Beyond problem-based learning: using dynamic PBL in chemistry
Tina L.
Overton
*a and
Christopher A.
Randles
b
aMonash University, Chemistry, Clayton Campus, Melbourne, Victoria, Australia. E-mail: tina.overton@monash.edu
bUniversity of Hull, Chemistry, Hull, UK
First published on 5th January 2015
Abstract
This paper describes the development and implementation of a novel pedagogy, dynamic problem-based learning. The pedagogy utilises real-world problems that evolve throughout the problem-based learning activity and provide students with choice and different data sets. This new dynamic problem-based learning approach was utilised to teach sustainable development to first year chemistry undergraduates. Results indicate that the resources described here motivated students to learn about sustainability and successfully developed a range of transferable skills.
Introduction
Problem-based learning (PBL) first appeared in 1969 as a new approach to medical education at McMaster University in Canada. It was developed as an educational approach drawing on philosophy, psychology, and educational research. According to Barrows (Barrows, 1996), PBL can be explained as “the learning that results from the process of working toward the understanding or resolution of a problem”. Savery and Thomas (Savery and Duffy, 1995) have used Barrow's model to demonstrate that PBL fits easily within the framework for effective learning described by the constructivist learning theory. Constructivism is a philosophy of learning founded on the premise that, by reflecting on our experiences, we construct our own understanding of the world we live in. PBL learning is a process of building on prior knowledge, problem solving, using critical thinking approaches and reflecting (Maudsley, 2000).According to Barrows (1996) PBL refers to learning which is facilitated by six core features:
(1) Learning is student-centred;
(2) Learning occurs in small peer-groups;
(3) Teachers act as facilitators;
(4) Problems provide the organising focus and stimulus for learning;
(5) Problems are the vehicle for the development of problem-solving skills;
(6) New information is acquired through self-directed learning.
In this way, PBL aims to facilitate students' self-learning (Johnstone and Otis, 2006). As a result of the problem solving process, the outcomes for students who learn through the PBL pedagogy are; that they are able to think critically and are able to recognise and solve complex, real-world problems by identifying and evaluating information sources, that they can work effectively in small groups, that they can demonstrate versatile communication skills and that they can use knowledge and intellectual skills to become independent and lifelong learners (Duch et al., 2001).
It has been suggested that problem-based curricula can lead to increased retention of knowledge, enhanced integration of basic scientific concepts, development of self-directed learning skills and increasing intrinsic interest in the subject matter being learned (Dolmans and Schmidt, 1996). PBL students have been found to be more likely to study for meaning than conventional students (Newble and Clarke, 1986), though Groves (Groves, 2005) suggests that this is unlikely to occur if the assessment is not supportive or the work load is excessive.
PBL is one of several related pedagogies that are designed to engage students in active learning and enhance learning outcomes. Like PBL, context-based learning (CBL) uses a real life context to motivate learners but, unlike PBL, may not involve problem solving. CBL has been used extensively in secondary science education as the underlying design principle of whole curricula (Ramsden, 1997; Bennett and Luben, 2006; Parchmann et al., 2006). Process-oriented guided inquiry learning (POGIL) has become popular in chemistry in higher education and uses an inquiry or problem-based approach, without necessarily using a real life context (Farrell et al., 1999).
The implementation of PBL in chemistry in higher education has been growing steadily. Belt et al. have produced a suite of PBL resources for analytical chemistry drawing on contexts in industrial, pharmaceutical, environmental and forensic chemistry. (Belt et al., 2002; Summerfield et al., 2003; Belt and Overton, 2007). These resources deliver learning outcomes in analytical chemistry as well as a range of transferable skills. Wang has also produced PBL resources for learning of analytical chemistry (Wang, 2003). Green chemistry has been used as a context for PBL in chemistry (Grant et al., 2004; Heaton et al., 2006) where the aim has been to raise the issue of green chemistry as it relates to the chemical industry. PBL has been employed to deliver learning on sustainable development where the focus has been on the chemistry and economics of energy generation (Belt et al., 2005; Williams and Parker, 2012) In another example, sport was used as the context to meet learning outcomes in biochemistry, simple thermodynamics and materials chemistry (Potter and Overton, 2006). Environmental chemistry is another context that lends itself to delivery of the chemistry curriculum (Kegley et al., 1996). It might be expected that the traditional branches of chemistry, inorganic, organic and physical, would be more difficult to deliver via problem based learning as the applications and real life contexts are less obvious. Some success has been achieved, however, and a collection of resources in these braches has been published by the Royal Society of Chemistry (RSC). The PBL approach has also been applied successfully to learning in the undergraduate chemistry laboratory. McGarvey has collaborated with industry to produce a suite of physical chemistry experiments (McGarvey, 2004) and McDonnell et al. have produced PBL mini-projects which utilise contexts such as cosmetics, food and forensic science (McDonnell et al., 2007). Tosun and Cenocak (2013) implemented PBL chemistry laboratories for trainee teachers finding enhanced motivation for the subject for those with a weak science background.
