Using animations in identifying general chemistry students' misconceptions and evaluating their knowledge transfer relating to particle position in physical changes
K.
Christopher Smith
*a and
Savannah
Villarreal
b
aDepartment of Chemistry, University of Texas-Pan American, 1201 W. University Drive, Edinburg, TX 78539-2999, USA. E-mail: kcsmith@utpa.edu
bScience Department, Michael E. Fossum Middle School, 7800 N. Ware Road, McAllen, TX 78504, USA
First published on 21st January 2015
Abstract
This article reports on the types of views and misconceptions uncovered after assessing 155 freshman general chemistry students on the concept of particle position during the reversible physical change of melting, using the Melting Cycle Instrument, which illustrates particulate-level representations of a melting–freezing cycle. Animations involving particulate-level representations of phase changes including melting and freezing were viewed and discussed, and the students were assessed a second time, on the concept of particle position during the reversible physical change of dissolving, using the Dissolving Cycle Instrument, which illustrates particulate-level representations of a dissolving-solvent evaporation cycle. Overall, the results of the assessments showed that some misconceptions did remain after viewing and discussing the animations, and that the use of the animations had no effect on the students' views on the movement of particles within the liquid.
The study of chemistry requires understanding and navigating between three sets of representations of chemistry: the macroscopic, sub-microscopic, and symbolic (Johnstone, 1991). The unseen sub-microscopic realm is a particularly challenging perspective for students, and there is much reported research in the literature on students' conceptions of aspects of the particulate nature of matter (Doran, 1972; Novick and Nussbaum, 1978, 1981; Stavy, 1990; Griffiths and Preston, 1992; Nakhleh, 1992; Lee et al., 1993; Nakhleh and Samarapungavan, 1999; Liu, 2001; Mulford and Robinson, 2002; Nakhleh et al., 2005; Yezierski and Birk, 2006; Löfgren and Helldén, 2008; Ayas et al., 2010; Rahayu and Kita, 2010; Özmen, 2011), which can be expressed as a set of statements describing the behavior of particles on the sub-microscopic level (de Vos and Verdonk, 1996; Ayas et al., 2010). The ideas contained within the particulate nature of matter are generally not taught collectively at a single point in a general chemistry course but are instead spread throughout general chemistry coursework. These ideas provide a good basis for a scientifically accepted conceptual framework when considering the behavior of particles.
The particulate nature of matter can be used to describe the behavior of particles involved in physical changes, and various studies have reported on students' conceptions of melting (Prieto et al., 1989; Griffiths and Preston, 1992; Lee et al., 1993; Ebenezer and Gaskell, 1995; Ebenezer and Erickson, 1996; Valanides, 2000; Goodwin, 2002; Uzuntiryaki and Geban, 2005; Pierri et al., 2008; Çalik et al., 2010; Durmuş and Bayraktar, 2010; Özmen, 2011; Smith and Nakhleh, 2011) and dissolving (Fensham and Fensham, 1987; Prieto et al., 1989; Haidar and Abraham, 1991; Longden et al., 1991; Lee et al., 1993; Ebenezer and Erickson, 1996; Blanco and Prieto, 1997; Ahtee and Varjola, 1998; Ebenezer, 2001; Pınarbaşi and Canpolat, 2003; She, 2004; Çalik, 2005, 2008; Çalik and Ayas, 2005; Çalik et al., 2007a, 2007b; Smith and Nakhleh, 2011; Naah and Sanger, 2012, 2013; Adadan, 2014) on the sub-microscopic level. Animations can be used by chemistry educators in order to help students visualize these types of processes involving particles occurring on the unseen sub-microscopic level (Özmen, 2011). Several articles have appeared in the literature on the use of particulate-level animations for processes involving physical changes, including phase changes (Yezierski and Birk, 2006; Özmen, 2011; Akaygun and Jones, 2013) and dissolving (Ebenezer, 2001; Kelly and Jones, 2007). These reports generally found that using animations increased the quality of students' explanations and the level of their performance on assessments.
Animations and illustrations are forms of external representations (Al-Balushi and Al-Hajri, 2014), which can help students visualize processes occurring on the sub-microscopic level, such as those described by the particulate nature of matter. In order for students to effectively make use of external representations, they must be able to make sense of the symbolic information presented in the representations, and use the representations to make predictions (Al-Balushi and Al-Hajri, 2014). Given that chemistry textbooks generally show molecules as two-dimensional illustrations (Al-Balushi and Al-Hajri, 2014), and the particulate-level representations used in this study were also two-dimensional, research on students' views and use of external two-dimensional particulate-level representations is useful and warranted.
One aspect of the particulate nature of matter is that in the liquid phase, particles move around within the volume occupied by the liquid (Ayas et al., 2010). One purpose of this study was to use illustrations to identify student views and misconceptions about the positions of particles during physical changes involving the liquid state. Another purpose was to evaluate how animations affected the extent to which students' knowledge of the positions of particles during physical changes involving the liquid state was transferred. One reason for this purpose was that in general chemistry classes, students are often taught that physical changes are reversible, and that at the end of the reversal one ends up with the substances that were there at the beginning; would students attribute this property to the particles involved in physical changes? Our research questions were: (a) How do students view illustrations of particles involved in physical changes? (b) What are students' conceptions about particle position in the initial and final states of physical changes? (c) How does the use of animations affect students' transfer of knowledge about particle position in the initial and final states of physical changes?
