Using concept mapping to uncover students' knowledge structures of chemical bonding concepts
Nikita L.
Burrows
and
Suazette Reid
Mooring
*
Department of Chemistry, Georgia State University, Atlanta, Georgia, USA. E-mail: smooring@gsu.edu
First published on 6th October 2014
Abstract
General chemistry is the first undergraduate course in which students further develop their understanding of fundamental chemical concepts. Many of these fundamental topics highlight the numerous conceptual interconnections present in chemistry. However, many students possess incoherent knowledge structures regarding these topics. Therefore, effective assessments are needed to identify these interconnections. The use of concept-mapping and think-aloud interviews to investigate the knowledge structures of undergraduate organic chemistry students' regarding bonding concepts is the focus of this research study. Herein, we spotlight the bonding concepts of electronegativity and polar covalent bonds. In essence, the study found that understanding of electronegativity was weak among students with low concept map scores (LS students) compared to students with high concept map scores (HS students). Additionally, several common misconceptions of electronegativity were revealed through student interviews. An examination of LS student interviews further revealed that a lack of understanding of electronegativity led to a misunderstanding of polar covalent bonding. The think-aloud interviews were a reflection of the connections students made with the concepts of electronegativity and polar covalent bonding in their concept maps. Implications for the chemistry curriculum are also presented.
Introduction and background
Chemistry courses are required for many students across science, technology, engineering and mathematics (STEM) fields. Many topics covered in general chemistry are fundamental to chemical understanding and are built upon as students advance to other courses such as organic chemistry and biochemistry. However, the research literature is clear that many students complete general chemistry but still lack conceptual understanding of several fundamental topics (Pickering, 1990; Sawrey, 1990; Nakhleh, 1993; Nakhleh and Mitchell, 1993; Mason et al., 1997; Cracolice et al., 2008). Conceptual difficulties have been uncovered in fundamental topics such as: (1) acids and bases (Calatayud et al., 2007; Lin and Chiu, 2007; Cartrette and Mayo, 2011; McClary and Talanquer, 2011a, 2011b), (2) Lewis structures (Nicoll, 2003; Cooper et al., 2010), and (3) chemical bonding (Peterson and Treagust, 1989; Peterson et al., 1989; Robinson, 1998; Birk and Kurtz, 1999; Tan and Treagust, 1999; Harrison and Treagust, 2000; Boo and Watson, 2001; Coll and Taylor, 2001; Coll and Treagust, 2001, 2002; Niaz, 2001, 2002; Nicoll, 2001a; Nahum et al., 2007; Othman et al., 2008; Luxford and Bretz, 2014).With these concerns in mind, chemical educators are giving more thought about what to teach, how to teach, and the appropriate order of topics in general chemistry (Lloyd and Spencer, 1994; Gillespie, 1997; Cooper, 2010; Cooper and Klymkowsky, 2013). Assessment of what students already know is an important component of making curriculum decisions (Ausubel, 1978; Holme et al., 2010). To this end, this study seeks to further investigate how concept maps can be used as an assessment of how students make connections among various interrelated concepts.
Knowledge structures
Chemistry is a complex subject that explores a number of abstract topics and concepts. The understanding of these topics necessitates that students make sense of a number of interrelated concepts and ideas; that is, that they develop coherent knowledge structures. In this study we define ‘knowledge structure’ as the schema in which students organize and relate various concepts in order to make sense of a particular topic (Novak and Cañas, 2006; Novak, 2010). Studies that compare novices and experts agree that experts have a more complex knowledge structure with many interconnections that are focused around fundamental concepts (Bransford et al., 2000). In contrast, novices tend to have limited knowledge structures with few connections and fewer cross connections. Consequently, if there are gaps in students' understanding or missing conceptual links, learning new material or incorporation of new concepts into a disjointed knowledge structure will be difficult (Taber and Coll, 2003).The notion of knowledge structure also emphasizes the complex nature of misconceptions. A misconception occurs when the understanding of a particular concept is different from the generally accepted scientific explanation (Taber, 2002). Much of chemistry education research has focused on student misconceptions (Singer et al., 2012). Additionally, there are several theories that attempt to describe the origin of misconceptions and how to elicit conceptual change. For example, Chi proposes that students' misconceptions can be put into three levels (Chi, 2008). These three levels are: (1) incorrect beliefs at the level of a single idea, (2) assigning concepts to incorrect categories, and (3) flawed mental models that apply to interrelated concepts. How these misconceptions are addressed depends on which level it resides. Misconceptions assigned to the third level are highly robust, resistant to change, and require the correction of several incorrect beliefs (Chi, 2005). Another perspective on misconceptions suggests that students' concepts are coherent, interrelated, and can be described as a naïve “theory” (Vosniadou, 1994). In contrast, diSessa proposed that students' concepts are not theory-like, but are fragments or pieces that are not put together in a coherent manner (diSessa and Sherin, 1998; diSessa, 2008). Regardless of which theory one ascribes to, they all suggest that an essential part of conceptual understanding is the relationship students make between concepts; that is, their knowledge structures. Essentially, the knowledge structure of a student gives insight into the organization and connections that a student has between various concepts (Novak and Cañas, 2006; Novak, 2010). Therefore tools that can correctly show a student's knowledge structure are beneficial to chemical educators.
Meaningful learning
Students do not arrive in the classroom with a clean slate to which new knowledge is added. Current research has moved towards a constructivist point of view that purports that knowledge is actively constructed by the learner (Bodner, 1986). In order for students' knowledge construction to be meaningful, three components are necessary: (1) the student must have some relevant prior knowledge to anchor to new knowledge, (2) the material to be learned must be meaningful in and of itself, and (3) the student must “consciously choose to non-arbitrarily incorporate this meaningful material into their existing knowledge” (Ausubel, 1978; Novak, 2010). If meaningful learning does not occur, rote learning takes precedence. As a result of rote learning, students are unable to effectively connect new information to their prior knowledge. Another consequence of rote learning is that new material is merely memorized, easily forgotten and not transferred (Novak and Gowin, 1984; Bretz, 2001). The theory of meaningful learning highlights the importance of general chemistry for upper level chemistry courses. Fundamentally, general chemistry provides crucial prior knowledge for the completion of other chemistry courses. One reason that students struggle in advanced courses such as organic chemistry and biochemistry is because their knowledge structures of fundamental chemistry concepts are lacking and incoherent.Assessments of what students already know is a critical component of curriculum change and design (Holme et al., 2010; Singer et al., 2012). Chemistry education researchers use a variety of tools to uncover students' conceptual understanding. These methods include think-aloud interviews (Bowen, 1994; Ericsson and Simon, 1998), concept inventories (Krause et al., 2004; Pavelich et al., 2004; Libarkin, 2008; McClary and Bretz, 2012; Barbera, 2013), and concept mapping (Ross and Munby, 1991; Nakhleh and Krajcik, 1994; Plotnick, 1997; Markow and Lonning, 1998; Nicoll et al., 2001; Ruiz-Primo et al., 2001a, 2001b; Francisco et al., 2002; Yin et al., 2005; Hay et al., 2008; Lopez et al., 2011; Greene et al., 2013).
