explanatory mechanism is given (E4).
As in the third edition of text L6, the marshmallow in Fig. 12.4 and the modification of the syringe of Fig. 12.3 are exploited, but this time, the action of the weight of lead is replaced by the action of blowing air into tires with a pump using a flask containing a syringe. It is interesting to note that both figures can be misleading because the external air present in the flask, which contains the syringe or marshmallow, does not follow Boyle's law, and the reason is very simple: the amount of outside air is variable and is not constant as Boyle's law states for particle mechanism collision (E4).
L8: In the sixth edition of Kotz, there were no changes from the fifth edition (L7) and Boyle's law was presented with the same text, the same figures and therefore the same mistakes as those of the text (E3 and E4) and figures (E4) of L7.
L9: The text includes the following sentence: “The volume of gas increases as the pressure thereon decreases, if the temperature remains constant”. This sentence expresses a pattern of causality, but the pressure reference is to the outer pressure (compression pressure) and not to the gas pressure, which is contrary to the particle collision mechanism (E4).
Fig. 13.3 in L9 shows the historical figure of Boyle's experiment of the column tube filled with liquid mercury. In this figure, the compression process of the occluded gas, as an effect of atmospheric pressure plus the pressure of the mercury gas, is indicated. Therefore, the physical compression process is used again but the internal gas particle collision mechanism is not used to explain Boyle's law (E4).
L10: The text includes the following sentence: “The product of pressure and volume of a given mass of gas is constant, if the gas temperature is maintained constant.” This sentence provides a list of natural variables, but no causality pattern is indicated, ignoring the implicit determinism of any law (E3) and any reference to the mechanism of molecular collisions (E4).
Fig. 6.2 in L10 shows the scheme of the piston compressing a gas by different masses acting on it. A positive aspect of the figure is that the pressures are referred to as internal gas, so no error was detected at this point.
Then, Fig. 6.2 shows a graph in which the y axis is the product of P × V and the x axis is P measured for one mole of water at different temperatures, showing that the slope is zero.
This figure shows a purely graphical view of measured values to present Boyle's law (E1). Moreover, the y axis is arbitrarily chosen as the product of P and V, with no reference to either a pattern of causality (E3) or an explanatory mechanism (E4).
L11: In this text (third edition), Boyle's law is presented as follows “At constant temperature, the volume V which occupies a given mass of gas is inversely proportional to the applied pressure P”. Here, Boyle's law is presented as a proportionality relationship with no causality pattern (E3). In contrast, it refers to the pressure of the compression mechanism but not to the gas pressure associated with the mechanism of particle collisions (E4). Another phrase found in this text is as follows:
“At a given temperature the product of pressure and volume of a gas is constant”. Here, a pattern of causality is not specified and Boyle's law is presented as a proportionality relationship between gas volume and pressure rather than as a law (E3).
Fig. 10-3 in L11 is misleading because it is presents pressure as an independent variable on the x axis and gas volume as a dependent variable, consistent with the concept of external pressure and the compressibility process but not with Boyle's law, in which the gas pressure is the effect and therefore the dependent variable (E4). Moreover, the figure presents Boyle's law as a graphical correlation of experimental points (E1) unrelated to any pattern of causality (E3).
L12: In this fifth edition, the book presented more errors because all references to a pattern of causality were removed (E3), presenting Boyle's law as a relationship of measured values of pressure and volume (E1). This error is demonstrated in the following phrase found in the text:
“…for a given sample gas at constant temperature, the product of the pressure and the volume P × V always gave the same number”; clearly, no explanatory mechanism is presented (E4).
Fig. 10-3 in L12 is exactly the same as in the third edition of L11, so the same errors can be found (E1; E3 and E4).
L13: In its eighth edition, this book presents Boyle's law in the same way as in the fifth edition of L13 regarding the use of the text and figures, so the same errors found in the fifth edition of L13 were detected (E1; E3 and E4).
L14: Boyle's law is presented as a relationship between gas pressure and gas volume, without a specification of the pattern of causality because it does not indicate which is the cause and which is the effect. This error can be found in this sentence from the book: “for a certain amount of gas at constant temperature, the volume of gas is inversely proportional to its pressure”. The text does not refer to the particle collision mechanism (E4).
The graphic in Fig. 6.6 shows pressure as the dependent variable (y) and volume on the x axis as the independent variable, which agrees with the presentation of Boyle's law. However, the label of the figure changes the causality pattern as follows: “If the temperature and quantity of a gas remain constant, the gas volume is inversely proportional to pressure: If the gas pressure is doubled, the volume is reduced to half the initial value” (E4), which refers to the compression mechanism instead of the collision mechanism (E4).