Theoretical perspective
The broad theory used for this research was constructivism. The key idea behind constructivism is that knowledge is not simply transmitted to the learner by others, but is instead actively constructed by the learner (Green and Gredler, 2002). Given this main idea, students' views and conceptions would not be an exact replica of material presented in the classroom. Instead, the views and conceptions expressed by students would be of their own construction. This perspective supported one of the research aims of this study, which was to examine the views and conceptions students held on particles and their positions during physical changes.One form of constructivism that was important for this research is Piagetian constructivism, which followed from Jean Piaget's theory of cognitive development (Piaget, 1970). Piaget held that one's cognitive development resulted from interaction with the external world, rather than from simply copying objects in the external world. As such, when students expressed their views and conceptions about particles and their positions during physical changes, they would not simply express a copy of the animations or classroom discussions involving physical changes. Instead, they would express their views and conceptions based on their internal cognitive integration and reconciliation of the animations and classroom discussions. This perspective supported one of the research aims of this study, which was to examine individual students' views and conceptions of physical changes, and would allow for various types of student misconceptions to be identified.
Another form of constructivism that was important for this research is social constructivism, which emphasized the influence of social aspects in constructing knowledge (Driver et al., 1994). Given that this study was carried out in a classroom setting with an instructor and a number of students, and illustrations and animations, which are social products, were used, this perspective acknowledged the social aspects and interactions of this study.
In sum, these forms of constructivism provided the framework for this study in underscoring the perspective that while knowledge is created by each learner, it is a socially mediated process, through an individual's interaction with instructors, peers, and social products such as illustrations, models, and animations. Constructivism informed this research through seeking to determine individual students' views of illustrations of particles as well as their conceptions about particle position in the initial and final states of physical changes.
Another theoretical perspective guiding this research was transfer. Chi and VanLehn (2012) presented transfer as “…the ability of individuals to “treat” a new concept, problem, or phenomenon as similar to one(s) they have experienced before.” Similarly, Georghiades (2000) defined transfer to include the use of a concept to solve a different problem in a different setting, and he argued that a student's ability to transfer knowledge was a generally accepted outcome of the educational process. Additionally, Schwartz et al. (2012) noted that transfer research was also concerned with “negative transfer”, or the overgeneralizing of prior learning. The theoretical perspective of transfer informed this research by examining the extent to which students' knowledge of the ability of particles to move around within the volume occupied by the liquid transferred when considering first physical changes involving melting, and subsequently, physical changes involving dissolving.
Method
Participants and context
This study took place in a large public university in the Southwestern United States after obtaining human subjects research approval from the Institutional Review Board. There were two experiments in the study: a first experiment involving students in a first-semester introductory general chemistry course during a spring semester, and a second experiment involving students in a subsequent first-semester introductory general chemistry course during a fall semester. The two experiments of the study differed in how animations were used in the two different courses; this difference is described in detail later.In Experiment 1 of the study there were 116 students enrolled in the first-semester introductory general chemistry course; 48 of these students took part in all aspects of the data collection for Experiment 1 as described later, so their results were included in the analysis. Twenty-five of these students were female (52.1%), while twenty-three students were male (47.9%). Twenty of the students were classified as freshman (41.7%), seventeen as sophomores (35.4%), seven as juniors (14.6%), and four as seniors (8.3%).
In Experiment 2 of the study there were 126 students enrolled in the first-semester introductory general chemistry course; 107 of these students took part in all aspects of the data collection as described later, so their results were included in the analysis. Seventy-five of these students were female (70.1%), while thirty-two students were male (29.9%). Fifteen of the students were classified as freshman (14.0%), fifty-seven as sophomores (53.3%), twenty-six as juniors (24.3%), and nine as seniors (8.4%). These first-semester introductory general chemistry courses were required by a variety of majors, so the students were enrolled in various majors, including chemistry, biology, nursing, dietetics, clinical laboratory sciences, and engineering.
Each experiment in the study involved two parts: the first part involved identifying student conceptions regarding particle position during a melting–freezing cycle, while the second part involved identifying student conceptions regarding particle position during a dissolving-solvent evaporation cycle.
Experiment 1: melting
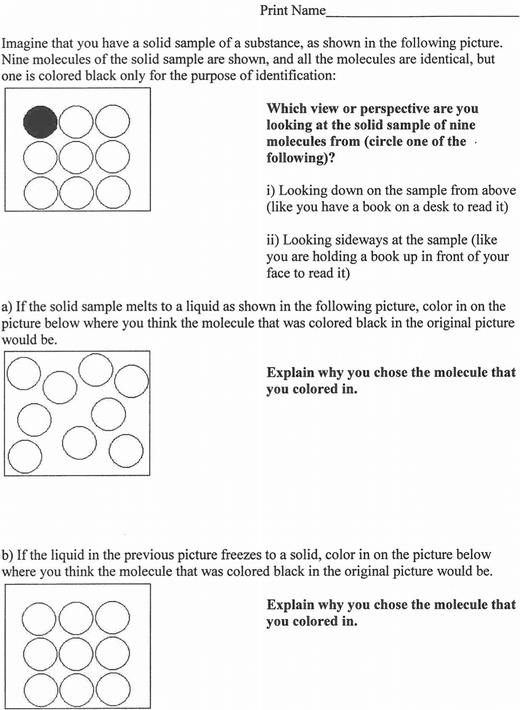
The data for Experiment 1: melting were collected four weeks into the semester during the first class exam, after phase changes had been discussed in the class, using textbook particulate-level figures but not animations. These particulate-level figures showed representations of the solid, liquid, and gas states, and were discussed in terms of the spacing, order, and movement of particles. At the end of their first exam, as a bonus question, students were provided with the Melting Cycle Instrument, which illustrates particulate-level representations of a melting–freezing cycle, shown in Fig. 1.The next class meeting, the exam and the Melting Cycle Instrument were discussed. Students were shown PhET animations of states of matter (available at http://phet.colorado.edu/en/simulation/states-of-matter), in which particulate-level representations of various elements and compounds depicted various phase changes. The PhET animations were produced at the University of Colorado at Boulder, and allow for the exploration of phase changes using a choice of different atoms and molecules, including Ne, Ar, O2, and H2O. The animations feature an interactive control to heat or cool the samples, and there is a temperature readout to accompany the simulations. The particulate-level representations show approximately 100 particles as the temperature is changed. The various aspects of the animations, including the speed, position, and arrangement of the particles, were discussed.
