Examining chemistry teachers' use of curriculum materials: in view of teachers' pedagogical content knowledge
Bo
Chen
* and
Bing
Wei
Faculty of Education, University of Macau, Macao. E-mail: njcb0128@aliyun.com
First published on 6th January 2015
Abstract
This paper aimed to explore how pedagogical content knowledge (PCK) of teachers influenced their adaptations of the curriculum materials of the new senior secondary chemistry curriculum, a standards-based science curriculum, in China. This study was based on the premise that the interaction of the teacher with the curriculum materials determines what happens in the classroom. An interpretive approach was adopted and five experienced chemistry teachers in four senior secondary schools participated in this study. Classroom observations and interviews were used as research methods. The data analysis revealed that there were four components of PCK that led to teachers' adaptations of curriculum materials and they were knowledge of students' understanding of science, knowledge of assessment in science, knowledge of instructional strategies, and knowledge of science curriculum. Among these four components of PCK, knowledge of students' understanding of science and knowledge of assessment in science were the factors that most influenced teachers' adaptations of curriculum materials. Furthermore, the ways that these four components of PCK influenced teachers' adaptations were different. The implications of the findings and the suggestions for the further studies were discussed in the last section of this paper.
Introduction
Since the late 1980s, science education at the school level has come to a new era with the documents of the standards-based science curriculum reform released (e.g., AAAS, 1989; NRC, 1996). In these documents, scientific literacy is claimed to be the central goal of science education. Initiated by the movement of scientific literacy, a large number of new curriculum materials have been developed around the world (Powell and Anderson, 2002). These curriculum materials are usually thought of as being standards-based, which means that they include inquiry as a part of science content, encourage a constructivist and student-centered approach to learning, and require long-term professional development for sustainable implementation (Powell and Anderson, 2002).Following the worldwide tendency, the latest round of science curriculum reform was initiated ten years ago in China (Wei, 2010). As part of science curriculum reform, the official chemistry curricula were established with the national standards of chemistry curriculum at the stages of junior and senior secondary schools promulgated by the Ministry of Education (MoE) in 2001 and 2003 respectively. Subsequently, new chemistry textbooks, aligned with these curriculum standards, have been published and put into use. Researchers have found that these curriculum standards and chemistry textbooks in China exhibit new features that are akin to those of the standards-based science curriculum materials (Wei and Thomas, 2006; Gao, 2007). Based on these studies, it could be concluded that the current chemistry textbooks in China have been transformed into the new type of curriculum materials.
In general, there exists a basic assumption that the standards-based approach to science education ensures the alignment among instruction, assessment, and the content standards in order to create equal opportunities for students to achieve expected learning outcomes (Herman and Webb, 2007). However, since the standards-based science curriculum requires the teachers to play a substantially different role in the classroom, to develop new knowledge and to change their original beliefs, most of the teachers are reluctant to implement this kind of curriculum in their classrooms (Aikenhead, 2006). As such, the current situation is that although the standards-based science curriculum materials are available, teachers are used to adapting these materials to some degree in the process of teaching (e.g., Schneider et al., 2005; Vos et al., 2011). As we know, it is generally agreed that teachers' actions in practice will be determined to a large extent by their pedagogical content knowledge (PCK) (Van Driel et al., 1998; Magnusson et al., 1999). Therefore, it can be inferred that teachers' PCK should be an important factor that leads to teachers' adaptations of curriculum materials. However, little international literature has examined teachers' adaptations of the standard-based curriculum materials in the view of teachers' PCK, especially in the countries like China where the centralized curriculum system is prevalent. In this paper, we are interested in exploring how teachers' PCK influences the enactment of the new curriculum materials in the classroom in China. Given that the component of PCK is complex and multiple (Grossman, 1990; Magnusson et al., 1999), the specific research questions of this study are posed as follows: (1) What components of PCK lead to teachers' adaptations of the standards-based senior secondary school chemistry curriculum materials? (2) What are the ways in which different components of PCK influence teachers' adaptations of the standards-based senior secondary school chemistry curriculum materials? And (3) which components of PCK most influence teachers' adaptations of the standards-based senior secondary school chemistry curriculum materials?
Literature review
Teachers and curriculum materials
Curriculum materials, which include instructional resources such as textbooks, teacher's guides, lesson plans, worksheet and other ancillary materials, are important resources upon which teachers rely to structure both their planned and enacted instruction (Powell and Anderson, 2002; Forbes and Davis, 2010). As we know, curriculum materials, particularly textbooks, have often determined the taught curriculum for many teachers, especially for young teachers (Ball and Feiman-Nemser, 1988; McNeil, 2003). Therefore, they have the potential to initiate and sustain reform in science education (Powell and Anderson, 2002; Aikenhead, 2006). However, curriculum materials themselves cannot generate changes in the classroom. It is dependent on teachers who can use them to enact changes in practice. This trait is called the ‘inert’ character of curriculum materials (Powell and Anderson, 2002). In this sense, while recognizing that curriculum materials provide no guarantee of actual change, Lloyd et al. (2009) argued that teachers are central players in the process of transforming curriculum ideas into reality. Based on this assumption, a growing body of literature on teachers' use of curriculum materials appears in recent years, especially in the field of mathematics education (e.g., Remillard et al., 2009).Curriculum materials use is different from curriculum materials implementation with the former emphasizing the two aspects: a teacher would not enact the curriculum precisely as envisioned by the designers and the process is not straightforward but involves substantial engagement, interpretation, and decision-making on the part of the teacher (Lloyd et al., 2009). That is to say, when employing the term of ‘curriculum materials use’, the subjectivity of teachers in the process of the enactment of curriculum materials is highlighted in a more intense way than using the term of ‘curriculum material implementation’. We take this point as the basic stance in this study. According to Brown (2009), the relationship between teachers and curriculum materials involves bi-directional influences in which curriculum materials influence teachers through their affordances and constraints, while teachers mobilize curriculum materials through their perceptions and decisions. Given the interactive relationship, Powell and Anderson (2002) argued that “the interaction of the teacher with the materials determines what happens in the classroom” (p. 112). In this study, we focus on the interaction between teachers and curriculum materials in the view of teachers' PCK. In the existing literature, while the impact of PCK on the adaptations of curriculum materials had been revealed (e.g., Brown, 2002; Bismack et al., 2014), what specific components of PCK influence adaptations and the ways that different components of PCK influence adaptations have not been well explored. This article attempted to bridge this research gap. We believe this exploration would be helpful to portray how teachers' PCK influences their adaptations of curriculum materials and inform teacher professional development on the use of the standards-based curriculum materials.