PBL is different from conventional forms of learning in chemistry in that the students work in teams throughout the process and move towards a solution to the problem scenario together by gathering and sharing information and ideas. There are several formal models of PBL and these are strictly adhered to in some disciplines, particularly medicine and associated professional disciplines, such as nursing (Boud and Feletti, 1998). As PBL is relatively new in the sciences, practitioners are developing flexible models and implementing them in ways that suit their own particular context. However, the common features of a PBL pedagogy are the use of a real world context for the problem scenario, group work, complex problem solving, the acquisition of new knowledge, and assessment through the presentation of outcomes or product. However, the problem scenarios used in PBL are usually static; that is, the problem that students produce a solution for is the same as the one that they were first presented with.
Usually, all groups of students work with the same scenario and same data sets. This situation is easy to manage in the classroom and is relatively easy to assess, but it is somewhat artificial and does not mirror real-world problem solving, where the context and scope of the problem may vary over time. The study described here utilized dynamic problem scenarios in order to simulate a more realistic problem solving experience for students and to develop problem solving skills that more closely resemble those used outside of education. Our new model of PBL, dynamic problem-based learning (dPBL), uses scenarios in which the parameters of the problem change over time, provide different groups of students with different data sets, or provide different routes through the problem for students. Thus, each group of students will have tackled an individualized problem.
PBL is most readily implemented in applied aspects of chemistry where the real-life contexts are easily identified. One such applied topic in chemistry is sustainable development which appears as a topic in many modern chemistry curricula. Sustainable development is defined as “development that meets the needs of the present without compromising the ability of future generations to meet their own needs” (UN, 1992). In the context of chemistry, green chemistry falls under this overarching definition. The principles of green chemistry are well established (Anastas, 2011) and the twelve principles of green chemistry were published nearly 20 years ago (Anastas and Warner, 1998). Applications of the principles of green chemistry have informed the development of undergraduate laboratory activities (Dicks, 2012; Landon et al., 2014) and some classroom activities (Andraos and Dicks, 2012). Much of the focus in the implementation of green chemistry in the curricula has been on more effective and efficient organic synthesis (Andraos, 2012), the replacement of solvents (Aktoudianakis et al., 2009) or catalysis (Clarke et al., 2012; Dicks and Batey, 2013). Andraos and Dicks (2012) state that quantification of energy consumption and costs for chemical reactions have still to receive attention from research and teaching perspectives.
Sustainable development in its broadest sense encompasses more than the principles of green chemistry. Issues still of interest to chemists include supply chain management (Foerstl et al., 2010), energy consumption (International Energy Agency, 2013), waste management (Christ, 2008), environmental impact, ecology and human living conditions. An implementation of dynamic PBL designed to deliver learning in sustainable development is described here.
Methodology
What is dynamic PBL?
The general pedagogy for PBL involves several steps;• students are divided into groups and presented with the problem,
• students brainstorm in order to clarify the nature of the problem and identify their learning needs,
• students delegate roles within the groups and share existing knowledge,
• outside the classroom the students engage in independent study,
• students come together in a group to share and critically evaluate the resources and information they have gathered,
• students work towards a solution.
This cycle of independent study, group interaction and critical analysis is repeated as many times as dictated by the problem. Eventually the students present their solution and reflect on the process and solution. Throughout the process the tutor's role is one of guidance and support.
This basic organizational structure was adhered to in the dPBL activity. However, at the outset of the activity the groups of students were presented with a choice of problem scenarios. Four scenarios were developed, each designed to deliver the same learning out comes, but the contexts were all different. In addition, each group was given the opportunity to select supporting information. Thus, each group commenced the information retrieval and problem solving stages with a different set of data. In between some of the cycles of independent study, group interaction, and critical analysis the groups were presented with additional information in the form of events that impacted upon the problem in some way, either positively or negatively. Each group received different sets of additional information. Consequently, groups had a choice of scenario, received different initial data and had to cope with different impacts on the problem.