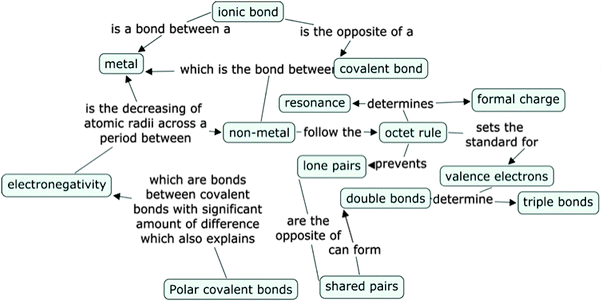
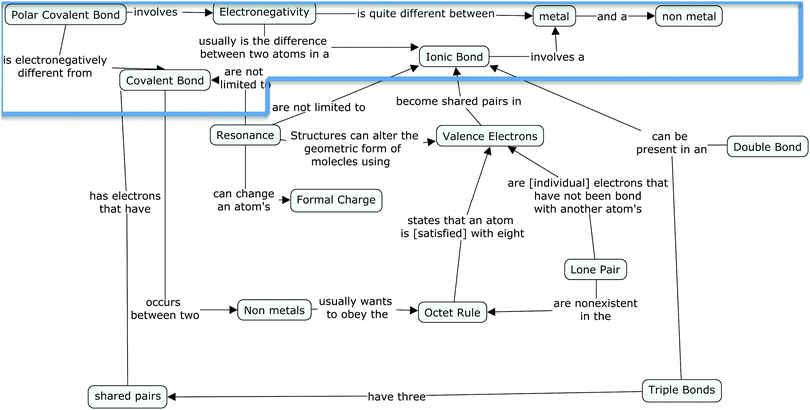
Concept mapping is an ideal tool for assessing the depth and breadth of students' knowledge structures; that is, concept maps can indicate how students organize information into their knowledge structure (Novak and Gowin, 1984). In addition, concept maps allow us to visualize how students relate various concepts to each other (Plotnick, 1997; Wheeldon and Faubert, 2009). Several studies have established the validity and utility of concept maps as an evaluation tool (Ross and Munby, 1991; Shavelson, 1993; Markham et al., 1994; Pendley et al., 1994; Markow and Lonning, 1998; Nicoll et al., 2001; Francisco et al., 2002; Van Zele et al., 2004; Shavelson et al., 2005; Lopez et al., 2011). Concept maps are graphical tools used to organize and represent an individual's knowledge by creating relationships between concepts in the form of propositions (Novak and Gowin, 1984; Novak and Cañas, 2006). Concept maps consist of three components – concept terms, linking arrows, and linking phrases. The linking arrows provide a directional relationship between two concepts while the linking phrases (words linking concepts) represent the specific relationships between a pair of concepts (Novak and Cañas, 2006) (Fig. 1).
There are several examples of the use of concept maps in chemistry in the research literature. For example, Nakhleh and Nicoll (Nakhleh and Krajcik, 1994; Nicoll et al., 2001) have used concept maps, generated by the researchers after open-ended interviews, to evaluate students' understandings of acid/base chemistry and bonding. However, our research study focuses on student-constructed concept maps in conjunction with interviews as a way of further probing students' responses on their concept maps. Assessment of student-generated concept maps has been extensively researched. For example, the Shavelson group has produced an extensive body of work establishing multiple ways of scoring concept maps and has validated their use in general chemistry and organic chemistry as assessment and research tools (Ruiz-Primo et al., 2001a, 2001b; Yin et al., 2005; Lopez et al., 2011; Szu et al., 2011). Their recent studies have demonstrated that concept maps can be used to represent students' knowledge structures in organic chemistry. Specifically, their studies showed that concept map scores were correlated with scores on problem sets and final course grades (Lopez et al., 2011) and that students knowledge structures as measured by concept maps was an indictor of success in organic chemistry (Lopez et al., 2014). There is still a need for additional studies, particularly in chemistry, that examine concept maps as an assessment tool. In other words, a determination of whether concept maps can measure students' knowledge structures of a particular topic. Therefore this present study was conducted by first having students' construct concept maps and then using think-aloud interviews to further investigate and verify the propositions they made in their concept maps.
Student understanding of chemical bonding
Without doubt, chemical bonding is an essential concept for chemists and is necessary for the understanding of chemistry. However, multiple studies have described numerous difficulties that students have with bonding concepts (Taber and Coll, 2003; Özmen, 2004). For instance, students have difficulty understanding why bonding occurs and provide incorrect explanations for bonding phenomena (Nicoll, 2001b). Many of these misconceptions are robust and remain even after instruction (Nicoll, 2001b; Taber and Coll, 2003; Özmen, 2004).In other studies, students confused ionic bonding with covalent bonding (Butts and Smith, 1987; Luxford and Bretz, 2014). In addition, others have shown that students were not clear about polar covalent bonding and covalent bonding and disregarded the role of electronegativity in polar covalent bonding (Peterson and Treagust, 1989). What is clear from these studies is that students' understanding of polar covalent bonding, bond polarity and related concepts such as intermolecular forces, bonding polarity, and electronegativity is fuzzy.
Some researchers have argued that the topic of polar covalent bonding is often presented in a problematic way, such that, students are left to interpret chemical bonding concepts in a multitude of ways (Teichert and Stacy, 2002; Bergqvist et al., 2013). Despite the widely understood notion that covalent bonding, polar covalent bonding and ionic bonding are a continuum, chemistry educators (Levy Nahum et al., 2010; Taber et al., 2012) and textbooks (Bergqvist et al., 2013) still present this information as three distinct types of bonding.
The students in this study are representative of those taught in a traditional chemistry curriculum in which bonding concepts are typically taught separately as ionic bonding, covalent bonding and polar covalent bonding. Hence, it is through these lenses that we are analyzing the data in this study as we explore students' understanding of bonding concepts. In this study, we are primarily interested in how students' knowledge structures regarding bonding concepts affect their explanations about bonding phenomena and whether we can use concept maps to ascertain students' knowledge structures.
We used the tools of concept mapping and think-aloud interviews to investigate students' knowledge structures of bonding concepts. We focused our study on students enrolled in the first-semester of an organic chemistry course, because we were interested in how their understanding of these topics has transferred from general chemistry. We employed a primarily qualitative research design (Bretz, 2008) to answer the following research questions:
(1) How well can concept maps uncover students' knowledge structures regarding aspects of chemical bonding?
(2) Are there differences in the explanations between students with high scoring concept maps (HS) and students with low scoring concept maps (LS) regarding aspects of chemical bonding?