Pedagogical content knowledge
The concept of PCK was first introduced by Shulman in a paper in which he argued that PCK was a fundamental component of content-specific knowledge for teaching (Shulman, 1986). According to Shulman (1987), PCK is what makes possible the interpretations and transformations of disciplinary content into forms that are accessible and attainable by students. Shulman emphasized that PCK is a specific category of knowledge “which goes beyond knowledge of subject matter per se to the dimension of subject matter knowledge for teaching” (Shulman, 1986, p. 9). This means that PCK is a specialized knowledge for teaching that distinguishes teachers from subject matter specialists. The key elements in Shulman's conception of PCK are knowledge of representations of subject matter on the one hand and understanding of specific learning difficulties and student conceptions on the other hand. Elaborating on Shulman's work, other scholars have extended the concept by including in PCK some other categories of knowledge (e.g.Grossman, 1990; Cochran et al., 1993; Magnusson et al., 1999). As a result, the boundaries of PCK are blurry and what exactly PCK comprises is not always clear and consistent in the literature. Fortunately, as Van Driel et al. (1998) noted, while there is no universally accepted conceptualization of PCK, there appears to be some consensus on the nature of PCK. First, as PCK refers to particular topics, it is distinct from knowledge of pedagogy, of educational purposes, or of learner characteristics; second, since PCK concerns the teaching of particular topics, it is to be different considerably from subject matter knowledge (SMK); and third, PCK is rooted in the classroom practice of teachers, implying that prospective or novice teachers will have relatively undeveloped PCK.According to Gess-Newsome (1999), the varied models of PCK could be generally divided into two groups, namely, ‘transformative’ and ‘integrative’. A transformative model defines PCK as new knowledge arising from the act of transforming subject matter, pedagogical and contextual knowledge for the purpose of instructing students (e.g., Shulman, 1987; Magnusson et al., 1999). In contrast, an integrative model does not recognize PCK as a separate knowledge component: instead, PCK is the term used to describe teacher knowledge as a whole, comprising SMK, pedagogy and context (e.g., Cochran et al., 1993; Koballa et al., 1999). We endorse transformative PCK models in that they can provide a clearer statement about how PCK develops and seem to be most useful for science teacher education. Among the transformative models, the model of Magnusson et al. (1999) was proposed specifically for science teaching and has been successfully employed to examine science teachers' practices (e.g., Hanuscin et al., 2011). Abell (2008) also noted that the discrete components of PCK in the model of Magnusson et al. (1999) can serve as useful tools for researchers. Based on this consideration, in the present study, we adopt the model of PCK of Magnusson et al. (1999) that consists of five components: (a) orientations toward teaching science (knowledge and beliefs about the purposes for teaching science at a particular grade level), (b) knowledge of science curriculum (knowledge about mandated goals and objectives, and specific curricular programs and materials), (c) knowledge of students' understanding of science (knowledge about students' prior knowledge, requirements for student learning, and areas of student difficulty), (d) knowledge of assessment in science (knowledge about dimensions of science learning to assess and methods of assessment), (e) knowledge of instructional strategies (knowledge about specific strategies that are useful for helping students comprehend specific science concepts).
Framework for examining curriculum use
Goodlad (1979) categorized curriculum representations into ‘ideal curriculum’, ‘formal curriculum’, ‘perceived curriculum’, ‘operational curriculum’, ‘experiential curriculum’, and ‘attained curriculum’. These categories provide a clue in examining teachers' use of curriculum materials. According to this theory, curriculum materials belong to the ‘formal curriculum’, which should be transformed to the ‘experiential curriculum’ and the ‘attained curriculum’ of students through the ‘perceived curriculum’ and ‘operational curriculum’ of teachers. Based on Goodlad's classification of curriculum representations and Van Hiele (1986)'s distinguishing on three ‘levels of thinking and acting’, Vos et al. (2010) developed a framework to examine the use of innovative context-based teaching materials by teachers in classroom. This analytical framework consists of a nine cell matrix, with ‘intended curriculum’, ‘perceived curriculum’, and ‘operational curriculum’ as the rows, and with ‘theoretical level’, ‘descriptive level’ and ‘grounded level’ as the columns (Vos et al., 2010). The rows from the intended to operational curriculum represent a process in which what is intended by curriculum designers in curriculum materials is perceived by teachers and then manifested by them in their classrooms. The columns distinguish concrete teaching activities on the ground level from teaching–learning strategies on the descriptive level and aims and vision on the theoretical level. This framework has been successfully employed to identify the characteristics of the interaction between innovative context-based teaching materials and teachers that hindered or facilitated classroom implementation as intended by the designers (Vos et al., 2011).As mentioned earlier, this study is concerned with how teachers adapted the curriculum materials to meet the needs of their classes, that is, how the intended curriculum is manifested in the classroom. Hence, we focus on two types of curriculum representations, i.e., ‘intended curriculum’ and ‘operational curriculum’. For each curriculum representation, according to the framework of Vos et al. (2010), we focus on the three ‘levels of thinking and acting’, which are ‘teaching objectives’, ‘teaching strategies’, and ‘teaching activities’.
Research method
If research aims to investigate how the intended curriculum can be implemented, as suggested by Anderson and Helms (2001), then conducting research in school settings is necessary. This reason justifies why we conducted this study in four senior secondary schools in Nanjing, the capital of Jiangsu province in eastern China. The research paradigm taken in this study can be classified as the interpretive. According to Erickson (1986), the interpretive approach is appropriate when one wants to know more about the meaning-perspective of the particular actors in the particular events. In the present study, the main focus was on the meaning-perspectives of chemistry teachers in their adaptations of the standards-based curriculum materials. Therefore, the interpretive approach was adopted. Next, the context of the study, the participating teachers and the procedure for data collection and analysis are described in detail.Context
The senior secondary school chemistry curriculum in China comprises required and selective course modules. Required course modules consist of Chemistry 1 and Chemistry 2 (Chemistry 1 precedes Chemistry 2), which are required for all senior secondary school students. The six selective course modules are Chemistry and Daily Lives, Chemistry and Technology, Particulate Structure and Properties of Substance, Chemical Reaction Mechanism, Basic Organic Chemistry, and Experimental Chemistry, which are provided for students according to their needs and interests (MoE, 2003). In China, there is a legacy that reformed-based curriculum materials, especially textbooks and accompanying teachers' guides, are used as a mechanism for school curriculum reforms. The circle of chemistry education in China specially recognizes that textbooks can be seen as the substantiation of the curriculum, and the ideas of the new curriculum should be delivered to practicing teachers through textbooks (Wang, 2010). In practice, chemistry teachers heavily rely on textbooks to determine their teaching content and sequences. Therefore, in the whole process of curriculum reform, no effort has been spared to compile materials and to publish new textbooks. Up to now, there have been three series of senior secondary school chemistry textbooks, which were written according to the national standards of the senior secondary school chemistry curriculum (MoE, 2003), have passed the official examination, and are currently used in schools.As mentioned at the beginning of this paper, the national curriculum system is centralized in China. Prior to the science education reform starting in 2001, there was a one-standard and one-textbook system in which all teachers in the country were expected to follow the same student textbook and teachers' guide in their teaching. Although the current science education reform intends to change this system by making available multiple textbooks and teachers' guides based on the same curriculum standards (e.g., senior secondary school chemistry), each province (e.g., Jiangsu) adopts one textbook and its accompanying teachers' guide. Therefore, different from their counterparts in western countries, Chinese teachers may still attach an inappropriately much higher authoritative status to textbooks and teachers' guides. In this context, identifying how teachers adapt curriculum materials (i.e., textbook and teachers' guide) fills a gap in the literature on Chinese science teachers' uses of curriculum materials and can potentially inform teacher professional development on the use of curriculum materials in China.
In Nanjing, the series of chemistry textbooks published by the People's Education Press (PEP), which has been designed as the national education press to produce the syllabi and textbooks directly under the leadership of the MoE since the 1950s, is mandated in all senior secondary schools. This series comprises eight textbooks, each of them representing each of the eight curriculum modules. Units and sections constitute the main body of the textbooks. Each unit has three to six sections, which are the basic teaching units in class. Teaching a section usually takes one to three class sessions (40 minutes for each session). In most cases, some special columns, such as ‘experiments’, ‘inquiry activities’, ‘scientific perspectives’, and ‘history of chemistry’, are inserted in the texts. Main knowledge points are summed in the ‘summary of this unit’. The units end with student exercises. Each textbook is accompanied by one teacher's guide, which is organized in the same sequence as the textbooks. For each unit, the general status and function of this unit, the teaching objectives of this unit, and the time allocation for each section of this unit are provided. For each section, the specific status and the function of the section, and pedagogical suggestions are given. The chemistry textbooks and the accompanying teacher's guides constitute the curriculum materials in this study.