The dynamic PBL activity
One of the major challenges for the twenty first century is sustainable development. Chemists have an important role to play in developing sustainable industries, renewable energy sources, recycling and waste management amongst other issues (Jenck et al., 2004; Foerstl et al., 2010). It is important that graduate chemists have some appreciation of the role they may play in a chemical industry that increasingly focusses on sustainable development and in ensuring a sustainable futures society. This resource was designed to give students this insight.The study described here was practise-based action research (Mills, 2003). The aim of the study was to investigate whether dynamic PBL was readily implementable, and how it impacted in students in terms of learning and attitudes to the activity. The learning activities were part of a module offered to first year undergraduate chemists. The undergraduates undertook the activities without any awareness that they were new or novel. Evaluation data was collected from all students at the end of the learning activities through a questionnaire with open and closed questions, but before the students had received the results of their assessed work. As this was part of a regular teaching activity no specific ethical clearance was required. However, students were informed in writing that their evaluation and assessment data would only be used anonymously and individuals would not be identified. They had the option to withdraw from the evaluation activities.
The cohort of students was 160 first year chemistry undergraduates per year. This paper resorts the outcomes of the first two years of implementation. They were divided into four groups of 40 students each of which was run by a facilitator and within each of these cohorts they were organised into smaller groups of 4–6 participants. The students were introduced to the activity with a short introduction to sustainability and were then given their brief. The brief informed them that they had been retained by a sustainable construction and consultancy company, Global Sustainable. They were to assist with a major contract and advise on design, economics, technology, energy and waste management. Each project had to be delivered within a constrained budget. Each 4–6 participant group then selected one of the four scenarios to work on.
Four different PBL scenarios that were developed.
Scenario 1 new development near the Physt River, USA
The task is to design a microgeneration sustainable village to house a population 480, implementing technology that minimises the impact on the local ecosystem.The constraints for this scenario included:
• no large scale industry can be moved into the area,
• care must be taken to reduce the environmental impact to both land and water,
• the site is an area of outstanding natural beauty, so no building can be more than two stories high,
• where possible local contracts must be used and 60% of all materials must be sourced locally,
• any repairs to the environment must be completed and not left for the local population,
• there is a budget of $160 million USD to complete the project.
Scenario 2 postgraduate campus in Hong Kong
The students are tasked to develop a self-sustaining postgraduate school in Hong Kong where all energy has to be produced and all waste processed on site. The school should provide education for at least 50 students and research facilities for 22 research scientists.The constraints for this scenario included:
• the site has been approved under the understanding that it will remain green and self-sustaining for both waste management and energy production,
• the school must have very low impact on the Nam Lang Shan environment,
• a centre of excellence in the development of green industrial processes must be created,
• there is a budget of HK$1 billion.
Scenario 3 green transport
In this scenario students must analyse the costs of producing biodiesel and bioethanol to be used in Midsummer's small fleet of 42 buses. Decide which biofuel is more productive and suitable to the company. Price the cost of reactants, plant and benefits of waste management.The constraints for this scenario included;
• This is a fleet of 240 buses which travel 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 miles a day,
000 miles a day,
• assess the feasibility of constructing their own biodiesel processing plant and the best reactants to use in its production,
• assess the feasibility of constructing their own bioethanol fermentation plant and the best reactants to use in its production,
• evaluate the best fuel mix,
• cost the production, implementation and running costs.
Scenario 4 greening Midsummer University
The aim of this activity is to develop a strategy for Midsummer University which has ambitions to be the world's greenest university.The chemistry department has been identified as having the highest water use. The accommodation department was identified as having the largest electricity consumption.
The constraints for this scenario included;
• Midsummer is a large community based university currently hosting around 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 students on various courses, with 3000 of them in university residency. Students are drawn from over 30 countries,
000 students on various courses, with 3000 of them in university residency. Students are drawn from over 30 countries,
• water and electrical usage are relatively high and wastage is increasing due the increase demand by the population for higher education,
• the chemistry department accounts for 30% of the university's annual water bill,
• the student accommodation has the largest bill for electricity consumption,
• the budget for implementation of new sustainable practices across the whole institution is £0.5 million.
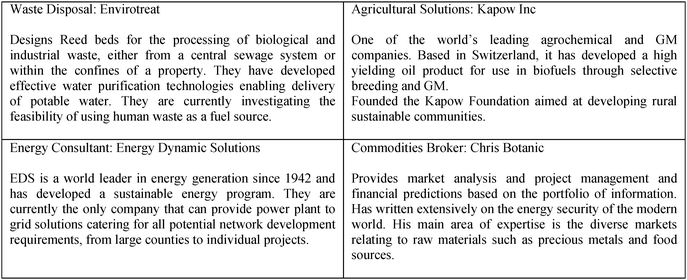
The four scenarios varied in popularity. The total number of students engaging with each scenario over the two cohorts was; scenario 1 N = 110, scenario 2 N = 50, scenario 3 N = 145 and scenario 4 N = 15. Once each group had chosen a scenario they were then invited to add six ‘experts’ to their team by selecting from a number of expert biographies (Fig. 1). Once selected, each ‘expert’ provided the students with a URL to an online paper of relevance to the activity and reflecting the chosen expertise. Thus each group began the activity with a different baseline set of supporting background information.