Methodology
Participants and setting
The study presented here represents one interview of a three-interview study conducted at a large, urban, research-intensive university in the southeast United States. All students were enrolled in a four-credit, first-semester organic chemistry course. Participants completed one interview on each of three topics – Lewis structure and bonding, molecular geometry, and acids and bases. Herein, we will only focus on the first interview regarding bonding concepts. A total of sixteen undergraduate students (N = 16), participated in the bonding concepts interview.Purposeful homogenous sampling was used in recruiting participants for this study. Homogenous sampling was used since our goal was to describe a specific group (first-semester organic chemistry students) in-depth (Patton, 2002). Participants were recruited from an announcement made by one of the researchers on the first day of the course and by a follow-up e-mail. Interviews were scheduled within the first one and a half weeks of the course. Our aim was to assess the prior knowledge that students brought into the organic chemistry course from their general chemistry courses. Essentially, these students had taken one year of general chemistry and were enrolled in the first-semester of an organic chemistry course for the first time. At the time of the interview students were just beginning a review of general chemistry topics. Of the 16 participants, nine were biology majors, three were chemistry majors, three were psychology majors, and one student was a nursing major. Students in the study were identified as Asian (6 students) or African–American (10 students). Student grades in the pre-requisite general chemistry course varied from ‘A’ to ‘C’. Student participation in the study was voluntary and informed consent was obtained. Each student received a $10 gift card for participating in the interview. To protect their identity, their names were replaced with pseudonyms. The Institutional Review Board of the University approved the study in August 2012.
The interview
The interviews took place within the first two weeks of the Spring 2013 semester. Each student was individually interviewed in a private room and had access to a laptop computer for concept-mapping. Students were allowed as much time as they needed to complete the concept map. They typically spent about 40 minutes constructing the concept maps and approximately 35 minutes on the think-aloud portion of the interview.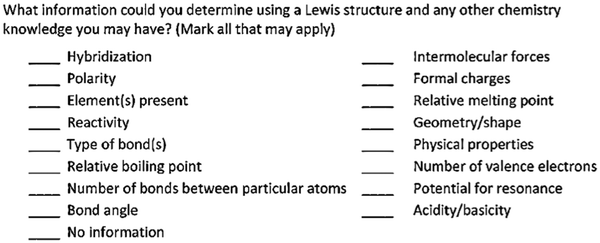 | ||
| Fig. 3 Implicit information from Lewis structures instrument (IILSI) (Cooper et al., 2012a). | ||
Data analysis
| Concept 1 | Linking phrase | Concept 2 | Grader 1 | Grader 2 | Average |
|---|---|---|---|---|---|
| Valence electrons | Can also be | Lone pair | 2 | 2 | 2 |
| Valence electrons | Can also be used to create a | Double bond | 2 | 2 | 2 |
| Valence electrons | Can also be used to create a | Triple bond | 2 | 2 | 2 |
| Valence electrons | Uses the extra electrons of a molecule called | Ionic bond | 0 | 0 | 0 |
| Lone pair | Is the opposite of a | Shared pair | 2 | 2 | 2 |
| Ionic bond | Deals with a | Metal | 2 | 2 | 2 |
| Ionic bond | Is the opposite of a | Covalent bond | 0 | 2 | 1 |
| Resonance | Structures use different types of bonds such as | Covalent bond | 2 | 1 | 1.5 |
| Covalent bond | Is related to | Polar covalent bond | 2 | 2 | 2 |
| Covalent bond | Deals with | Non-metal | 2 | 2 | 2 |
| Metal | Can be | Electronegativity | 0 | 0 | 0 |
| Metal | Have a positive | Formal charge | 1 | 0 | 0.5 |
| Electronegativity | Determines an atom's | Formal charge | 1 | 1 | 1 |
| Total score | 18 |
Results and discussion
Concept maps
Since only 16 students participated in the study, only the descriptive statistics are presented (Table 2). The average concept-map score and the salience score were obtained for each student.| Map components | Mean | SD | Minimum score | Maximum score |
|---|---|---|---|---|
| # of propositions | 14 | 3.0 | 10 | 21 |
| # of accurate prop (≥2) | 7 | 3.4 | 2 | 12 |
| Sum score | 23.6 | 7.0 | 12 | 35.5 |
| Salience score | 0.5 | 0.2 | 0.17 | 0.92 |
Students made an average of 14 propositions of which half were accurate. The average sum score on the participants' concept maps was 24. Concept maps scores ranged from 12 to 35.
The sums of the concept map scores were used to partition the students into high, medium and low scorers in three terciles (thirds). These terciles provided an equal distribution of the students into three groups. The cut off points for the 33rd percentile and 66th percentile were 19.8 and 28.0 respectively (Table 3). The qualitative data also verified that these students belonged to the groups assigned by the terciles. These divisions gave us a way of comparing students with low scoring concept map scores (LS) to students with high scoring concept map scores (HS) (Fig. 4).
| Student | Concept map score | Correct answer before distractors shown? | Correct answer after distractors shown? |
|---|---|---|---|
| Lori | 12 | No | No |
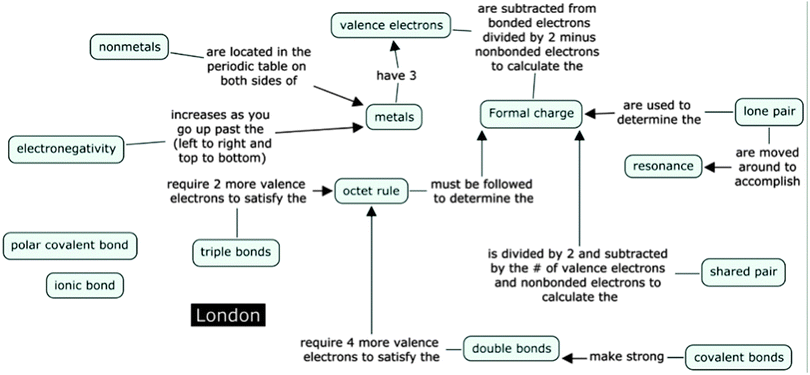
| London | 14 | No | No |
| Linda | 16.5 | No | Yes |
| Liza | 18 | No | No |
| Luanne | 19.5 | Yes | No |
| Alexa | 20 | Yes | Yes |
| Angel | 20 | Yes | No |
| Ashley | 23 | Yes | Yes |
| Ana-Marie | 24 | No | No |
| Abby | 24 | Yes | Yes |
| Ayden | 27.5 | Yes | No |
| Harper | 29 | Yes | Yes |
| Haley | 31 | Yes | Yes |
| Helen | 31 | Yes | Yes |
| Hilda | 32.5 | Yes | Yes |
| Holly | 35.5 | Yes | Yes |
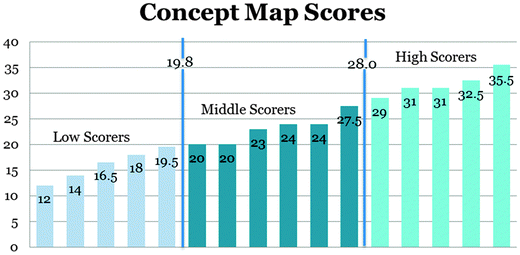 | ||
| Fig. 4 Graph showing the distribution of concept map scores for the 16 participants into low, medium and high scoring concept maps. | ||
Think-alouds
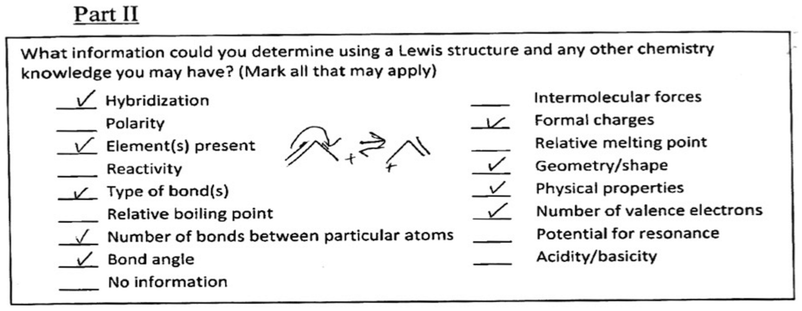
A number of recurring themes emerged regarding the topics of electronegativity and polar covalent bonds. The concept maps for all of the participants were evaluated for the connections they made with these concepts. Additionally, each student's interview was evaluated to determine which interviews from the LS and HS groups had the richest data.During the interview each participant was presented with a question from the Peterson and Treagust bonding concept inventory (Peterson and Treagust, 1989; Peterson et al., 1989) to probe their understanding of electronegativity and polar bonding (Fig. 5). Students were familiar with this type of representation, since it was used in their general chemistry course and in their textbook. They were initially presented with the main question without the four distractors and asked to predict the position of a shared electron pair between the HF molecule. After their initial explanation, students were shown the distractors and asked to choose an answer.