Participants
For the interpretive study, the major principle of sampling is maximizing the scope and range of information obtained (Lincoln and Guba, 1985). In order to meet this principle of sampling to some extent, we employed the sampling strategy of ‘maximum variation’ (Marshall and Rossman, 2006) to select the participants. That is to say, teachers are varied in terms of school type,† teaching experience, and gender. In addition, teachers are also different in the content they taught, e.g., the type of chemistry curriculum (compulsory or elective), and the nature of the content of the unit (theoretical or descriptive chemistry‡). Furthermore, given that experienced teachers usually have relative developed and rich PCK (Van Driel et al., 1998), the teachers whom we invited are all experienced ones with more than 10 years of teaching experiences. A total of eight chemistry teachers were contacted by the authors and five of them participated in the present study on a voluntary basis. The five teachers are from four middle schools, namely, Jiankang, Yingtian, Moling and Tianjing. These four schools are all public ones. Among the four schools, Jiankang and Yingtian are exemplary schools, and Moling and Tianjing are ordinary schools. Table 1 shows the demographic information for the five teachers.| Teacher name | School name | School type | Educational background | Teaching experience | Teaching grade |
|---|---|---|---|---|---|
| Note: both teachers' and schools' names are anonymous. | |||||
| Ms Ai | Jiankang | Exemplary | BS/chemistry education | 25 years | Grade 10 |
| Ms Chang | Yingtian | Exemplary | BS/chemistry education | 20 years | Grade 11 |
| Mr Dong | Moling | Ordinary | BS, ME/chemistry education | 14 years | Grade 10 |
| Mr En | Tianjing | Ordinary | BS, ME/chemistry education | 12 years | Grade 10 |
| Mr Fang | Tianjing | Ordinary | BS/chemistry education | 15 years | Grade 11 |
Data collection
For each teacher, the research was focused on a whole unit to obtain a relatively complete picture of the related curriculum materials and the teacher's practice. The details of the curriculum materials we observed for each teacher are shown in Table 2.| Unit | Sections | Module | |
|---|---|---|---|
| Ms Ai | Metals and their compounds | (1) Chemical properties of metals | Chemistry 1 |
| (2) Several important metallic compounds | |||
| (3) Metallic materials with wide usages | |||
| Ms Chang | Basic organic substances in lives | (1) Grease | Basic organic chemistry |
| (2) Saccharide | |||
| (3) Protein and nucleic acid | |||
| Mr Dong | Chemistry reactions and energy | (1) Chemical energy and thermal energy | Chemistry 2 |
| (2) Chemical energy and electric energy | |||
| (3) The rate and the limitation of chemical reaction | |||
| Mr En | Chemistry reactions and energy | (1) Chemical energy and thermal energy | Chemistry 2 |
| (2) Chemical energy and electric energy | |||
| (3) The rate and the limitation of chemical reaction | |||
| Mr Fang | Ionic equilibrium in aqueous solution | (1) The ionization of weak electrolytes | Chemical reaction mechanism |
| (2) Water ionization and the acidity and alkalinity of solution | |||
| (3) Hydrolysis of salts | |||
| (4) The dissolution equilibrium of insoluble electrolytes | |||
For each unit, the teaching objectives, teaching strategies in the teacher's guide, and the special column, ‘inquiry activity’, in the textbooks, were selected as the components of the intended curriculum. To find teachers' adaptations of the intended curriculum, and the various components of PCK that led to these adaptations, classroom observations and interviews were used as research methods. Each teacher was observed through the whole unit, which lasted approximately from two to four weeks. During each observation, the first author took field notes, recording what teaching strategies were used and how the special column, “inquiry activities”, was implemented in classroom. All the observations were also videotaped by using an electronic camera. In addition, semi-structured interviews were conducted after each section. The interviews were focused on these questions: (1) What were the teaching objectives the teacher set for the section? If they were different from those set in the teacher's guide, what were the reasons? (2) If the teaching strategies the teacher used were different from those set in the teacher's guide, what were the reasons? (3) When ‘inquiry activity’ is set in the textbook, but the actual procedures (experiments, activities) in classroom or lab were different from those in the textbook, what were the reasons? The interviews were audio-taped, and then transcribed into Chinese after the interview. The transcripts were returned to each of the teachers for their scrutiny, confirmation, or criticism. Overall, classroom observations were data sources of ‘teaching strategies’ and ‘teaching activities’ in teacher practices, and interviews were data sources of ‘teaching objectives’ in the operational curriculum and teachers’ explanations for adaptations.
Data analysis
In the present study, data analysis comprises two steps. In the first one, classroom observations and the interviews about teaching objectives were used as sources to compare with curriculum materials to identify teachers’ adaptations of the intended curricula at the three levels of ‘teaching objectives’, ‘teaching strategies’, and ‘teaching activities’. The analysis of this step provided the clues for us to investigate the factors that influenced teachers' adaptations of curriculum materials.In the second step, the interviews about teachers' explanations for adaptations were used as sources to trace the factors that led to teachers' adaptations of curriculum materials. Additionally, the data of classroom observations were used appropriately as supplements to verify teachers' interviews so as to enhance the validity of our analysis. It should be noted that not all of the interview data can be verified by the observational data. In this study, we used the data of classroom observations to triangulate with teachers' interview whenever possible. In qualitative research, the coding is usually grounded in the data (Glaser and Strauss, 1967). In this study, the coding was derived from the interviews with each teacher. Specifically, we read the transcripts of the interviews line by line repeatedly to get ourselves familiarized with these data, and then we coded the data and added background information about the context of the discussion on every excerpt in order to clarify the classification. We constructed the codes from quotations and categorized items into one or more categories. In the end, 13 initial coding categories emerged. These coding categories were knowledge about teaching strategy, knowledge about content of examination, knowledge about teaching objective, knowledge about students' prior knowledge, knowledge about requirements for learning new knowledge, belief about science, insufficient teaching time, saving lesson hours, lack of experimental apparatus, insufficient laboratory, big size of class, colleague's suggestion, and safety of experiment. Furthermore, ‘constant comparative technique’ was employed (Charmaz, 2000), which means initial coding categories were compared and integrated to generate the different types of factors that led to the adaptations. Finally, seven factors were identified, and they were teacher's PCK, teacher's belief about science, time constraint, teaching resources, class size, peer coaching, and lab safety. This article only focused on the factors related to teachers' PCK and then judged what components of PCK they belong to on the basis of the model of PCK of Magnusson et al. (1999).
To ensure the credibility, the two authors analyzed the data together. Overall, eight discrepancies were noted in the categorizations of 78 codes and the high level of inter-rater agreement was reached at about 90%. In the process of analysis, whenever disagreements occurred at any stage of analysis, we discussed our differences, eventually agreeing on one's ideas or a merging of both ideas. Another way we sought to ensure validity in this study was that we sent the findings back to the participants in this study so as to seek their recognition and confirmation of these findings (member checking). The participants agreed that the findings reflected what they had told us. In addition, in order to answer the second and third question, we tabulated the data in two ways. Firstly, the various components of PCK that led to the adaptations at the three levels were tabulated to identify the numbers of teachers that each component exerted influence at each level. Secondly, these factors were tabulated to identify how many teachers were influenced by each one.