The activity then proceeded in the same way as a regular PBL activity. The students brainstormed in order to identify what they were being asked to achieve, the information requirements of the problem and their own knowledge gaps. They engaged in independent study and information retrieval outside the classroom and met together, both inside and outside classroom sessions, to share and critically evaluate the information found. When they came back together as a group in the classroom the tutor checked their progress and provided support and feedback.
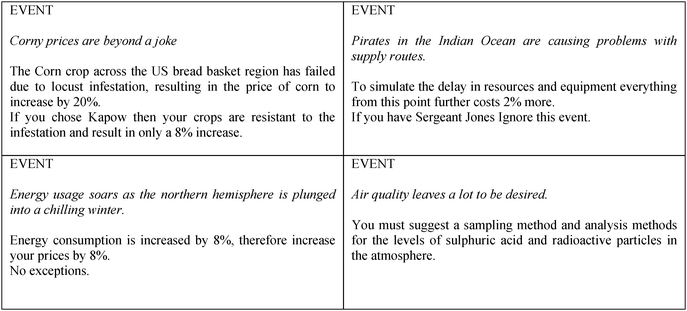
After the first cycle of independent study, reflection and reporting back, the tutor provided each group with a set of cards which contained information that had an impact on how the activity progressed (Fig. 2). The imposed changes covered a range of issues, for example, there were changes to economic conditions, new logistical issues introduced, changes to national or international legislation or issues related to personnel. The groups had to carefully consider how this additional information impacted on their scenario and adjust their thinking and their plans accordingly.
The activity ran over a period of five one-hour classroom sessions. The classroom sessions were supplemented by a resource bank which was made available on the virtual learning environment. The resource bank was used to provide information that is difficult to find, such as site-specific ecological and geological information, energy consumption and carbon footprint data, employment densities and a budget spreadsheet. This enabled the students to focus on finding the readily available information, using and critically evaluating that information and their plans. Students were given access to group online forums and some groups used these quite heavily, although most set up groups on social media. The schedule for the activity is shown in Table 1.
| Classroom session | Activity |
|---|---|
| 1 | Introduce activity. Students choose a scenario and select a ‘team’ of six experts. |
| 2 | Students report on progress. Tutor provides feedback and support. |
| 3 | Students report on progress. Tutor hands each group six external event cards. |
| 4 | Students report on progress. Tutor provides feedback and support. |
| 5 | Each group gives five minute presentation. Each groups submits a report and supporting material. Individuals complete peer assessment. |
The outcomes of the activity were assessed via a five minute summary group presentation (25%) and a group final report (75%). For the oral presentation students were limited to using a maximum of five slides and were assessed for the quality of the presentation and a summary of their outcomes (Table 2). For the final group report students were required to discuss site design, environmental impact of their design, full financial costings, methods of energy generation and energy use, employment, materials used, waste management and transport (Table 3). Students were encouraged to be creative and some groups produced maps and models to accompany their report. Marks for individual students were scaled by the use of peer assessment of each group member's contribution to the group activity (Conway et al., 1993). At the end of the activity each students scored the other members of their group on a scale of 0–100% for the individual students' contribution to the group activity. The group mark derived from the oral presentation and the report was then multiplied by the percentage obtained from the peer assessment to give an individual score for each student.