 | ||
| Fig. 5 Electronegativity probing question (Peterson and Treagust, 1989). | ||
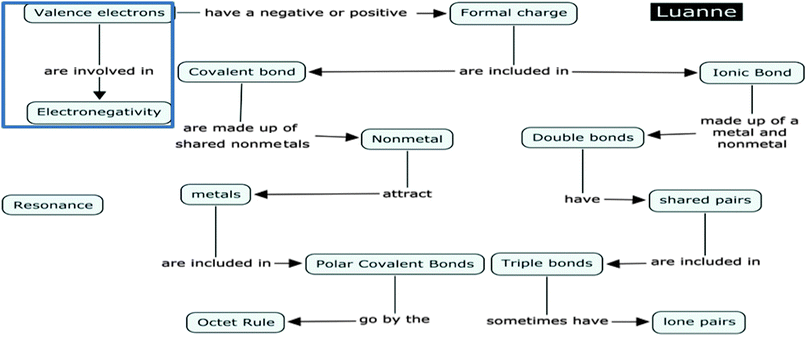
Understanding of electronegativity was weak among the LS students compared to the HS students. Several common misconceptions of electronegativity were revealed through student interviews. The most prevalent misconception was that “electronegativity is determined by the number of electrons around an atom.” This particular misconception was also uncovered by the Peterson and Treagust (1989) study. One example of this misconception comes from a senior undergraduate student, Luanne. Luanne had a concept map score of 19.5 and circled the first answer. This indicated that she believed the shared electron pair in the HF molecule would gravitate more towards the fluorine atom. Further probing revealed that despite her correct response, she possessed flawed ideas. After seeing the distractors she responded:
Luanne: I chose D because it says, ‘Fluorine is the larger of the two atoms and hence exerts greater control of the shared electron pair.’ I chose that because according to the number of valence electrons, it has seven and hydrogen has one, so therefore, when you're thinking of electronegativity, it pulls more [directs hands in a pulling motion] – it pretty much, like, since they are non-metal, it wants more electrons than hydrogen does. The hydrogen always gives away and the fluorine always gets because they're trying to fulfill the octet rule.
Here we see that Luanne views electronegativity as a property that has to do with the number of valence electrons. Closer inspection of her concept map regarding electronegativity also indicated that Luanne had this misconception of electronegativity that involves valence electrons. Her concept map proposition states: ‘Valence Electrons are involved in Electronegativity’ (Fig. 6).
Further examination of Luanne's interview reveals a lack of understanding of electronegativity, which in turn leads to a misunderstanding of polar and ionic bonding. In her interview she stated, “The hydrogen always gives away and the fluorine always gets because they're trying to fulfill the octet rule.” Here Luanne seems to be categorizing HF as an ionic bond rather than a polar covalent bond. Her concept map also highlights her confusion between ionic and polar covalent bonds. Her concept map proposition linking formal charge was: “Formal charge are included in ionic bond”. This proposition received a score of 0.5 and seems to imply that she associates formal charge with ionic bonding.
Another common misconception revealed during the interviews was the belief that shared electron pairs should be centrally located. As observed in previous studies (Peterson et al., 1986; Nicoll, 2001a), the position of the shared electron pair was often stated as centrally located by LS students. A good example of this comes from London, a senior pre-medical student with a low scoring concept map score of 14. During the interview, London circled the HF molecule with the shared pair centrally located and defended his answer by saying:
London: [points to picture with electron equally between fluorine and hydrogen] I'm thinking it's this one because it just like – because there's nothing over here at all [points to picture that has electrons closer to fluorine]. But yeah, I mean I've never seen anything like quite like this before though. Like I've never seen this before or like that. Because like I think H is just there, and like I don't know.
Interviewer: What do you mean by the H is just there [referencing first drawing]?
London: Like it's [H molecule] over by itself. That's why I would think it's this [points to centrally located pair drawing] because like over here in this thing [referencing first drawing], you kind of don't even see this. It's supposed to be HF, but this is – I don't know, I'll say that. I don't know.
Interviewer: [Turn over paper to show distractors] So similarly you can choose the best reason or fill in your own.
London: Yeah, this sound about right [circles B – As hydrogen and fluorine form a covalent bond the electron pair must be centrally located].
Interviewer: Why did you choose B?
London: Because B looks like – B like bread just like this sounds the same [as my reasoning] like because it said that the electron must be centrally located for him to form a covalent bond and that's what exactly what this looks like. Because the electron pair is centrally located, so I guess they're about to form a covalent bond.
Throughout the entire interview London never made any mention of electronegativity despite being questioned about polarity. London made no connections with the term ‘polarity’ on his concept map (see Fig. 7). In addition, London did not tick the word ‘polarity’ on the IILSI (Fig. 8). When probed as to why ‘polarity’ was not checked on the ILLSI London responded:
London: Because like on the last thing [referencing the concept map construction], I'm not like really familiar with that.
Interviewer: So in regards to, what do you know about polarity?
London: Like with water, like –
Interviewer: You can elaborate?
London: Like hydrophobic, hydrophilic and stuff like that. And polar like – because if something is polar that means it likes water. Yeah, so.
Interviewer: So polarity you don't associate with Lewis structure?
London: I don't, no. But I'm pretty sure that it's somewhere in there but I don't know.
Overall, London's interview confirms a limited understanding of electronegativity and polarity. The combination of interviews, problem sets and concept mapping highlighted students' inability to make meaningful connections among and between those concepts. London, like other LS students, did not have a clear understanding of the concept of electronegativity, which in turn connects to their limited understanding of polar covalent bonds and polarity.