Results
This section presents the five teachers one by one. For each one, we first introduce the relevant context information, and then describe how the teacher's PCK led to the discrepancies between the operational and intended curricula at the three levels of ‘teaching objectives’, ‘teaching strategies’, and ‘teaching activities’. At the end of each one, we sum up what components of PCK that influenced his/her adaptations of curriculum materials and what ‘levels of thinking and acting’ at which each component of PCK exerted influence. In the last part of this section, the various components of PCK that led to the teachers’ adaptations at the three levels will be tabulated to identify the tentative ways that these factors influenced the teachers' adaptations and the factors that most influenced teachers' adaptations.Ms Ai
Ms Ai is from Jiankang middle school, which is an exemplary school. She achieved the bachelor's degree, majoring in chemistry education. She has 25 years of experience in teaching. During this study, she taught in Grade 10. There were 52 students in her class. The unit we observed for Ms Ai is ‘metals and their compounds’, which belongs to the module of Chemistry 1. The main teaching content in this unit includes chemical properties of several metals (sodium, aluminum, iron, copper) and their compounds, and the application of metallic materials.Ms Ai did not adapt the curriculum materials at the level of ‘teaching objectives’. At the level of ‘teaching strategies’, in the section of ‘several important metallic compounds’ in the unit of ‘metals and their compounds’, Ms, Ai disregarded the strategies of ‘picture’ and ‘relating’ suggested in the teacher's guide. For the ‘picture’ – ‘guiding students to observe the pictures in the textbook carefully and to find a wealth of information contained in the pictures’ (PEP, 2007a, p. 44), Ms Ai pointed out that students had observed these phenomena in the process of experiments, which was painted in the pictures in the textbook, so it was not necessary to guide students to observe the pictures in the textbook. As we observed, in the teaching of this section, Ms, Ai arranged some demonstration experiments and student experiments about the properties of several metallic compounds. In the process of experiments, those experimental phenomena painted in the textbook indeed had been observed by students. This adaptation was based on the teacher's knowledge about instructional strategies.
As for the ‘relating’ – ‘strengthening the relationship between the properties of metallic compounds and the sequence of metal reactivity’ (PEP, 2007a, p. 44), Ms Ai explained in this way:
As you know, there is no relationship between the acidity or alkalinity of the metal hydroxides and the sequence of metal reactivity, all right? If you emphasize the relationship between the properties of metallic compounds and the sequence of metal reactivity, I think it is possible for students to be confused, so I do not adopt this strategy.
As shown in the excerpt, the reason that Ms Ai did not adopt the strategy of ‘relating’ was that this strategy might have negative effect on student learning. This belonged to the factor of her knowledge about instructional strategies.
In the same section, Ms Ai added a teaching strategy of ‘graph’ – ‘drawing the graphs of the reactions of two types of binary mixtures (sodium carbonate and sodium hydroxide, and sodium carbonate and sodium bicarbonate) and hydrochloric acid’. When asked for the reason, she gave the following comments:
The advantage of the graph is intuitive and quantitative, and it provides students with a way of thinking, that is model! It can help students develop rational thinking and think about issues from sensibility to rationality.
According to Ms Ai, the fact that she added the strategy of ‘graph’ was due to her thought that the graph can provide students with a way of thinking and make students understand the issues intuitively, quantitatively and rationally. This reason belonged to the factor of the teacher's knowledge about instructional strategies.
In the section of ‘metallic materials with wide usages’, Ms Ai did not adopt the teaching strategy of ‘social survey’ – ‘to organize students to carry out social surveys so as to enhance their understanding of metallic materials’ (PEP, 2007a, p. 50) in her classes. She gave the following explanation:
Well, you can say that our teaching is for examinations. Yes, you are right at this point. As it [referring to ‘social survey’] is not involved in the examination, therefore, I would not put emphases on it.
As indicated above, Ms Ai did not adopt the strategy of ‘social survey’ because it is not involved in the examination. This adaptation was based on her knowledge about assessment.
In the section of ‘chemical properties of metals’, there is an ‘inquiry activity’, titled ‘the reaction of iron and water vapor’ (Song, 2007a). But Ms Ai arranged it as a teacher's demonstration rather than as student experiments. When asked why she did not arrange this as an inquiry activity as suggested in the textbook, she gave the following comments:
As you know, the reaction of metals with water produces hydrogen, which is a common property of metals. Students have had this knowledge from learning the reactions of sodium with water and magnesium with water. For this activity, the compound of iron cannot be explored for students. The only part of possible exploration is that the production might be hydrogen. However, students have known this. Do you think it is necessary for students to make an inquiry here?
According to Ms Ai, the reason that she replaced inquiry activity with teaching demonstration was that students had known this knowledge in the previous study. As we have observed, in the previous lesson, Ms Ai did teach the reactions of sodium with water and magnesium with water, and stress that the reaction of metals with water will produce hydrogen. Hence, her explanations were consistent with the classroom observations. The reason for this adaptation could be classified as Ms Ai's knowledge about students' understanding of science.
For the ‘inquiry activity’, ‘the properties of sodium carbonate and sodium bicarbonate’ in the section of ‘several important metallic compounds’ (Song, 2007a), Ms Ai added a procedure, that is ‘the same mass of solid sodium carbonate and solid sodium bicarbonate reacting with hydrochloric acid separately’. For this adaptation, she gave the following explanation:
You see, for the same mass of sodium carbonate and sodium bicarbonate, the amount of hydrochloric acid which they consume is not the same. The amount of carbon dioxide which they react with hydrochloric acid to produce is not the same. The rate and the energy change of these two reactions are also not the same. The purpose of this set of experiments is to let students learn to observe the differences from different sides in one set of experiments. We hope it can cultivate the students' abilities of observation.
As explained by Ms Ai, the reason that she added this procedure was to cultivate the students' abilities of observation, whereas cultivating the students' abilities of observation is just a teaching objective suggested in the teacher's guide in this unit. In other words, Ms Ai added this procedure was to achieve the teaching objective. This decision was based on the teacher's knowledge about curriculum.
In sum, there were four components of PCK that led to Ms Ai's adaptations of curriculum materials, and they were knowledge of science curriculum, knowledge of students' understanding of science, knowledge of assessment in science and knowledge of instructional strategies. Specifically, knowledge of science curriculum and knowledge of students' understanding of science exerted the influence at the level of ‘teaching activities’, and knowledge of assessment in science and knowledge of instructional strategies exerted the influence at the level of ‘teaching strategies’.
Ms Chang
Ms Chang teaches in Yingtian middle school, which is an exemplary school. She achieved the bachelor’s degree, majoring in chemistry education. She has 20 years' teaching experience. During this study, she taught in Grade 11. There were 48 students in her class. The unit we observed for Ms Chang is ‘basic organic substances in lives’, which belongs to the module of Basic Organic Chemistry. The main teaching content in this unit contains the structure and properties of grease, the structure and properties of saccharide (monosaccharide, disaccharide, and polysaccharide), the structure and chemical properties of amino acid and protein, the relationship between protein and human health, and the structure of nucleic acid.In the unit of ‘basic organic substances in lives’, at the level of ‘teaching objectives’, Ms Chang did not mention the teaching objective in the interview, which is suggested in the teacher's guide: ‘to enable students to further experience the processes of investigating chemical substances, understand the meaning of scientific inquiry, learn the basic methods of scientific inquiry, and enhance the abilities of doing scientific inquiry through the investigative experiments of monosaccharide, disaccharide, and polysaccharide’ (PEP, 2007b, p. 84). When talking about this objective, Ms Chang gave the following comments:
In my mind, I don't consider them [referring to the experiments in this objective] investigative experiments, as our students have known the results a long time ago. They knew the reducibility of fructose, sucrose, maltose from their biology classes. They had known it! They [experiments] would only be funny for them [students] if we did these experiments in our class. Did we really cultivate students' abilities of scientific inquiry? No! In fact, these experiments in the textbook are counterfeit inquiries and none of them can arouse students' interest. If you do not know the result, you will be interested in investigating it; if you already know the answer, you will not be interested in. Am I right?