The results in Table 5 show that in university chemistry textbooks, the most frequent error in the presentation of Boyle's law is disconnection from the particle collision mechanism (E4). It is impossible to establish the correct causal relationship between volumes of gas (V) as the cause and gas pressure (P) as the effect without considering the particle collision mechanism, which is microscopic. For this reason, most of the texts present Boyle's law under the compression mechanism, which is macroscopic, mainly referring to pressure on a piston as a cause rather than the pressure exerted by the gas as an effect, or have removed claims of causal relationships or simply present the pressure–volume relationship as a mathematical proportionality.
Regarding type one errors associated with an empiricist conception of Boyle's law, these errors were not as widespread in the texts. From an ontological point of view, Boyle's law is not presented as an abstract proportionality in any of the texts. All of the texts referred to x and y as natural variables, volume and pressure, so no type 2 errors were present in any of the texts. Thus, we can see that the problem for the presentation of Boyle's law is deterministic rather than ontological.
We can see from Table 5 that the errors that occurred in the texts tended to be the same as those presented in the figures and that the presentation of Boyle's law was deficient in most of the texts analyzed, except for L3.
Regarding the change in the presentation of Boyle's law through editions of the same text, the tendency is to repeat the same mistakes. For example, over the evolution of Chang's books (L2 and L3), Kotz's books (L6 and L8) and Whitten's books (L11 and L14), errors have been relatively constant through the editions. The only exception is Atkins' book, which introduced more serious mistakes in its fifth edition than in its third edition.
Finally, it is clear that most of the figures are functional to the text and contextualize variables in a theoretical framework. However, all of the contexts and examples indicate the macroscopic–symbolic connection, ignoring the connection with the microscopic level, which is closely related to the type 4 error, indicating disconnection with the underlying microscopic particle collision mechanism.
Conclusions
In the context of Boyle's law and its presentation in textbooks, based on our results we can conclude the following.• The epistemological and ontological account of what a scientific law represents is a complex issue that requires clarification for the correct presentation of Boyle's law or any other natural science law. In this work, we have developed a tool for detecting errors or omissions in the presentation natural laws applied to the Boyle's law presentation based on the philosophical system of Mario Bunge's scientific realism.
• Most of the general chemistry textbooks analyzed here contained several errors in their texts and illustrations. When applying the developed tool, we were able to detect errors of the following types: epistemological (confusion between law and correlation and between cause and effect) and ontological (confusion between internal gas pressure and the external pressure exerted on the piston).
• It is necessary to relate Boyle's law to the context of the kinetic theory of gases to identify correctly the cause, which is the gas volume, and the effect, which is the internal gas pressure. In this case, most textbooks are disconnected from this theory. This disconnection from the kinetic theory of gases generates an incorrect pattern of causality between gas pressure and gas volume.
• The connection between gas volume as the cause and gas pressure as the effect requires knowledge of the microscopic mechanism of collision. The microscopic mechanism of particle collisions is fundamental to understanding the origin of a gas's internal pressure and its final macroscopic effect and to be able to differentiate between the pressure of the gas and the external pressure exerted on the system. Confusion between compression vs. collision mechanisms is the most common error in the presentation of Boyle's law. We can conclude that the source of errors in the presentation of Boyle's law in most of the texts analyzed was disconnection from the microscopic mechanisms, in which multiple representations, such as in figures and tables, reinforce this error.
According to our analysis, we can say that the introduction of a law in textbooks required specifying the pattern of causality and the underlying mechanism to explain the variable causes and effects. This requirement applies to mathematized laws, such as the Lambert–Beer law, as well as non-mathematized laws, such as stochastic laws like Mendel's segregation laws. We believe that further study of general chemistry textbooks at the secondary school and university levels should be conducted with regard to the presentation of these and other laws, such as Charles' law, the laws of multiple and definite proportions, the law of mass conservation, and the laws of classical thermodynamics and so on.
Finally, regarding multiple representations of research into the presentation of scientific laws, based on our results, we can advise the following: the multiple representations of the presentation of scientific laws must consider the mechanisms that connect causes with effects, with special attention paid to the mechanisms that originate at the microscopic level, which are the majority mechanisms of chemistry laws. Exclusively emphasizing past experiences or known phenomena can be counterproductive to laws related to microscopic mechanisms because humans experience only the macroscopic level.