In contrast, HS students displayed a good understanding of the concepts of electronegativity and polar bonding. Unlike the LS students, the HS students all checked the term polarity on their IILSI indicating that they understood that polarity was an implicit concept relating to Lewis structures. Table 4 shows a list of all the links made with polar covalent bond by the HS students. The majority of their propositions received a scored 2 or greater.
| Concept 1 | Linking phrase | Concept 2 | Link score |
|---|---|---|---|
| Polar covalent bonds | Is electronegatively different from | Covalent bond | 2 |
| Polar covalent bonds | Involves | Electronegativity | 2 |
| Electronegativity | Determines polarity | Polar covalent bond | 3 |
| Polar covalent bond | Are between two polar | nonmetals | 1 |
| Covalent bond | Has a sub group called | Polar covalent bond | 2 |
| Polar covalent bonds | Have | Lone pair | 1.5 |
| Covalent bond | With a net dipole moment is considered a | Polar covalent bond | 3 |
| Electronegativity | Determines whether or not a bond is a | Polar covalent bond | 2.5 |
| Polar covalent bond | Has between 0.4 and 2.0 in | Electronegativity | 2 |
Holly is a HS student with a concept map score of 35.5. Holly, unlike the LS students, has a clear understanding of the role electronegativity plays in the formation of different bond. This understanding is uncovered in her concept map (see Fig. 9 below) where she not only differentiates metal and non-metal electronegativity, but she also links electronegativity to polar covalent bonds and ionic bonds. She further identifies that the difference between a polar covalent bond and a covalent bond is electronegativity. Thus her concept map shows that she identifies that electronegativity is a deciding factor in the type of bond that would be formed.
During the interview Holly correctly chose the HF molecule with the shared electron pair closest to the fluorine atom (see Fig. 1). When asked about her reason for choosing that answer she responded:
Holly: [points to HF molecule with the shared electron pair closest to the fluorine] This one. Well, oh yeah [fluorine] is more electronegative, so fluorine would be more electronegative than hydrogen, therefore the electrons are pulled towards the fluorine atom, therefore this would be closer, meaning it's this one [circles HF molecule with the shared electron pair closest to the fluorine].
Interviewer: Okay. So why did you choose that?
Holly: Because the – in this one the electrons look like they're equally distributed between these two atoms, when it's – because this [fluorine] is more electronegative, it's [points to electron pair] more toward the more electronegative atom.
Interviewer: Okay. Based on this question can you choose an answer?
Holly: Okay [circles C – fluorine has a stronger attraction for the shared electron pair].
Interviewer: Okay, why didn't you choose D [fluorine is the greater of the two atoms and hence exerts more control over the electron pair]?
Holly: Oh, actually I didn't even read it yet. So, maybe I should read it. Can I just read it? Okay, I don't think size has to do with any effects of the electrical pull between two atoms. I think it's just really more of how polar the different atoms are.
Holly, unlike the LS students, has a clear understanding of the role electronegativity plays in directing the position of the shared electron pair in the HF molecule. Her understanding of electronegativity is further magnified by her ability to sort through why the distractor D (fluorine is the larger of the two atoms and thus exerts greater control over the shared electron pair) is incorrect.
Additionally, many LS students were confused between the periodic trend of size and electronegativity. For example, Lacy could not distinguish between size and electronegativity when looking at answers C (fluorine has a stronger attraction for the electron pair) and D (fluorine is the larger of the two atoms and hence exerts a great control over the shared electron pairs). Specifically, Lacy stated:
C and D is similar to me just kind of based on the fluorine. Not only is it larger, I mean, it is stronger. It has a stronger attraction…Fluorine would be – it does have a stronger attraction and a higher electronegativity. So I think that it would take – I was going to say it would take the H. But these answers are similar, I mean to me, just kind of – it's the larger of the two and it's exerts greater control. So I would change D and I'll use C instead because it does have a stronger attraction, which will bring the electron to the F.
The clarity to which HS students understand electronegativity is further exemplified in their recognition of the concepts examined in the study. In the probing HF question, Helen was able to recognize the concepts being assessed despite her initial misinterpretation of the problem. Initially, Helen chose the incorrect answer based on her literal interpretation of the word ‘share.’ This misconception was also reflected in a study by Luxford and Bretz (2014) in which students demonstrated a similar idea that there is equal sharing of electrons between atoms with slightly different electronegativities. Thus initially when questioned about the position of the shared pair in the HF molecule Helen responded:
Interviewer: So, on to the next question. Which of the following best represents the position of the shared electron pair in the HF molecule?
Helen: The position of it? Okay. This one [points HF molecule with the shared pair centrally located].
Interviewer: Okay. Now why did you choose that one?
Helen: Because it's [referencing shared electron pair] in the middle, and you can see that they're sharing it.
Interviewer: Okay. So what do you mean by that?
Helen: Honestly, I'm just going off of the word sharing. So well shared, and for me, I would write it in the middle to show that they're sharing it. And over here, it looks like this one, the F, has it more. Like it's just hogging it. And it's just for that and that this is on its own like they're two separate things.
Interviewer: Okay. Okay. So what is your reasoning [Turn over page and shows distractor answers]?
However, when Helen saw the distractors, the meaning of the question became clearer:
Helen: Okay, now that I see what you want [looks at the options and points to the word electronegativity] – well, I don't know. I'm going to put my own reasoning, but it's because how I took the question literally. Like, yeah. Not based off of how much one pulls electrons toward it. So I'm going to say because. But that's because – oh, because I said the first image doesn't seem like they are sharing the electrons. And that's because when I read the sentence, or you read the sentence, I thought you just meant literally does the image look like they share the electrons. But reading these, I think what you wanted more is to see if the F pulls the electrons more towards itself, or does the hydrogen pull them? Or do they share them equally?
Interviewer: So, what do you think, based on that interpretation?
Helen: Based on that, then I think it would be the first one [first picture in the problem] because F is more electronegative than the H. And then hydrogen only has one electron, and it's usually more positive.
A number of misconceptions were revealed during this interview and Table 5 below shows a summary of the three major electronegativity misconceptions revealed during the interviews along with an example of that code.
| Code name | Code description | Example |
|---|---|---|
| Valence electron determines electronegativity | The amount of valence electrons surrounding an atom determines how electronegative an atom will be | Angel: Well, the one single electron is taken from the hydrogen and shared with the F molecule. Since it's stronger… I mean, more electrons making it stronger than the hydrogen. |
| Larger equal more electronegative | The larger the atom the more electronegative | Harper: Fluorine? Fluorine is bigger, right? I think it's from physics: the greater a mass, the greater the attraction. So it does make sense too. |
| electronegativity has no effect on bonding | When molecules form a covalent bond, despite the presence of electronegativity, there is no effect on the position of the shared electron pair | Ana-Marie: Well, I know fluorine has a higher electronegativity than hydrogen, but I don't think that affects like the position…when you draw the Lewis structure, if one's stronger. you don't draw like a longer line because that one's stronger…I still feel like it would be this one because they're sharing it |
Conclusions
This study contributes to previous research on bonding misconceptions and on the use of concept mapping as an assessment and research tool. Some of the misconceptions presented have been documented in the literature; however this study is focused on students' knowledge structures. This factor is of particular importance in chemistry since individual concepts are inextricably connected. This work provides additional evidence that students can continue with flawed understanding and misconceptions beyond the general chemistry course, since all students interviewed were enrolled in organic chemistry. This study has allowed us to answer our two research questions.(1) How well can concept maps uncover students' knowledge structures regarding aspects of chemical bonding concepts?