| 90–100 | Outstanding presentation, outstanding and focussed summary of main points. |
| 80–89 | Excellent presentation, excellent and focussed summary of main points. |
| 70–79 | Very good presentation, very good summary of main points. |
| 60–69 | Good presentation and summary of main points. |
| 50–59 | Adequate presentation and summary of main point s. Maybe unfocused or present too much detail. |
| 40–49 | Barely adequate presentation, poor focus on main points or includes too much or too little information. |
| 35–39 | Poor presentation, lacking organisation and information or presents too much detail. |
| 20–34 | Very poor attempt at presentation, very poor quality of information presented. |
| 1–19 | Unacceptable attempt. |
| 0 | No submission. |
| 90–100 | Creative, comprehensive, outstanding presentation, polished and fluent writing, extremely well argued and critical, demonstrating outstanding understanding of principles of sustainable development. Difficult to improve at this level. |
| 80–89 | Comprehensive, critical and coherently argued assignment with polished and fluent writing, excellent presentation, demonstrating excellent understanding of principles of sustainable development. |
| 70–79 | Sound, critical and coherently argued assignment, lacking the fluency or polish of a higher grade. Demonstrating very good understanding of principles of sustainable development. |
| 60–69 | Good all-round assignment, but may lack innovation or originality, average quality of writing and presentation. Demonstrating good understanding of principles of sustainable development. |
| 50–59 | Adequate assignment, but descriptive rather than analytical, adequate quality of writing and presentation, demonstrating average understanding of principles of sustainable development. |
| 40–49 | Descriptive assignment, may be factually correct but have substantial errors in interpretation, demonstrating weak understanding of sustainable development. Some problems with fluency of writing and presentation. |
| 35–39 | Incomplete assignment, very brief, poor writing and presentation, demonstrates poor understanding of sustainable development. |
| 20–34 | Incomplete assignment, very poor writing and presentation, with fundamental misunderstandings of sustainable development. |
| 1–19 | Unacceptable and/or incomplete, with little attempt made. |
| 0 | No submission. |
Results
The activity has been run for two successive years and the combined results for those two cohorts are described here.The four scenarios all supported learning around the principles of sustainable development. They each had a slightly different focus and some were more interdisciplinary than others. Students had free choice of scenario and could therefore follow their own interests.
Scenario 1, the Physt River development, required students to think carefully about renewable energy production, balancing the needs of the community with possible energy generated. There were constraints imposed by the are being designation of outstanding natural beauty and its particular ecological and geological features. Students also had to plan a living habitable environment for 450 residents and had to consider their requirements, balancing their quality of life against cost to build. This scenario proved very popular with students with 35% of the students over both years choosing to tackle it.
Scenario 2, the postgraduate centre in Hong Kong, proved less popular with 15% of students choosing to tackle this activity. The main focus was on the use of renewable energy generation, waste processing and the use of innovative building materials. What made this development challenging was the mountain environment and lack of available cleared building land.
Scenario 3 was the biofuels challenge. This scenario was clearly the most chemistry-related and proved the most popular with the chemistry undergraduates with 45% choosing this one. The focus of this activity was supply chain management; choosing synthetic routes based on energy use and atom efficiency and waste management, very much fulfilling the criteria mentioned by Andraos and Dicks (2012).
Scenario 4, the greener university activity, required students to consider a range of issues including waste management, recycling, energy use, renewable energy production, transport and living accommodation. This scenario proved very unpopular with only 5% of students choosing it. This scenario was obviously not very engaging for undergraduate chemistry students, even though the context was very relevant to them. Incidentally, this scenario has since been used with student teachers in a course on sustainable schools and was engaged with very enthusiastically. For those students the context is directly relevant to that module.
The quality of the outputs produced by the students was very high on each occasion, with very creative designs and in depth discussion of the issues involved. The marks awarded averaged around 65% each year and ranged from 50% to 80%. For example, a typical report receiving 80% for the Physt River project was presented in a very professional was and provided a detail plan of the proposed village development, made detailed calculations of the costs and energy efficiencies of different types of housing, opting for a mixed housing stock. This group calculated the energy needs of the community and proposed biomass energy generation with the energy shortfall being provided by solar energy. Local amenities and services were planned that provided enough employment for all local residents, thus reducing the need for commuting. There were impressive plans in place for water treatment and substantial recycling in order to keep landfill to a minimum. Consideration was given to the environment and wildlife. The financial costings were detailed and the project was delivered under budget. By contrast, a report that received 50% was not very well presented and used a very superficial treatment. The proposals were descriptive rather than quantitative with energy generation and use calculations not being carried out.
Of the four possible choices, it is perhaps not surprising that scenario 3 was the most popular choice with chemistry undergraduates as it was clearly the most chemistry-based and they are already interested in and committed to chemistry. However, scenario 1 was not far behind in terms of popularity and qualitatively produced some of the most creative outcomes. It is pleasing that students tackling scenarios 1, 2 and 4 were happy to move outside of their tightly defined discipline area and tackle something more interdisciplinary, as they will have to in the workplace.