As explained by Ms Chang, students had already known the results of these experiments from their biology classes, so it was not necessary to conduct such investigative work in the chemistry class. This adaptation was based on the teacher's knowledge about students' understanding of science.
In the section of ‘saccharide’, there is an ‘inquiry activity’, titled ‘the conditions of chemical hydrolysis of starch’ (Song, 2007b). In practice, Ms Chang provided the procedures of the experiments instead of requiring students to design the experiments arranged in the textbook, and the tasks of students were to conduct the experiments according to these procedures. For this adaptation, she gave the following explanation:
In this section, students should know that starch can be hydrolyzed. It's enough! But now, it [referred to this activity] is exploring the conditions of chemical catalyzing. Does this section aim to investigate the catalyst? Of course, not. So, what is its purpose? Put it simply, the purpose is letting students know that starch can be hydrolyzed. So, I can tell them directly. It does not need a kind of investigation.
According to Ms Chang, she thought that investigating the conditions of chemical hydrolysis of starch was not consistent with the teaching content of this section. That is to say, it was not appropriately set in this section. This belonged to the factor of her knowledge about the curriculum.
In sum, there were two components of PCK that led to Ms Chang's adaptations of curriculum materials, and they were knowledge of science curriculum and knowledge of students' understanding of science. Specifically, knowledge of science curriculum exerted the influence at the level of ‘teaching activities’, and knowledge of students' understanding of science exerted the influence at the level of ‘teaching objectives’.
Mr Dong
Mr Dong is from Moling middle school, which is an ordinary school. He achieved the master's degree, majoring in chemistry education. He has 14 years of experience in teaching. During this study, he taught in Grade 10. There were 44 students in his class. The unit we observed for Mr Dong is ‘chemistry reactions and energy’, which belongs to the module of Chemistry 2. The main teaching content in this unit includes the transformation of chemical energy and thermal energy and its application, the transformation of chemical energy and electric energy, the application and development of galvanic cell, the rate of chemical reaction and its influencing factors, the limitation of chemical reaction, and the characteristics of chemical equilibrium.In the unit of ‘chemistry reactions and energy’, at the level of ‘teaching objectives’, comparing the operational curriculum with the intended curriculum, we found that Mr Dong did not mention the teaching objective in the interview, which is suggested in the teacher's guide: ‘to realize the method and value of experiment in investigating the principles of chemical reactions’ (PEP, 2007c, p. 15). Thus, he did not take ‘method of experiment’ as his teaching objective. Specifically, the two experiments about the two factors that affect the rate of chemical reaction in the section of ‘the rate and the limitation of chemical reaction’ were not implemented in his class. When asked why, Mr Dong explained:
If I would do them [the two experiments], I could. But, I feel that they have no sense. Why? As for the experiment about catalyst, it was taught that the rate of decomposing potassium chlorate would speed up when adding manganese dioxide as a catalyst in the junior secondary school. Students already have a deep impression on this matter. The same principle can be applied to this experiment. So, it is not necessary to repeat it. As for the experiment about temperature, some examples can be taken from the daily lives, and thus, there is no need to do the experiment any more.
As explained by Mr Dong, the reason that he did not take the ‘method of experiment’ as his teaching objective was that he thought the students had already known the results of the experiments. Thus, there was no necessary to do the experiments any more. This belonged to the factor of the teacher's knowledge about students' understanding of science.
At the level of ‘teaching strategies’, in the section of ‘chemical energy and thermal energy’, Mr Dong did not adopt the strategy of ‘model’ – ‘using the simulated courseware to visualize the abstract knowledge that breakdown and formation of chemical bonds is the main reason for energy changes in chemical reactions’ (PEP, 2007c, p. 19) suggested in the teacher's guide. When asked for the reason, he gave the following comments:
I explained this knowledge in the form of blackboard writing. I feel that students are more receptive to this knowledge in the form of blackboard writing. In the early years of teaching, I have ever tried to use the simulated courseware, but the effect of teaching is not very good. That is to say, most students do not have a good understanding of this knowledge.
As shown in the excerpt, Mr Dong did not adopt the strategy of ‘model’ because he thought that simulated courseware was not conducive to students' understanding of the reason for energy changes in chemical reactions from the perspective of chemical bonds based on his teaching experience. This adaptation was based on the teacher's knowledge about instructional strategies.
In the section of ‘chemical energy and electric energy’, Mr Dong added the strategy of ‘exercise’ – ‘making students complete the exercises related to galvanic cell’. For this adaptation, he gave the following explanation:
As you know, galvanic cell is a focal point in the examination. Therefore, I arranged the section of exercise to solidify students' skills and knowledge. I hope that the exercise can detect students' misunderstanding about galvanic cell and thus I can make some correction and remedy in the subsequent lessons.
As indicated above, the reason that Mr Dong added the strategy of ‘exercise’ was that a galvanic cell is a focal point in the examination. This belonged to the factor of the teacher's knowledge about assessment.
Overall, there were three components of PCK that influenced Mr Dong's adaptations of curriculum materials, and they were knowledge of students' understanding of science, knowledge of assessment in science and knowledge of instructional strategies. Specifically, knowledge of students' understanding of science exerted the influence at the level of ‘teaching objectives’, knowledge of assessment in science and knowledge of instructional strategies exerted the influence at the level of ‘teaching strategies’.
Mr En
Mr En teaches in Tianjing middle school, which is an ordinary school. He achieved the master's degree, majoring in chemistry education. He has 12 years' teaching experience. During this study, he taught in Grade 10. There were 45 students in his class. The unit we observed for Mr En is ‘chemistry reactions and energy’, which belongs to the module of Chemistry 2. The main teaching content in this unit contains the transformation of chemical energy and thermal energy and its application, the transformation of chemical energy and electric energy, the application and development of galvanic cell, the rate of chemical reaction and its influencing factors, the limitation of chemical reaction, and the characteristics of chemical equilibrium.In the unit of ‘chemistry reactions and energy’, at the level of ‘teaching objectives’, Mr En did not take the objective of ‘to be aware of the applications of the transform of chemistry energy to thermal energy in industries and daily lives’ (PEP, 2007c, p. 15) suggested in the teacher's guide as his objective. He gave the following explanation:
Of course, we should have taken this as our objective in the view of chemistry education at a higher level so as to help students recognize this kind of energy change in nature. However, as you know, it is not included in the examination, so I would not take it seriously.
As explained by Mr En, the reason that he did not take the applications of the transform of chemistry energy to thermal energy in industries and daily lives as his objective was that this kind of knowledge is not included in the examination. This belonged to the factor of the teacher's knowledge about assessment.
At the level of ‘teaching strategies’, in the section of ‘chemical energy and thermal energy’, Mr En added the strategy of ‘graph’ – ‘using the graph to explain the energy changes of the two reactions (magnesium reacting with hydrochloric acid and crystal of barium hydroxide reacting with crystal of ammonium chloride)’. When asked for the reason, he gave the following comments:
As you know, the comparison of the amounts of energy of reactants and products determines whether the reaction will absorb energy or release energy. This kind of knowledge is a bit abstract. Given that the graph is more intuitive, I believed it would make easier for students to understand this abstract knowledge. This is why I added this strategy.
As indicated above, the fact that Mr En added the strategy of ‘graph’ was due to his thought that this strategy can make students understand the knowledge more intuitively. This decision was based on his knowledge about instructional strategies.