In this study students sum concept map scores were an indication of how well they understood bonding concepts overall. The concept maps gave us insight into their overall knowledge structures and allowed us to pinpoint specific gaps in students' knowledge. For example students who had low scoring concept maps overall, also had specific problems understanding the concept of electronegativity itself or how electronegativity was linked to the polarity of a bond. Students understanding or lack thereof as indicated in their concept maps was corroborated by the explanations they gave when solving problems relating to these concepts. Therefore, we conclude that concept maps, to some extent, can uncover the students' knowledge structures regarding chemical bonding concepts.
(2) Are there differences in the knowledge structures between students with high scoring concept maps (HS) and students with low scoring concept maps (LS) regarding aspects of chemical bonding concepts?
The findings of the study reveal a distinction in the knowledge structures of LS students and HS students. More specifically, LS students had gaps in their understanding of the concept of electronegativity itself and also had difficulty connecting electronegativity to the concept of polar covalent bonding. These gaps were apparent in their concept map propositions and/or their inability to make any meaningful links between and among those concepts. In contrast, HS students were able to make meaningful relationship between the concepts of electronegativity and polar covalent bonding and other concepts. In addition, the concept map scores were reflected in their problem solving ability when addressing these concepts. HS students seemed to have a clearer understanding of electronegativity and polar covalent bonds, while LS students often presented flawed reasoning when trying to explain their incorrect answers. Table 6 compares HS students to LS students.
| Theme | High scoring students | Low scoring students |
|---|---|---|
| Electronegativity | Understood the periodic trend of electronegativity |
– Confused the periodic trend of electronegativity with size
– Attributed electronegativity to the number of valance electrons |
| Polar covalent bond | Associated bond polarity with electronegativity differences | Confused covalent bond with ionic bond |
| Effect of electronegativity on bond polarity | Understood that electronegativity affects the position of the shared pair in a covalent bond | Thought that electronegativity has no effect on the position of the shared electron pair in a covalent bond |
| Concept map construction | Made meaningful connections with the concepts of electronegativity and polar bond | Either made no connection or incorrect connections with the concepts of electronegativity and polar bond |
Implications for teaching
The findings of this study demonstrate that many students have difficulty making meaningful relationships betweem the concepts of electronegativity and polar covalent bonding. This concept is fundamental to chemical understanding and has implications for future courses such as organic chemistry and biochemistry. Therefore, this study has implications for what we teach and how we teach general chemistry.Examining students' prior knowledge in terms of their overall knowledge structures will help chemical educators design more meaningful curriculum materials. Concept maps can be used as a pre-assessment and formative assessment tool to analyze students' knowledge structures regarding a group of related concepts. Chemical educators can determine which concepts and connections need to be more explicitly taught and can address common misconceptions and knowledge gaps.
When considering general chemistry curriculum reform, chemistry instructors may need to consider spending more time focusing on fundamental concepts that are built upon and that need to be transferrable to other courses. It is important that students grasp these fundamental concepts and how concepts are linked together. There is certainly a need for more structured learning progression that focus on explicit transfer of concepts across courses and disciplines. Several authors have proposed the use of learning progression as a promising tool to design such a structured curriculum in chemistry (Boo and Watson, 2001; Johnson and Tymms, 2011; Cooper et al., 2012b; Cooper and Klymkowsky, 2013; Wolfson et al., 2014). Furthermore, to facilitate reform efforts increased conversations with general chemistry, organic chemistry and biochemistry instructors are essential to better coordinate and align the concepts that students need to be successful in these courses and to ensure that students can develop more coherent knowledge structures regarding fundamental topics.
We are using a similar research protocol to examine student knowledge structures regarding additional fundamental concepts such as molecular shape and acid–base chemistry. We are also expanding the sample size of our study so we can do more quantitative studies on how students' knowledge structures are related to their success in chemistry courses. We hope to use the research results as a springboard for designing a more meaningful curriculum for general chemistry.
Limitations of the study
This research was conducted with a small number of students (N = 16) at a large urban research university. Therefore, the research results and conclusions may have limited generalizability. The use of concept mapping has limitations, in that it may not reflect every connection that a student can make. Think-aloud interviews also have limitations because we may be unable to uncover the students' thoughts regarding particular concepts despite additional probing. However, in this study concept mapping was used in conjunction with think-aloud interviews to reduce some of the limitations that each method may have when used alone. Despite these limitations, this study provides general trends among students' conceptual understanding of the bonding concepts of electronegativity and polar bonding and opens the door for similar studies in other settings.References
- Ausubel D. P., (1978), Educational psychology: a cognitive view, New York: Holt Rinehart and Winston.
- Barbera J., (2013), A Psychometric Analysis of the Chemical Concepts Inventory, J. Chem. Educ., 90(5), 546–553.
- Bergqvist A., Drechsler M., De Jong O. and Rundgren S.-N. C., (2013), Representations of chemical bonding models in school textbooks – help or hindrance for understanding?, Chem. Educ. Res. Pract., 14(4), 589–606.
- Birk J. P. and Kurtz M. J., (1999), Effect of Experience on Retention and Elimination of Misconceptions about Molecular Structure and Bonding, J. Chem. Educ., 76(1), 124.
- Bodner G. M., (1986), Constructivism: a theory of knowledge, J. Chem. Educ., 63(10), 873.
- Boo H.-K. and Watson J. R., (2001), Progression in high school students' (aged 16–18) conceptualizations about chemical reactions in solution, Sci. Educ., 85(5), 568–585.
- Bowen C. W., (1994), Think-aloud methods in chemistry education: understanding student thinking, J. Chem. Educ., 71(3), 184.
- Bransford J. D., Brown A. L. and Cocking R. R., (2000), How people learn, Washington, DC: National Academy Press.
- Bretz S. L., (2001), Novak's Theory of Education: human Constructivism and Meaningful Learning, J. Chem. Educ., 78(8), 1107.
- Bretz S. L., (2008), Qualitative Research Designs in Chemistry Education Research, Nuts and Bolts of Chemical Education Research, American Chemical Society, pp. 79–99.
- Butts B. and Smith R., (1987), HSC chemistry students' understanding of the structure and properties of molecular and ionic compounds, Res. Sci. Educ., 17(1), 192–201.
- Calatayud M.-L., Barcenas S. L. and Furio-Mas C., (2007), Surveying Students' Conceptual and Procedural Knowledge of Acid-Base Behavior of Substances, J. Chem. Educ., 84(10), 1717.
- Cartrette D. P. and Mayo P. M., (2011), Students' understanding of acids/bases in organic chemistry contexts, Chem. Educ. Res. Pract., 12(1), 29–39.
- Chi M. T., (2005), Commonsense conceptions of emergent processes: why some misconceptions are robust, J. Learn. Sci., 14(2), 161–199.
- Chi M. T., (2008), Three types of conceptual change: Belief revision, mental model transformation, and categorical shift, International handbook of research on conceptual change, pp. 61–82.
- Coll R. K. and Taylor N., (2001), Alternative conceptions of chemical bonding held by upper secondary and tertiary students, Res. Sci. Tech. Educ., 19(2), 171–191.
- Coll R. K. and Treagust D. F., (2001), Learners' mental models of chemical bonding, Res. Sci. Educ., 31(3), 357–382.