Experiment 1: dissolving
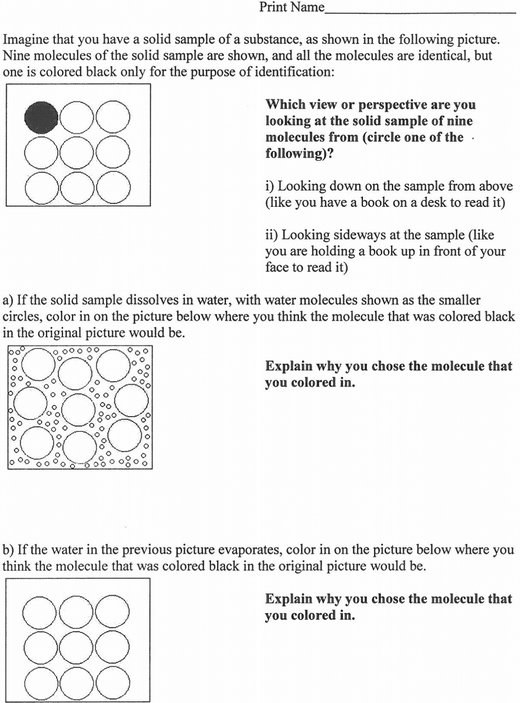
The data for Experiment 1: dissolving were collected four weeks later during the second class exam, after the dissolving process had been introduced in the class, using particulate-level representations but not animations. At the end of their second exam, as a bonus question, students were provided with the Dissolving Cycle Instrument, which illustrates particulate-level representations of a dissolving-solvent evaporation cycle, shown in Fig. 2.Experiment 2: melting
The data for Experiment 2: melting were collected in a subsequent introductory general chemistry course. The data for Experiment 2: melting were collected four weeks into the semester during the first class exam, after phase changes had been discussed in the class, using the textbook particulate-level figures as well as the PhET states of matter animations that were described previously. At the end of their first exam, as a bonus question, students were provided with the Melting Cycle Instrument. The next class meeting, the exam and the Melting Cycle Instrument were discussed, without the use of the PhET states of matter animations.Experiment 2: dissolving
The data for Experiment 2: dissolving were collected four weeks later during the second class exam, after the dissolving process had been introduced in the class, using particulate-level representations but not animations. At the end of their second exam, as a bonus question, students were provided with the Dissolving Cycle Instrument.The data collection in Experiment 1 and Experiment 2 is summarized as follows: Experiment 1: Class discussion of phase changes without PhET animations → Melting Cycle Instrument → Class discussion of Melting Cycle Instrument with PhET animations → Class discussion of dissolving without PhET animations → Dissolving Cycle Instrument.
Experiment 2: Class discussion of phase changes with PhET animations → Melting Cycle Instrument → Class discussion of Melting Cycle Instrument without PhET animations → Class discussion of dissolving without PhET animations → Dissolving Cycle Instrument.
The data collected in Experiment 1 and Experiment 2 were analyzed using a combination of quantitative and qualitative methods. The data on the students' perspectives from which they were viewing the solid sample represented on the Melting and Dissolving Cycle Instruments as well as the data on which molecules the students colored in on the various parts of the Melting and Dissolving Cycle Instruments were analyzed quantitatively, through frequency counts. The students' written explanations on the various parts of the Melting and Dissolving Cycle Instruments were first analyzed qualitatively, by reading through them and grouping them into emergent categories; this process was carried out by the two authors of this paper. After the categories were established, frequency counts were carried out in order to determine the number and proportion of written explanations falling into each category. In order to establish the reliability of this analysis, the first author of this paper and a chemistry graduate student researcher independently coded a sample of 21% (N = 64) of the Melting and Dissolving Cycle Instruments included in the analysis for the entire study. These Melting and Dissolving Cycle Instruments were selected randomly and represented the melting and dissolving parts of Experiment 1 and Experiment 2. The inter-rater reliability using the weighted Cohen's K statistic was 0.74, indicating substantial agreement (Landis and Koch, 1977).