In the section of ‘chemical energy and electric energy’, Mr En added the teaching strategy of ‘exercise’ to provide more time for student to complete the exercises related to galvanic cell. He explained this adaptation as follows:
You see, galvanic cell is usually a focal point either in our daily test or university entrance examination. Therefore, I organized students to do the exercises so as to solidify their knowledge.
According to Mr En, he added the exercises related to galvanic cell because this kind of knowledge is included in the examination. The reason for this adaptation could be classified as his knowledge about assessment.
In sum, there were two components of PCK that led to Mr En's adaptations of curriculum materials, and they were knowledge of assessment in science and knowledge of instructional strategies. Specifically, knowledge of assessment in science exerted the influence at the levels of ‘teaching objectives’ and ‘teaching strategies’, and knowledge of instructional strategies exerted the influence at the level of ‘teaching strategies’.
Mr Fang
Mr Fang is from Tianjing middle school, which is an ordinary school. He achieved the bachelor’s degree, majoring in chemistry education. He has 15 years of experience in teaching. During this study, he taught in Grade 11. There were 49 students in his class. The unit we observed for Mr Fang is ‘ionic equilibrium in aqueous solution’, which belongs to the module of Chemical Reaction Mechanism. The main teaching content in this unit includes ionization equilibrium of weak electrolytes, water ionization, calculation of pH, hydrolysis of salts and its application, dissolution equilibrium of insoluble electrolytes, and the nature of the transformation of precipitation.In the unit of ‘ionic equilibrium in aqueous solution’, at the level of ‘teaching objectives’, Mr Fang added an objective concerning factors that affect the shift of the ionization equilibrium. In the interview, he gave the following explanation:
It is should be said that this kind of knowledge is really important. If students do not acquire this knowledge, then it will have a negative impact on the subsequent learning of the ionization of water. Of course, there is another reason. As it is tested in the university entrance examination, you know, we will give more emphasis to it.
As shown in the above excerpt, Mr Fang added the objective of ‘factors that affect the shift of the ionization equilibrium’ for two reasons. First, this kind of knowledge is the basis for learning subsequent knowledge. Second, this kind of knowledge would be included in university entrance examinations. These two reasons could be classified as the teacher's knowledge about students' understanding of science and the teacher's knowledge about assessment in science.
Also at the level of ‘teaching objectives’, Mr Fang added an objective of ‘learning to determine the relationship between the concentrations of various ions in the solution’. He explained this adaptation as follows:
Frankly, our teaching is related with the examination. It is involved in the examination and the item in the exam is usually a bit difficult for students. Thus, we inevitably put emphases on it in practice.
According to Mr Fang, he added the objective of ‘learning to determine the relationship between the concentrations of various ions in the solution’ because it is involved in the examination. This adaptation was based on the teacher's knowledge about assessment in science.
At the level of ‘teaching strategies’, in the section of ‘the ionization of weak electrolytes’, Mr Fang added the strategy of ‘prior knowledge activation’ – ‘leading students to review the concepts of electrolytes and non-electrolytes before teaching the new lesson’. For this adaptation, he gave the following comments:
As we know, the concepts of strong electrolytes and weak electrolytes are subordinate to the concept of electrolytes. Therefore, the intention of reviewing the concepts of electrolytes and non-electrolytes is to make students better understand the concepts of strong electrolytes and weak electrolytes in the new lesson. I consider that it is necessary for students to build new knowledge based on their existing knowledge.
As shown above, the intention of Mr Fang's addition of the strategy of ‘prior knowledge activation’ is to make students better learn new knowledge on the basis of their existing knowledge. This adaptation was based on the teacher's knowledge about student’ understanding of science.
In the section of ‘hydrolysis of salts’, there is an ‘inquiry activity’, titled ‘inquiring factors that affect the degree of hydrolysis of salts’ (Song, 2007c). However, Mr Fang disregarded this ‘inquiry activity’ arranged in the textbook. When asked why he did not carry out this activity in his class, he gave the following explanation:
As you know, in the first section, we analyzed the shift of the ionization equilibrium of weak electrolytes, and in the second section, we analyzed the shift of the ionization equilibrium of water. Based on these lessons, it has been clear that it [the shift of the hydrolysis equilibrium] is a shift of chemical equilibrium in its nature. That is to say, it is similar to the previous two sections. The influencing factors include the temperature, the concentration of substances, and additional acids, bases, and salts. In its nature, this lesson also indicates the issue of chemical equilibrium and its shift. Students have already known these pieces of knowledge. So, I say that it is not necessary to do these kinds of things in this section.
As explained by Mr Fang, he did not adopt this ‘inquiry activity’ because he thought students had already acquired this knowledge. Thus, engaging the students in this activity was unnecessary. According to our observations, in the previous two sections, Mr Fang did explain the shift of ionization equilibrium and the factors that affect the shift of equilibrium. Hence, his explanations coincided with the classroom observations. The reason for this adaptation belonged to the factor of Mr Fang's knowledge about students' understanding of science.
Overall, there were two components of PCK that influenced Mr Fang's adaptations of curriculum materials, and they were knowledge of students' understanding of science and knowledge of assessment in science. Specifically, knowledge of students' understanding of science exerted the influence at the level of ‘teaching objectives’, ‘teaching strategies’ and ‘teaching activities’, and knowledge of assessment in science exerted the influence at the level of ‘teaching objectives’.
Summary
In general, we found four components of PCK that led to teachers' adaptations of curriculum materials at the three levels, ‘teaching objectives’, ‘teaching strategies’, and ‘teaching activities’. They were knowledge of students' understanding of science, knowledge of assessment in science, knowledge of instructional strategies, and knowledge of science curriculum. We are interested to know how many teachers were influenced by each component of PCK at each level. The results are shown in Table 3.| Knowledge of students' understanding of science | Knowledge of assessment in science | Knowledge of instructional strategies | Knowledge of science curriculum | |
|---|---|---|---|---|
| Note: numbers in the table represent the number of teachers (0 ≤ n ≤ 5). | ||||
| Teaching objectives | 3 | 2 | 0 | 0 |
| Teaching strategies | 1 | 3 | 3 | 0 |
| Teaching activities | 2 | 0 | 0 | 2 |
As indicated in Table 3, at the level of ‘teaching objectives’, knowledge of students' understanding of science influenced three teachers, knowledge of assessment in science influenced two teachers, and knowledge of instructional strategies and knowledge of science curriculum did not influenced any teacher. At the level of ‘teaching strategies’, knowledge of assessment in science and knowledge of instructional strategies influenced three teachers respectively, knowledge of students' understanding of science influenced one teacher, and knowledge of science curriculum did not influenced any teacher. At the level of ‘teaching activities’, knowledge of students' understanding of science and knowledge of science curriculum influenced two teachers respectively, and knowledge of assessment in science and knowledge of instructional strategies did not influenced any teacher. Based on the above findings, the ways that the four components of PCK influenced the teachers' adaptations could be tentatively built. Specifically, knowledge of students' understanding of science exerted the influence at all three levels, and major at the level of ‘teaching objectives’; knowledge of assessment in science exerted the influence at two levels of ‘teaching objectives’ and ‘teaching strategies’; knowledge of instructional strategies only exerted the influence at one level of ‘teaching strategies’; and knowledge of science curriculum only exerted the influence at one level of ‘teaching activities’. We describe the tentative ways that the four components of PCK influenced the teachers' adaptations of curriculum materials in Fig. 1.