Other questions remaining for the continuation of this line of research include the following. What are the origins of these errors and omissions in the presentation of Boyle's law? Are there bad translations or alternative conceptions? What effects arise from these errors and omissions among the students using these texts? The application of scientific realism as a philosophical system appears to be a promising framework for addressing these questions.
Acknowledgements
Scientific product derived from FONDECYT research 1150659. The authors gratefully acknowledge the sponsor from CONICYT – CHILE.References
- Abd-El-Khalick F., Waters M. and Le A. P., (2008), Representations of nature of science in high school chemistry textbooks over the past four decades, J. Res. Sci. Teach., 45, 835–855.
- Arellano M., Jara R. and Merino C., (2014), Macroscopic, submicroscopic and symbolic representations of matter, Educ. Quim., 25, 46–55.
- Bunge M., (1974a), Semantics II: Interpretation and Truth: Semantics II: Interpretation and Truth, Springer.
- Bunge M., (1974b), Treatise on Basic Philosophy: Semantics I: Sense and Reference, Netherlands: Springer.
- Bunge M., (1979), Treatise on Basic Philosophy: Ontology II, Netherlands: Springer.
- Bunge M., (1983a), Epistemology & Methodology I: Epistemology & Methodology I: Exploring the World, Netherlands: Springer.
- Bunge M., (1983b), Treatise on Basic Philosophy: Volume 6: Epistemology & Methodology II: Understanding the World, Netherlands: Springer.
- Bunge M. A., (1985), Treatise on Basic Philosophy, Netherlands: Springer.
- Bunge M. A., (1989), Treatise on basic philosophy, Dordrecht: D. Reidel.
- Bunge M., (1997), Epistemología, México: Siglo Veintiuno.
- Bunge M., (2000), La investigación científica: su estrategia y su filosofía, México: Siglo Veintiuno.
- Bunge M., (2012), Causality and Modern Science, New York: Third Revised Edition Dover Publications.
- Coll R. K., Ali S., Bonato J. and Rohindra D., (2006), Investigating first-year chemistry learning difficulties in the South Pacific: A case study from Fiji, Int. J. Sci. Math. Educ., 4, 365–390.
- de Berg K. C., (1995), Revisiting the pressure-volume law in history-what can it teach us about the emergence of mathematical relationships in science?, Sci. Educ., 4, 47–64.
- Faikhamta C., (2013), The Development of In-Service Science Teachers' Understandings of and Orientations to Teaching the Nature of Science within a PCK-Based NOS Course, Res. Sci. Educ., 43, 847–869.
- Harrison A. and Treagust D., (2003), in Chemical Education: Towards Research-based Practice, ed. Gilbert J., De Jong O., Justi R., Treagust D. and Van Driel J., Netherlands: Springer, vol. 17, ch. 9, pp. 189–212.
- Kang H., Shin S. and Noh T., (2008), Exploring the causes of students' connecting errors induced in learning Boyle's law and Charles's law with multiple external representations, Journal of the Korean Chemical Society, 52, 550–560.
- Lederman N. G., Abd-El-Khalick F., Bell R. L. and Schwartz R. S., (2002), Views of Nature of Science Questionnaire: Toward Valid and Meaningful Assessment of Learners' Conceptions of Nature of Science, J. Res. Sci. Teach., 39, 497–521.
- Niaz M. and Fernández R., (2008), Understanding quantum numbers in general chemistry textbooks, Int. J. Sci. Educ., 30, 869–901.
- Niaz M. and Maza A., (2011), Nature of Science in General Chemistry Textbooks, Springer.
- Perales J. and Jiménez J. D., (2002), Las ilustraciones en la enseñanza-aprendizaje de las ciencias. Análisis de libros de texto, Enseñanza de las Ciencias, 20, 369–386.
- Popper K. R., (2008), La lógica de la investigación científica, Editorial Tecnos.
- Sharma B. D., (1982), Boyle's law – a different view, J. Chem. Educ., 59, 827.
- Siddiquee M. N. E. A. and Ikeda H., (2012), Science teachers' views on nature of science: a case of bangladeshi secondary schools, Chemistry, 21, 865–887.
- Smith B. L., (1971), The inert gases: model systems for science, Wykeham.
- Talanquer V., (2011), Macro, submicro, and symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33, 179–195.
- West J. B., (1999), The original presentation of Boyle's law, J. Appl. Physiol., 87, 1543–1545.
| This journal is © The Royal Society of Chemistry 2015 |