Results and discussion
Students' views of illustrations of particles involved in physical changes
A summary of the numbers and percentages of students viewing the illustrations in the Melting Cycle Instrument and the Dissolving Cycle Instrument from View i (looking down on the sample from above (like you have a book on a desk to read it)) and from View ii (looking sideways at the sample (like you are holding a book up in front of your face to read it)) is presented in Table 1.| View i | View ii | |||
|---|---|---|---|---|
| Number of students | Percentage of students (%) | Number of students | Percentage of students (%) | |
| Experiment 1: melting | 30 | 63 | 18 | 37 |
| Experiment 1: dissolving | 27 | 56 | 21 | 44 |
| Experiment 2: melting | 65 | 61 | 42 | 39 |
| Experiment 2: dissolving | 65 | 61 | 42 | 39 |
These results showed that in the various parts of the study, 56–63% of the students viewed the illustrations from View i, while 37–44% of the students viewed the illustrations from View ii. In our experience, the majority of particulate-level animations and static representations in general chemistry show three-dimensional perspectives or sideways perspectives of the sample (View ii), even if the animations or representations do not explicitly instruct students to use these perspectives. These present results on students' views of the illustrations highlight the importance of guidance and instruction on perspective when students are viewing animations and representations. There were also 39 students who changed their perspective from View i to View ii, or from View ii to View i, within the two experiments. This result further underscored the importance of guidance when students are viewing animations and representations.
Students' conceptions of particle position in physical changes: Experiment 1: melting and Experiment 2: melting
In Experiment 1: melting and Experiment 2: melting student conceptions on particle position in the melting–freezing cycle depicted in the Melting Cycle Instrument were investigated. Students in Experiment 1: melting were exposed to only textbook particulate-level figures while discussing phase changes before completing the Melting Cycle Instrument, while students in Experiment 2: melting were exposed to textbook particulate-level figures as well as the PhET states of matter animations while discussing phase changes before completing the Melting Cycle Instrument.Table 2 shows the number and percentage of students in Experiment 1: melting and Experiment 2: melting who colored in the black molecule in the topmost and leftmost position in Part a and Part b of the Melting Cycle Instrument.
| Experiment 1: melting | Experiment 2: melting | |||
|---|---|---|---|---|
| Number of students | Percentage of students (%) | Number of students | Percentage of students (%) | |
| Part a | 33 | 69 | 62 | 58 |
| Part b | 31 | 65 | 54 | 50 |
These results indicated that a large proportion of the students viewed the black molecule as being near to the same position after melting as it was before melting, and being in the position it was originally in after the liquid froze back to the solid. There were no significant differences between any of the sets of results in Table 2, as Pearson Chi-square analyses of each of the sets of results in Table 2 showed that the proportion of students coloring in the black molecule in the topmost and leftmost position was always statistically significantly greater than would be expected from a random distribution at the 99% confidence level. In these analyses χ2 and p values were as follows: Experiment 1 Part a (χ2 = 164.148, p = 0.000), Experiment 1 Part b (χ2 = 138.961, p = 0.000), Experiment 2 Part a (χ2 = 243.150, p = 0.000), Experiment 2 Part b (χ2 = 172.495, p = 0.000). These results indicated that the PhET animations had no effect on students' views on the ability of particles to move around within the volume of the liquid.
The students' choices for the position of the black molecule in Part a and Part b of the Melting Cycle Instrument were supported by their explanations, as shown in Table 3.
| Experiment 1: melting | Experiment 2: melting | |||
|---|---|---|---|---|
| N | % | N | % | |
| a This is the scientifically accepted response. b There was one student whose response included both of these explanations. | ||||
| Explanation for the position of the black molecule in the Melting Cycle Instrument: Part a | ||||
| (1) The molecule can be anywhere/the molecules move arounda | 6 | 13 | 17 | 16 |
| (2) The molecule doesn't move far from its original position | 21 | 44 | 44b | 41 |
| (3) The sample expands, the molecule is pushed outwards/away | 10b | 21 | 18 | 17 |
| (4) The molecule moves down when the sample melts | 4 | 8 | 16b | 15 |
| (5) Liquids take the shape of their container | 1b | 2 | 3 | 3 |
| (6) No response/nonmeaningful response | 7 | 15 | 10 | 9 |
| Explanation for the position of the black molecule in the Melting Cycle Instrument: Part b | ||||
| (1) The molecule can be anywhere/the molecules move arounda | 7b | 15 | 17 | 16 |
| (2) The molecule ends up near where it was positioned in the liquid | 30b | 63 | 35b | 33 |
| (3) The molecule goes back to its original position in the solid | 8 | 17 | 25b | 23 |
| (4) The sample freezes and/or the molecule moves up/to the center | 0 | 0 | 24 | 22 |
| (5) No response/nonmeaningful response | 4 | 8 | 7 | 7 |
The results for the Melting Cycle Instrument: Part a indicated that a relatively small proportion of students from both Experiment 1: melting and Experiment 2: melting (13–16%) held the scientifically accepted view that the molecules move around within the volume occupied by the liquid. Conversely, a relatively large proportion of students from both experiments (41–44%) held the view that the black molecule would not move far from its original position in the solid when the sample melted.
The results for the Melting Cycle Instrument: Part a also indicated that students mixed macroscopic-level thinking with sub-microscopic-level thinking when considering the Melting Cycle Instrument: Part a. Some of the students indicated the sample expanded as it melted, which would result in the black molecule being pushed outwards or away. Other students indicated that the black molecule would move down when the sample melted, possibly due to their experience with seeing a macroscopic object (such as a cube of ice) melt, with the bulk of the material going down as it melts. These explanations do not take into consideration the molecules moving around within the volume occupied by the liquid. These types of findings where students mix macroscopic-level thinking with sub-microscopic-level thinking have also been reported previously in the literature, with students holding the misconception that molecules of a solid expand when the sample is heated (Griffiths and Preston, 1992), and students holding the misconception that molecules themselves were heated or frozen when a sample was heated or frozen (Lee et al., 1993).