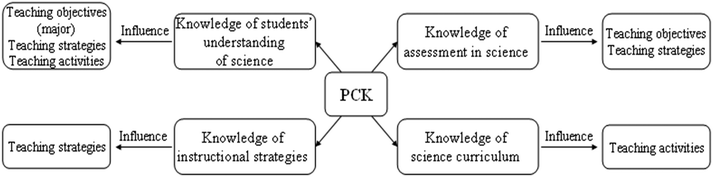 | ||
| Fig. 1 The tentative ways that the four components of PCK influenced the teachers' adaptations of curriculum materials. | ||
As presented above, it can be seen that the four components of PCK influenced the five teachers differently. The influences of each component of PCK on each teacher are shown in Table 4.
| Knowledge of students' understanding of science | Knowledge of assessment in science | Knowledge of instructional strategies | Knowledge of science curriculum | |
|---|---|---|---|---|
| Ms Ai | √ | √ | √ | √ |
| Ms Chang | √ | √ | ||
| Mr Dong | √ | √ | √ | |
| Mr En | √ | √ | ||
| Mr Fang | √ | √ |
As shown in Table 4, knowledge of students' understanding of science and knowledge of assessment in science influenced four teachers respectively, knowledge of instructional strategies influenced three teachers, and knowledge of science curriculum influenced two teachers.
Taking Tables 3 and 4 into consideration together, we can see that knowledge of students' understanding of science and knowledge of assessment in science were the factors that most influenced teachers' adaptations among the four factors. That is to say, these two factors were the key ones that influenced teachers' adaptations of curriculum materials.
Conclusion and discussions
This study was conceptually grounded in Magnusson et al. (1999) model of PCK. Our empirical research supported the applicability of this model to characterize and examine teachers' adaptations of curriculum materials. In particular, the five components of PCK in the model of Magnusson et al. (1999) have been clearly defined, thus we could easily judge the factors that influenced teachers' adaptations belong to which component of PCK. In this paper, we have identified four components of PCK that lead to chemistry teachers' adaptations of the standards-based curriculum materials, and they are knowledge of students' understanding of science, knowledge of assessment in science, knowledge of instructional strategies, and knowledge of science curriculum. More importantly, we have disclosed that the tentative ways that the four components of PCK influence the teachers' adaptations of curriculum materials. In this sense, this study provided a specific scenario that portrayed the interaction between the teachers and the curriculum materials in the process of the implementation of the standards-based science curriculum in the view of teachers' PCK. As mentioned earlier, although the impact of PCK on the use of reform-based curriculum materials had been indicated in the previous studies (e.g., Brown, 2002; Bismack et al., 2014), we revealed what components of PCK had really led to teachers' adaptations and tentatively identified the ways that different components of PCK influenced teachers' adaptations for the first time. In this regard, we believe that this study makes a contribution to the international literature of curriculum use in the field of science education.It can be seen from this study that the PCK of teachers was a constant factor that led to their adaptations of curriculum materials. As mentioned earlier, for the nature of PCK, it is closely related to the teaching of particular topics and novice teachers usually have a relatively undeveloped PCK (Van Driel et al., 1998). Given that the focus of this study was on the teaching of specific units and all of the participants in this study can be thought of as experienced teachers (with twelve or more years of teaching chemistry), we think this finding is understandable. Furthermore, this finding also echoes the vision emphasized in the literature, that is, the personal factors of teachers exert important influences on the use of curriculum materials (Remillard, 2005; Lloyd, 2008). Since this study was conducted in China, where the national curriculum system is centralized; in this sense, we can say that the centralized curriculum system itself cannot guarantee the alignment between the instruction and content standards. Therefore, we suggest that more interactions should be undertaken with experienced science teachers in designing curriculum materials and in preparing textbooks. The PCK of these experienced teachers should be represented in the curriculum materials and science textbooks. Specifically, setting teaching objectives, recommending teaching strategies, and designing teaching activities should consider and link to the PCK of teachers. In this way, the objectives, strategies, and activities in the curriculum materials could be more feasible and useful in the classroom. Of course, less experienced teachers should also be consulted in that the problems they experience and the successes they have may provide a unique insight into our understanding of curriculum implementation. Thus, teacher professional development could be appropriately provided to less experienced teachers so as to help them make use of reform-based curriculum materials better.
Furthermore, we have found that knowledge of students' understanding of science and knowledge of assessment in science are the two key factors that lead to teachers' adaptations. The first one as the key factor shows that chemistry teachers are very concerned about the needs of students in the process of the designs of teaching in China. It also indicates that the modes of teaching of teachers have been shifted from the teacher-centered to the student-centered direction with the influence of the ideas embedded in the standards-based curriculum materials. As for ‘knowledge of assessment in science’, we argue that it was identified as a key factor for two reasons. First, the high-stakes public assessment, particularly university entrance examination, dominates science teaching in China, where the ‘culture of examination’ is prevalent (Gu, 2004). In teachers' minds, teaching science is for the examination, which mainly refers to university entrance examination. In practice, teachers usually pay high attention to university entrance examination and take the responsibility to ensure students to achieve the exam requirements (Gao and Watkins, 2002). Second, there exists a significant discrepancy between the science curriculum materials and the standardized test in China (Liu et al., 2009). As a result, teachers have to adapt curriculum materials to meet the requirements of the examination based on their knowledge about assessment.
In spite of some theoretical and practical implications discussed above, there is a distinct limitation in the present study. The participants in this study only represent a specific group of senior secondary school chemistry teachers from Nanjing, an economically developed region in China. It is still unknown whether the findings presented in the current study can be applied to the teachers in other parts of China. Hence, further studies should involve more teachers and be extended to economically underdeveloped regions, for example, western provinces of China, to test if the conclusion of this study can be generalized to other areas in China.
References
- Abell S. K., (2008), Twenty years later: dose pedagogical content knowledge remain a useful idea? Int. J. Sci. Educ., 30(10), 1405–1416.
- Aikenhead G. S., (2006), Science education for everyday life, New York: Teachers College Press.
- American Association for the Advancement of Science (AAAS), (1989), Science for all American: a project 2061 report on goals in science, mathematics, and technology, Washington, DC: American Association for the Advancement of Science (AAAS).
- Anderson R. D. and Helms J. V., (2001), The ideal of standards and the reality of schools: needed research, J. Res. Sci. Teach., 38(1), 3–16.
- Ball D. L. and Feiman-Nemser S., (1988), Using textbooks and teachers' guides: a dilemma for beginning teachers and teacher educators, Curriculum Inquiry, 18, 401–423.
- Bismack A. S., Arias A. M., Davis E. A. and Palincsar A. S., (2014), Connecting curriculum materials and teachers: elementary science teachers' enactment of a reform-based curricular unit, J. Sci. Teach. Educ., 25, 489–512.
- Brown M. W., (2002), Teaching by design: understanding the interactions between teacher practice and the design of curricular innovation, Unpublished doctoral dissertation, Evanston, IL: Northwestern University.
- Brown M. W., (2009), The teacher-tool relationship: theorizing the design and use of curriculum materials, in Remillard J. T., Herbel-Eisenmann B. A. and Lloyd G. M. (ed.), Mathematics teachers at work: connecting curriculum materials and classroom instruction, New York: Routledge, pp. 17–36.
- Charmaz K., (2000), Grounded theory: objectivist and constructivist methods, in Denzin N. K. and Lincoln Y. S. (ed.), Handbook of qualitative research, 2nd edn, Thousand Oaks, CA: Sage, pp. 509–535.
- Cochran K. F., DeRuiter J. A. and King R. A., (1993), Pedagogical content knowing: an integrative model for teacher preparation, J. Teach. Educ., 44, 263–272.