- Coll R. K. and Treagust D. F., (2002), Exploring tertiary students' understanding of covalent bonding, Res. Sci. Tech. Educ., 20(2), 241–267.
- Cooper M., (2010), The Case for Reform of the Undergraduate General Chemistry Curriculum, J. Chem. Educ., 87(3), 231–232.
- Cooper M. and Klymkowsky M., (2013), Chemistry, Life, the Universe, and Everything: A New Approach to General Chemistry, and a Model for Curriculum Reform, J. Chem. Educ., 90(9), 1116–1122.
- Cooper M. M., Grove N., Underwood S. M. and Klymkowsky M. W., (2010), Lost in Lewis Structures: An Investigation of Student Difficulties in Developing Representational Competence, J. Chem. Educ., 87(8), 869–874.
- Cooper M. M., Underwood S. M. and Hilley C. Z., (2012a), Development and validation of the implicit information from Lewis structures instrument (IILSI): do students connect structures with properties? Chem. Educ. Res. Pract., 13(3), 195–200.
- Cooper M. M., Underwood S. M., Hilley C. Z. and Klymkowsky M. W., (2012b), Development and assessment of a molecular structure and properties learning progression, J. Chem. Educ., 89(11), 1351–1357.
- Corbin J. and Strauss A., (2008), Basics of qualitative research: Techniques and procedures for developing grounded theory, Los Angeles: Sage.
- Cracolice M. S., Deming J. C. and Ehlert B., (2008), Concept learning versus problem solving: a cognitive difference, J. Chem. Educ., 85(6), 873.
- diSessa A., (2008), A bird's-eye view of the “pieces” vs. “coherence” controversy (from the “pieces” side of the fence), International handbook of research on conceptual change, pp. 35–60.
- diSessa A. A. and Sherin B. L., (1998), What changes in conceptual change? Int. J. Sci. Educ., 20(10), 1155–1191.
- Ericsson K. A. and Simon H. A., (1998), How to Study Thinking in Everyday Life: Contrasting Think-Aloud Protocols With Descriptions and Explanations of Thinking, Mind Cult. Act., 5(3), 178–186.
- Francisco J. S., Nakhleh M. B., Nurrenbern S. C. and Miller M. L., (2002), Assessing student understanding of general chemistry with concept mapping, J. Chem. Educ., 79(2), 248.
- Gillespie R. G., (1997), Commentary: reforming the general chemistry textbook, J. Chem. Educ., 74(5), 484.
- Glaser B. G. and Strauss A. L., (1967), The Discovery of Grounded Theory: Strategies for Qualitative Research.
- Greene B., Lubin I., Slater J. and Walden S., (2013), Mapping Changes in Science Teachers' Content Knowledge: Concept Maps and Authentic Professional Development, J. Sci. Educ. Technol., 1–13.
- Harrison A. G. and Treagust D. F., (2000), Learning about atoms, molecules, and chemical bonds: a case study of multiple-model use in grade 11 chemistry, Sci. Educ., 84(3), 352–381.
- Hay D., Kinchin I. and Lygo-Baker S., (2008), Making learning visible: the role of concept mapping in higher education, Stud. High. Educ., 33(3), 295–311.
- Holme T., Bretz S. L., Cooper M., Lewis J., Paek P., Pienta N., Stacy A., Stevens R. and Towns M., (2010), Enhancing the role of assessment in curriculum reform in chemistry, Chem. Educ. Res. Pract., 11(2), 92–97.
- IHMC, (2013), CMap tools January 27, 2013.
- Johnson P. and Tymms P., (2011), The emergence of a learning progression in middle school chemistry, J. Res. Sci. Teach., 48(8), 849–877.
- Krause S., Birk J., Bauer R. Jenkins B. and Pavelich M. J., (2004), Development, testing, and application of a chemistry concept inventory, Presented at Frontiers in Education, FIE 2004. 34th Annual.
- Levy Nahum T., Mamlok-Naaman R., Hofstein A. and Taber K. S., (2010), Teaching and learning the concept of chemical bonding, Stud. Sci. Educ., 46(2), 179–207.
- Libarkin J., (2008), Concept inventories in higher education science. Prepared for the National Research Council Promising Practices in Undergraduate STEM Education Workshop 2, (Washington, DC). http://www.nationalacademies.org/bose/PP_Commissioned_Papers.html.
- Lin J.-W. and Chiu M.-H., (2007), Exploring the characteristics and diverse sources of students mental models of acids and bases, Int. J. Sci. Educ., 29(6), 771–803.
- Lloyd B. W. and Spencer J. N., (1994), The Forum: New Directions for General Chemistry: recommendations of the Task Force on the General Chemistry Curriculum, J. Chem. Educ., 71(3), 206.
- Lopez E., Kim J., Nandagopal K., Cardin N., Shavelson R. J. and Penn J. H., (2011), Validating the use of concept-mapping as a diagnostic assessment tool in organic chemistry: implications for teaching, Chem. Educ. Res. Pract., 12(2), 133–141.
- Lopez E. J., Shavelson R. J., Nandagopal K., Szu E. and Penn J., (2014), Factors Contributing to Problem-Solving Performance in First-Semester Organic Chemistry, J. Chem. Educ., 91(7), 976–981.
- Luxford C. J. and Bretz S. L., (2014), Development of the Bonding Representations Inventory To Identify Student Misconceptions about Covalent and Ionic Bonding Representations, J. Chem. Educ., 91(3), 312–320.
- Markham K. M., Mintzes J. J. and Jones M. G., (1994), The concept map as a research and evaluation tool: further evidence of validity, J. Res. Sci. Teach., 31(1), 91–101.
- Markow P. G. and Lonning R. A., (1998), Usefulness of concept maps in college chemistry laboratories: students' perceptions and effects on achievement, J. Res. Sci. Teach., 35(9), 1015–1029.
- Mason D. S., Shell D. F. and Crawley F. E., (1997), Differences in problem solving by nonscience majors in introductory chemistry on paired algorithmic-conceptual problems, J. Res. Sci. Teach., 34(9), 905–923.
- McClary L. and Talanquer V., (2011a), College chemistry students' mental models of acids and acid strength, J. Res. Sci. Teach., 48(4), 396–413.
- McClary L. and Talanquer V., (2011b), Heuristic reasoning in chemistry: making decisions about acid strength, Int. J. Sci. Educ., 33(10), 1433–1454.
- McClary L. M. and Bretz S. L., (2012), Development and assessment of a diagnostic tool to identify organic chemistry students' alternative conceptions related to acid strength, Int. J. Sci. Educ., 34(15), 2317–2341.
- McMurry J., (2007), Organic Chemistry, Boston: Cengage Learning.
- Nahum T. L., Mamlok-Naaman R., Hofstein A. and Krajcik J., (2007), Developing a new teaching approach for the chemical bonding concept aligned with current scientific and pedagogical knowledge, Sci. Educ., 91, 579.
- Nakhleh M. B., (1993), Are Our Students Conceptual Thinkers or Algorithmic Problem Solvers? Identifying Conceptual Students in General Chemistry, J. Chem. Educ., 70(1), 52.