Some students also exhibited a misconception of the idea of molecules moving around within the volume occupied by the liquid by when considering the Melting Cycle Instrument: Part a by coloring all of the molecules black and explaining that the black molecule would spread among the other molecules or stain the other molecules. These students appeared to have focused on the idea of the black molecule moving within the liquid, but instead of considering it as an intact molecule these students broke apart the black molecule so that all parts of the black molecule were distributed throughout the liquid.
In addition, the results for the Melting Cycle Instrument: Part a included some blank or nonmeaningful responses, such as students indicating that liquids take the shape of their container, which is one of the main defining characteristics of liquids as is taught to students.
The results for the Melting Cycle Instrument: Part b indicated that a relatively small proportion of students from both Experiment 1: melting and Experiment 2: melting (15–16%) held the scientifically accepted view that the molecules move around within the volume occupied by the liquid, and so the black molecule could end up in any position once the liquid froze back to the solid. Conversely, a relatively large proportion of students from both Experiment 1: melting and Experiment 2: melting (33–63%) held the view that the molecule ends up in a position in the solid state near where it was positioned in the liquid state.
The results for the Melting Cycle Instrument: Part b indicated as well that students mixed macroscopic-level thinking with sub-microscopic-level thinking when considering the Melting Cycle Instrument: Part b. Some of the students indicated that the black molecule goes back to its original position in the solid state after the melting–freezing cycle, possibly due to their macroscopic-level view of the melting–freezing cycle of an object (such as a cube of ice) which looks similar at the start and end of the cycle. Other students indicated that the black molecule would move up or to the center when the sample froze, possibly due to their viewing freezing as the opposite of melting, and if a macroscopic object (such as an ice cube) melts with the bulk of the material going down or outwards, then when it freezes the material should go up or to the center. These explanations do not take into consideration the molecules moving around within the volume occupied by the liquid.
Interestingly, none of the students from Experiment 1: melting indicated that the black molecule would move up or to the center when the sample froze, but there were some students from Experiment 2: melting who gave this response. This result indicated that this explanation may have been due to an interpretation of the PhET animations, as the students in Experiment 2: melting were exposed to the PhET animations before completing the Melting Cycle Instrument, whereas the students in Experiment 1: melting were not.
In addition, the results for the Melting Cycle Instrument: Part b included some blank or nonmeaningful responses, such as students indicating that the sample freezes.
Students' conceptions of particle position in physical changes: Experiment 1: dissolving and Experiment 2: dissolving
In Experiment 1: dissolving and Experiment 2: dissolving student conceptions on particle position in the dissolving-solvent evaporation cycle depicted in the Dissolving Cycle Instrument were investigated. Students in Experiment 1: dissolving discussed the Melting Cycle Instrument with the PhET animations, while students in Experiment 2: dissolving discussed the Melting Cycle Instrument without the PhET animations, although they had viewed the PhET animations earlier in the course.Table 4 shows the number and percentage of students in Experiment 1: dissolving and Experiment 2: dissolving who colored in the black molecule in the topmost and leftmost position in Part a and Part b of the Dissolving Cycle Instrument.
| Experiment 1: dissolving | Experiment 2: dissolving | |||
|---|---|---|---|---|
| Number of students | Percentage of students (%) | Number of students | Percentage of students (%) | |
| Part a | 11 | 23 | 35 | 33 |
| Part b | 12 | 25 | 31 | 29 |
These results indicated that a minor proportion of the students viewed the black molecule as being near to the same position after dissolving as it was before dissolving, and being in the position it was originally in after the water evaporated away leaving the solid once again. There were no significant differences between any of the sets of results in Table 4, as Pearson Chi-square analyses of each of the sets of results in Table 4 showed that the proportion of students coloring in the black molecule in the topmost and leftmost position was always statistically significantly greater than would be expected from a random distribution at the 99% confidence level. In these analyses χ2 and p values were as follows: Experiment 1 Part a (χ2 = 7.398, p = 0.007), Experiment 1 Part b (χ2 = 164.148, p = 0.000), Experiment 2 Part a (χ2 = 56.453, p = 0.000), Experiment 2 Part b (χ2 = 40.254, p = 0.000). These results indicated that the PhET animations had no effect on students' transfer of knowledge of the movement of particles in the liquid state to the dissolving-solvent evaporation cycle.
The students' choices for the position of the black molecule in the Dissolving Cycle Instrument: Part a and the Dissolving Cycle Instrument: Part b were supported by their explanations, as shown in Table 5.
| Experiment 1: dissolving | Experiment 2: dissolving | |||
|---|---|---|---|---|
| N | % | N | % | |
| a This is the scientifically accepted response. b There was one student whose response included both of these explanations. c There was one student whose response included both of these explanations. | ||||
| Explanation for the position of the black molecule in the Dissolving Cycle Instrument: Part a | ||||
| (1) The molecule can be anywhere/the molecules move arounda | 34 | 71 | 48 | 45 |
| (2) The molecule doesn't move far from its original position | 5b | 10 | 25 | 23 |
| (3) The sample expands, the molecule is pushed outwards/away | 4b | 8 | 3 | 3 |
| (4) The molecule moves down when the sample dissolves | 1 | 2 | 12 | 11 |
| (5) No response/nonmeaningful response | 5 | 10 | 19 | 18 |
| Explanation for the position of the black molecule in the Dissolving Cycle Instrument: Part b | ||||
| (1) The molecule can be anywhere/the molecules move arounda | 23 | 48 | 31b | 29 |
| (2) The molecule ends up near where it was positioned in the solution | 5 | 10 | 16b | 15 |
| (3) The molecule goes back to its original position in the solid | 4 | 8 | 11 | 10 |
| (4) The molecule settles on the bottom | 3 | 6 | 13c | 12 |
| (5) The molecule can be anywhere since it is in the gas state | 2 | 4 | 8 | 7 |
| (6) The molecule moves up | 0 | 0 | 4 | 4 |
| (7) The sample remains a solid/reforms a solid/did not dissolve | 1 | 2 | 11c | 10 |
| (8) No response/nonmeaningful response | 10 | 21 | 15 | 14 |
The results for the Dissolving Cycle Instrument: Part a indicated that a large proportion of students from both Experiment 1: dissolving and Experiment 2: dissolving (45–71%) held the scientifically accepted view that the molecules move around within the volume occupied by the liquid. In addition, a relatively small proportion of students from both Experiments (10–23%) held the view that the black molecule would not move far from its original position in the solid when the sample dissolved.