- Erickson F., (1986), Qualitative methods in research on teaching, in Wittrock M. C. (ed.), Handbook of research on teaching, New York: Macmillan, pp. 119–161.
- Forbes C. and Davis E. A., (2010), Curriculum design for inquiry: preservice elementary teachers' mobilization and adaptation of science curriculum materials, J. Res. Sci. Teach., 47, 820–839.
- Gao L., (2007), Reform and challenge: an review of new science curriculum reform in China, in Sio Y. and Cheung M. H. (ed.), The research of science education in Chinese society, Hong Kong: Seedland Publishing Limited, pp. 17–28 (in Chinese).
- Gao L. and Watkins D. A., (2002), Conceptions of teaching held by school science teachers in P. R. China: identification and cross-cultural comparisons, Int. J. Sci. Educ., 24(1), 61–79.
- Gess-Newsome J., (1999), Pedagogical content knowledge: an introduction and orientation, in Gess-Newsome J. and Lederman N. G. (ed.), Examining pedagogical content knowledge: the construct and its implications for science education, Boston: Kluwer, pp. 3–20.
- Glaser B. G. and Strauss A., (1967), The discovery of grounded theory: strategies for qualitative research, Chicago, IL: Adline.
- Goodlad J. I., (1979), The scope of curriculum field, in Goodlad J. I., et al. (ed.), Curriculum inquiry: the study of curriculum practice, New York: McGraw-Hill, pp. 17–41.
- Grossman P. L., (1990), The making of a teacher: teacher knowledge and teacher education, New York: Teacher College Press.
- Gu M., (2004), The cultural foundation to the Chinese education, Taiyuan: Shanxi Education Press (in Chinese).
- Hanuscin D. L., Lee M. H. and Akerson V. L., (2011), Elementary teachers' pedagogical content knowledge for teaching the nature of science, Sci. Educ., 95, 145–167.
- Herman J. L. and Webb N. M., (2007), Alignment methodologies, Applied Measurement in Education, 20(1), 1–5.
- Koballa T. R., Graber W., Coleman D. and Kemp A. C., (1999), Prospective teachers' conceptions of the knowledge base for teaching chemistry at the gymnasium, J. Sci. Teach. Educ., 10(4), 269–286.
- Lincoln Y. S. and Guba E. G., (1985), Naturalistic inquiry, Beverly Hills, CA: Sage Publications.
- Liu X., Zhang B., Liang L., Fulmer G., Kim B. and Yuan H., (2009), Alignment between the physics content standard and the standardized test: a comparison among the United States – New York State, Singapore, and China – Jiangsu, Sci. Educ., 93, 777–797.
- Lloyd G. M., (2008), Curriculum use while learning to teach: one student teacher's appropriation of mathematics curriculum materials, J. Res. Math. Educ., 39(1), 63–94.
- Lloyd G. M., Remillard J. T. and Herbel-Eisenmann B. A., (2009), Teachers' use of curriculum materials: an emerging field, in Remillard J. T., Herbel-Eisenmann B. A. and Lloyd G. M. (ed.), Mathematics Teachers at work: connecting curriculum materials and classroom instruction, New York: Routledge, pp. 3–14.
- Magnusson S., Krajcik J. and Borko H., (1999), Nature, sources, and development of pedagogical content knowledge for science teaching, in Gess-Newsome J. and Lederman N. G. (ed.), Examining pedagogical content knowledge: the construct and its implications for science education, Boston: Kluwer, pp. 95–132.
- Marshall C. and Rossman G. B., (2006), Designing qualitative research, 4th edn, Thousand Oaks: Sage Publications.
- McNeil J. D., (2003), Curriculum: the teacher's initiative, 3rd edn, New Jersey: Pearson Education.
- Ministry of Education (MoE), (2001), The chemistry curriculum standard of compulsory education of full-time system, Beijing: Beijing Normal University (in Chinese).
- Ministry of Education (MoE), (2003), National standards of general senior secondary school chemistry curriculum, Beijing: People's Education Press (in Chinese).
- National Research Council (NRC), (1996), National science education standards, Washington, DC: National Research Council (NRC).
- People Education Press (PEP), (2007a), Chemistry 1: teachers' guide, 3rd edn, Beijing: People Education Press (in Chinese).
- People Education Press (PEP), (2007b), Basic organic chemistry: teachers' guide, 3rd edn, Beijing: People Education Press (in Chinese).
- People Education Press (PEP), (2007c), Chemistry 2: teachers' guide, 3rd edn, Beijing: People Education Press (in Chinese).
- Powell J. C. and Anderson R. D., (2002), Changing teachers' practice: curriculum materials and science education reform in the USA, Stud. Sci. Educ., 37, 107–136.
- Remillard J. T., (2005), Examining key concepts in research on teachers' use of mathematics curricula, Rev. Educ. Res., 75, 211–246.
- Remillard J. T., Herbel-Eisenmann B. A. and Lloyd G. M. (ed.), (2009), Mathematics Teachers at work: connecting curriculum materials and classroom instruction, New York: Routledge.
- Schneider M. R., Krajcik J. and Blumenfeld P., (2005), Enacting reform-based science materials: the range of teacher enactments in reform Classrooms, J. Res. Sci. Teach., 42, 283–312.
- Shulman L. S., (1986), Those who understand: knowledge growth in teaching, Educ. Res., 15(2), 4–14.
- Shulman L. S., (1987), Knowledge and teaching: foundations of the new reform, Harv. Educ. Rev., 57(1), 1–22.
- Song X. Q. (ed.), (2007a), Chemistry 1, 3rd edn, Beijing: People Education Press (in Chinese).
- Song X. Q. (ed.), (2007b), Basic organic chemistry, 3rd edn, Beijing: People Education Press (in Chinese).
- Song X. Q. (ed.), (2007c), Chemical reaction mechanism, 3rd edn, Beijing: People Education Press (in Chinese).
- Van Driel J. H., Verloop N. and De Vos W., (1998), Developing science teachers' pedagogical content knowledge, J. Res. Sci. Teach., 33(6), 673–695.
- Van Hiele P. M., (1986), Structure and insight: a theory mathematics education, New York: Academic Press.
- Vos M. A. J., Taconis R., Jochems W. M. G. and Pilot A., (2010), Teachers implementation context-based teaching materials: A framework for case-analysis in chemistry, Chem. Educ. Res. Pract., 11, 193–206.
- Vos M. A. J., Taconis R., Jochems W. M. G. and Pilot A., (2011), Classroom implementation of context-based chemistry education by teachers: the relation between experiences of teachers and the design of materials, Int. J. Sci. Educ., 33, 1407–1432.
- Wang L., (2010), Progress and reflection of secondary chemistry curriculum reform in the past ten years (part A), Chin. J. Chem. Educ., 4, 15–21 (in Chinese).
- Wei B., (2010), The changes in science curricula in China after 1976: a reflective review, in Lee Y. J. (ed.), Handbook of research in science education research in Asia, Netherlands: Sense Publishers, pp. 89–102.
- Wei B. and Thomas G., (2006), An examination of the change of the junior secondary school chemistry curriculum in the P. R. China: in the view of scientific literacy, Res. Sci. Educ., 36, 403–418.
Footnotes |
| † In China, senior secondary schools are classified into exemplary schools and ordinary schools. Exemplary schools have more resources and are able to recruit more competent students than ordinary schools. |
| ‡ Theoretical chemistry mainly refers to chemical concepts, laws, theories, principles and models; descriptive chemistry mainly refers to chemical properties of substances and chemical reactions. |
| This journal is © The Royal Society of Chemistry 2015 |