A questionnaire was delivered to students at the end of the activity. The students were all first year undergraduates. The students' ethnicity or gender was not included in the questionnaire so the data could not be analysed for this variables. Also, the questionnaire treated the activity as one learning experience so did not differentiate between the four different scenarios and did not take account of other differing dynamic elements, such as external events. The questionnaire contained closed likert-type questions with some open response questions (Table 4). Analysis of the questionnaire indicated that the majority of the students (94%) understood the relevance of sustainable development and appreciated the role that chemistry plays in it. They recognised that they had developed problem solving and communication skills with over 80% agreeing that these skills had been enhanced.
| Question | SA/N (%) | A/N (%) | N/N (%) | D/N (%) | SD/N (%) |
|---|---|---|---|---|---|
| Creating a sustainable community was interesting. | 198 (62) | 102 (32) | 0 (0) | 16 (5) | 4 (1) |
| Sustainability is an important subject. | 198 (62) | 102 (32) | 0 (0) | 20 (6) | 0 |
| Chemistry plays an important role in sustainability. | 198 (62) | 102 (32) | 0 | 20 (6) | 0 |
| I liked having a having choice of project. | 198 (62) | 102 (32) | 0 | 16 (5) | 4 (1) |
| I have learnt about sustainability in more depth than studying in a convention way. | 198 (62) | 102 (32) | 0 | 16 (5) | (1) |
| The problems were more like real-life than in a conventional course. | 198 (62) | 102 (32) | 0 | 20 (6) | 0 |
| We came up with more than one solution? | 221 (69) | 29 (9) | 0 | 70 (22) | 0 |
| This project developed my problem solving skills? | 170 (53) | 105 (33) | 0 | 45 (14) | 0 |
| This project developed my communication skills? | 170 (53) | 105 (33) | 0 | 45 (14) | 0 |
| This project developed my group work skills? | 170 (53) | 105 (33) | 0 | 45 (14) | 0 |
In terms of the dPBL pedagogy, they thought that the problem scenarios were realistic and that they had undertaken deep learning compared to deep methods. The dynamic elements were well received. Over 90% of the students liked having a choice of project. The introduction of the changes to the problem context was dealt with very well by the students. The tutors anticipated that introducing these changes might not be well received but the students understood the value of them making comments such as ‘we were apprehensive, but it was good to see how adaptive our ideas were’, ‘they made the project more interesting’, ‘it was good, we had to adapt’ and ‘I liked it and had to think about what to do’.
The students were asked ‘Has this activity developed critical and analytical skills?’. Their responses were very positive with typical comments such as ‘definitely more than regular lectures’, ‘It’s been fantastic’. Some students, whilst being positive about the approach and seeing its value in this topic, did not advocate large scale implementation: ‘Yes its great but wouldn't want to do everything this way’.
Discussion
This paper discusses the development of a resource used to teach undergraduates the relevance and importance of sustainable development and chemistry's role within it. It also discusses the first implementation of dynamic problem-based learning pedagogy.In terms of sustainable development education the activity has successfully engaged students with the issues with students' understanding its relevance and having experienced a simulated sustainable development project. Students have gained an appreciation of issues such as renewable energy, energy cost and usage, waste management, recycling, atom economy, employment densities, planning and budgeting. The activities required students to take a quantitative rather than descriptive approach, emphasising for students the fact that sustainability makes quantitative differences to industry, the environment and quality of life. The requirements for an education in sustainable chemistry as discussed by Andreas and Dicks (2012) have been largely met and even filled some of the knowledge gaps that they identified in their review.
There were three elements to the dynamic problem-based learning pedagogy: students' choice of scenario, different initial background data and the shift in problem context. The choice in scenario was appreciated by the students with an overwhelming majority of them reporting positively on this aspect. It has been previously reported that allowing students to have choice allows them to feel in control of their own learning and develops self-motivation, persistence and responsibility (McCombs and Miller, 2007). The fact that each group of students, even when tackling the same scenario, had a slightly different set of initial background data increased the individualized learning experiences of the students and modelled ‘real’ problem solving for them in the classroom (Smith et al., 1995). Further realism was introduced by the events cards which were administered during the activity. The events cards exposed students to issues that may impact on chemists in the workplace, such as economics, legislation, logistics and supply chain. Students dealt with this sudden introduction of uncertainty very well and provided very positive feedback on the experience. This aspect of the dynamic problem-based learning is the one that gives students the best insight into how chemists solve real-life problems, as complex problems often evolve over time, due to internal or external factors.
Problem-based learning is well understood to develop enhanced transferable skills in students (Savin-Baden, 2003) and this variant was no exception to that. Students self-reported enhanced communication, group work and problem solving skills.
This paper reports an initial implementation of a new pedagogy. The aim of the study was to investigate whether dynamic PBL was readily implementable, and how it impacted in students in terms of learning and attitudes to the activity. The dynamic elements of the activity were implemented without adverse effects on students' attitudes or motivation. The usual outcomes of PBL in terms of skills development were also observed. So as a ‘proof of concept’ study it has been successful. However, this is a small scale study with limited data. It would be desirable to investigate new implementations of dPBL in other areas of chemistry and to more fully explore learning gains for students’’ in terms of knowledge and skills.