The results for the Dissolving Cycle Instrument: Part a also indicated that students mixed macroscopic-level thinking with sub-microscopic-level thinking when considering the Dissolving Cycle Instrument: Part a, similar to the Melting Cycle Instrument. Some of the students indicated the sample expanded as it dissolved, which would result in the black molecule being pushed outwards or away. Other students indicated that the black molecule would move down when the sample dissolved, possibly due to their experience with seeing a macroscopic-level solution process, such as dissolving sugar in water, with the sugar sinking to the bottom of the water as it is added. These explanations do not take into consideration the molecules moving around within the volume occupied by the liquid.
Some students also exhibited a misconception of the dissolving process by coloring either none or all of the molecules black and indicating that the black particle was dissolved evenly. Those students who colored none of the molecules black appeared to have also mixed macroscopic-level thinking with sub-microscopic-level thinking, possibly due to their experience with seeing a macroscopic-level solution process, such as dissolving sugar in water, with the sugar disappearing from sight. Those students who colored all of the molecules black appeared to have also mixed macroscopic-level thinking with sub-microscopic-level thinking, possibly due to their experience with seeing a macroscopic-level solution process involving a substance with color, such as a powdered beverage, dissolving in water, with the entire solution taking on the color of the powdered beverage.
In addition, the results for the Dissolving Cycle Instrument: Part a included some blank or nonmeaningful responses, such as students indicating that the sample would not dissolve.
The results for the Dissolving Cycle Instrument: Part b indicated that a relatively large proportion of students from both Experiment 1: dissolving and Experiment 2: dissolving (29–48%) held the scientifically accepted view that the molecules move around within the volume occupied by the liquid, and so the black molecule could end up in any position once the water evaporated leaving the solid. In addition, a relatively small proportion of students from both Experiments (10–15%) held the view that the molecule ends up in a position in the solid state near where it was positioned in the solution.
The results for the Dissolving Cycle Instrument: Part b indicated as well that students mixed macroscopic-level thinking with sub-microscopic-level thinking when considering the Dissolving Cycle Instrument: Part b. Some of the students indicated that the black molecule goes back to its original position in the solid state after the dissolving-solvent evaporation cycle, possibly due to their macroscopic-level view of the dissolving-solvent evaporation cycle of a system (such as sugar in water) which looks similar at the start and end of the cycle. Other students indicated that the black molecule would move up or settle to the bottom when the water evaporated, again possibly due to their macroscopic-level view of the dissolving-solvent evaporation cycle of a system. Other students indicated that the black molecule would move up when the water evaporated, possibly due to their viewing solvent evaporation as the opposition of dissolving, and if the black molecule moved down when the sample dissolved, then it should move up when the water evaporated. These explanations do not take into consideration the molecules moving around within the volume occupied by the liquid.
In addition, the results for the Dissolving Cycle Instrument: Part b included some blank or nonmeaningful responses, such as some of the students responding that the water evaporated. Furthermore, some students indicated that the molecule could be anywhere since it was in the gas state, which indicated confusion with viewing the water evaporating and leaving behind the solid solute.
Conclusions
This study revealed that students view illustrations of particles involved in physical changes from various perspectives, and these perspectives may change when viewing different illustrations. This finding highlights the importance of guidance and instruction when students view illustrations and animations, and was supported by the theoretical perspective of constructivism. This finding also supported previous research on the use of animations to investigate students' understanding of dissolving salt in water, in which the authors reported that instructors must explicitly connect the animations to the dissolving process (Ebenezer, 2001), and that students sometimes missed essential features of the animations (Kelly and Jones, 2007).This study also revealed that when considering a melting–freezing cycle on the particulate level, students held various types of misconceptions regarding the position of particles within the sample, identified with guidance by the theoretical perspective of constructivism. These misconceptions paid little consideration to the aspect of molecules moving around within the entire volume occupied by the liquid, and mixed macroscopic-level thinking with sub-microscopic-level thinking when considering the melting–freezing cycle. This type of finding has been reported previously in the literature, with students holding the misconception that molecules of a solid expand when the sample is heated (Griffiths and Preston, 1992), and students holding the misconception that molecules themselves were heated or frozen when a sample was heated or frozen (Lee et al., 1993). The use of animations had no effect on the students' views on the molecules moving around within the entire volume occupied by the liquid. This finding was contrary to previous quantitative research which indicated that animations involving various states of matter as well as phase changes had a positive effect on students' scores on multiple-choice question particulate nature of matter instruments involving misconceptions (Yezierski and Birk, 2006; Özmen, 2011). This finding was also contrary to previous qualitative research which indicated that animations had a positive effect on students' explanations of the process of dissolving salt in water (Kelly and Jones, 2007).