- Nakhleh M. B. and Krajcik J. S., (1994), Influence of levels of information as presented by different technologies on students' understanding of acid, base, and pH concepts, J. Res. Sci. Teach., 31(10), 1077–1096.
- Nakhleh M. B. and Mitchell R. C., (1993), Concept learning versus problem solving: there is a difference, J. Chem. Educ., 70(3), 190.
- Niaz M., (2001), A rational reconstruction of the origin of the covalent bond and its implications for general chemistry textbooks, Int. J. Sci. Educ., 23(6), 623–641.
- Nicoll G., (2001a), A report of undergraduates' bonding misconceptions, Int. J. Sci. Educ., 23(7), 707–730.
- Nicoll G., (2001b), A report of undergraduates' bonding misconceptions, Int. J. Sci. Educ., 23(7), 707–730.
- Nicoll G., (2003), A Qualitative Investigation of Undergraduate Chemistry Students' Macroscopic Interpretations of the Submicroscopic Structures of Molecules, J. Chem. Educ., 80(2), 205.
- Nicoll G., Francisco J. S. and Nakhleh M., (2001), An investigation of the value of using concept maps in general chemistry, J. Chem. Educ., 78(8), 1111.
- Novak J. D., (2010), Learning, Creating, and Using Knowledge: Concept Maps as Facilitative Tools in Schools and Corporations, Routledge.
- Novak J. D. and Cañas A. J., (2006), The theory underlying concept maps and how to construct and use them, Technical Report IHMC Cmap Tools 2006-01 Rev 01-2008, Florida Institute for Human and Machine Cognition, 2008.
- Novak J. D. and Gowin B. D., (1984), Learning how to learn, New York: Cambridge University Press.
- Othman J., Treagust D. F. and Chandrasegaran A. L., (2008), An investigation into the relationship between students' conceptions of the particulate nature of matter and their understanding of chemical bonding, Int. J. Sci. Educ., 30(11), 1531–1550.
- Özmen H., (2004), Some Student Misconceptions in Chemistry: A Literature Review of Chemical Bonding, J. Sci. Educ. Technol., 13(2), 147–159.
- Patton M. Q., (2002), Qualitative Research and Evaluation Methods, Thousand Oaks, California, USA: SAGE Publications.
- Pavelich M., Jenkins B., Birk J., Bauer R. and Krause C., (2004), Development of a chemistry concept inventory for use in chemistry, materials and other engineering courses, Presented at Proceedings of the 2004 American Society for Engineering Annual Conference & Exposition, Paper.
- Pendley B. D., Bretz R. L. and Novak J. D., (1994), Concept maps as a tool to assess learning in chemistry, J. Chem. Educ., 71(1), 9.
- Peterson R. F. and Treagust D. F., (1989), Grade-12 students' misconceptions of covalent bonding and structure, J. Chem. Educ., 66(6), 459.
- Peterson R., Treagust D. and Garnett P., (1986), Identification of secondary students' misconceptions of covalent bonding and structure concepts using a diagnostic instrument, Res. Sci. Educ., 16(1), 40–48.
- Peterson R. F., Treagust D. F. and Garnett P., (1989), Development and application of a diagnostic instrument to evaluate grade-11 and -12 students' concepts of covalent bonding and structure following a course of instruction, J. Res. Sci. Teach., 26(4), 301–314.
- Pickering M., (1990), Further studies on concept learning versus problem solving, J. Chem. Educ., 67(3), 254.
- Plotnick E., (1997), Concept mapping: a graphical system for understanding the relationship between concepts: an ERIC digest, Clearinghouse on Information & Technology.
- Robinson W. R., (1998), An alternative framework for chemical bonding, J. Chem. Educ., 75(9), 1074.
- Ross B. and Munby H., (1991), Concept mapping and misconceptions: a study of high-school students' understandings of acids and bases, Int. J. Sci. Educ., 13(1), 11–23.
- Ruiz-Primo M. A., Schultz S. E., Li M. and Shavelson R. J., (2001a), Comparison of the reliability and validity of scores from two concept-mapping techniques, J. Res. Sci. Teach., 38(2), 260–278.
- Ruiz-Primo M. A., Shavelson R. J., Li M. and Schultz S. E., (2001b), On the Validity of Cognitive Interpretations of Scores From Alternative Concept-Mapping Techniques, Educ. Assess., 7(2), 99–141.
- Sawrey B. A., (1990), Concept learning versus problem solving: revisited, J. Chem. Educ., 67(3), 253.
- Shavelson R. J., (1993), On Concept Maps as Potential “Authentic” Assessments in Science. Indirect Approaches to Knowledge Representation of High School Science, Los Angeles: National Center for Research on Evaluation, Standards, and Student Testing.
- Shavelson R. J., Ruiz-Primo M. A. and Wiley E. W., (2005), Windows into the mind, High. Educ., 49(4), 413–430.
- Singer S. R., Nielsen N. R. and Schweingruber H. A., (2012), Discipline-Based Education Research, National Academies Press.
- Szu E., Nandagopal K., Shavelson R. J., Lopez E. J., Penn J. H., Scharberg M. and Hill G. W., (2011), Understanding Academic Performance in Organic Chemistry, J. Chem. Educ., 88(9), 1238–1242.
- Taber K. S., (2002), Chemical Misconceptions—Prevention, Diagnosis and Cure, vol. I: Theoretical Background.
- Taber K. S. and Coll R. K., (2003), Bonding, Chemical education: Towards research-based practice, Springer, pp. 213–234.
- Taber K. S., Tsaparlis G. and Nakiboğlu C., (2012), Student conceptions of ionic bonding: patterns of thinking across three European contexts, Int. J. Sci. Educ., 34(18), 2843–2873.
- Tan K.-C. D. and Treagust D. F., (1999), Evaluating Students' Understanding of Chemical Bonding, Sch. Sci. Rev., 81(294), 75–83.
- Teichert M. A. and Stacy A. M., (2002), Promoting understanding of chemical bonding and spontaneity through student explanation and integration of ideas, J. Res. Sci. Teach., 39(6), 464–496.
- Tro N. J., (2010), Principles of chemistry: a molecular approach, New York: Prentice Hall.
- Van Zele E., Lenaerts J. and Wieme W., (2004), Improving the usefulness of concept maps as a research tool for science education, Int. J. Sci. Educ., 26(9), 1043–1064.
- Vosniadou S., (1994), Capturing and modeling the process of conceptual change, Learn. Instr., 4(1), 45–69.
- Wheeldon J. P. and Faubert J., (2009), Framing experience: concept maps, mind maps, and data collection in qualitative research, Int. J. Qual. Meth., 8(3), 52–67.
- Wolfson A. J., Rowland S. L., Lawrie G. A. and Wright A. H., (2014), Student conceptions about energy transformations: progression from general chemistry to biochemistry, Chem. Educ. Res. Pract., 15(2), 168–183.
- Yin Y., Vanides J., Ruiz-Primo M. A., Ayala C. C. and Shavelson R. J., (2005), Comparison of two concept-mapping techniques: implications for scoring, interpretation, and use, J. Res. Sci. Teach., 42(2), 166–184.
| This journal is © The Royal Society of Chemistry 2015 |