Conclusions
This paper describes the successful implementation of a variant of problem-based learning, referred to here as dynamic problem-based learning. In this variant the students experienced ownership of their learning through a choice of problem scenario and individualised data. Their experience of solving a ‘real-life’ problem was enhanced by introducing external change factors within the problem-based learning cycle. Students dealt effectively with these dynamic elements and reported enhanced problem solving, communication and group working skills.The scenarios focused on sustainable development. The scenarios required students to consider energy generation and use, energy costs, synthetic routes, supply chain, waste management and recycling. Of the four scenarios the most chemistry-focussed one was the most popular, but was closely followed by one which had a very multidisciplinary context.
The use of problem-based learning in chemistry is steadily increasing. One of the common features of it is that students often feel that they are all working on the same problem and can produce different but similar products for assessment. The dynamic variant of problem-based learning means students have more ownership of their learning and experience more life-like problems solving. Such dynamic elements would be relatively simple to introduce into existing problem-based learning scenarios.
References
- Aktoudianakis E., Chan E., Edward A. R., Jarosz I., Lee V., Mui L., Thatipamala S. S. and Dicks A. P., (2009), Comparing the traditional and the modern – a greener, solvent-free dihydropyrimidone synthesis, J. Chem. Educ., 86, 730–732.
- Anastas P., (2011), Twenty years of green chemistry, Science, 259, 1538–1541.
- Anastas P. T. and Warner J. C., (1998), Green chemistry: theory and practice, New York: Oxford University Press.
- Andraos J., (2012), Designing a green organic chemistry lecture course, in Dicks A. P., (ed.), Green organic chemistry in lecture and laboratory, Boca Raton, FL: CRC Press, pp. 29–68.
- Andraos J. and Dicks A. P., (2012) Green chemistry teaching in higher education: a review of effective practices, Chem. Educ. Res. Pract., 13, 69–70.
- Barrows H. S., (1996), Problem-based learning in medicine and beyond: a brief overview, New Directions for Teaching and Learning, 1996, 68, 3–12.
- Belt S. T., Evans E. H., McCreedy T., Overton T. L. and Summerfield S., (2002), A problem based learning approach to analytical and applied Chemistry', Univ. Chem. Educ., 6(2), 65–72.
- Belt S. and Overton T. (2007), Context-based Case Studies in Analytical Chemistry, in Marbrouk P. A. (ed.), Active Learning: Models from the Analytical Sciences, Washington: ACS.
- Belt S. T., Leisvik M. J., Hyde A. J. and Overton T. L., (2005), Using a context-based approach to undergraduate chemistry teaching – a case study for introductory physical chemistry, Chem. Educ. Res. Pract., 6, 166–179.
- Bennett J. And Luben F., (2006), Context-based chemistry: the Slaters approach, Int. J. Sci. Educ., 28(9), 999–1015.
- Boud D. and Feletti G. (1998), The challenge of problem-based learning, London: Kogan Page.
- Christ C. (ed.), (2008), Production-integrated environmental protection and waste management in the chemical industry, Weinheim: Wiley VCH.
- Clarke R. A., Stock A. E. and Zovinka E. P., (2012), Metalloporphyrins as oxidation catalysts: moving towards “greener” chemistry in the inorganic teaching laboratory, J. Chem. Educ., 89, 271–275.
- Conway R., Kember D., Sivan A. and Wu M., (1993), Peer Assessment of an Individual 's Contribution to a Group Project, Assessment and Evaluation in Higher Education, 18(1), 45–56.
- Dicks A. P. (ed.), (2012), Green organic chemistry in lecture and laboratory, Boca Raton, FL: CRC Press.
- Dicks A. P. and Batey R. A., (2013), Greening the Organic Curriculum: Development of an Undergraduate Catalytic Chemistry Course, J. Chem. Educ., 2013, 90(4), 519–520.
- Dolmans D. and Schmidt H., (1996), The advantages of problem-based curricula, Postgraduate Medical Journal, 72, 535–538.
- Duch B. J., Groh S. E. and Allen D. E., (2001), in Duch B. J., Groh S. E. and Allen D. E. (ed.), The Power of Problem-based Learning: a practical “how to” guide for teaching undergraduate courses in any discipline, Virginia: Stylus, pp. 3–12.
- Farrell J.J., Moog R. S. and Spencer J. N., (1999), A Guided Inquiry Chemistry Course, J. Chem. Educ., 76, 570–574.