After viewing and discussing particulate-level animations of a melting–freezing cycle students considered a dissolving-solvent evaporation cycle on the particulate level. The same types of misconceptions revealed in the melting–freezing cycle were still present in the dissolving-solvent evaporation cycle, which gave an indication of the resilience of these misconceptions. In addition, the use of animations had no effect on students' transfer of knowledge of the movement of particles in the liquid state to the dissolving-solvent evaporation cycle.
One limitation of this study was that the demographics of the students in the two experiments in the study were not identical. However, since this was not primarily a quantitative study, and similar categories of qualitative data were found in both experiments, the differences in the demographics should not detract from the findings, and might in fact aid in the generalizability of the findings to other student populations. Another limitation was in the short answer format of the data collection instruments. More in-depth data, such as interviews, may have provided additional information; this further in-depth data collection might be a future line of research. A further limitation is that general chemistry textbooks and instruction usually present the dissolving process as resulting in a solution in which the solute and solvent are evenly dispersed. Combined with the research finding that students often associate stirring and/or heating with the dissolving process (Prieto et al., 1989; Haidar and Abraham, 1991; Ebenezer and Erickson, 1996; Blanco and Prieto, 1997; Smith and Nakhleh, 2011), these aspects might cause students to be more likely to envision a more random distribution of particles when considering dissolving versus melting in general.
Acknowledgements
We would like to acknowledge the chemistry students for taking part in the study. We would also like to acknowledge and thank John Hamilton for his help in the inter-rater reliability determination.References
- Adadan E., (2014), Investigating the influence of pre-service Chemistry teachers' understanding of the particulate nature of matter on their conceptual understanding of solution Chemistry, Chem. Educ. Res. Pract., 15, 219–238.
- Ahtee M. and Varjola I., (1998), Students' understanding of chemical reaction, Int. J. Sci. Educ., 20, 305–316.
- Akaygun S. and Jones L. L., (2013), Research-based design and development of a simulation of liquid-vapor equilibrium, Chem. Educ. Res. Pract., 14, 324–344.
- Al-Balushi S. M. and Al-Hajri S. H., (2014), Associating animations with concrete models to enhance students' comprehension of different visual representations in organic chemistry, Chem. Educ. Res. Pract., 15, 47–58.
- Ayas A., Özmen H. and Çalik M., (2010), Students' conceptions of the particulate nature of matter at secondary and tertiary level, Int. J. Sci. Math. Educ., 8, 165–184.
- Blanco A. and Prieto T., (1997), Pupils' views on how stirring and temperature affect the dissolution of a solid in a liquid: a cross-age study (12–18), Int. J. Sci. Educ., 19, 303–315.
- Çalik M., (2005), A cross-age study of different perspectives in solution chemistry from junior to senior high school, Int. J. Sci. Math. Educ., 3, 671–696.
- Çalik M., (2008), Facilitating students' conceptual understanding of boiling using a four-step constructivist teaching method, Res. Sci. Technol. Educ., 26(1), 59–74.
- Çalik M. and Ayas A., (2005), A comparison of level of understanding of grade 8 students and science student teachers related to selected chemistry concepts, J. Res. Sci. Teach., 42(6), 638–667.
- Çalik M., Ayas A. and Coll R. K., (2007a), Enhancing pre-service primary teachers' conceptual understanding of solution chemistry with conceptual change text, Int. J. Sci. Math. Educ., 5(1), 1–28.
- Çalik M., Ayas A., Coll R. K., Ünal S. and Coştu B., (2007b), Investigating the effectiveness of a constructivist-based teaching model on student understanding of the dissolution of gases in liquids, J. Sci. Educ. Technol., 16(3), 257–270.
- Çalik M., Ayas A. and Coll R.C., (2010), Investigating the effectiveness of teaching methods based on a four-step constructivist strategy, J. Sci. Educ. Technol., 19, 32–48.
- Chi M. T. H. and VanLehn K. A., (2012), Seeing deep structure from the interactions of surface features, Educ. Psychol., 47, 177–188.
- de Vos W. and Verdonk A., (1996), The particulate nature of matter in science education and in science, J. Res. Sci. Teach., 33, 657–664.
- Doran R., (1972), Misconceptions of selected science concepts held by elementary school students, J. Res. Sci. Teach., 9, 127–137.
- Driver R., Asoko H., Leach J., Mortimer E. and Scott P., (1994), Constructing scientific knowledge in the classroom, Educ. Res., 23, 5–12.
- Durmuş J. and Bayraktar S., (2010), Effects of conceptual change texts and laboratory experiments on fourth grade students' understanding of matter and change concepts, J. Sci. Educ. Technol., 19, 498–504.
- Ebenezer J. V., (2001), A hypermedia environment to explore and negotiate students' conceptions: animation of the solution process of table salt, J. Sci. Educ. Technol., 10, 73–92.
- Ebenezer J. V. and Erickson G. L., (1996), Chemistry students' conceptions of solubility: a phenomenography, Sci. Educ., 80, 181–201.
- Ebenezer J. V. and Gaskell P. J., (1995), Relational conceptual change in solution chemistry, Sci. Educ., 79, 1–17.
- Fensham P. and Fensham N., (1987), Description and frameworks of solutions and reactions in solutions, Res. Sci. Educ., 17, 139–148.
- Georghiades P., (2000), Beyond conceptual change learning in science education: focusing on transfer, durability and metacognition, Educ. Researcher, 42, 119–139.
- Goodwin A., (2002), Is salt melting when it dissolves in water? J. Chem. Educ., 79, 393–396.
- Green S. K. and Gredler M. E., (2002), A review and analysis of constructivism for school-based practice, School Psych. Rev., 31, 53–70.
- Griffiths A. K. and Preston K. R., (1992), Grade-12 students' misconceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach., 29, 611–628.
- Haidar A. H. and Abraham M. R., (1991), A comparison of applied and theoretical knowledge of concepts based on the particulate nature of matter, J. Res. Sci. Teach., 28, 919–938.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J Comput. Assist. Lear., 7, 75–83.
- Kelly R. M. and Jones L. L., (2007), Exploring how different features of animations of sodium chloride dissolution affect students' explanations, J. Sci. Educ. Technol., 16, 413–429.
- Landis J. R. and Koch G. G., (1977), The measurement of observer agreement for categorical data, Biometrics, 33, 159–174.
- Lee O., Eichinger D. C., Anderson C. W., Berkheimer G. D. and Blakeslee T. D., (1993), Changing middle school students' conceptions of matter and molecules, J. Res. Sci. Teach., 30, 249–270.
- Liu X., (2001), Synthesizing research on student conceptions in science, Int. J. Sci. Educ., 23, 55–81.
- Longden K., Black P. and Solomon J., (1991), Children's interpretation of dissolving, Int. J. Sci. Educ., 13, 59–68.
- Löfgren L. and Helldén G., (2008), Following young students' understanding of three phenomena in which transformations of matter occur, Int. J. Sci. Math. Educ., 6, 481–504.
- Mulford D. R. and Robinson W. R., (2002), An inventory for alternate conceptions among first-semester general chemistry students, J. Chem. Educ., 79, 739–744.
- Naah B. M. and Sanger M. J., (2012), Student misconceptions in writing balanced equations for dissolving ionic compounds in water, Chem. Educ. Res. Pract., 13, 186–194.
- Naah B. M. and Sanger M. J., (2013), Investigating students' understanding of the dissolving process, J. Sci. Educ. Technol., 22, 103–112.
- Nakhleh M. B., (1992), Why some students don't learn chemistry: chemical misconceptions, J. Chem. Educ., 69, 191–196.
- Nakhleh M. B. and Samarapungavan A., (1999), Elementary school children's beliefs about matter, J. Res. Sci. Teach., 36, 777–805.
- Nakhleh M. B., Samarapungavan A. and Saglam Y., (2005), Middle school students' beliefs about matter, J. Res. Sci. Teach., 42, 581–612.
- Novick S. and Nussbaum J., (1978), Junior high school pupils' understanding of the particulate nature of matter: an interview study, Sci. Educ., 62, 273–281.
- Novick S. and Nussbaum J., (1981), Pupils' understanding of the particulate nature of matter: a cross-age study, Sci. Educ., 65, 187–196.
- Özmen H., (2011), Effect of animation enhanced conceptual change texts on 6th grade students' understanding of the particulate nature of matter and transformation during phase changes, Comput. Educ., 57, 1114–1126.
- Piaget J., (1970), Piaget's theory, in Mussen P. H. (ed.), Carmichael's manual of child psychology, 3rd edn, New York: John Wiley & Sons, Inc., vol. 1, pp. 703–732.
- Pierri E., Karatrantou A. and Panagiotakopoulos C., (2008), Exploring the phenomenon of ‘change of phase’ of pure substances using the Microcomputer-Based-Laboratory (MBL) system, Chem. Educ. Res. Pract., 9, 234–239.
- Pınarbaşi T. and Canpolat N., (2003), Students' understanding of solution chemistry Concepts, J. Chem. Educ., 80, 1328–1332.
- Prieto T., Blanco A. and Rodriguez A., (1989), The ideas of 11 to 14-year-old students about the nature of solutions, Int. J. Sci. Educ., 11, 451–463.
- Rahayu S. and Kita M., (2010), An analysis of Indonesian and Japanese students' understandings of macroscopic and submicroscopic levels of representing matter and its changes, Int. J. Sci. Math. Educ., 8, 667–688.
- Schwartz D. L., Chase C. C. and Bransford J. D., (2012), Resisting overzealous transfer: coordinating previously successful routines with needs for new learning, Educ. Psychol., 47, 204–214.
- She H., (2004), Facilitating changes in ninth grade students' understanding of dissolution and diffusion through DSLM instruction, Res. Sci. Educ., 34, 503–525.
- Smith K. C. and Nakhleh M. B., (2011), University students' conceptions of bonding in melting and dissolving phenomena, Chem. Educ. Res. Pract., 12, 398–408.
- Stavy R., (1990), Children's conception of changes in the state of matter: from liquid (or solid) to gas, J. Res. Sci. Teach., 27, 247–266.
- Uzuntiryaki E. and Geban O., (2005), Effect of conceptual change approach accompanied with concept mapping on understanding of solution concepts, Instr. Sci., 33, 311–339.
- Valanides N., (2000), Primary student teachers' understanding of the particulate nature of matter and its transformations during dissolving, Chem. Educ.: Res. Pract. Eur., 1, 249–262.
- Yezierski E. J. and Birk J. P., (2006), Misconceptions about the particulate nature of matter: using animations to close the gender gap, J. Chem. Educ., 83, 954–960.
| This journal is © The Royal Society of Chemistry 2015 |