- Foerstl K., Reuter C., Hartmann E. and Blome C., (2010), Managing supplier sustainability risks in a dynamically changing environment – sustainable supplier management in the chemical industry, Journal of Purchasing and Supply Management, 16, 118–130.
- Grant S., Freer A. A., Winfield J. M., Gray C., Overton T. L. and Lennon D., (2004), An undergraduate teaching exercise that explores contemporary issues in the manufacture of titanium dioxide on the industrial scale, Green Chem., 6, 25–32.
- Groves M., (2005), Problem-based learning and learning approach: is there a relationship? Adv. Health Sci. Educ., 10, 315–326.
- Heaton A., Hodgson S., Overton T. and Powell R., (2006), The challenge to develop CFC (chlorofluorocarbon) replacements: a problem-based learning case study in green chemistry, Chem. Educ. Res. Pract., 7, 280–287.
- International Energy Agency, (2013), New roadmap explores technologies that improve chemical industry energy use, emissions intensity, http://www.iea.org/newsroomandevents/news/2013/june/name,38965,en.html, Accessed 21st November 2014.
- Jenck J. F., Agterberg F. and Droescher M. J., (2004), Products and processes for a sustainable chemical industry: a review of achievements and prospects, Green Chem., 6, 544–556.
- Johnstone A. H. and Otis K. H., (2006), Concept mapping in problem-based earning: a cautionary tale, Chem. Educ. Res. Pract., 7, 84–95.
- Kegley S., Stacy A. M. and Carroll M. K., (1996), Environmental chemistry in the general chemistry laboratory, Part I: A context-based approach to teaching chemistry, Chem. Educ., 1, 1–14.
- Landon J. G., Korolok K. J., Golmakani M. and Dicks A. P., (2014), Green chemistry decision-making in an upper-level undergraduate organic laboratory, J. Chem. Educ., 91, 1040–1043.
- Maudsley G., (2000), Promoting professional knowledge, experiential learning and critical thinking for medical students, Med. Educ., 34, 535–544.
- McCombs B. L. and Miller L., (2007), Learner-centered classroom practices and assessments: maximizing student motivation, learning, and achievement, Thousand Oaks, CA: Corwin Press.
- McDonnell C., O'Connor C. and Seery M. K., (2007), Developing practical chemistry skills by means of student-driven problem-based learning mini-projects, Chem. Educ. Res. Pract., 8, 130–139.
- McGarvey D. J., (2004), Experimenting with undergraduate practicals, Chem. Educ. Res. Pract., 8, 54–65.
- Mills G. E., (2003), Action Research: A Guide for the Teacher Researcher, New Jersey: Prentice Hall.
- Newble D. I. and Clarke R. M., (1986), The approaches to learning of students in a traditional and in an innovative problem-based medical school, Med. Educ., 20, 267–273.
- Parchmann I., Gräsel C., Baer A., Nentwig P., Demuth R. and Ralle B., (2006), “Chemie im Kontext”: a symbiotic implementation of a context-based teaching and learning approach, Int. J. Sci. Educ., 28(9), 1041–1062.
- Potter N. and Overton T. L., (2006), Chemistry in sport – context-based e-learning in chemistry, Chem. Educ. Res. Pract., 7, 195–202.
- Ramsden J. M., (1997), How does a context-based approach influence understanding of key chemical ideas at 16+? Int. J. Sci. Educ., 19, 697–710.
- Savery J. R. and Duffy T. M., (1995), Problem-based learning: an instructional model and its constructivist framework, Educ. Tech., 35, 31–37.
- Savin-Baden M., (2003), Facilitating Problem-based Learning, Milton Keynes: Open University Press.
- Smith C. A., Powell S. P. and Wood E. J., (1995), Problem-based learning and problem-solving skills, Biochem. Educ., 23(3), 149–152.
- Summerfield S., Overton T. and Belt S., (2003), Problem-solving case studies. Anal. Chem., 75(7), 181–182.
- Tosun C. and Cenocak E., (2013), The effects of problem-based learning on metacognitive awareness and attitudes toward chemistry of prospective teachers with different academic backgrounds, Aust. J. Teach. Educ., 38(3), 61–73.
- United Nations Conference on Environment and Development, Agenda 21, 1992.
- Wang Y., (2003), Using problem-based learning in teaching analytical chemistry, The China Papers, 2, 18–32.
- Williams D. and Parker K., (2012), The chemistry of energy, Learn Chemistry, London: Royal Society of Chemistry, http://www.rsc.org/learn-chemistry/content/filerepository/CMP/00/001/340/The%20Chemistry%20of%20Energy%20-%20Student%20Guide.pdf, accessed 21st November 2014.
| This journal is © The Royal Society of Chemistry 2015 |